Abstract
The role of nitric oxide (NO)• in the development of the metastatic properties of nasopharyngeal carcinoma (NPC) is not fully understood. Previous studies proposed that interleukin-6 (IL-6) would act as regulator of matrix metalloprotease activation in NPC. Recently, we showed that (NO)• was a critical mediator of tumor growth in patients. The aim of this study was to determine the implication of IL-6 in the progression of NPC pathology via MMPs activation and their possible correlation with (NO)• production. We observed a significant increase in IL-6 and nitrites (NO2−) synthesis in patients (n=17) as well as a strong expression of IL-6 and nitric oxide synthase 2 (NOS2) in the analyzed tumors (n=8). In patients’ plasma, a negative correlation associated IL-6 with circulating nitrites (r=−0.33). A negative correlation associated the H-scores of these signals in the tumors (r=−0.47). In patients’ plasma, nitrites synthesis was positively associated with MMP-9 activation (r=0.45), pro-MMP-2 expression (r=0.37) and negatively correlated with MMP-2 activation (r=−0.51). High nitrite levels was associated with better recurrence free survival RFS (p=0.02). Overall our results suggest that the IL-6/NOS2 inflammatory signals are involved in the regulation of MMP-9 and MMP-2 dependent metastatic activity, and that high circulating nitrite levels in NPC patients may constitute a prognostic predictor for survival.
Keywords: IL-6, Nitric oxide, MMP, Nasopharyngeal carcinoma, Inflammation
Introduction
Nasopharyngeal carcinoma (NPC) is a highly metastatic malignancy which has been associated with EBV infections in North Africa [1, 2]. In susceptible individuals, the risk to develop NPC is increased following prolonged exposure to domestic fumes, marijuana, tobacco and consumption of food containing nitrosamines [3]. These environmental triggers act as inducers of chronic inflammation of the nasopharyngeal tissue and promote cell transformation. The pathology of the NPC tumor microenvironment (TME) is characterized by an 1) important immune cell infiltrates which promote tumor development via the release of a plethora of inflammatory messengers such as cytokines (TNF-α, IL-6), reactive oxygen species (ROS) and nitric oxide (NO)• [4–7] and 2) the synthesis of matrix metalloproteases which support NPC metastatic spread [8].
IL-6 is widely incriminated in chronic inflammation and cancer [9, 10]. In several malignancies including NPC, IL-6 promotes tumor cell proliferation, migration, invasion and associates with poor-prognosis [11–14]. Even though its function is predominantly associated with pro-tumoral activities, several reports indicate that these effects may be counterbalanced by its anti-tumoral functions by promoting active T cell responses against the tumor [15]. In a number of tumors, the pro-tumoral effects directly correlate with the activity of the inducible nitric oxide synthase (NOS2), an aggressive tumor biomarker [16–18]. In NPC patients, nitric oxide synthesis is associated with tumor growth [4].
Nitric oxide (NO)•, the product of the NOS genes, is a critical mediator of inflammation-dependent tumorigenesis but also of tumor clearance; this complexity of function occurs as a result of the level and time of exposure to (NO)• [19, 20]. Among the three NOS isoforms, NOS2 is solely responsible for the synthesis of μMolar amounts of (NO)• [20]. NOS2 expression is positively regulated by NF-κB activation induced by multiple pro-inflammatory cytokines (ex. TNF-α, IL-6) and by low molecular (NO)• concentrations [47]. In contrast, NOS2 expression is inhibited by immunoregulatory cytokines like TGF-β and large amounts of (NO)• through the inhibition of NF-κB activity [21, 47]. Functionally, several lines of evidence suggest that (NO)• is an important effector of angiogenesis and metastatic diffusion [22].
Cell metastasis from a primary tumor location to distant niches involves several complex events including processing of the components of the extracellular matrix (ECM) by metalloproteases (MMPs) [23]. These molecules constitute a family of zinc-dependent endopeptidases which also mediate processing of a number of cytokines and chemokines to regulate inflammation [24]. MMPs are primarily synthesized as zymogens (pro-MMP). These molecules may be induced by NF-κB transcriptional activity [25] and can be activated by (NO)• and reactive nitrogen species (RNS) by disruption of a cysteine–zinc bond and degradation of their prodomain [26–28]. In patients with advanced undifferentiated NPC, pro-MMP-2 and MMP-9 accumulation associates with poor-prognosis [29, 30].
Recently, IL-6 secretion has been associated with MMP-9 activity and was described to promote the acquisition of an invasive behaviour in squamous cell carcinoma of the head and neck (SCCHN) [31]. IL-6 and its receptor have also been shown to contribute to the up-regulation of MMP-2 and MMP-9 in NPC cell lines [32]. Although the direct influence of IL-6 on NOS2 activation has been demonstrated in NPC [33], it is still unclear how this inflammatory response would be involved in the activation of MMP-2/MMP-9 and influence NPC patients’ outcome.
Here we report the analysis of the associations between IL-6, (NO)• synthesis and MMP activation in NPC patients in order to explore the role of the IL-6/NOS2 pathway in the acquisition of a MMP dependent invasive phenotype and its repercussions on NPC outcome.
Patients and methods
Characteristics of the study population
In this study, a total of 17 untreated NPC patients and 8 healthy controls were enrolled (median age 49 and 40.5 years old, respectively). All subjects were recruited from the otolaryngology service of Algiers University Hospital M. Pacha. All the recruited patients presented a late stage (III/IV) NPC according to the American Joint Committee on Cancer (AJCC, 2009) and tumors were diagnosed as UC/WHO type 3. This study was approved by the ethic committee of the national agency for research development in health (ATRSS). All participants gave their informed consent before enrollment in the study.
