Abstract
Inhibition of interleukin–6 (IL-6) holds significant promise as a therapeutic approach for triple negative breast cancer (TNBC). We previously reported that phenylmethimazole (C10) reduces IL-6 expression in several cancer cell lines. We have identified a more potent derivative of C10 termed COB-141. In the present work, we tested the hypothesis that C10 and COB-141 inhibit TNBC cell expressed IL-6 and investigated the potential for classical IL-6 pathway induced signaling within TNBC cells. A panel of TNBC cell lines (MDA-MB-231, Hs578T, MDA-MB-468) was used. Enzyme linked immunosorbent assays (ELISA) revealed that C10 and COB-141 inhibit MDA-MB-231 cell IL-6 secretion, with COB-141 being ∼6.5 times more potent than C10. Therefore, the remainder of the study focused on COB-141 which inhibited IL-6 secretion, and was found, via quantitative real time polymerase chain reaction (QRT-PCR), to inhibit IL-6 mRNA in the TNBC panel. COB-141 had little, if any, effect on metabolic activity indicating that the IL-6 inhibition is not via a toxic effect. Flow cytometric analysis and QRT-PCR revealed that the TNBC cell lines do not express the IL-6 receptor (IL-6Rα). Trans-AM assays suggested that COB-141 exerts its inhibitory effect, at least in part, by reducing NF-κB (p65/p50) DNA binding. In summary, COB-141 is a potent inhibitor of TNBC cell expressed IL-6 and the inhibition does not appear to be due to non-specific toxicity. The TNBC cell lines do not have an intact classical IL-6 signaling pathway. COB-141's inhibitory effect may be due, at least in part, to reducing NF-κB (p65/p50) DNA binding.
Keywords: Breast cancer, methimazole, interleukin-6, cytokine
1. Introduction
Approximately 10-20% of women diagnosed with breast cancer have triple negative breast cancer (TNBC) (Lehmann et al., 2011), which is particularly aggressive and difficult to treat (Hudis and Gianni, 2011). Several lines of evidence suggest that the pleiotropic inflammatory cytokine interleukin-6 (IL-6), which can be produced by many cell types, including tumor cells, plays a significant role in TNBC [e.g. (Barbie et al., 2014; Hartman et al., 2013; Knupfer and Preiss, 2007; Zhang and Adachi, 1999)]. Thus, inhibition of IL-6 activity has emerged as an important therapeutic target for TNBC [e.g. (Clinical Trials.gov Identifier:NCT02041429, 2014)].
IL-6 inhibition could be achieved by disrupting the production of IL-6 and/or disrupting IL-6 signaling. In regard to the latter approach, IL-6 signal transduction is initiated when IL-6 binds to either the membrane-bound (classical pathway) or soluble (trans pathway) form of the IL-6 receptor (IL-6Rα) (Goldberg and Schwertfeger, 2010). The resulting complex associates with glycoprotein 130 (gp130), which homodimerizes, triggering Janus kinase (JAK) activation that leads to tyrosine phosphorylation of signal transducers and activators of transcription (STAT) proteins (Wolf et al., 2014). IL-6 can also signal through the mitogen activated protein kinase (MAPK) as well as the phosphatidyl-inositol-3-kinase (PI3K) cascades (Eulenfeld et al., 2012).
Several strategies for disrupting IL-6 signaling are being pursued. Ruxolitinib, a selective JAK1/2 inhibitor, and related compounds (e.g. INCB24360), are being investigated for use in solid tumors including TNBC (Clinical Trials.gov Identifier:NCT02041429, 2014). While this approach holds promise, the development of the JAK1/2 inhibitor AZD1480 was recently discontinued due to dose-limiting toxicities and lack of clinical activity observed in targeting solid tumors (Plimack et al., 2013). Biologics targeting IL-6Rα or IL-6 have also been investigated for use as cancer therapeutics (Rossi et al., 2010; Zheng et al., 2014). However, a clinical study investigating CNTO328, a mAb to IL-6, found an increase in IL-6 with CNTO328 treatment (Dorff et al., 2010).
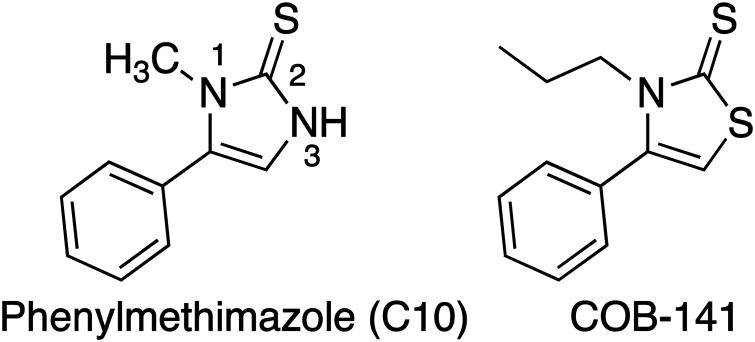
Thus, there is significant interest in targeting IL-6 and developing complementary approaches, such as inhibiting the production of IL-6, is clearly warranted. Previously we found that phenylmethimazole [C10 (Fig. 1); (4-phenyl-3-methyl-1, 3 imidazole-2-thione)], inhibits estrogen receptor α+ breast, melanoma and pancreatic cancer cell IL-6 expression in vitro (Schwartz et al., 2009, 2016) and tumor growth in vivo (Schwartz et al., 2009). We have identified a potentially more potent derivative of C10 [COB-141 (Fig. 1)] through a general compound screening program. Specifically, the heterocyclic ring of C10 contains a slightly acidic unsubstituted NH in the 3 positon. We altered C10 by extending the N-1 alkyl chain from a methyl to an n-propyl which may facilitate cell membrane transit, and changing the N-3 to an S providing the thiazole derivative (Fig. 1). Thiazoles are important anti-inflammatory agents (Pattan et al., 2009; Siddiqui et al., 2009) and previous studies have suggested that thiazoles may exhibit higher efficacy than imidazoles in certain settings (Unangst et al., 1994).
Figure 1.
Compounds used in the present study. Methimazole (MMI) is used clinically for the treatment of autoimmune disease (The number 1 next to the nitrogen indicates the 1-nitrogen). Addition of a phenyl group to the 5th position of the imidazolidinone ring of MMI yields phenyl methimazole (C10). Substitution of the imidazolidinone ring of C10 for a thiazolidinone and extension of the methyl on the 1-nitrogin to a propyl yields COB-141.
