Abstract
Background
There is a need to identify new prognostic factors that may be used in addition to the known risk factors in gastrointestinal adenocarcinomas. In this study, we aimed to determine the expression of Necl 4 and RNase 5 biomarkers in gastric and colon adenocarcinomas, as well as the prognostic efficacy of these biomarkers in gastric and colon adenocarcinomas.
Material/Methods
Ninety-two cases resected due to stomach and colon adenocarcinoma were included in the study. The expression of Necl 4 and RNase 5 biomarkers was evaluated by immunohistochemical staining of the stomach and colon normal mucosa and adenocarcinoma areas.
Results
In colon adenocarcinomas, there was a significant association between Necl 4 and lymphovascular invasion, vascular invasion, and perineural invasion (p<0.05). There was a significant association between RNase 5 and histological differentiation in colon adenocarcinomas (p<0.05). There was no association between RNase 5 and Necl 4 in gastric or colon adenocarcinomas.
Conclusions
Necl 4 may have prognostic value in colon adenocarcinomas, but it is difficult to ascertain in gastric adenocarcinomas.
MeSH Keywords: Colonic Neoplasms, Prognosis, Stomach Neoplasms
Background
Gastrointestinal cancers are responsible for one-third of all deaths due to malignant neoplasms worldwide, and colon and stomach cancers are the most common [1,2]. Understanding the molecular mechanisms in the tumorigenesis and angiogenesis leading to mortality and morbidity, as well as common colon and gastric cancers, has led to the development of new treatment strategies and new molecular tests. It has been reported that Necl 4 (Alternative names: CADM 4 antibody/IGSF4C antibody/TSLL2 antibody/synCAM4 antibody), a biomarker for immunohistochemical studies, is expressed in the brain, prostate, kidney, liver, lung, and other organs [3]. It has been also reported that Necl 4 is presented at the plasma membrane, and is responsible for cell-to-cell interaction as well as tumor suppression [3]. Understanding the role of this molecule in embryogenesis, tumor formation, and development will be helpful in understanding the physiological and pathological effects in epithelium [3].
Angiogenesis plays a key role in tumor progression [4,5]. Ribonuclease 5 antibody (alternative names, angiogenin antibody/RNase 5/ALS9 antibody) has limited documentation in terms of nuclear expression in neoplastic cells for immunohistochemical studies [5–8]. Angiogenin activates vascular-originated endothelial and smooth muscle cells by binding to membrane proteins and by entrance into nuclear translocation. This molecule also induces cellular responses (cell migration, invasion, proliferation, and formation of tubular structures) [5–8].
Immunohistochemical studies and molecular pathologic analyses provide histological and molecular information for diagnosis, appropriate treatment, and prognosis. In this study, we used immunohistochemical staining to evaluate expression of Necl 4 and RNase 5 biomarkers in gastric and colon adenocarcinoma areas and in normal mucosa. Furthermore, the prognostic utility of these biomarkers in the gastric and colon adenocarcinomas was investigated.
Material and Methods
The study included 92 patients who underwent resection due to stomach and colon adenocarcinoma between 2008 and 2015 at Erzincan University Medical Faculty and Dışkapı Yıldırım Beyazıt Educational Research Hospital. Of the 92 adenocarcinoma cases, 51 were localized in the stomach and 41 were localized in the colon. The fixed tissue samples from each case were removed from the archive and re-examined. These patients were re-evaluated in terms of the parameters of histological differentiation (well/moderate/poor), invasion depth (submucosa, muscularis propria, serosa), lymphovascular invasion (positive/negative), perineural invasion (positive/negative), vascular invasion (positive/negative), and metastasis (positive/negative). Four-micron sections were taken and placed on slides by selecting appropriate blocks. The slides were subjected to alcohol and xylene for 5 min each and were deparaffinized in an oven for 15 min. The specimen slides were subjected to immunohistochemical staining using Necl 4 (Rabbit polyclonal, code: ab69605, Abcam, dilution rate: 1/100) and RNase 5 antibodies (Rabbit polyclonal, code: ab125231; Abcam, dilution rate: 1/100). For this, a fully automated immunohistochemical device (Leica Bond-Max, Melbourne, Australia) was used. After the specimen slides were stained, they were examined according to their respective staining patterns using a light microscope (Olympus BX53, Tokyo, Japan). Membrane staining for Necl-4 and nuclear staining for RNase 5 were assessed. According to this, the intensity was scored as: absence 0, light intensity +1, medium intensity +2, and severe intensity + 3. The percentage of staining was accepted as none (0) if it was less than 5%; focal (1+) if between 5% and 50%; and (2+) diffuse if 50% or more. As a positive control for biomarkers, specific tissues in the data sheet as well as normal colonic mucosa and stromal inflammatory cells were used.
