Abstract
Border zone disorders involve neurological disorders with psychiatric symptoms and signs as well as psychiatric disorders with soft neurological features. This becomes a cause for great diagnostic and therapeutic concerns. We, in this paper, analyzed some of the imitators such as epilepsy, dementia, some forms of encephalitis, and pure psychiatric diseases which produce problems in decision making due to soft neurological features and the utility of electroencephalography (EEG) as a simple diagnostic tool in differentiating some of these conditions from each other as well as the therapeutic role of EEG in some of these disorders. We retrospectively took index cases which produced problems for us in decision making in the last 5 years and correlated with the final diagnosis, EEG parameters as well as literature available by PubMed search using specific key words based on the conditions identified. EEG can be normal in organic diseases and abnormal in psychiatric diseases. Typical EEG findings in neuropsychiatric syndromes point to specific diagnosis. Soft EEG changes are common in psychiatric disorders and do not indicate organicity. EEG can be used to assess efficacy and toxicity of therapeutic agents in psychiatry. Biofeedback-based training to keep the brain in particular rhythm is of use in psychiatric disorders as a pharmaco-sparing agent.
Keywords: Electroencephalography, neuropsychiatric border zone syndromes, therapeutics
INTRODUCTION
Human brain is an amazing creation of divinity and as Hippocrates has mentioned “man ought to know that from nothing else but the brain comes emotions and their disorders.” When they occur without associated structural changes, it becomes difficult to decide if it is organic or nonorganic. Psychiatric symptoms caused by organic brain disorders could be called “neuropsychiatric border zone symptoms.” Cognition is the process by which we understand and affect is the way by which we feel about the various happenings.[1] The brain is a well-connected amazing structure and breaking down of any part of connectivity can result in a group of border zone symptoms which are difficult to characterize. Electroencephalography (EEG) being a functional assessment tool it is of relevance in the assessment of these disorders. Changes in emotions and behavior are considered an inherent part of psychiatric disorders but are mainly a limbic function modified by social and cultural practices of a particular society. In Vedic literature, mind is a channel between the material world and universal consciousness. Sense organs act as tentacles to scan the world and feed the superficial mind. The deeper part links with universal consciousness. “Chintha” is the process to think, “Vicharya” is to accept or reject. “Uhya” is logic, “Dhyeya” is emotion and “Sankalpa” is a decision and these are triaging instruments of the mind. Its experiences may be measurable in a macro scale and stretches to abstractness.[3,4,5] Individual variations depend on development, environment, epigenetic and genetics. Instinct-based impulsive behavior is a limbic function. With the development of mirror neurons and rapid ascend in the evolutionary scale cortical monitoring and inhibitions over limbic functions helped to differentiate human behavior from other animals and primates.
NEURAL BASIS OF PSYCHOPATHOLOGY
The existence of dedicated emotion circuits in the brain was postulated by Damasio et al. in 2000.[2] Limbic system is a highly connected region of the brain involving structures around the interventricular foramen and extends up to brainstem. Dorsolateral prefrontal cortex monitors its function with reference to culture based social cognition and behavior. Amygdala, ventral striatum including nucleus accumbens, insula, anterior cingulate cortex, medial prefrontal cortex, and orbitofrontal cortex form key structures in the emotion processing circuit. This involves detection and evaluation of salient stimuli, experience, and regulation of affective response. Cortical monitoring of limbic functions is not only circuit based but also chemical. Serotonergic circuits in prefrontal cortex when dysfunctional cause aggression.[6] Central nucleus of amygdala to nucleus pontine reticularis is called as the “startle circuit.” Fear is appreciated better by amygdala, anger by orbitofrontal cortex and nucleus accumbens. Circuits are involved in automatic emotional regulatory responses. Ventromedial prefrontal cortex is involved in reverse learning based on previous experience. Suppression of negative responses is by inhibitory connections from prefrontal cortex to amygdala. Disruption of serotonin system is linked to aggression and violence. Abnormal adaptive circuits cause abnormal emotions.[6]
NEUROPSYCHIATRIC BORDER ZONE SYMPTOMS
These are situations where one is not sure if a particular symptom is organic or nonorganic because of the nature of the phenomena where psychiatric symptoms are the result of neurological insult or psychiatric disorders where soft electrophysiological features confuse the diagnosis.
Organic disorders which are sometimes capable of producing border zone symptoms are epilepsy, dementia, delirium, immune-mediated encephalopathies, prion disease and slow virus diseases.
There are also the phenomena of “over read EEGs” in patients with psychiatric illness and carry serious consequences for patient. Therefore correlating with clinical picture is very important.