Plasma collection
Plasma was collected from (4 ml) fresh blood using lithium heparin as anticoagulant and immediately transferred to the laboratory. After centrifugation at 1800 rpm for 10 min, plasmas were stored at −20 C until use.
NPC biopsy culture
Biopsies were taken from the postnasal cavity of NPC patients and processed as soon as possible. Prior to culture, biopsies were rinsed in PBS and the blood clots and the underlying connective tissue were teased away. The tumors were cut into fine standardised 3mm3 biopsy cuts pieces and cultured on flat-bottom plates with complete RPMI at 37°C, 5% CO2. Incubation was performed over-night in 200μl. Supernatants were stored at −20 C until use.
Determination of nitrite concentrations
Nitric oxide production in plasma and culture supernatants was assessed by the quantification of its stable metabolite nitrites (NO2−) [34]. Nitrites levels were determined by the modified Griess reaction and read at λ=543nm using a spectrophotometer.
Enzyme Linked Immunosorbent Assay (ELISA)
IL-6 levels in plasma and culture supernatants were analyzed using a human IL-6 ELISA kit (Invitrogen Co., Ltd., Camarillo, CA, USA) according to the manufacturer’s instructions.
Immunohistochemistry (IHC)
Paraffin embedded tumor sections (5 μm) from untreated NPC patients (n=8) were used to evaluate IL-6 and NOS2 expression. All IHC stainings were performed by Avidin-Biotin Complex (ABC) method with standardized VECTASTAIN ABC Kit (Vector Lab., USA, PK4000). Prior to primary antibody application, antigen retrieval was performed with 10 mmol/L citrate buffer, pH 6.0 (DAKO, USA, S1699) in a steamer for 30 min. Tissue sections were stained with monoclonal mouse anti-human IL-6 (clone 3G9; cat numb: NBP1-47810; 1/200, Novusbio, USA), anti-NOS2 (clone 13F5.1; Cat numb: MABN527; 1/5000, EMD, USA) and incubated in moisture chamber overnight at 4 C. After PBS buffer washing, the slides were treated with biotinylated anti-mouse antibody (7.5 μg/ml, Vector Lab, USA, BA-2000) for 30min at room temperature (RT). After wash with PBS, avidin-biotin complex (Vector Lab. USA) was applied for 30min at RT. The slides were developed with DAB (3, 3′-diaminobenzidine). Mouse IgG isotype was used as negative control. Immunostaining evaluation was conducted following the H-scoring method [35] to take into account the number of positive cells and the intensity of staining following this equation: H score= (% weak positive cells x1)+ (% moderate positive cells x2)+ (% strong positive cells x3)= 300. Five non-overlapping, randomly selected, high power elds were analyzed for quanti cation per patient. The tissue sections were read and scored independently by two investigators.
Gelatin zymography
Plasmatic MMP-2 and MMP-9 levels in NPC patients were assessed by gelatin zymography. In brief, equal protein concentrations of plasma were applied to 8% SDS-polyacrylamide gel containing 0.2% gelatin. After electrophoresis, gels were washed twice in 2.5% Triton X-100 for 20min and then incubated overnight in 10mM CaCl2 buffer at 37°C. The gels were stained in Coomassie Brilliant blue R250 solution and destained with a solution containing 30% methanol/10% acetic acid followed by several washing steps. Images were analysed by densitometry using ImageJ software.
Statistical analysis and Recurrence free survival calculation (RFS)
The Kaplan–Meyer actuarial method was used to estimate recurrence free survival over 36 months for plasmatic nitrites (median = 28.5μM) and IL-6 (median = 21.2pg/ml). A log-rank test was used to identify the prognostic factors predicting for RFS. A p-value ≤0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism software version 6.0.1. Data were analyzed using GraphPad Prism software version 6.0.1. Data are shown as (Mean±SD). Statistical analysis between two groups was evaluated using the Mann Whitney or the unpaired t test with Welch’s correction depending on the normality test. Correlation analysis was conducted with Spearman or Pearson’s test with respect to data type and distribution. p value≤0.05 was accepted as statistically significant.
Results
Low plasmatic IL-6 levels are associated with high nitrite synthesis in NPC patients
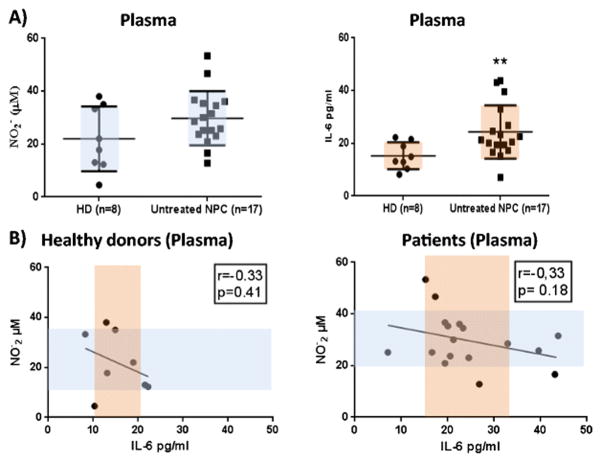
We initially examined the expression of IL-6 and nitrites levels in the plasma of NPC patients. Consistent with our previous findings [4], we observed an increase in nitrites in the plasma of NPC patients (n=17) in comparison with the healthy donors (n=8)(29.74±10.22 vs. 21.97±12.26)(Fig. 1A). The same patients showed a significant increase in the levels of plasmatic IL-6 compared to the control group (24.31±10.09 vs. 15.51±5.14; p≤0.05)(Fig. 1A). To determine the effect of IL-6 on NOS2 activity, plasmatic IL-6 values were plotted against nitrites concentrations for both healthy and NPC patients. As shown in Fig. 1B, Pearson correlation analysis indicated a trend, although not statistically significant, towards a linear inverse correlation between NOS2 activity and IL-6 release in both analyzed groups (r=−0.33, p=0.18 vs. p=0.41). Interestingly we observed that the most notable nitrite levels elevations were linked to IL-6 concentrations falling in the range below the median value of the group.