Thus, based on our previous findings, and the importance of IL-6 in TNBC, we explored the hypothesis that C10 and COB-141 potently reduce TNBC cell expression of IL-6.
2. Materials and Methods
2.1 Cell culture and treatment
The TNBC cell lines MDA-MB-231 [American Type Culture Collection, Manassas, VA (ATCC); HTB-26], MDA-MB-468 (ATCC HTB-132), and the ERα+ breast cancer cell line MCF7 (ATCC HTB-22), were cultured in Dulbecco's Modified Eagle Medium (DMEM) with high glucose (Thermo Scientific; Waltham, MA), 10% heat inactivated fetal bovine serum (FBS) (Lonza; Walkersville, MD), and 1% penicillin/streptomyocin (Lonza). The Hs578T cell line (ATCC HTB-126) was cultured in DMEM with 0.01 mg/ml bovine insulin (Sigma-Aldrich; St. Louis, MO), 10% heat inactivated FBS, and 1% penicillin/streptomycin. Undifferentiated THP-1 cells (ATCC TIB-202) were cultured in medium RPMI-1640 (ATCC) supplemented with 10% non-heat inactivated FBS and 0.05 mM β-mercaptoethanol (Gibco, Grand Island, NY). For compound treatment, cells were treated with fresh media containing C10, COB-141 or 0.25% dimethyl sulfoxide (DMSO – solvent control) (Sigma). For studies lasting 48 h, culture media containing the appropriate treatment was replaced at 24 h.
2.2 Quantitative real-time polymerase chain reaction (QRT-PCR)
Post-treatment, cells were lysed directly on the growth surface. Lysates were homogenized using QIAshredder microcentrifuge spin-column homogenizers and were purified using the RNeasy Mini Kit, both purchased from Qiagen (Valencia, CA) (Schwartz et al., 2009). Genomic DNA was eliminated using the RNase-free DNase Set (Qiagen) (Schwartz et al., 2009). Purified mRNA samples were quantified using the Nanodrop 2000 Micro-volume UV-Vis Spectrophotometer (Thermo). cDNA was subsequently synthesized from 1000 ng of each sample with the High Capacity cDNA Reverse Transcription Kit purchased from Applied Biosystems (Carlsbad, CA). Real-time PCR was performed using Taqman® Gene Expression Assays, Taqman® Gene Expression Master Mix, and the Step-One-Plus™ Real-Time PCR System (Applied Biosystems). HPRT-1 was used as the endogenous control primer alongside target primers in a multiplex format. Results were calculated using the ΔΔCT method, and represented as fold expression relative to 0.25% DMSO or THP-1 control samples.
2.3 Enzyme linked immunosorbent assays (ELISA) and MTS assays
Post-treatment, the supernatants from cells were harvested and centrifuged to remove cells and cellular debris, and stored at -20°C until used in the ELISA. The BD OptEIA Human IL-6 and IL-8 ELISA Set and Reagent set B (BD Biosciences) were conducted according to manufacturer specifications. Results are represented as averages of percent inhibition of IL-6 or IL-8 relative to solvent control (0.25% DMSO). The CellTiter 96 Aqueous Non-Radioactive Cell Proliferation Kit (Promega; Madison, WI) was used to determine metabolic activity according to the manufacturer's protocol and as previously described (Alapati et al., 2015). OD 490 nm was determined using a Synergy HT Multi-Mode Microplate Reader (BioTek, Winooski, VT). Results are given as a percent (%) MTS signal relative to the signal observed with 0.25% DMSO. The MTS signal from wells containing media alone was subtracted from all readings prior to determining the %MTS signal.
2.4 Flow cytometry analysis (FCA)
FCA was performed as previously described (Burdick et al., 2006). In brief, TNBC cells were harvested non-enzymatically (Thermo), washed, aliquoted (∼ 4 × 105 / aliquot), washed again, treated with a mouse anti-human IL-6Rα monoclonal antibody (mAb) (IgG; Abcam; Cambridge, UK) or mouse anti-human gp130 mAb (IgG; BD Biosciences), treated with a biotinylated goat anti-mouse IgG polyclonal antibody (pAb) (SouthernBiotech; Birmingham, AL) and finally treated with fluorescein isothiocyanate (FITC) conjugated streptavidin (SA) (BD Biosciences). Mouse IgGκ (BD Biosciences) was the isotype control for the mAbs to IL-6Rα and gp130. All antibodies were diluted in growth media containing 1% FBS. Treatments were 30 min on ice followed by washes. The final wash was done with Hank's Balanced Saline Solution (Lonza) HBSS+, 1% BSA (Sigma) and subsequently the cells were re-suspended in HBSS+ containing 1% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA). Samples were analyzed using a FACSAria SO (BD Biosciences).
2.5 Detection of NF-κB (p65/p50) DNA-binding activity
NF-κB (p65/p50) DNA binding activity in nuclear protein extracts of the TNBC cells was quantified with an ELISA-based assay. Nuclear proteins were extracted using NE-PER nuclear and cytoplasmic extraction kit (Thermo) then quantified using the micro BCA protein assay kit (Thermo) and Nanodrop 2000 Micro-volume UV-Vis Spectrophotometer (Thermo). Trans-AM NF-κB (p65/p50) transcription factor ELISA assays (Active Motif; Carlsbad, CA) were performed on 10 μg of extracted nuclear proteins according to manufacturer's protocol and analyzed using a Synergy HT Multi-Mode Microplate Reader (BioTek).
3 Results
3.1 A panel of TNBC cell lines express significant levels of interleukin 6 (IL-6)
Earlier work has shown that TNBC cell lines constitutively express IL-6 [e.g. for MDA-MB-231 cell line (Armenante et al., 1999)]. Thus, we sought to determine if the TNBC cell lines MDA-MB-231, MDA-MB-468 and Hs578T cultured in our laboratory secrete IL-6. As a comparison, we determined the level of IL-6 secretion by the ER+ breast cancer cell line MCF7. As shown in Fig. 2, the level of IL-6 protein secreted by the TNBC cell lines is significantly higher than the MCF7 cell line with the MDA-MB-231 and Hs578T cell lines secreting the highest levels and the MDA-MB-468 the lowest. Strikingly, the levels of IL-6 protein secreted by MDA-MB-231 and Hs578T cells is >250 fold higher than that expressed by MCF7 cells and even the MDA-MB-468 cells secrete nearly 10 fold higher levels of IL-6 compared to the MCF7 cells (Fig. 2).