Statistical analysis
Mean, standard deviation, median lowest, median highest, frequency, and ratio values were used in the descriptive statistics of the data. The distribution of variables was measured by the Kolmogorov-Smirnov test. The Mann-Whitney U test was used in the analysis of quantitative data. The chi-square test was used to analyze qualitative data, and the Fischer test was used when chi-square test conditions were not met. The McNemar test was used to analyze recurrent measurements. In the compliance analysis, the Kappa compliance test was used. We used the SPSS 22.0 program for all analyses.
Results
The mean age was 67 years in gastric adenocarcinoma cases and 66 in colon adenocarcinoma cases. Demographic findings of gastric and colon adenocarcinomas are shown in Table 1.
Table 1.
Demographic findings in stomach and colon.
| Gastric-Colon | Min–Max | Median | Mean ±s.s./n-% | |||
|---|---|---|---|---|---|---|
| Gastric | Colon | Gastric | Colon | Gastric | Colon | |
| Age | 37–90 | 35–89 | 67 | 66 | 67.1±11.0 | 65.6±11.5 |
| Sex | Male | 38 74.5% | 27 65.9% | |||
| Female | 13 25.5% | 14 34.1% | ||||
| Lymphovascular invasion | − | 8 15.7% | 17 41.5% | |||
| + | 43 84.3% | 24 58.5% | ||||
| Depth | Submucosa | 4 7.8% | 3 7.3% | |||
| Muscle | 4 7.8% | 8 19.5% | ||||
| Serosa | 43 84.3% | 30 73.2% | ||||
| Perineural invasion | − | 15 29.4% | 23 56.1% | |||
| + | 36 70.6% | 18 43.9% | ||||
| Vascular invasion | − | 29 56.9% | 24 58.5% | |||
| + | 22 43.1% | 17 41.5% | ||||
| Metastasis | − | 9 17.9% | 18 43.9% | |||
| + | 42 82.4% | 23 56.1% | ||||
| Differentiation | Well | 9 17.6% | 12 29.3% | |||
| Moderate | 25 49.0% | 17 41.5% | ||||
| Necl 4 tumor tissue | Low | 17 33.3% | 12 29.3% | |||
| No staining | 7 13.7% | 0 0.0% | ||||
| Less staining | 5 9.8% | 6 14.6% | ||||
| High staining | 39 76.5% | 35 85.4% | ||||
| RNase 5 tumor tissue | No staining | 11 21.6% | 2 4.9% | |||
| Less staining | 18 35.3% | 10 24.4% | ||||
| High staining | 22 43.1% | 29 70.7% | ||||
Patient age, sex distribution, lymphovascular invasion, perineural invasion, vascular invasion, metastasis rate, and differential distribution did not differ significantly from the Necl 4 staining (Figure 1) rate in stomach adenocarcinomas (p>0.05).
Figure 1.
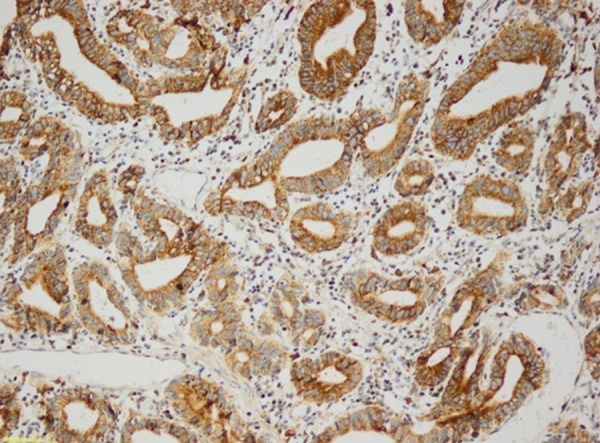
Gastric adenocarcinoma (Necl 4 ×200).
Necl 4 staining (Figure 2) was significantly higher (p<0.05) when lymphovascular invasion was present in the colon adenocarcinomas. In addition, Necl 4 staining was significantly higher in the presence of vascular invasion (p<0.05). Necl 4 staining was also significantly higher (p<0.05) in the presence of perineural invasion (Tables 2, 3). There was no significant difference between Necl 4 staining rate and age, sex distribution, metastasis rate, and differential distribution of the patients (p>0.05).
Figure 2.
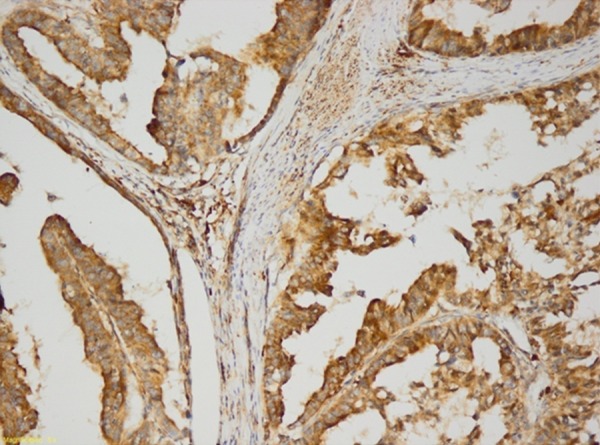
Colon adenocarcinoma (Necl 4 ×200).