Fig 1. Analysis of plasmatic nitrite (NO2-) and IL-6 synthesis in healthy donors and untreated NPC patients.
A) Analysis of plasmatic NO2- and IL-6 levels in healthy donors (HD) (n=8) and untreated NPC patients (n=17). The means of the groups were compared with unpaired t test with Welch correction. B) Correlation analysis between plasmatic NO2− and IL-6 concentrations in NPC patients and controls. NO2- values were assessed using the Griess test and IL-6 concentrations were measured by ELISA. NO2- values obtained from healthy donors (n=8) and untreated NPC patients (n=17) were plotted against the respective IL-6 values. Pearson’s tests coefficients (r=−0.33).
These data suggest that NPC patients with the lowest levels of IL-6 would tend to associate with high levels of nitrites secretions.
NOS2 and IL-6 expression are increased in inflammatory NPC tumors
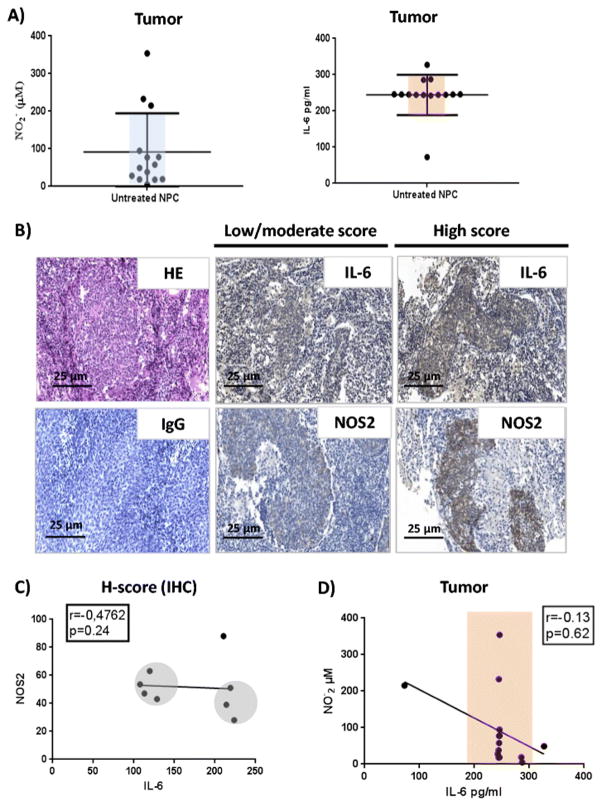
As several types of cells may generate nitrites and secrete IL-6 in the nasopharyngeal TME [5, 13], we assessed the levels of these molecules in supernatant of NPC tumor explants following 24h culture. Griess and ELISA tests showed that the biopsies released high amounts of nitrites and IL-6 in the culture supernatants (Fig. 2A). Consistent with this finding, we observed massive immunostaining of IL-6 and NOS2 in the stroma and in the tumor nests of the analyzed biopsies (n=8)(Fig. 2B). To clarify the levels of association between these molecular expressions, we performed a histological evaluation based on the histo-score (H-score). We observed a strong negative, but not statistically significant, correlation between IL-6 and NOS2 expression in the TME (r=−0.47, p=0.24). The analysis discriminated two groups of tumors in which, the lowest IL-6 expression associated with the highest NOS2 staining and the highest IL-6 H-scores associated with the weakest NOS2 scores (Fig. 2C). These differences were not observed by ELISA analysis (r=0.13, p=0.62) (Fig. 2D).
Fig 2. Analysis of IL-6 and NOS2 signals in NPC patient’s tumors.
A) Analysis of nitrites and IL-6 levels in biopsy supernatants of untreated patients cultured biopsies (n=14). The horizontal lines show the Mean ± SD of the groups. B) IL-6 expression NOS2 expression in untreated NPC patients’ biopsies. The tumor nests and the tumor immune infiltrating cells express IL-6 with more intensity in the stroma (Magnification x20). NOS2 is expressed by tumor and by the immune cells infiltrating the tumor microenvironment (Magnification x20). HE stainings and isotype control antibody IgG labelling were used as negative controls. C) Correlation between IL-6 and NOS2 staining in NPC patients (H-score). A negative correlation was observed between NOS2 and IL-6 among patients (n=8; r=−0.47). D) Correlation analysis between NO2- and IL-6 concentrations in NPC tumor explants supernatants. No correlation was found between nitrites and IL-6 among NPC patients (n=12) (Spearman’s test coefficient r=0.13).
Taken together these results indicate that IL-6 and NOS2 expression would be increased in the inflammatory TME of nasopharyngeal carcinoma and point toward a negative linear association between IL-6 expression and nitrite synthesis.