Figure 2. A panel of TNBC cell lines have significant basal expression of IL-6.
A panel of TNBC cell lines was analyzed for basal IL-6 secretion via ELISA. Each TNBC cell line exhibited a significantly higher secretion of IL-6 compared to the MCF7 ER+ breast cancer cell line. Graph is the average of n=2 separate experiments with each value determined in triplicate. *p≤0.05 compared to each other bar as determined by a Games-Howell post-hoc test.
3.2 C10 and COB-141 inhibit IL-6 expression by MDA-MB-231 cells
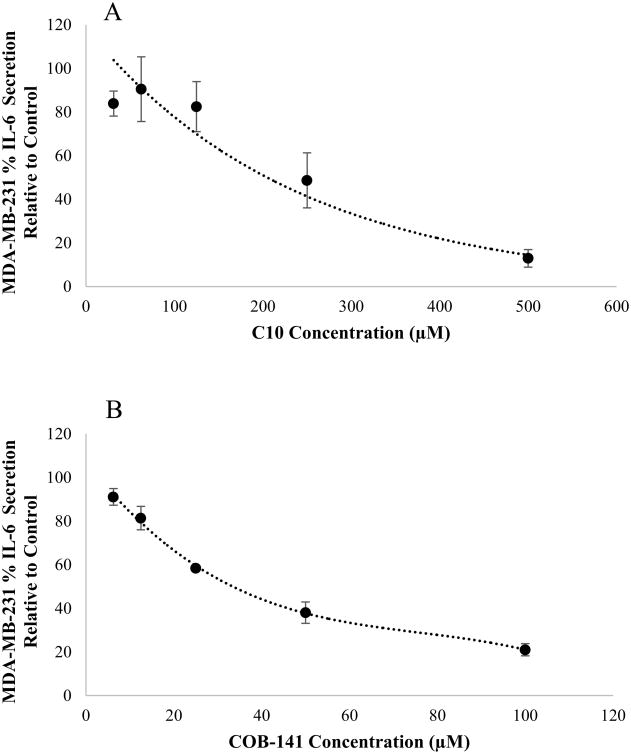
Our first step was to determine if C10 would inhibit IL-6 expression in MDA-MB-231 cells. In addition, we previously created a derivative of C10 by substituting the imidazolidinone ring of C10 for a thiazolidinone ring and extending the alkyl chain on N-1 from a methyl to a propyl affording COB-141 (Fig. 1). We have found that COB-141 has greater potency than C10 in several in vitro assays (not shown). Thus, we also investigated the effect of COB-141 on IL-6 expression by MDA-MD-231 cells. MDA-MB-231 were treated with varying concentrations of C10 (20 to 500 μM) or COB-141 (6.25 to 100 μM) for 24 h and, subsequently, the level of IL-6 present in the supernatant determined via ELISA. The lower concentrations utilized for COB-141, relative to C10, was due to the greater potency of COB-141.
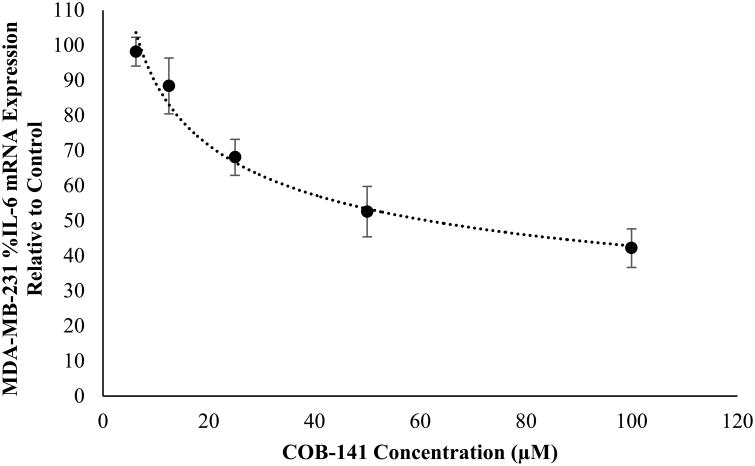
Both C10 and COB-141 significantly inhibited IL-6 secretion (Fig. 3). At concentrations > 125 μM, as the concentration of C10 increased, the level of IL-6 secretion decreased (Fig. 3A). As the concentration of COB-141 increased, the level of IL-6 secretion decreased (Fig. 3B). To compare the potencies of C10 and COB-141, we estimated IC50 for each compound, i.e. the concentration needed to inhibit 50% of basal IL-6 secretion. A best fit regression of the data was performed and the estimated IC50 determined from the resulting curve. The IC50 for C10 was estimated to be 230 μM and the IC50 for COB-141 was estimated to be 35 μM. Since COB-141 is more potent than C10, we focused the remainder of the study on COB-141. We sought to determine if the inhibition of IL-6 was occurring at the mRNA level. MDA-MB-231 cells were treated with COB-141 at concentrations ranging from 6.25 to 100 μM for 24 h and, subsequently, the IL-6 mRNA was quantified using QRT-PCR. IL-6 mRNA decreased as the concentration of COB-141 increased (Fig. 4). The IC50 for COB-141 inhibition of IL-6 mRNA was estimated to be 61 μM.
Figure 3. C10 and COB-141 inhibit MDA-MB-231 cell secretion of IL-6.
MDA-MB-231 cells were treated with 0.25% DMSO (solvent control), treated with varying levels of C10 (A) or treated with varying levels of COB-141 (B). 24 hrs later, the level of secreted IL-6 was quantified by ELISA. The dose response was determined in three separate experiments. These averages (+/- the S.E.M.) were plotted vs the concentration of inhibitor to arrive at the figures shown. A best fit regression of this data was performed and the IC50 determined from the resulting curve.
Figure 4. COB-141 inhibits MDA-MB-231 expression of IL-6 mRNA.
MDA-MB-231 cells were treated with a range of COB-141 concentrations to determine the dose dependent effect on IL-6 mRNA expression and the IC50. The dose response was determined in three separate experiments. These averages (+/- the S.E.M.) were plotted vs the concentration of COB-141 to arrive at the figures shown. A best fit regression of this data was performed and the IC50 determined from the resulting curve.