BORDER ZONE SYMPTOMS IN EPILEPSY
Charaka Samhita chapters 8 and 10 characterizes epilepsy as Abasmara and classifies as Akshepaka (simple type), Apantantraka (status epilepticus). Daruna apatanraka (psychogenic). He also described Purvarupa or aura and Rupa as the ictus itself. Complex partial type is described as “Pitta” dominant and as refractory “sannipataja” type.[7,8] Epilepsy for a long time in history remained a psychiatric nosology until it was radically redefined by Hugling Jackson. Brutus and Casca, who plotted to murder Caesar had the falling sickness and were labeled lunatics. Still epilepsy sits firmly as a bridge zone disease in view of its close associations with psychiatric symptomatology.[9]
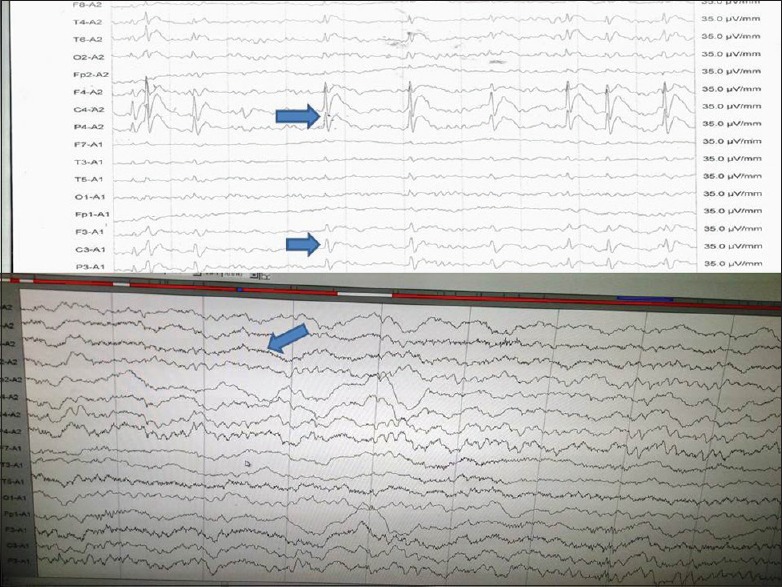
Border zone symptoms are commonly a cause of concern in localization-related epilepsy and in patients with nonepileptic attack disorders. Patients with nonepileptic attacks also can have true seizures, major or minor mental illness, etc., In general, ictal EEG [Figure 1] is useful in this situation apart from other features like females in the early 2nd decade. Attacks are commonly seen in the presence of others, mostly during wakefulness or just on falling asleep not deep sleep retained inner awareness, out of phase movements, pelvic thrust, absence of eye signs or closed eyes, no incontinence, and injury at the tip of tongue not sides, etc., Ictal EEG in true seizures shows beta fast activity, spikes or spikes and waves starting from the area of onset. It builds up in amplitude and frequency and becomes generalized, whereas in nonepileptic attacks, it will show movement artifacts only.
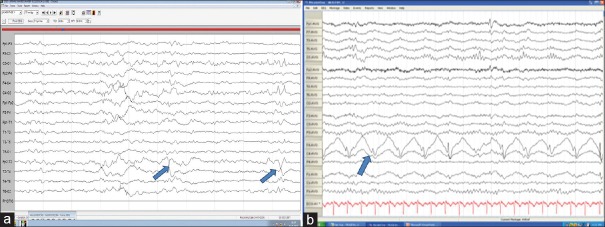
Figure 1.
Electroencephalography shows ictal build up from T2 with increasing frequency and amplitude and generalization (blue arrow)
FRONTAL LOBE SEIZURES AND BORDER ZONE SYMPTOMS
They may be confused with nonepileptic attacks, parasomnias, or behavioral disorder in view of the semiology but the clue will be stereotyped and nocturnal attacks. With dominant hemisphere involvement they repeatedly call names of Gods, speak illegible words, laughing, screaming, and swearing also occurs.[9] Supplementary motor area involvement produces ipsilateral, contralateral or bilateral tonic posturing of arms and legs in adduction with moan or cry and preserved consciousness. Facial grimacing, violent kicking, pelvic thrust, laughing, rolling over, rocking, arching, pedaling movements, thrashing, gestural automatisms and complex behavior, whistling, retching, crying, laughing, and bizarre attacks is seen in mesial frontal, cingulate, orbitofrontal, and frontopolar origin.[10] Figure of four appearances of limbs, coprolalia, genital manipulation without postictal drowsiness and some attacks are indistinguishable from parasomnias.[11,12]
Forced thinking can occur in frontopolar foci, olfactory hallucination, gestural automatisms, unformed or formed speech is seen in orbitofrontal, fear, mastication, salivation, laryngeal symptoms in opercular region, etc., EEG both ictal and interictal is helpful by showing features of focal slow waves, spike and waves, phase reversals, etc., [Figure 2]. Mutations in cholinergic receptor nicotinic alpha 4 subunit (CHRNA4) in chromosome 21q13 and 1q21 (CHRNB2 manifests as autosomal dominant frontal lobe seizures which present with gasping, groaning, vocalization, etc.)