Nitrites formation positively influences MMP-9 activation and pro-MMP-2 expression, and negatively influences MMP-2 activation in NPC patients
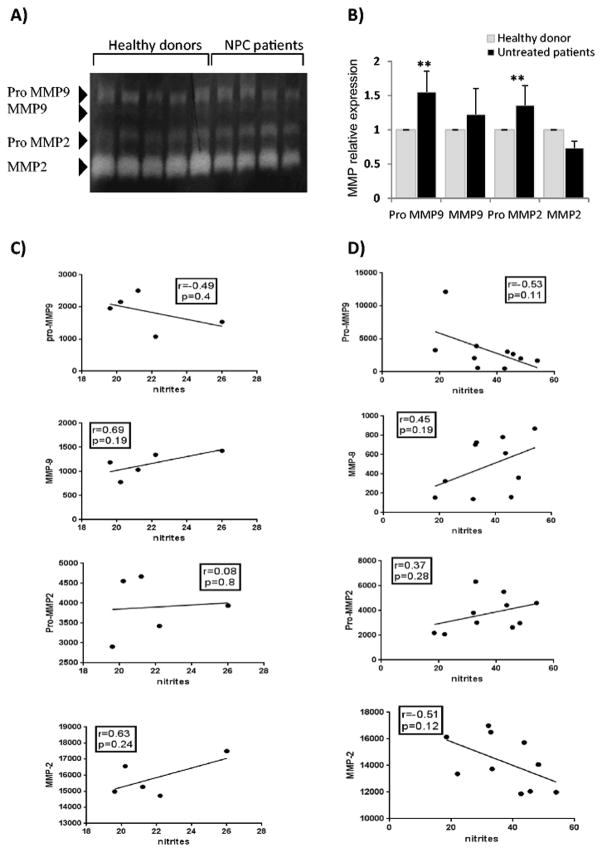
To determine the impact of nitric oxide synthesis on the acquisition of a metastatic phenotype in patients, we next conducted a gelatine zymography and a densitometric analysis to evaluate the impact of the circulating nitrites levels on the plasmatic MMP-2 and MMP-9 profile. The relationship between nitrites, pro-MMP and MMP values was examined using the Pearson or the Spearman correlation depending on the normality test. Our results showed that the levels of pro-MMP-9 and pro-MMP-2 in patients’ plasma (n=10) were significantly increased in comparison to the controls (n=5). The zymogram revealed presence of bands corresponding to the active forms of the analyzed gelatinases. Interestingly, the densitometric analysis revealed that except for MMP-2, all the other MMPs were upregulated in patients’ plasma compared to healthy donors (Fig. 3A and 3B). A strongest trend toward a negative correlation was observed between circulating pro-MMP-9 and plasma nitrites levels in patients compared to healthy donors (r=−0.53, p=0.11 vs. r=−0.49, p=0.4). We also found that circulating pro-MMP-2 levels strongly increased in patients compared to the controls (r=0.37, p=0.28 vs. r=0.08, p=0.8). Conversely, elevation in plasma nitrites levels correlated with a reduction in MMP-2 activation in patients but not in control individuals (r=−0.51, p=0.12 vs. r=0.63, p=0.24) (Fig. 3C and 3D).
Fig 3. Analysis of gelatinase activities in function of circulating nitrite (NO2-) concentration in NPC.
A) MMP activation profile in NPC patients compared to controls. B) Histogram presentation of MMP expression levels among NPC patients and controls. C and D) Analyses of the correlations between plasmatic MMP and nitrite (NO2-) levels in healthy donors and in NPC patients. Each circle represents one patient. Values of the densitometric analysis of MMP-9 and MMP-2 were plotted against the respective plasmatic NO2- value. Healthy donors (n=5) and patients (n=10). Correlation coefficients pro-MMP-9(r=0.49 vs. r=−0.53), MMP-9 (r=0.69 vs. r=0.45), pro-MMP-2 (r=0.08 vs. 0.37) and MMP-2 (r=0.63 vs. r=−0.51). All the correlations were analyzed using the Pearson test except for pro-MMP-9 values, in patients, which did not follow a normal distribution.
These findings indicate that nitrites formation can lead to MMP-9 activation and pro-MMP-2 expression, and negatively impact MMP-2 activation in NPC patients.
High plasma (NO)• levels associate with better prognosis in NPC patients
To understand how the different combined profiles associating NOS2 and IL-6 activities may affect disease recurrence and patients’ survival, we next assessed the RFS prognostic values for our cohort after 36 months follow-up. The plasmatic nitrites and IL-6 values of the cancer patients were stratified by the median (Low (NO)• vs. High (NO)• and Low IL-6 vs. High IL-6). Kaplan-Meier analysis showed that nitrite levels variations, but not those of IL-6, significantly influenced NPC recurrence-free survival (p= 0.02 vs. p= 0.51). Indeed, 50% of the analyzed patients with low nitrite levels [below the median: Low (NO)•] showed tumor recurrence after 20 months following the first diagnosis. Patients with Low IL-6 [below the median] had a slight survival advantage compared with high IL-6 patients at the last follow-up (10%). Strikingly, 90% of the tested patients with nitrites levels above the median (High (NO)•) survived without evidence of the disease after 36 months. In comparison only 35% of the analyzed patients with low (NO)• completed the study without disease recurrence (Fig. 4).
Fig 4. Crude analyses of mortality.