Table 2.
Necl 4 and RNase 5 staining rate and the association with prognostic factors in gastric adenocarcinomas.
| Gastric (chi-square test/Mann-Whitney U test) | No or less staining | High staining | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ±s.s./n-% | Mean ±s.s./n-% | ||||||||||
| Necl 4 | RNase 5 | Necl 4 | RNase 5 | Necl 4 | RNase 5 | ||||||
| Lymphovascular invasion | − | 2 | 16.7% | 6 | 20.7% | 6 | 15.4% | 2 | 9.1% | 1.000 | 0.259 |
| + | 10 | 83.3% | 23 | 79.3% | 33 | 84.6% | 20 | 90.9% | |||
| Depth | Submucosa | 0 | 0.0% | 2 | 6.9% | 4 | 10.3% | 2 | 9.1% | 1.000 | 1,000 |
| Muscle | 1 | 8.3% | 3 | 10.3% | 3 | 7.7% | 1 | 4.5% | |||
| Serosa | 11 | 91.7% | 24 | 82.8% | 32 | 82.1% | 19 | 86.4% | |||
| Perineural invasion | − | 3 | 25.0% | 9 | 31.0% | 12 | 30.8% | 6 | 27.3% | 0.701 | 0.770 |
| + | 9 | 75.0% | 20 | 69.0% | 27 | 69.2% | 16 | 72.7% | |||
| Vascular invasion | − | 9 | 75.0% | 16 | 55.2% | 20 | 51.3% | 13 | 59.1% | 0.147 | 0.780 |
| + | 3 | 25.0% | 13 | 44.8% | 19 | 48.7% | 9 | 40.9% | |||
| Metastasis | − | 3 | 25.0% | 6 | 20.7% | 6 | 15.4% | 3 | 13.6% | 0.445 | 0.513 |
| + | 9 | 75.0% | 23 | 79.3% | 33 | 84.6% | 19 | 86.4% | |||
| Differentiation | No staining | 4 | 33.3% | 3 | 10.3% | 5 | 15.4% | 6 | 27.3% | 0.261 | 0.276 |
| Less staining | 5 | 41.7% | 16 | 55.2% | 20 | 15.4% | 9 | 40.9% | |||
| High staining | 3 | 25.0% | 10 | 34.5% | 14 | 15.4% | 7 | 31.8% | |||
Table 3.
Necl 4 and RNase 5 staining rate and the association with prognostic factors in colon adenocarcinomas.
| Colon (chi-square test/Mann-Whitney U test) | No or less staining | High staining | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ±s.s./n-% | Mean ±s.s./n-% | ||||||||||
| Necl 4 | RNase 5 | Necl 4 | RNase 5 | Necl 4 | RNase 5 | ||||||
| Lymphovascular invasion | − | 0 | 0.0% | 6 | 50.0% | 6 | 15.4% | 11 | 37.9% | 0.026 | 0.475 |
| + | 6 | 100.0% | 6 | 50.0% | 18 | 51.4% | 18 | 62.1% | |||
| Depth | Submucosa | 0 | 0.0% | 0 | 0.0% | 3 | 8.6% | 3 | 10.3% | 1,000 | 0,820 |
| Muscle | 1 | 16.7% | 3 | 25.0% | 7 | 20.0% | 5 | 17.2% | |||
| Serosa | 5 | 83.3% | 9 | 75.0% | 25 | 71.4% | 21 | 72.4% | |||
| Perineural invasion | − | 1 | 16.7% | 8 | 66.7% | 22 | 62.9% | 15 | 51.7% | 0.035 | 0.380 |
| + | 5 | 83.3% | 4 | 33.3% | 13 | 37.1% | 14 | 48.3% | |||
| Vascular invasion | − | 1 | 16.7% | 8 | 66.7% | 23 | 65.7% | 16 | 55.2% | 0.024 | 0.497 |
| + | 5 | 83.3% | 4 | 33.3% | 12 | 34.3% | 13 | 44.8% | |||
| Metastasis | − | 1 | 16.7% | 7 | 58.3% | 17 | 48.6% | 11 | 37.9% | 0.146 | 0.231 |
| + | 5 | 83.3% | 5 | 41.7% | 18 | 51.4% | 18 | 62.1% | |||
| Differentiation | No staining | 1 | 16.7% | 7 | 58.3% | 11 | 31.4% | 5 | 17.2% | 0.227 | 0.002 |
| Less staining | 2 | 33.3% | 0 | 0.0% | 15 | 42.9% | 17 | 58.6% | |||
| High staining | 3 | 50.0% | 5 | 41.7% | 19 | 25.7% | 7 | 24.1% | |||
On the other hand, there were no significant difference in RNase 5 staining rate with age, sex distribution, lymphovascular invasion, perineural invasion, vascular invasion, and metastasis rate (p>0.05) in gastric and colonic adenocarcinomas. There was no such significant relationship in gastric adenocarcinomas (Figure 3). The RNase 5 staining (Figure 4) rate was higher when histological differentiation was increased in colon adenocarcinomas. The difference was statistically significant (p<0.05) (Tables 2, 3).