3.3 COB-141 inhibits IL-6 expression in two other TNBC cell lines
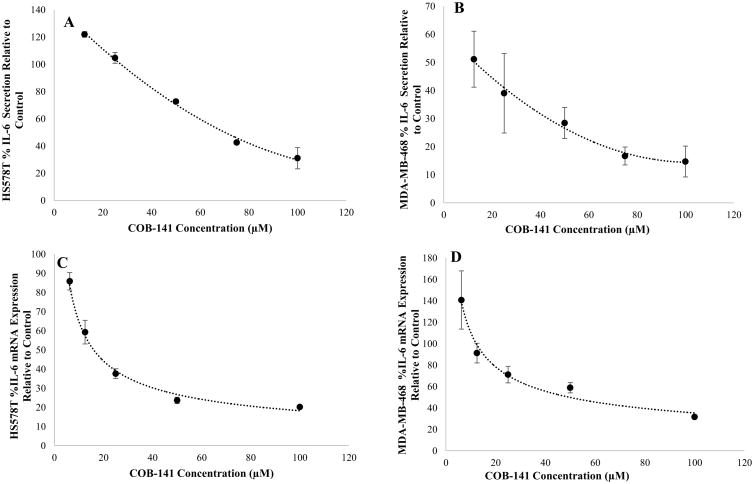
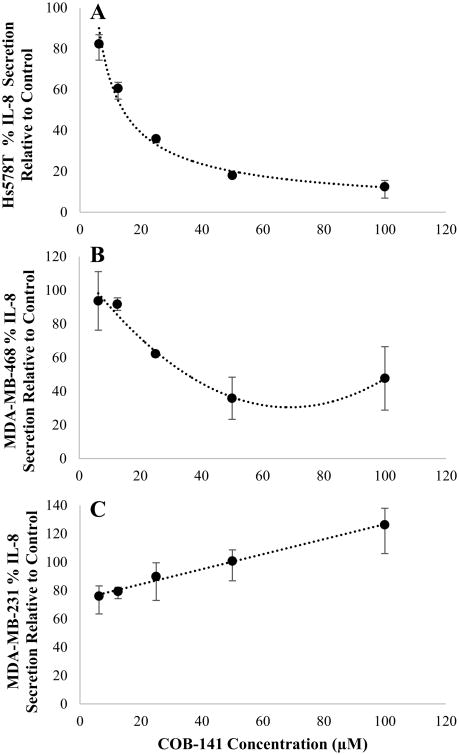
To determine if the results with the MDA-MB-231 cell line are generalizable to other TNBC cell lines, we investigated the effect of COB-141 on the TNBC cell lines, Hs578T and MDA-MB-468. As described in the above sections for the MDA-MB-231 cell line, the effect of COB-141 on IL-6 secretion and mRNA expression was determined via ELISA and QRT-PCR. COB-141 significantly reduced IL-6 secretion (Fig. 5A,B) and mRNA expression (Fig. 5C,D) in both the Hs578T (Fig. 5A,C) and MDA-MB-468 cell lines (Fig. 5B,D). The IC50 for IL-6 secretion was estimated to be 70 μM for the Hs578T and 13 μM for the MDA-MB-468 cells. The IC50 for IL-6 mRNA was estimated to be 16 μM for the Hs578T and 50 μM for the MDA-MB-468 cells. Thus, COB-141 inhibition of basal IL-6 expression was generalizable to other TNBC cell lines.
Figure 5. COB-141 inhibits Hs578T and MDA-MB-468 cell expression of IL-6.
Hs578T and MDA-MB-468 cells were treated with a range of COB-141 concentrations to determine the dose dependent effect on IL-6 protein secretion (A and B) and mRNA expression (C and D) which allowed estimation of an IC50 value for each. For each study, the dose response was determined in three separate experiments. The results at each dose were averaged for the three experiments. These averages (+/- the S.E.M.) were plotted vs the concentration of COB-141 to arrive at the figures shown. A best fit regression of the data was performed and the IC50 determined from the resulting curves.
3.4 COB-141 exhibits a relatively limited, if any, effect on the metabolic activity of the panel of TNBC cell lines at the concentrations tested for IL-6 inhibition
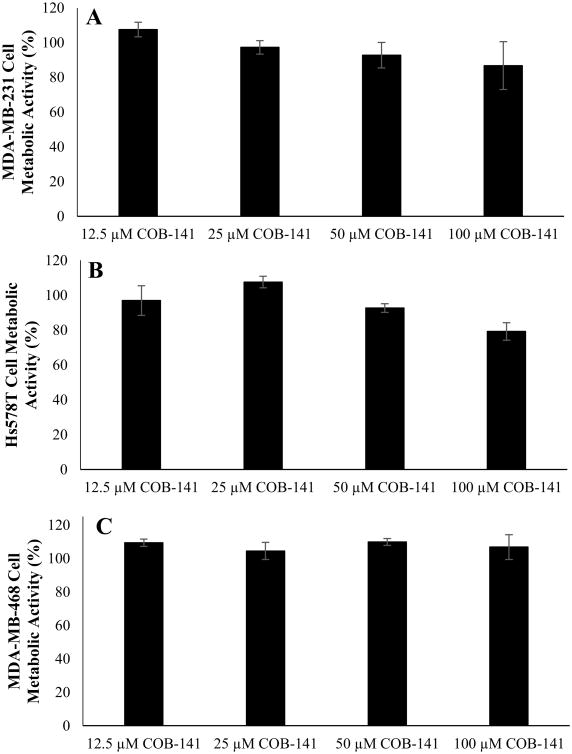
Inhibition of IL-6 expression could be due to a “non-specific” toxic effect on the TNBC cells. To gain insight into this possibility, we sought to determine if COB-141 treatment has a significant effect on the TNBC cell lines' viability as measured by a metabolic (MTS) assay. 48 h treatment with COB-141 had a quite limited effect on the metabolic activity of the TNBC cell lines (Fig. 6) that did not parallel the rather dramatic inhibitory effect of COB-141 on IL-6 expression (Figs. 3B, 4, 5). For example, at 50 μM, there is little effect on metabolic activity (Fig. 6) despite the fact that this concentration of COB-141 yields a significant reduction in IL-6 secretion and mRNA expression (Figs. 3B, 4, 5). There might be a slight effect of COB-141 on the metabolic activity of the Hs578T cell line (Fig. 6) at moderate concentrations, but even in this case, the effect is not as dramatic as the effect on IL-6 (Fig. 5A,C). Note that we chose to use 48 h for this assay, as opposed to 24 h used in the IL-6 inhibition analysis, reasoning that toxic effects might not manifest themselves until a later time point. Thus, it appears that the inhibitory effect of COB-141 on IL-6 expression is not due to “non-specific” cell toxicity.