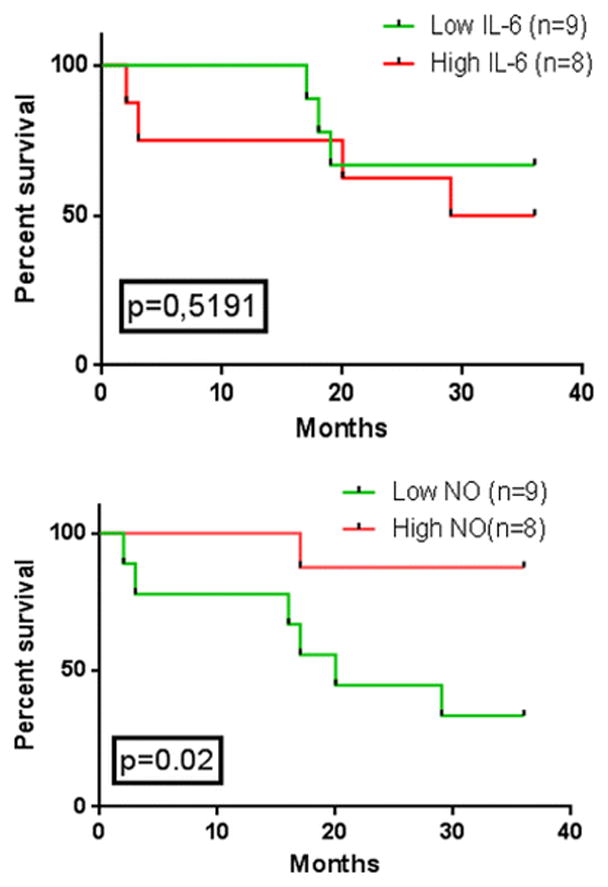
Kaplan–Meyer estimates of recurrence free survival for all patients (n=17) as a function of median plasma nitrite (NO2-) and IL-6 levels. p values were calculated using the log-rank test (High IL-6 vs. Low IL-6, p=0.51; High NO vs. Low NO, p=0.02).
These results indicate that low NOS2 activity in patients can frequently lead to tumor recurrence or death, and that high NOS2 activity supports patients’ survival.
Discussion
IL-6 and nitric oxide play a prominent role in inflammation induced carcinogenesis by altering cell metabolism, promoting tumor growth, angiogenesis and metastasis [36–38]. Our previous studies showed that the TNF-α/NOS2 inflammatory pathway supported tumor growth in-vitro and in patients, and that TNF-α was not the sole responsible for NOS2 activation [4, 5]. We speculated then on the significance of the large differences in nitrite production observed among the tested patients and their possible role in the acquisition of metastatic features or tumor elimination. Considering the dual roles of IL-6 and (NO)• and the association of their functions in tumorigenesis, we here explored the impact of their signals on the acquisition of an MMP dependent invasive phenotype and the outcome of NPC patients. In the present study we show that in NPC patients a negative correlation associates IL-6 to NOS2 activity, as reflected by the negative relation associating plasma IL-6 to NOS2 expression and nitrites synthesis. We also report that nitrite synthesis in patients correlate with MMP-9 activation, pro-MMP-2 expression and the inhibition of MMP-2 activation. Importantly we show that developing a strong nitrosative response (High (NO)•) against NPC augments significantly the chances for the patient to survive and to avoid tumor recurrence.
Increase in plasma IL-6 levels among patients with cancer has been demonstrated in several studies. Its importance has been stressed by the effects of IL-6 production on metastasis development, resistance to treatment and poor disease outcome [37]. In NPC, IL-6 was found to be a useful marker for the assessment of treatment outcome [39]. In our study we observe that NPC patients displayed high levels of the cytokine in their tumors and at the plasma level. This expression was concomitant with the activation of the IL-6 and of the NOS2 genes in the TME, an indicator of nitrosative stress and the principal source of nitric oxide in nasopharyngeal tumors. Possibly, the activated tumor associated macrophages (TAM) infiltrating the nasopharyngeal TME would constitute, beside the tumor cells, the major source of both molecules as indicated by the strong signals detected by immunohistochemistry in the tumor and the stroma, and by our recent observations reporting massive CD68 staining in the TME and increased capacity of monocytes/macrophages from cancer patients to synthesize nitrites [5].
To date, several mechanisms have been described to be at the origin of IL-6 and NOS2 expression in NPC. These processes appear to be intricate in a way to ensure their mutual amplification in a cross-talk involving the tumor and cells infiltrating the TME. Indeed several cross-amplifying signals have been described to associate a) NOS2 expression to NF-κB activation and IL-6 synthesis; b) NF-κB activation to EBV LMP1 oncogene expression; c) LMP1 signaling to IL-6 induction and Stat-3 activation; d) IL-6 mediated perpetuation of Stat-3 activity to LMP1 expression and e) IL-6 signaling to NF-κB mediated NOS2 induction [5, 33, 40–42].
Therefore we propose that the complex escape mechanisms accompanying nasopharyngeal tissue carcinogenesis would take advantage of IL-6 and NOS2 induction to promote tumor growth and lead to the acquisition of pro-metastatic proprieties instead of supporting an anti-tumoral response [43–45].
In line with the work of Ma et al. [33] showing that IL-6 induced Stat-3 activation plays a key role in the upregulation of NOS2 activity in NPC tumors and the work of Villavicencio et al. showing that NOS2 overexpression could in turn suppress IL-6 induced Stat-3 activation [46], we hypothesize that NPC tumor growth would engage low/moderate levels of IL-6 and nitrites [33, 40] and limited activation levels of Stat-3 and NF-κB [47, 48] to ensure its perpetual development. This hypothesis is supported by our results showing presence of a negative regulatory loop associating IL-6 to NOS2 activity in NPC patients.
With respect to previous studies stressing the prognostic value of MMP-9 and pro-MMP-2 in NPC [29, 30] and as (NO)• may directly regulate MMP transcription and activation [27], we next analyzed the effect of (NO)• production on the regulation of MMPs’ dependent metastatic activities. Our results indicate a trend for (NO)• synthesis to be associated with pro-MMP-9 expression induction and of its conversion to active MMP-9; thereby, this result suggests that NOS activation in NPC patients would lead to MMP-9 transcription and to the acquisition of a MMP-9 dependent gelatinolytic activity. This result is in agreement with the recent reports indicating an increase in MMP-9 activity in NPC cell lines [32]. Interestingly we observed that MMP-9 activation occurred preferentially in patients with low to intermediate levels of (NO)•, this could be explained by the implication of NF-κB in the regulation of MMP-9 expression [49]. At the opposite of MMP-9, we did not find that IL-6/NOS signals could induce pro-MMP-2 activation; instead, and in agreement with Chen et al. reporting that nitric oxide inhibits MMP-2 expression, we observed that nitrites production negatively impacted MMP-2 activation [50]. This inhibition would probably be responsible for the increased levels of pro-MMP-2 observed in patients. Considering the recent demonstration of Kesanakurti et al. showing that MMP-2 down-regulation may support IL-6/Stat-3 signaling and promote cell survival [51], future studies should analyse the implication of a such process in NPC development and define the probable participation of TIMP metallopeptidase inhibitor 2 (TIMP-2) in nitric oxide dependent inhibition of MMP-2 [52].