Figure 2.
(a) Medial temporal epilepsy with phase reversals across T2 (blue arrow). (b) Frontal PLEDS (blue arrow)
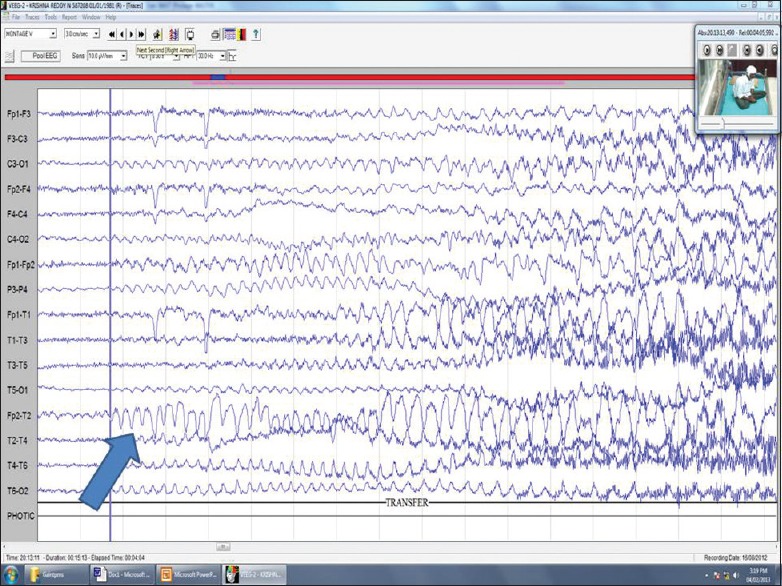
TEMPORAL LOBE SEIZURES
Nonconvulsive status epilepticus can present as prolonged twilight state with partial responsiveness, impaired speech, quasi-purposive automatisms etc.[13] The aura which is the earliest part of the seizure for which awareness is retained can manifest as anxiety, panic, Deja vu-a state of forced thinking, Jamai vu, Deja vecu, Deja etendu where familiar places are distressingly misfeldt as unfamiliar or vice versa, feeling of having heard some words before or having lived through an era. Simple partial seizures can just present as abrupt onset psychic symptoms with abrupt subsidence. Ictal and interictal rage is common. Bursts of laughter without mirth are called gelastic attacks, cry without sadness is dacrytic attack. Anterior temporal epilepsy can just manifest as episodes of strange smells, mesial temporal epilepsy can present with olfactory, gustatory hallucinations, fear, pallor, fullness of face, flushing, nausea, autonomic features, hyperventilation, postictal cough, and EEG shows anterior temporal changes in the form of phase reversals, spikes, slow waves or spike and waves. BiPLED and PLED's can also be seen [Figure 2a and b]. Landau–Kleffner syndrome is a condition where patients present with mute state due to ictal aphasia. Imaging will be normal when a structural pathology in the dominant hemispheres is suspected but EEG is diagnostic showing dominant discharges over the left hemisphere during wakefulness and generalized discharges during sleep [Figure 3]. Lateral temporal epilepsies present with auditory hallucinations, illusion, dreamy state, visual misperceptions and language changes in the dominant side. EEG shows unilateral or bilateral spikes in the middle or posterior temporal leads. Autosomal Dominant Partial Epilepsy With Auditory Features is a special seizure type which presents with hearing unformed sounds, buzzing, change in volume or pitch of sounds or formed sounds like sounds of specific singers or sounds from the past.[12] This is linked to LG11 gene and shows discharges in temporal or occipital leads.
Figure 3.