Figure 6. At the concentrations tested for IL-6 inhibition, COB-141 has a limited effect on the metabolic activity of the panel of TNBC cell lines.
MDA-MB-231 (A), Hs578T (B) and MDA-MB-468 (C) cells were treated with various concentrations of COB-141 for 48 hrs. Subsequently, an MTS assay was used to determine the effect of the compound on metabolic activity. For each study, the dose response was determined in three separate experiments. The results at each dose were averaged for the three experiments. These averages (+/- the S.E.M.) were plotted vs the concentration of COB-141 to arrive at the figures shown.
3.5 The panel of TNBC cells lack expression of IL-6 receptor α (IL-6Rα) but do express gp130
A reduction in IL-6 expression could exert a therapeutic result via a direct effect on the tumor cells themselves or by acting on the non-tumor cells present in the tumor microenvironment. To investigate the former possibility, we sought to determine if the panel of TNBC cell lines expresses the IL-6 signaling molecules (receptors) IL-6Rα and gp130. The panel of TNBC cell lines expressed little, if any, IL-6Rα mRNA compared to that expressed by THP-1 cells [positive control (Vermes et al., 2002)] (Fig. 7). In addition, IL-6Rα protein was not detected on the panel of TNBC cell lines (Fig. 7) via flow cytometric analysis (FCA). In contrast, IL-6Rα protein was detected on THP-1 cells consistent with previous reports (Vermes et al., 2002). These results (Fig. 7) provide compelling evidence that the panel of TNBC cell lines do not express IL-6Rα. Interestingly, FCA (Fig. 7) and QRT-PCR (Table 1) did reveal that all three cell lines express gp130.
Figure 7. The panel of TNBC cell lines does not express the IL-6 receptor α.
(Left Panel) MDA-MB-231, Hs578T and MDA-MB-468 cells were tested alongside THP-1 cells (as a positive control) for the presence of IL-6Rα mRNA expression via QRT-PCR. Significant message was observed in THP-1 cells while little, if any, message was observed in the panel of TNBC cell lines. Results are expressed relative to THP-1 cells and represent three separate experiments. (Right Panels) MDA-MB-231, Hs578T and MDA-MB-468 cells (upper 3 panels) and THP-1 cells (lowest panel) were tested for membrane expression of IL-6Rα and gp130 using flow cytometric analysis. The panel of TNBC cell lines treated with a mAb to IL-6Rα exhibit nearly identical fluorescence (solid grey line) as cells treated with an isotype control (dotted line) suggesting that no IL-6Rα is present on the TNBC cell lines. In contrast, THP-1 cells treated with a mAb to IL-6Rα show a definite shift in fluorescence (solid grey line) compared to THP-1 cells treated with an isotype control (dotted line) demonstrating that THP-1 cells express IL-6Rα, consistent with the literature (Vermes et al., 2002), and that the mAb for IL-6Rα works as expected. All three TNBC cell lines were positive for gp130 (solid black line) consistent with the results from the QRT-PCR (Table 1). Results representative of three separate experiments.
Table 1. Multiplex Real-Time PCR Results (Ct Values) for gp130 and HPRT1 Expression.
The panel of TNBC cell lines express gp130 signal transduction protein. MDA-MB-231, HS578T Hs578T and MDA-MB-468 cells were tested for the presence of gp130 mRNA expression via QRT-PCR. THP-1 cells served as a positive control. Significant message was observed in all cell lines.
| Housekeeping Gene | Gene of Interest | |||
|---|---|---|---|---|
|
|
|
|||
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | |
|
|
|
|||
| HPRT1 | HPRT1 | gp130 | gp130 | |
| MDA-MB-231 Sample 1 | 20.8542 | 21.0557 | 20.7459 | 21.0018 |
| MDA-MB-231 Sample 2 | 20.5117 | 20.8309 | 20.6094 | 20.9634 |
| MDA-MB-231 Sample 3 | 20.4699 | 20.648 | 20.8029 | 20.8053 |
| Hs578T Sample 1 | 21.6563 | 21.911 | 20.113 | 20.4201 |
| Hs578T Sample 2 | 21.477 | 21.7132 | 19.7947 | 19.9812 |
| Hs578T Sample 3 | 21.6563 | 21.8859 | 20.0868 | 20.5735 |
| MDA-MB-468 Sample 1 | 20.8628 | 21.2024 | 22.2648 | 22.6831 |
| MDA-MB-468 Sample 2 | 21.3967 | 21.6432 | 22.7584 | 22.9019 |
| MDA-MB-468 Sample 3 | 21.2865 | 21.6053 | 22.6902 | 22.9063 |
| THP-1 Sample 1 | 21.1378 | 21.2445 | 24.2374 | 24.2065 |
| THP-1 Sample 2 | 20.9466 | 21.2468 | 23.886 | 24.1176 |
| THP-1 Sample 3 | 21.0651 | 21.1598 | 24.0519 | 24.2497 |
3.6 COB-141 treatment of the panel of TNBC cells reduces NF-κB (p65/p50) DNA binding
We next initiated an investigation into the possible mechanism(s) by which COB-141 inhibits IL-6 expression. For this we focused on the transcription factor NF-κB, specifically the canonical p65/p50 pathway, since it plays a key role in inflammatory cytokine regulation, including IL-6 (Lawrence, 2009; Libermann and Baltimore, 1990), and has been implicated in IL-6 expression by TNBC cells (Hartman et al., 2013). p65/p50 is normally sequestered in the cytoplasm under the regulation of IκB. Upon activation, IκB is phosphorylated and degraded (Lawrence, 2009). The degradation of IκB frees p65/p50 to translocate to the nucleus where it can bind to its DNA binding motifs and initiate transcription (Lawrence, 2009). Although the mobilization of p65/p50 to the nucleus is typically in the context of activation of the cells with a stimulus, we reasoned that probing p65/p50 was a rational place to start our investigation into mechanism.