To define the consequences of NOS2 and IL-6 activities on patients’ survival, we next assessed the RFS prognostic values for our cohort after 36 months follow-up. We showed for the first time that low NOS2 activities in patients led frequently to tumor recurrence or death. On the contrary, patients developing a high NOS2 activity survived in almost 90% of the cases and showed no sign of tumor recurrence. In agreement with the literature we observed that the inverse situation prevailed for IL-6 activity [14]. These findings support the idea of a mutual regulation existing between IL-6 and NOS2 signals in NPC, and strongly indicate that whereas low/moderate NOS2 responses may lead to tumor growth, recurrence and patients’ death, strong NOS2 responses can be detrimental to tumor development and favor the rehabilitation of a potent anti-tumoral response [53]. Future experiments should verify that possibility by TUNEL analysis and confirm our preliminary observations indicating presence of morphological features of cell death in few high (NO)• producing tumors (data not shown).
Another probable mechanism through which (NO)• may exert its protective effects in NPC, may involve high levels of activation of NF-κB, which have been shown to be able to inhibit the immunosuppressive and oncogenic functions of Stat-3 [54]. The impact of high (NO)• on Stat-3 activation require further investigations but it is possible that the Stat-3 dependent cross-talk existing in the TME may be affected in such conditions and that the plethora of events controlled by Stat-3 signaling may be counterbalanced [55].
Thus, it is important that future analyses in patients verify the impact of high NOS/low IL-6 signals on NPC tumors growth and verify their impact on the rehabilitation of a potent Th1 anti-tumoral response by characterising a) the modulation of a tolerogenic T-reg cell response [56] and the inhibition of the expansion of the myeloid derived suppressor cells (MDSC) [57, 58], b) the promotion of dendritic cells maturation and expression of MHC class II molecules (CD80, CD86) [59], c) the rehabilitation of the anti-tumour CD8+ and natural killer (NK) response [60] and d) the inhibition of the synthesis of angiogenic factors (ex. VEGF) [61].
In conclusion, our results demonstrate for the first time that the acquisition of the metastatic capacities of NPC, correlates with high levels of IL-6 and is accompanied with production of low/moderate levels of (NO)•, and that such combined profile could be used to predict risks of tumor recurrence and early death of NPC patients; our results also demonstrate that high circulating levels of (NO)• associating with low plasma IL-6 levels may be used as good predictors of patient’ survival. Expanding these observations to a larger cohort should strengthen the statistical significance of our results and allow us to define with more precision the range of (NO)• values which could be used in clinics to identify the patients with the highest survival chances and those with the lowest survival chances. Further explorations must follow this study to clarify the implication of Stat-3 and NF-κB in such responses, but most of all, define if the modification of the levels of the cited transcription factors in the TME would be accompanied with a rehabilitation of a potent cytotoxic Th1 inflammatory response directed against the tumor. Such a result will support the idea that induction of a potent anti-tumoral response against NPC may rely in part on the induction of (NO)• synthesis. In case of verification of our hypotheses, future interventional and clinical approaches should take into account the possibility of manipulating the nitrosative stress in-vivo, by directly delivering (NO)• or NOS2 substrate bearing molecules, such as nanoparticles, to the tumor site or to (NO)• producing cells, to increase nitric oxide bioavailability and possibly inhibit NPC metastatic activity and induce tumor cell clearance [62, 63].
Highlights.
Plasma IL-6 correlates negatively with nitrite synthesis in cancer patients.
In the NPC tumor microenvironment, IL-6 expression negatively correlates with NOS2 expression.
Nitrites formation associates with MMP-9 activation, pro-MMP-2 expression and inhibition of MMP-2 activation in cancer patients.
Patients with high NOS activity have better RFS than patients with low/moderate NOS activity.