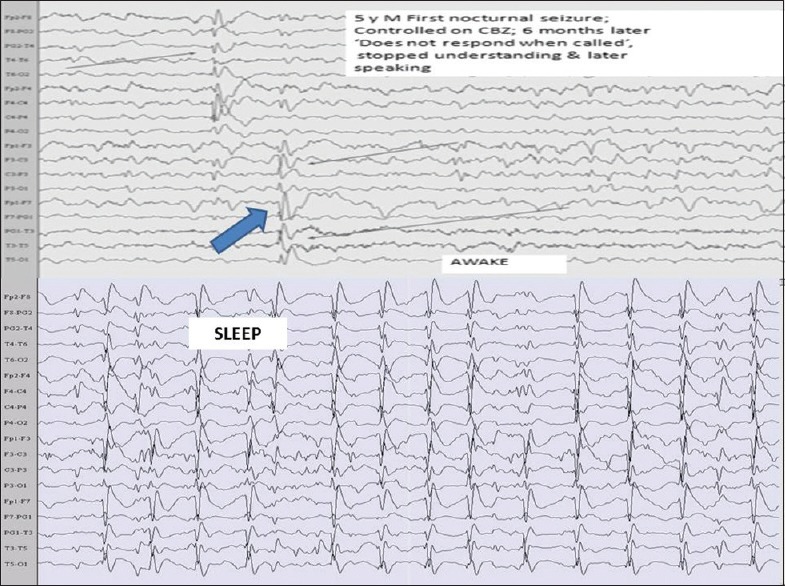
Landau–Kleffner syndrome showing dominant focal discharges on the left side during wakefulness which generalizes during sleep (blue arrow)
PARIETAL LOBE SEIZURES
Anterior parietal lobe seizures can present with tingling, electricity like feeling, desire to move a body part or that a body part is being moved, loss of awareness of a part or asomatagnosia, macrosomatognosia (feeling big), microsomatognosia (feeling a body part small), elongated hyperschematica, shortened or hyperschematica, supernumerary limbs, etc., Face or tongue pain, torturing or stabbing feeling and pilomotor attacks characterized by episodes of gooseflesh, etc., along with formed hallucinations, metamorphopsia, and confusion in posterior parietal lobe; vertigo, disorientation in time and abdominal sensation in inferior parietal lobe; paracentral seizures can present as contralateral genital sensations, etc., Repetitive language disturbance can happen in dominant side, gustatory hallucinations in operculum, psychoparetic limb characterized by feeling of inability to move the limb occurs in suprasylvian region. EEG shows changes in parietal region or can be normal [Figure 4].
Figure 4.
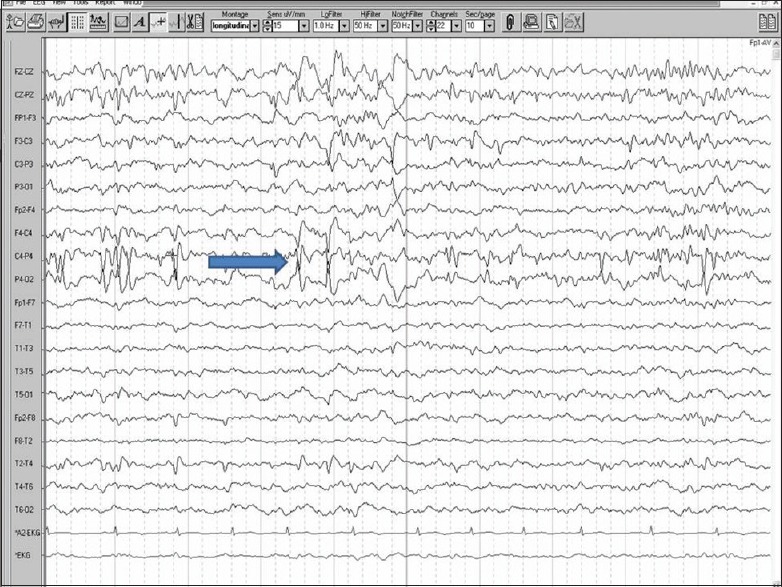
Electroencephalography showing epileptic discharges in the parietal leads (blue arrow)
OCCIPITAL LOBE EPILEPSY
Seeing bright or colored lights, amaurosis, scotomas, change in size and shape of objects, formed scenes like seeing movies, teleopsia is feeling of inappropriate orientation of objects, epileptic autoscopy where mirror image of one's own self is seen, etc., which are episodic. Figure 5 shows epileptiform abnormalities in occipital region.
Figure 5.