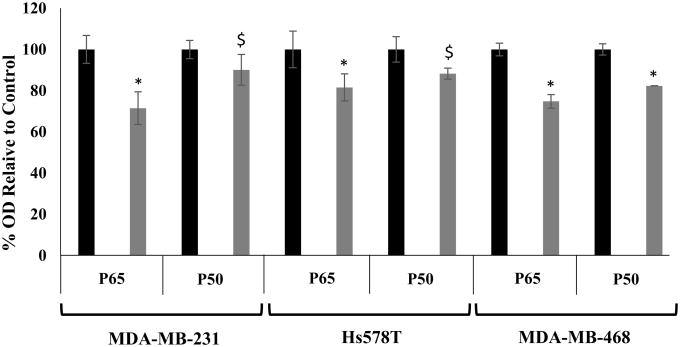
Thus, we treated the panel of TNBC cells with COB-141 or DMSO (carrier control), subsequently isolated the nuclear proteins from the cells and used a Trans-AM assay to measure p65/p50 binding to its DNA binding motifs. As shown in Fig. 8, COB-141 did appear to reduce the level of p65/p50 DNA binding in all three TNBC cells lines with the size of the effect ranging from distinct (∼30% reduction) to modest (∼10% reduction). Therefore, it appears that COB-141 may inhibit IL-6 expression by the panel of TNBC cells, at least in part, by reducing p65/p50 DNA binding.
Figure 8. COB-141 treatment reduces NF-κB DNA binding.
MDA-MB-231, Hs578T and MDA-MB-468 were treated with 50 μM COB-141 or 0.25% DMSO (carrier control). 24 h later, the nuclear protein fraction was harvested and the binding of the protein extracts to DNA determined via a NF-κB (p65/p50) specific Trans-AM assay. The OD observed for treatment with 0.25% DMSO was set to 100% (black bars) and the OD observed for treatment with COB-141 (grey bars) was determined relative to the DMSO control. Two biological replicates were performed with duplicate determinations for each replicate. These four values were averaged to give the means shown (+/- S.E.M.). * P < 0.05; $ P < 0.073 as determined by a Student's t-test.
3.7 COB-141 inhibits IL-8 expression in the Hs578T and MDA-MB-468 but not the MDA-MB-231 TNBC cell lines
Previous work has linked TNBC IL-8 and IL-6 expression mechanistically through NF-κB (Hartman et al., 2013). Thus, we sought to investigate COB-141's effect on IL-8. As shown in Fig. 9 A&B, COB-141 did have a significant effect on IL-8 protein secretion by the Hs578T (IC50 14 μM) and MDA-MB-468 (IC50 35 μM) cell lines. In striking contrast to these results, COB-141 did not inhibit MDA-MB-231 cell secretion of IL-8 (Fig. 9C). Thus, the results obtained for IL-6 cannot necessarily be extrapolated to predict what effect COB-141 will have on the secretion of the mechanistically related cytokine IL-8. The discordant results between the cell lines for IL-8 do, however, underscore the significance of the IL-6 findings presented in Figs. 3-5 in that a similar result was observed for three different TNBC cell lines.
Figure 9. COB-141 inhibits Hs578T and MDA-MB-468 but not MDA-MB-231 cell secretion of IL-8.
Hs578T (A), MDA-MB-468 (B) and MDA-MB-231 (C) cells were treated with a range of COB-141 concentrations to determine the dose dependent effect on IL-8 protein secretion. For each study, the dose response was determined in three separate experiments using ELISA. The results at each dose were averaged for the three experiments. These averages (+/- the S.E.M.) were plotted vs the concentration of COB-141 to arrive at the figures shown. A best fit regression of the data was performed and the IC50 determined from the resulting curves for the Hs578T and MDA-MB-468 cell lines.
4. Discussion
Our team has worked extensively with a derivative of MMI termed C10 [e.g. (Alapati et al., 2015; Dagia et al., 2004; Harii et al., 2005; McCall et al., 2010; Schwartz et al., 2009)]. C10 differs from MMI by the presence of a hydrophobic phenyl group (Fig. 1), which presumably facilitates transport into the cell thereby increasing activity. We have recently provided in vitro evidence that C10 exhibits greater activity than MMI (Alapati et al., 2015). Directly related to the present work, research pioneered by the McCall lab revealed that C10 reduces IL-6 expression by several cancer cell lines (McCall et al., 2007; Schwartz et al., 2009) including, most recently, the ERα+ breast cancer cell line MCF7 (Schwartz et al., 2016), and inhibits tumor growth in murine models (Schwartz et al., 2009). In addition, our compound screening program identified a C10 derivative, COB-141, that appears to be more potent than C10. Both of these compounds were tested in this study.
Our initial results revealed that both C10 and COB-141 inhibit basal IL-6 expression by MDA-MB-231 cells (Fig. 3) and that COB-141 is ∼6.5 times more potent than C10. Thus, we focused the remainder of our study on COB-141. We found that COB-141 is a potent inhibitor of IL-6 secretion by a panel of TNBC cell lines and COB-141 acts at the mRNA level (Figs. 4 and 5). The inhibition of IL-6 does not appear to be due to a non-specific toxic effect as an MTS assay revealed little, if any, decrease in metabolic activity upon treatment with COB-141 (Fig. 6). In addition, the data presented in Fig. 8, indicate that COB-141 exerts its inhibitory effect, at least in part, by decreasing the level of nuclear NF-κB (p65/p50) DNA binding; a finding that is congruent with COB-141 inhibiting IL-6 transcription (Figs. 4 and 5). The regulation of NF-κB is complex [e.g. (Hartman et al., 2013; Lawrence, 2009)] and future studies will be needed to further elucidate the details by which COB-141 might inhibit p65/p50 DNA binding activity. Interestingly, the inhibition of IL-6 was not generalizable to another closely related cytokine IL-8 (Hartman et al., 2013). Specifically, while we found that COB-141 inhibited IL-8 secretion by Hs578T and MDA-MB-468 cells, it did not inhibit, and may have actually increased, IL-8 secretion by MDA-MB-231 cells (Fig. 9).