Acknowledgments
This works was supported by the Agence Thématique de Recherche Scientifique en Santé (ATRSS, Algeria) and the MSKCC (Memorial Sloan-Kettering Cancer Center, New York, USA).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Reference List
- 1.Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235:323–333. doi: 10.1002/path.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan G, Hashim MJ. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990–2010. Infect Agent Cancer. 2014;9:38. doi: 10.1186/1750-9378-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng BJ, Khyatti M, Ben Ayoub W, Dahmoul S, Ayad M, Maachi F, Bedadra W, Abdoun M, Mesli S, Bakkali H, Jalbout M, Hamdi-Cherif M, Boualga K, Bouaouina N, Chouchane L, Benider A, Ben Ayed F, Goldgar DE, Corbex M. Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer. 2009;101:1207–1212. doi: 10.1038/sj.bjc.6605281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourouba M, Boukercha A, Zergoun AA, Zebboudj A, Elhadjan M, Djenaoui D, Asselah F, Touil-Boukoffa C. Increased production of nitric oxide correlates with tumor growth in Algerian patients with nasopharyngeal carcinoma. Biomarkers. 2012;17:618–624. doi: 10.3109/1354750X.2012.706643. [DOI] [PubMed] [Google Scholar]
- 5.Bourouba M, Zergoun AA, Maffei JS, Chila D, Djennaoui D, Asselah F, Amir-Tidadini ZC, Touil-Boukoffa C, Zaman MH. TNFalpha antagonization alters NOS2 dependent nasopharyngeal carcinoma tumor growth. Cytokine. 2015;74:157–163. doi: 10.1016/j.cyto.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Gourzones C, Barjon C, Busson P. Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol. 2012;22:127–136. doi: 10.1016/j.semcancer.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan YY, Hsiao JR, Chang KC, Chang JS, Chen CW, Lai HC, Wu SY, Yeh TH, Chang FH, Lin WH, Su IJ, Chang Y. Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J Virol. 2012;86:6656–6667. doi: 10.1128/JVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landskron G, De la FM, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One. 2013;8:e61901. doi: 10.1371/journal.pone.0061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukaszewicz M, Mroczko B, Szmitkowski M. Clinical significance of interleukin-6 (IL-6) as a prognostic factor of cancer disease. Pol Arch Med Wewn. 2007;117:247–251. [PubMed] [Google Scholar]
- 13.Liao Q, Zeng Z, Guo X, Li X, Wei F, Zhang W, Li X, Chen P, Liang F, Xiang B, Ma J, Wu M, Tang H, Deng M, Zeng X, Tang K, Xiong W, Li G. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33:2098–2109. doi: 10.1038/onc.2013.161. [DOI] [PubMed] [Google Scholar]
- 14.Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu AS, Huang YJ, Wu MS. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008;14:428–434. doi: 10.1158/1078-0432.CCR-07-1032. [DOI] [PubMed] [Google Scholar]
- 15.Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagares-Garcia JA, Moore RA, Collier B, Heggere M, Diaz F, Qian F. Nitric oxide synthase as a marker in colorectal carcinoma. Am Surg. 2001;67:709–713. [PubMed] [Google Scholar]
- 17.Grimm EA, Ellerhorst J, Tang CH, Ekmekcioglu S. Constitutive intracellular production of iNOS and NO in human melanoma: possible role in regulation of growth and resistance to apoptosis. Nitric Oxide. 2008;19:133–137. doi: 10.1016/j.niox.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, Martin DN, Switzer CH, Hudson RS, Wink DA, Lee DH, Stephens RM, Ambs S. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 20.Pfeilschifter J, Eberhardt W, Beck KF. Regulation of gene expression by nitric oxide. Pflugers Arch. 2001;442:479–486. doi: 10.1007/s004240100586. [DOI] [PubMed] [Google Scholar]
- 21.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA, Lee ME. Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem. 2000;275:36653–36658. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- 22.Zaragoza C, Balbin M, Lopez-Otin C, Lamas S. Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int. 2002;61:804–808. doi: 10.1046/j.1523-1755.2002.00224.x. [DOI] [PubMed] [Google Scholar]
- 23.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 26.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroncke KD. Cysteine-Zn2+ complexes: unique molecular switches for inducible nitric oxide synthase-derived NO. FASEB J. 2001;15:2503–2507. doi: 10.1096/fj.01-0240hyp. [DOI] [PubMed] [Google Scholar]
- 28.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong TS, Kwong DL, Sham JS, Wei WI, Kwong YL, Yuen AP. Clinicopathologic significance of plasma matrix metalloproteinase-2 and -9 levels in patients with undifferentiated nasopharyngeal carcinoma. Eur J Surg Oncol. 2004;30:560–564. doi: 10.1016/j.ejso.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Li L, Yang Z, Luo W, Li X, Yang H, Yao K, Wu B, Fang W. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer. 2010;10:270. doi: 10.1186/1471-2407-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Tubergen EA, Banerjee R, Liu M, Vander BR, Light E, Kuo S, Feinberg SE, Willis AL, Wolf G, Carey T, Bradford C, Prince M, Worden FP, Kirkwood KL, D’Silva NJ. Inactivation or loss of TTP promotes invasion in head and neck cancer via transcript stabilization and secretion of MMP9, MMP2, and IL-6. Clin Cancer Res. 2013;19:1169–1179. doi: 10.1158/1078-0432.CCR-12-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun W, Liu DB, Li WW, Zhang LL, Long GX, Wang JF, Mei Q, Hu GQ. Interleukin-6 promotes the migration and invasion of nasopharyngeal carcinoma cell lines and upregulates the expression of MMP-2 and MMP-9. Int J Oncol. 2014;44:1551–1560. doi: 10.3892/ijo.2014.2323. [DOI] [PubMed] [Google Scholar]
- 33.Ma N, Kawanishi M, Hiraku Y, Murata M, Huang GW, Huang Y, Luo DZ, Mo WG, Fukui Y, Kawanishi S. Reactive nitrogen species-dependent DNA damage in EBV-associated nasopharyngeal carcinoma: the relation to STAT3 activation and EGFR expression. Int J Cancer. 2008;122:2517–2525. doi: 10.1002/ijc.23415. [DOI] [PubMed] [Google Scholar]
- 34.Touil-Boukoffa C, Bauvois B, Sanceau J, Hamrioui B, Wietzerbin J. Production of nitric oxide (NO) in human hydatidosis: relationship between nitrite production and interferon-gamma levels. Biochimie. 1998;80:739–744. doi: 10.1016/s0300-9084(99)80027-3. [DOI] [PubMed] [Google Scholar]