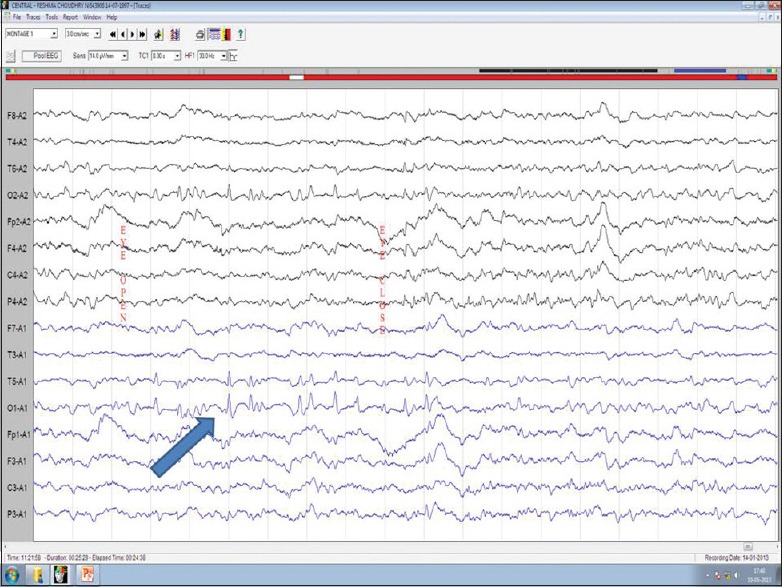
Electroencephalography showing epileptic discharges across occipital leads (blue arrow)
BORDER ZONE SYMPTOMS IN DEMENTIA AND ROLE OF ELECTROENCEPHALOGRAPHY
Frontotemporal dementia
As memory impairment is relatively less (11.1% in our study and 25% in literature) and severe psychiatric symptoms are seen early, these patients do not qualify for dementia as per Diagnostic and Statistical Manual of Mental Disorders-IV in the early stages. This results in significant diagnostic delay resulting in problems in treatment and prognostication. The incidence of aggression and agitation is seen in 85.7%, apathy 35%; disinhibition 50%. Stereotyped behavior is also seen in 25%, eating problems, delusions, and paranoia and a spectrum of environmental dependency related features such as hoarding, utilization, imitation, hypersexual, antisocial obsessed and wandering, etc., This interesting combination of neuropsychological symptoms makes it more difficult to distinguish organicity from purely late-onset psychosis in these patients’. Neuropsychiatric symptoms in AD are not uncommon. Helkala et al. (1991) reported greater decline in praxis functions in Alzheimer's disease patients with abnormal EEG early in the course of illness and most apraxias are often mistaken as psychological as patients hold common objects as if they have never held them before and show strange behavior and get lost in familiar environment and confuse with dressing. Application of Jonkman score clearly establishes organicity in this situation in a simple cost-effective manner.[14,15]
The well-known EEG abnormalities in Alzheimer's disease include slowing of the dominant posterior alpha rhythm; appearance of theta and delta activity; and generalized bursts of slow activity that are usually maximal in frontal and temporal leads.[15] EEG changes in cortical dementia (both Alzheimer's disease and frontotemporal dementia) shows correlation between EEG abnormalities, clinical severity of dementia and global grey matter volume assessed by volumetric magnetic resonance imaging of brain.[15] Psychiatric symptoms are presenting features in Creutzfeldt–Jakob disease (CJD) variant and sporadic type. Eighty percent of the cases demonstrated psychiatric symptoms depression, anxiety, psychosis, behavior dyscontrol, sleep disturbances within the first 100 days of illness, with 26% occurring at presentation occurring before formal diagnosis and EEG is diagnostic [Figure 6].
Figure 6.
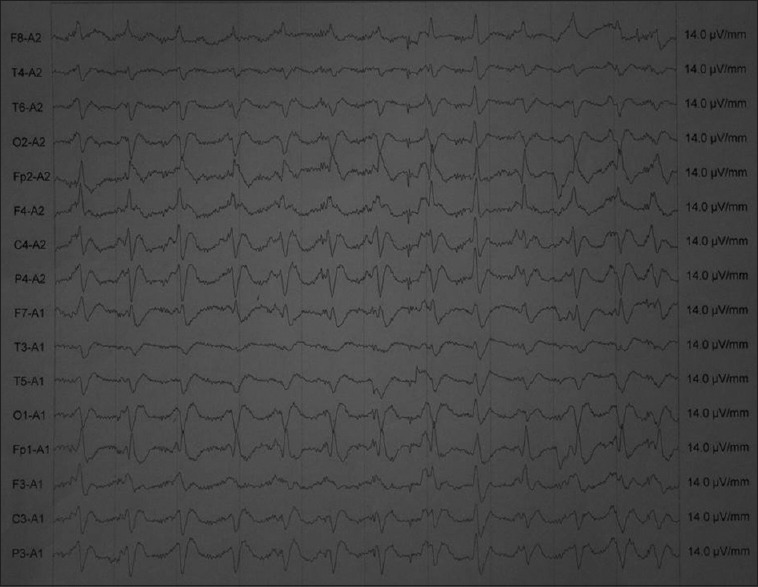
Electroencephalography showing 1/s discharges typical of Creutzfeldt–Jakob disease (blue arrow)
Diffuse Lewy body disease
Sustained periods of staring into space, decreased attention, improved memory, episodes of disorganized speech, dramatic fluctuations and recurrent visual hallucinations of animals and humans is seen. EEG shows low frequency, low amplitude poorly reactive alpha.