The results for IL-6 (and to a certain extent IL-8) reveal that COB-141 defines a chemical design space which contains potential therapeutics for TNBC. To exert an inhibitory effect on tumor growth and progression, it is reasonable to postulate that a reduction in IL-6 would achieve a therapeutic effect by directly affecting the tumor cells and/or diminishing the growth promoting characteristics of the microenvironment. Our data suggest that inhibition of IL-6 may not have a direct effect on tumor cells themselves via the classical IL-6 signaling pathway, at least in some cases. First, we observed limited, if any, effect on TNBC cell metabolic activity at COB-141 concentrations that resulted in significant inhibition of IL-6 (Fig. 6 compared to Figs. 3B, 4, 5). In this regard, it is important to note that treatment with COB-141 did not yield complete inhibition of IL-6 raising the possibility that a compound that reduced IL-6 to nil might achieve an effect on metabolic activity. That said, the second piece of evidence supporting no direct effect on the tumor cells via the classical IL-6 pathway is that the panel of TNBC cells used in this study did not appear to have IL-6Rα as assessed by both flow cytometric analysis and QRT-PCR (Fig. 7). There is the possibility that IL-6 signaling could occur through the trans pathway since these cells do express gp130 (Fig. 7 and Table 1). In the case of trans-signaling it would appear that the sIL-6Rα would need to be provided by another cell within the tumor since the panel of TNBC cell lines tested did not express IL-6Rα mRNA (Fig. 7). If it is true that a particular TNBC tumor cell's growth is not dependent on a direct effect of IL-6 on the tumor cells themselves via either the classical or trans pathways, an IL-6 inhibitor could still decrease tumor growth in vivo through actions on the tumor microenvironment. Indeed, inhibition of IL-6 signaling has been shown to reduce tumor growth in vivo, even under the circumstance where there is no direct effect on tumor cell growth and viability in vitro [e.g. (Chang et al., 2013)]. Thus, continuing a concerted effort to identify, characterize and develop potent IL-6 inhibitors is clearly warranted.
While our study has focused on IL-6 expression, it is possible that COB-141 has other effects as well. One intriguing possibility would be COB-141 altering the “stemness” of the cell population. For breast cancer, cancer stem cell markers (CSCs) include the canonical CSC markers CD24, CD44, and ALDH1 (Akbari-Birgani et al., 2016; Cieslar-Pobuda et al., 2015; Farahani et al., 2014; Sansone et al., 2016). Thus, in a preliminary study we utilized flow cytometric analysis with immunolabeling and an Aldefuor assay to investigate the effects of COB-141 on the expression of CD24/CD44 and ALDH1 activity, respectively, in the panel of TNBC cell lines. COB-141 did not appear to change the expression of these stem markers in any of the TNBC cell lines tested. Given the complexity of this issue [e.g. there are other markers of stemness and the type of cell culture (2D vs 3D) can influence stemness], there is still much that could be explored regarding the effect of COB-141 on stemness. Other possible effects of COB-141 include altering key regulators of the cell cycle [e.g. PFKFB3 (Cieslar-Pobuda et al., 2015)] that might manifest at time points after those investigated in the MTS study presented here (Fig. 6).
In conclusion, we found that (i) C10 and COB-141 inhibit IL-6 secretion by a TNBC cell line, (ii) COB-141 is significantly more potent than C10, (iii) COB-141 inhibits IL-6 expression in a panel of TNBC cell lines at both the protein and mRNA level, (iv) a panel of TNBC cell lines do not appear to have an intact classical IL-6 signaling pathway, and (v) COB-141 may exert its inhibitory effect, at least in part, by reducing NF-κB (p65/p50) DNA binding.
Acknowledgments
We thank Dr. Grady Carlson and Mr. Alexander Ostermann (Department of Chemical and Biomolecular Engineering, Ohio University) for their assistance. This work was supported, in part, by NIH grant R15 GM110602-01A1 (DJG/KDM/SCB) and NSF grant CBET 1106118 (MMB).
Footnotes
Conflict of Interest: SPD was a paid employee of Interthyr Corporation and DJG is a shareholder in the Interthyr Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbari-Birgani S, Paranjothy T, Zuse A, Janikowski T, Cieslar-Pobuda A, Likus W, Urasinska E, Schweizer F, Ghavami S, Klonisch T, Los MJ. Cancer stem cells, cancer-initiating cells and methods for their detection. Drug Discov Today. 2016;21:836–842. doi: 10.1016/j.drudis.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Alapati A, Deosarkar SP, Lanier OL, Qi C, Carlson GE, Burdick MM, Schwartz FL, McCall KD, Bergmeier SC, Goetz DJ. Simple modifications to methimazole that enhance its inhibitory effect on tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression by human endothelial cells. Eur J Pharmacol. 2015;751:59–66. doi: 10.1016/j.ejphar.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenante F, Merola M, Furia A, Tovey M, Palmieri M. Interleukin-6 repression is associated with a distinctive chromatin structure of the gene. Nucleic Acids Res. 1999;27:4483–4490. doi: 10.1093/nar/27.22.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbie TU, Alexe G, Aref AR, Li S, Zhu Z, Zhang X, Imamura Y, Thai TC, Huang Y, Bowden M, Herndon J, Cohoon TJ, Fleming T, Tamayo P, Mesirov JP, Ogino S, Wong KK, Ellis MJ, Hahn WC, Barbie DA, Gillanders WE. Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J Clin Invest. 2014;124:5411–5423. doi: 10.1172/JCI75661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick MM, Chu JT, Godar S, Sackstein R. HCELL is the major E- and L-selectin ligand expressed on LS174T colon carcinoma cells. J Biol Chem. 2006;281:13899–13905. doi: 10.1074/jbc.M513617200. [DOI] [PubMed] [Google Scholar]
- Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, Cotari J, Alpaugh ML, de Stanchina E, Manova K, Li M, Bonafe M, Ceccarelli C, Taffurelli M, Santini D, Altan-Bonnet G, Kaplan R, Norton L, Nishimoto N, Huszar D, Lyden D, Bromberg J. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslar-Pobuda A, Jain MV, Kratz G, Rzeszowska-Wolny J, Ghavami S, Wiechec E. The expression pattern of PFKFB3 enzyme distinguishes between induced-pluripotent stem cells and cancer stem cells. Oncotarget. 2015;6:29753–29770. doi: 10.18632/oncotarget.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Trials.gov Identifier: NCT02041429. Ruxolitinib W/ Preop Chemo For Triple Negative Inflammatory Brca Clinical Trials.gov Identifier: NCT02041429. 2014 https://clinicaltrials.gov.