- 35.Levidou G, Tzenou T, Kyrtsonis MC, Nikolaou E, Kavantzas N, Maltezas D, Xirokosta K, Koulieris E, Sepsa A, Bitsanis K, Pessach I, Bartzis V, Dimou M, Panayiotidis P, Pangalis A, Patsouris E, Korkolopoulou P. The Role of CXC-Chemokine IL-8, IL-6 and CXCR2 Receptor in Lymphoplasmacytic Lymphoma: Correlations with Microvascular Characteristics and Clinical Features. Current Angiogenesis. 2013;2:110–118. [Google Scholar]
- 36.Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 37.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Chang CF, Diers AR, Hogg N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med. 2015;79:324–336. doi: 10.1016/j.freeradbiomed.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan EL, Selvaratnam G, Kananathan R, Sam CK. Quantification of Epstein-Barr virus DNA load, interleukin-6, interleukin-10, transforming growth factor-beta1 and stem cell factor in plasma of patients with nasopharyngeal carcinoma. BMC Cancer. 2006;6:227. doi: 10.1186/1471-2407-6-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Hutt-Fletcher L, Cao L, Hayward SD. A positive autoregulatory loop of LMP1 expression and STAT activation in epithelial cells latently infected with Epstein-Barr virus. J Virol. 2003;77:4139–4148. doi: 10.1128/JVI.77.7.4139-4148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson P, Jansson A, Ruetschi U, Rymo L. Nuclear factor-kappaB binds to the Epstein-Barr Virus LMP1 promoter and upregulates its expression. J Virol. 2009;83:1393–1401. doi: 10.1128/JVI.01637-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliopoulos AG, Gallagher NJ, Blake SM, Dawson CW, Young LS. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 43.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, Doja S, Jiang W, Subleski J, Shorts L, Haines D, Laubach VE, Wiltrout RH, Djurickovic D, Harris CC. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 44.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19:711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 45.Yu CR, Dambuza IM, Lee YJ, Frank GM, Egwuagu CE. STAT3 regulates proliferation and survival of CD8+ T cells: enhances effector responses to HSV-1 infection, and inhibits IL-10+ regulatory CD8+ T cells in autoimmune uveitis. Mediators Inflamm. 2013;2013:359674. doi: 10.1155/2013/359674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villavicencio RT, Liu S, Kibbe MR, Williams DL, Ganster RW, Dyer KF, Tweardy DJ, Billiar TR, Pitt BR. Induced nitric oxide inhibits IL-6-induced stat3 activation and type II acute phase mRNA expression. Shock. 2000;13:441–445. doi: 10.1097/00024382-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 48.Siednienko J, Nowak J, Moynagh PN, Gorczyca WA. Nitric oxide affects IL-6 expression in human peripheral blood mononuclear cells involving cGMP-dependent modulation of NF-kappaB activity. Cytokine. 2011;54:282–288. doi: 10.1016/j.cyto.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Chen HH, Wang DL. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol Pharmacol. 2004;65:1130–1140. doi: 10.1124/mol.65.5.1130. [DOI] [PubMed] [Google Scholar]
- 51.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with alpha5beta1 integrin in glioma. Oncogene. 2013;32:327–340. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simeone AM, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM, Tari AM. TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res. 2008;10:R44. doi: 10.1186/bcr2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snyder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS One. 2009;4:e7059. doi: 10.1371/journal.pone.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lui VW, Wong EY, Ho Y, Hong B, Wong SC, Tao Q, Choi GC, Au TC, Ho K, Yau DM, Ma BB, Hui EP, Chan AS, Tsang CM, Tsao SW, Grandis JR, Chan AT. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int J Cancer. 2009;125:1884–1893. doi: 10.1002/ijc.24567. [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fogg M, Murphy JR, Lorch J, Posner M, Wang F. Therapeutic targeting of regulatory T cells enhances tumor-specific CD8+ T cell responses in Epstein-Barr virus associated nasopharyngeal carcinoma. Virology. 2013;441:107–113. doi: 10.1016/j.virol.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsukamoto H, Nishikata R, Senju S, Nishimura Y. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res. 2013;1:64–76. doi: 10.1158/2326-6066.CIR-13-0030. [DOI] [PubMed] [Google Scholar]
- 58.Li J. The expansion and activity of myeloid-derived suppressor cells in nasopharyngeal carcinoma mediated by up-regulating COX-2. J Immunol. 2015;194:141.12. [Google Scholar]
- 59.Chang CS, Chang JH, Hsu NC, Lin HY, Chung CY. Expression of CD80 and CD86 costimulatory molecules are potential markers for better survival in nasopharyngeal carcinoma. BMC Cancer. 2007;7:88. doi: 10.1186/1471-2407-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zanussi S, Vaccher E, Caffau C, Pratesi C, Crepaldi C, Bortolin MT, Tedeschi R, Politi D, Barzan L, Tirelli U, De Paoli P. Interferon-gamma secretion and perforin expression are impaired in CD8+ T lymphocytes from patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother. 2003;52:28–32. doi: 10.1007/s00262-002-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang KP, Chang YT, Wu CC, Liu YL, Chen MC, Tsang NM, Hsu CL, Chang YS, Yu JS. Multiplexed immunobead-based profiling of cytokine markers for detection of nasopharyngeal carcinoma and prognosis of patient survival. Head Neck. 2011;33:886–897. doi: 10.1002/hed.21557. [DOI] [PubMed] [Google Scholar]
- 62.Kudo S, Nagasaki Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J Control Release. 2015;217:256–262. doi: 10.1016/j.jconrel.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 63.Munaweera I, Shi Y, Koneru B, Patel A, Dang MH, Di Pasqua AJ, Balkus KJ., Jr Nitric oxide- and cisplatin-releasing silica nanoparticles for use against non-small cell lung cancer. J Inorg Biochem. 2015;153:23–31. doi: 10.1016/j.jinorgbio.2015.09.002. [DOI] [PubMed] [Google Scholar]