Contingent negative variation
Richard Caton in 1875 first used the term negative variation while describing electrical activity of gray matter, while Walter (1964) coined the term contingent negative variation (CNV). During expectancy and response preparation a slowly rising negative shift appears before stimulus onset. The first stimulus gives a preparatory signal for a motor response to be carried out at the time of a second stimulus. The phenomena of expectation producing a documentable wave of unknown origin. CNV can be elicited by a standard reaction time paradigm (S1–S2-motor response) or only by paired stimuli without any motor response (S1–S2 paradigm). The first stimulus (S1) is a preparatory signal for the imperative stimulus (S2) to which the patient makes a response. In the S1–S2 interval, there are early and late CNV components. Early CNV indicates arousal processes, and late CNV attention to the experimental task. Presence of the expectancy wave or CNV indicates pseudodementia.[16]
Limbic encephalitis
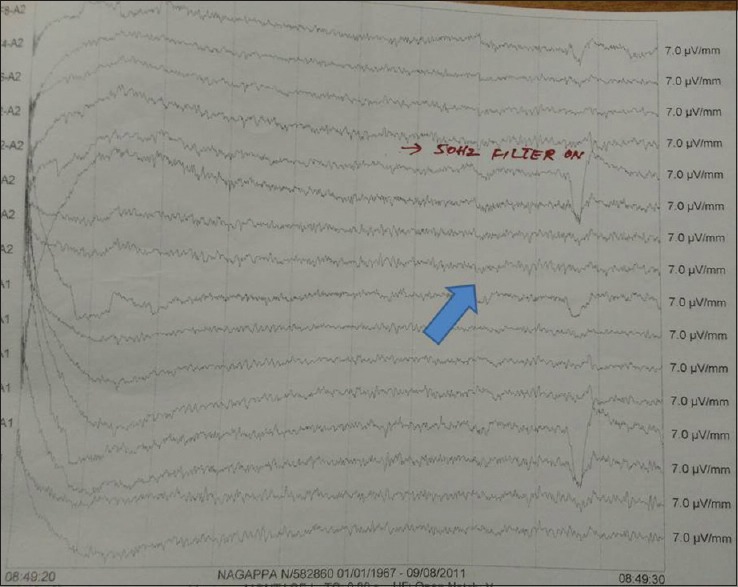
When inflammation occurs in these regions patients present with a subacute onset syndrome of confusion, psychiatric symptoms, seizures, memory problems, apathy, panic, autistic, and schizophreniform features.[17] EEG showed an abnormality in the maximum number of patients: 95.5%. Periodic lateralized epileptiform discharges were seen 18.5%. Diffuse epileptiform discharges were seen in 18.5% suggestive of probable nonconvulsive status. Mild positive triphasic waves were seen in 13.6% and nonspecific abnormality in the form of excessive theta and multifocal sharp waves were seen in 45.45% of patients.[17] PLEDS, BIPLEDS, TRIPHASIC WAVES, and DELTA BRUSH as well as nonspecific EEG changes are common. Anti-NMDA receptor (NMDAR) encephalitis is an increasingly recognized etiology of previously unexplained encephalopathy and encephalitis. The majority of children with anti-NMDAR encephalitis demonstrate diffuse slowing or anteriorly predominant slowing. A unique electrographic pattern characterized by rhythmic delta activity at 1–3 Hz with superimposed bursts of rhythmic 20–30 Hz beta frequency activity “riding” on each delta wave were noticed recently and termed “extreme delta brush” (EDB). An important practical implication is that detection of EDB may be a unique electrographic marker for a subset of patients with more severe disease. Although it is not yet known whether the EDB pattern is specific for anti-NMDAR encephalitis, its presence in the correct clinical context should raise a strong suspicion of the diagnosis.[17]
Figure 7.
BIPLEDS and DELTA BRUSH pattern in electroencephalography (blue arrow)
UNRESPONSIVE PATIENT
Unresponsive patient may be suffering from catatonia, structural brain disease, drug overdose for which EEG serves as a simple and useful screening tool. Patients with catatonia have normal EEG. Drug overdose shows diffuse beta activity and therefore called beta coma [Figure 8]. Spindle coma shows diffuse spindles and localizes pathology in thalamo-hypothalamic region. Alpha coma shows diffuse unreactive alpha and indicates pathology in brainstem. Delta coma shows delta activity and indicates severe structural or metabolic disease [Figure 9].
Figure 8.
Diffuse beta activity due to drug overdose and therefore called beta coma (blue arrow)
Figure 9.
