The unsolved problem of how the pollen and pistil components of angiosperm self-incompatibility (SI) are inherited has a long history (Lewis, 1960). A recent paper provides evidence, based on transgenic experiments in Solanum chacoense, that a single amino acid difference between proteins encoded by two very similar SI-specifying (S) alleles can result in plants that reject the pollen of both alleles (Matton et al., 1999). The authors propose that this result may help solve the difficult problem of how new S allele specificities could arise over evolutionary time if there are separate (but linked) loci for pollen and pistil specificities. The purpose of the present Letter is to suggest, however, that this proposal is implausible.
The first step in the proposed pathway from one allele (Sx) to a new functional allele (Sy) could be a change in the pistil component of recognition, from an allele that recognizes its own corresponding pollen specificity (Sx), to a dual-function allele (designated SxyF for specificities x and y in the female function) that recognizes both Sy and Sx pollen. The Sy component of such a dual-recognition allele should be effectively “neutral” to the extent that no corresponding SyM (male) function would preexist in the population. As pointed out by Matton et al. (1999), the SxyF allele could therefore persist in the population, and would not suffer the evident disadvantage (in a two-gene system) that a changed pollen or pistil specificity, without a change in the other component, would simply cause self-compatibility (Charlesworth, 1995). Matton et al. (1999) propose that this first change, to dual specificity, might later be followed by changes in the male function, creating a new specificity haplotype with female and male alleles SxyF and SyM, respectively. Finally, loss of the dual specificity by replacement of SxyF by SyF could lead to a fully functional system of SI based on the novel S haplotype, SyF–SyM. (The opposite order of the changes, i.e., pollen reaction changed first, followed by changed pistil reaction, would also be possible, and everything below can also be applied to this version.)
On closer examination, this attractive scenario appears less easy to accept. Consider a two-locus model, as hypothesized by Matton et al. (1999). In a population in which the first change has occurred, so that the population contains both the initial SxF–SxM haplotype and the new SxyF–SxM one, the requisite change to generate SyM must subsequently happen in the very same haplotype that carries the SxyF allele at the female function gene. Otherwise, if the SyM allele appeared in a different haplotype, say the Sa haplotype, the new “y-type” specificity would encounter two disadvantages. Table 1 shows how the model of Matton et al. (1999) would behave in this case and illustrates the difficulties. First, SaF–SyM plants would have the disadvantage of being self-compatible; the disadvantage that new specificities cause loss of self-incompatibility thus appears at this stage of the evolutionary process, rather than at the first step, and is not eliminated by allowing dual-specificity alleles. Second, the new SyM pollen would be incompatible with unrelated plants carrying SxyF, leading to lower fertility for this pollen type than for other pollen types. (SxM would also manifest this problem, but would confer self-incompatibility.) Thus, SyM would be a cross-incompatibility allele, not a new SI allele, and would be more likely to be eliminated from the population than to be selectively advantageous. In view of the two disadvantages of the SaF–SyM haplotype, it seems that SyM could be an evolutionary successful mutation only if it were to occur in the haplotype that carries the SxyF allele. It is therefore unnecessary to discuss mechanisms by which the two “y-type” components might subsequently be brought together into a single SyF–SyM haplotype.
Table 1.
Step-Wise Process leading to New SI Specificitya
| Step No. | Mutation | Haplotype | Phenotype |
|---|---|---|---|
| SxF−SxM | Self-incompatible (specificity x), cross-compatible with all non-x alleles | ||
| 1 | SxF⇒SxyF | ⇓ | |
| SxyF−SxM | Self-incompatible (specificity x), cross-compatible with all non-x alleles | ||
| 2 | SaM⇒SyM | ⇓ | |
| SaF−SyM | Self-compatible, pistil cross-compatible with all non-a alleles, but pollen incompatible with SxyF | ||
| 3 | SaF⇒SyF | ⇓ | |
| SyF−SyM | Self-incompatible (specificity y), cross-compatible with all non-y alleles, complete new specificity |
An evolutionary model for SI is depicted. The model assumes separate but tightly linked pollen and pistil genes; note that the second mutation does not occur in the same haplotype as the first (but in a haplotype with pistil allele SaF).
Can we then envisage the evolution of an SyF–SyM haplotype by assuming that the SyM mutation arises from the SxyF–SxM haplotype? This would produce self-incompatibility and cross-compatibility, as required, but the difficulties are not eliminated. We still have a process requiring three successive mutations all within the same haplotype (loci affected shown in bold):
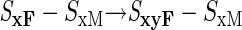 |
(1) |
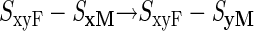 |
(2) |
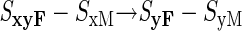 |
(3) |
Note that the second of these mutations must create a male determinant that is rejected by the pistil y specificity of the haplotype (but not by its x specificity, because the new haplotype ultimately generated by these mutations should not be rejected by pistils with x specificity). The other two mutations (steps 1 and 3), moreover, must both occur in the same gene. This might be plausible, given the large amounts of time available for self-incompatibility to evolve, but it is difficult to accept that this process could occur for each new specificity, given the very high numbers of specificities in some species (sometimes as many as a hundred or more; e.g., Bernatzky et al., 1988; Okazaki et al., 1997).
Given the mounting evidence that separate pollen and pistil genes exist in a self-incompatible Brassica species (Schopfer et al., 1999), along with the clear implication of two-gene systems in fugal incompatibility (see Casselton, 1997, 1998), there is a pressing need to solve the puzzle of how new specificities arise. It seems, however, that the possibility of dual specificities does not provide an easy solution to this puzzle.
References
- Bernatzky, R., Anderson, M.A., and Clarke, A.E. (1988). Molecular genetics of self-incompatibility in flowering plants. Dev. Genet. 9 1–12. [Google Scholar]
- Casselton, L.A. (1997). Molecular recognition in fungal mating. Endeavour 21 159–163. [DOI] [PubMed] [Google Scholar]
- Casselton, L.A. (1998). Molecular genetics of mating recognition in Basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, D. (1960). Genetic control of specificity and activity of the S antigen in plants. Proc. Roy. Soc. Lond. B 151 468–477. [DOI] [PubMed] [Google Scholar]
- Matton, D.P., Luu, D.T., Xike, Q., Laublin, G., O'Brien, M., Maes, O., Morse, D., and Cappadocia, M. (1999). Production of an S RNase with dual specificity suggests a novel hypothesis for the generation of new S alleles. Plant Cell 11 2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, K., Kusaba, M., Ockendon, D., and Nishio, T. (1997). Characterization of S-tester lines in Brassica oleracea: polymorphism of restriction fragment length of SLG homologues and isoelectric points of S-locus glycoproteins. Theor. Appl. Genet. 98 1329–1334. [Google Scholar]
- Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (1999). The male determinant of self-incompatibility in Brassica. Science 286 1697–1700. [DOI] [PubMed] [Google Scholar]