- Dagia NM, Harii N, Meli AE, Sun X, Lewis CJ, Kohn LD, Goetz DJ. Phenyl methimazole inhibits TNF-a induced VCAM-1 expression in an IFN regulatory factor-1-dependent manner and reduces monocytic cell adhesion to endothelial cells. J Immunol. 2004;173:2041–2049. doi: 10.4049/jimmunol.173.3.2041. [DOI] [PubMed] [Google Scholar]
- Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PNJ, Van Veldhuizen PJJ, Quinn DI, Vogelzang NJ, Thompson IMJ, Hussain MHA. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res. 2010;16:3028–3034. doi: 10.1158/1078-0432.CCR-09-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenfeld R, Dittrich A, Khouri C, Muller PJ, Mutze B, Wolf A, Schaper F. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol. 2012;91:486–495. doi: 10.1016/j.ejcb.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P, Wiechec E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis. 2014;35:747–759. doi: 10.1093/carcin/bgu045. [DOI] [PubMed] [Google Scholar]
- Goldberg JE, Schwertfeger KL. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr Drug Targets. 2010;11:1133–1146. doi: 10.2174/138945010792006799. [DOI] [PubMed] [Google Scholar]
- Harii N, Lewis CJ, Vasko V, McCall K, Benavides-Peralta U, Sun X, Ringel MD, Saji M, Giuliani C, Napolitano G, Goetz DJ, Kohn LD. Thyrocytes express a functional toll-like receptor 3: overexpression can be induced by viral infection and reversed by phenylmethimazole and is associated with Hashimoto's autoimmune thyroiditis. Mol Endocrinol. 2005;19:1231–1250. doi: 10.1210/me.2004-0100. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck SG, Mills GB, Brown PH. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73:3470–3480. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. The Oncologist. 2011;16(1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall KD, Harii N, Lewis CJ, Malgor R, Kim WB, Saji M, Kohn AD, Moon RT, Kohn LD. High basal levels of functional toll-like receptor 3 (TLR3) and noncanonical Wnt5a are expressed in papillary thyroid cancer and are coordinately decreased by phenylmethimazole together with cell proliferation and migration. Endocrinology. 2007;148:4226–4237. doi: 10.1210/en.2007-0459. [DOI] [PubMed] [Google Scholar]
- McCall KD, Holliday D, Dickerson E, Wallace B, Schwartz AL, Schwartz C, Lewis CJ, Kohn LD, Schwartz FL. Phenylmethimazole blocks palmitate-mediated induction of inflammatory cytokine pathways in 3T3L1 adipocytes and RAW 264.7 macrophages. J Endocrinol. 2010;207:343–353. doi: 10.1677/JOE-09-0370. [DOI] [PubMed] [Google Scholar]
- Pattan SR, Hullolikar RL, Dighe NS, Ingalagi BN, Hole MB, Gaware VM, Chavan PA. Synthesis and Evaluation of some New Phenyl Thiazole Derivatives for their Anti-Inflammatory Activites. J Pharm Sci Res. 2009;1:96–102. [Google Scholar]
- Plimack ER, Lorusso PM, McCoon P, Tang W, Krebs AD, Curt G, Eckhardt SG. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. The Oncologist. 2013;18:819–820. doi: 10.1634/theoncologist.2013-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Ceccarelli C, Berishaj M, Chang Q, Rajasekhar VK, Perna F, Bowman RL, Vidone M, Daly L, Nnoli J, Santini D, Taffurelli M, Shih NNC, Feldman M, Mao JJ, Colameco C, Chen J, DeMichele A, Fabbri N, Healey JH, Cricca M, Gasparre G, Lyden D, Bonafe M, Bromberg J. Self-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancer. Nat Commun. 2016;7:10442. doi: 10.1038/ncomms10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, Dickerson E, Dagia NM, Malgor R, McCall KD. TLR signaling inhibitor, phenylmethimazole, in combination of tamoxifen inhibits human breast cancer cell viability and migration. Oncotarget. 2016 doi: 10.18632/oncotarget.10358. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AL, Malgor R, Dickerson E, Weeraratna AT, Slominski A, Wortsman J, Harii N, Kohn AD, Moon RT, Schwartz FL, Goetz DJ, Kohn LD, McCall KD. Phenylmethimazole decreases Toll-like receptor 3 and noncanonical Wnt5a expression in pancreatic cancer and melanoma together with tumor cell growth and migration. Clin Cancer Res. 2009;15:4114–4122. doi: 10.1158/1078-0432.CCR-09-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui N, Arshad MF, Ahsan W, Alam MS. Thiazoles: A valuable insight into the recent advances and biological activities. Int J Pharm Sci Drug Res. 2009;1:136–143. [Google Scholar]
- Unangst PC, Connor DT, Cetenko WA, Sorenson RJ, Kostlan CR, Sircar JC, Wright CD, Schrier DJ, Dyer RD. Synthesis and Biological Evaluation of 5-[[3,5-Bis(1,1-Dimethylethyl)-4-Hydroxyphenyl]methylene]oxazoles, -Thiazoles, and -Imidazoles - Novel Dual 5-Lipoxygenase and Cyclooxygenase Inhibitors with Antiinflammatory Activity. J Med Chem. 1994;37:322–328. doi: 10.1021/jm00028a017. [DOI] [PubMed] [Google Scholar]
- Vermes C, Jacobs JJ, Zhang J, Firneisz G, Roebuck KA, Glant TT. Shedding of the interleukin-6 (IL-6) receptor (gp80) determines the ability of IL-6 to induce gp130 phosphorylation in human osteoblasts. J Biol Chem. 2002;277:16879–16887. doi: 10.1074/jbc.M200546200. [DOI] [PubMed] [Google Scholar]
- Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70:11–20. doi: 10.1016/j.cyto.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res. 1999;19:1427–1432. [PubMed] [Google Scholar]
- Zheng Y, Basel D, Chow SO, Fong-Yee C, Kim S, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ. Targeting IL-6 and RANKL signaling inhibits prostate cancer growth in bone. Clin Exp Metastasis. 2014;31:921–933. doi: 10.1007/s10585-014-9680-3. [DOI] [PubMed] [Google Scholar]