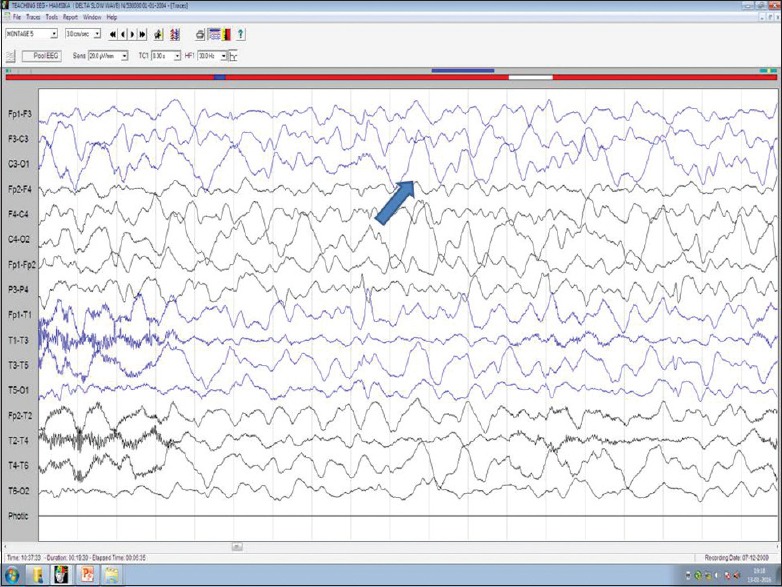
Delta activity and indicates severe structural or metabolic disease (blue arrow)
ELECTROENCEPHALOGRAPHY IN DELIRIUM
Delirium is a state of consciousness where patient is unable to think with the usual speed and clarity with disturbed consciousness, memory, orientation, language, perceptual changes of short duration and often general medical condition are present with or without dementia. EEG shows unreactive alpha with drop out, generalized slowing, occipital slowing, etc., Persistent and prolonged slowing indicate delirium complicating dementia (beclouding).
INDICATION FOR ELECTROENCEPHALOGRAPHY IN APPARENTLY PSYCHIATRIC DISORDERS IS AS FOLLOWS
Late onset nonfamilial psychosis, refractory behavioral problems, first episode of schizophrenia after 4th decade, cognitive, and developmental disorders. Quantitative EEG is useful in differentiating delirium, dementia, and depression. Healthy people with abnormal EEG in 10%–15%. Schizophrenia in 25% bipolar affective disorder with 20%.[18]
EEG in schizophrenia shows increased frontal beta, increased slow waves and decreased sleep spindles. Choppiness is the term applied to low amplitude, disorganized fast activity, with reduced or absent alpha and sometimes excess of slow activity, high amplitude beta waves.
Epileptiform discharges are seen in the following conditions. Panic attacks attention deficit hyperactivity syndromes, aggression, and autism. Slowing is seen in acute confusional state, depression, alcohol intoxication, and postconcussion syndromes. Commonly seen EEG changes in psychiatric diseases are as follows. Schizophrenia shows left temporal theta, mood disorders shows right temporal theta, and 6/s spike or waves [Figure 10], positive spikes, small sharp spikes. Chronic alcoholism shows irregular bilateral spikes; impulsive person shows more positive spikes, dyslexic patients show a variety of abnormal patterns.
Figure 10.
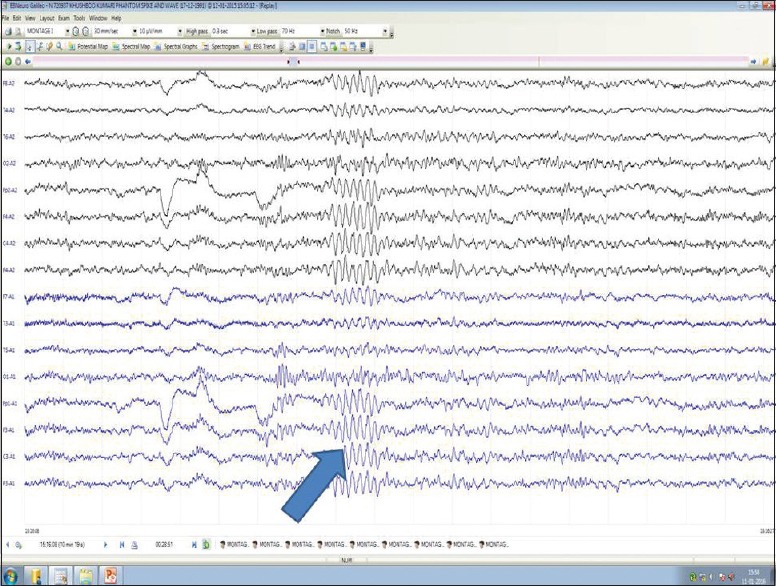
6/s spike and waves in mood disorders (blue arrow)
ROLE OF ELECTROENCEPHALOGRAPHY IN PSYCHIATRIC PHARMACOLOGY
It is useful in monitoring psychotropic drug toxicity and prediction of response to treatment. Clozapine use needs close monitoring looking for epileptic discharges. Beta coma will indicate drug overdose; lithium overdose will cause slow waves, sharp waves, and midpositive waves resembling CJD. Other uses of EEG in therapeutics are neurofeedback which works like natural AED. Rewards brain for holding in a particular frequency and patients learn to produce the desirable brain wave pattern. It corrects deficits in regulatory function related to attention, arousal, and vigilance. Beta state improves arousal and depression. Sensory motor rhythm (SMR) produces calmness and relaxation. Left side SMR and right neurofeedback correct dysregulations and right side training helps in social and emotional conduct disorder.[15,16,17,18]
CONCLUSION
EEG can be normal in organic diseases and abnormal in psychiatric diseases. Typical EEG findings in neuropsychiatric syndromes point to a specific diagnosis. Soft EEG changes are common in psychiatric disorders and do not indicate organicity. EEG can be used to assess efficacy-toxicity of therapeutic agents in psychiatry. Biofeedback-based training to keep the brain in particular rhythm is of use in psychiatric disorders as a pharmaco-sparing agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–68. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 2.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 3.Chandra SR, Ragavan S. Endogenous Event Related Potentials in Patients with Pseudo Seizures. Abstracts of Annals of Indian Academy of Neurology. IAN Conference, Ranchi. 1990 [Google Scholar]
- 4.Chandra SR. Is brain the seat of the soul? Reviews in Neurology of IAN, Continuing medical education programme of Indian academy of Neurology. 2002:11–9. [Google Scholar]
- 5.Chandra SR, Ramachandran A. Anasognosia for memory loss in patients with dementia. A descriptive study Indian experience. Original article. TAPIJ. 2011;3(3):6–8. [Google Scholar]
- 6.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – A possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Tandon PN. Ayurvedic medicine and Indian literature on epilepsy. Neurol Asia. 2004;9:57–8. [Google Scholar]
- 8.Tandon PN. Epilepsy in India: Report Based on a Multicentric Study on Epidemiology of Epilepsy Carried Out as a PL 480 Funded Project of the Indian Council of Medical Research. The Council. 1989 [Google Scholar]
- 9.Trimble MR, Reynolds EH. Epilepsy, Behaviour and Cognitive Function. In order to produce this book, a symposium was organized at Stratford-upon-avon in late 1987 at which a number of specialists in epilepsy who also have a specific interest in cognitive function and behaviour, were brought together. An invited audience also attended, and the final production represents papers catalysed by that meeting and some of the more interesting discussion. New Jersey, USA: John Wiley and Sons; 1988. [Google Scholar]
- 10.Wallace SJ, Farrell K. Epilepsy in Children. 2nd ed. CARDIFF, UK: CRC Press; 2004. p. 449. [Google Scholar]
- 11.Shih JJ, LeslieMazwi T, Falcao G, Van Gerpen J. Directed aggressive behavior in frontal lobe epilepsy: A video-EEG and ictal spect case study. Neurology. 2009;73:1804–6. doi: 10.1212/WNL.0b013e3181c2933f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagla R, Skidmore CT. Frontal lobe seizures. Neurologist. 2011;17:125–35. doi: 10.1097/NRL.0b013e31821733db. [DOI] [PubMed] [Google Scholar]
- 13.Duncan JS, Fish DR, Shorvon SD. Clinical Epilepsy (in Advances in Neurology series) Edinburgh: Churchill Livingstone; 1995. p. 408. ISBN 0 443 04936 X. [Google Scholar]
- 14.Claus JJ, Strijers RL, Jonkman EJ, Ongerboer de Visser BW, Jonker C, Walstra GJ, et al. The diagnostic value of electroencephalography in mild senile Alzheimer's disease. Clin Neurophysiol. 1999;110:825–32. doi: 10.1016/s1388-2457(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 15.Chandra SR, Issac TG, Korada SK, Teja KV, Philip M. Neuropsychiatric symptoms in a cohort of patients with frontotemporal dementia: Our experience. Indian J Psychol Med. 2016;38:326–30. doi: 10.4103/0253-7176.185960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sur S, Sinha VK. Event-related potential: An overview. Ind Psychiatry J. 2009;18:70–3. doi: 10.4103/0972-6748.57865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra SR, Seshadri R, Chikabasaviah Y, Issac TG. Progressive limbic encephalopathy: Problems and prospects. Ann Indian Acad Neurol. 2014;17:166–70. doi: 10.4103/0972-2327.132616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: A unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]