Figure 3.
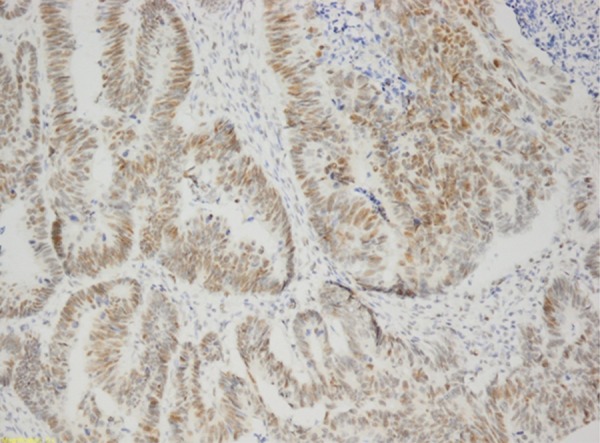
Gastric adenocarcinoma (RNase 5 ×200).
Figure 4.
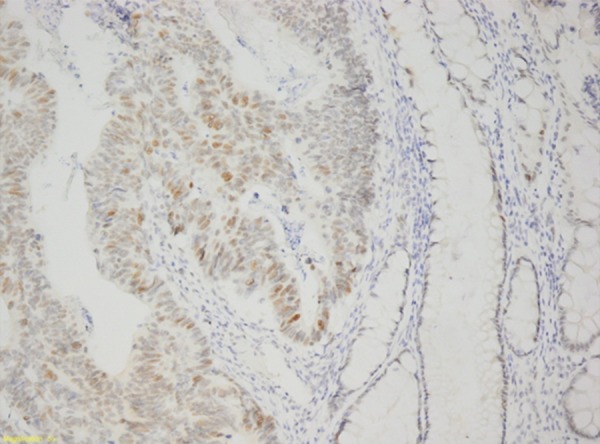
Colon adenocarcinoma (RNase 5 ×200).
Interestingly, there was a significant difference (p<0.05) between normal tissue and tumor tissue staining of Necl 4 and RNase 5 in the stomach and the colon. On the other hand, there was no significant association (p>0.05) between the RNase 5 and Necl 4 staining in stomach and colon adenocarcinomas.
Discussion
Adhesion molecules function specifically in the regulation of cells, such as orientation toward the tissues, recognition of each other, embryogenesis, cell growth, cell differentiation, and inflammation [9–11]. Adhesion molecules are studied in 4 classes today: integrins, selectins, and adhesion molecules, including immunoglobulin family and cadherins [9–11]. In addition, adhesion molecules that function as adhesives are not classified in the above groups [3]. Although previous studies have examined Necl 4 in the prostate and brain, immunohistochemical studies are very limited within these organs. Interestingly, we did not find any immunohistochemical studies in the literature investigating this biomarker in gastric adenocarcinomas [12,13].
On the other hand, a previous report demonstrated that expression of Necl 4 in colon tumors correlated with tumor size, mucinous tumor type, lymph node metastasis, poor differentiation, and high stage [13]. Consistent with this, there was a significant correlation between increased expression of Necl 4 in tumor cells of colon tumors and lymphovascular invasion, vascular invasion, and perineural invasion in our study. When the invasion depth of colon tumors increased, the staining intensified. It is also noteworthy that Necl 4 stained stomach and colon adenocarcinomas at a higher rate compared to normal mucosa in our study. This is an important observation that supports results of several previous reports.
Cell adhesion mutations and angiogenic genes play important roles in invasive and metastatic behavior of gastric and colon carcinomas [14–17]. Angiogenesis is not only associated with the growth of solid tumors, but also with the distant metastases of primary tumors [18–20]. It has an influence on increased cancer progression and poor prognosis in many cancers [18–23]. Furthermore, it has been reported that angiogenin inhibition suppressed and inhibited the growth in tumor cells in rodent models [14,15]. Our study shows that increased nuclear expression of tumor cells may also contribute to tumorigenesis. However, we observed a statistically significant association between this biomarker and histologic differentiation in colon tumors. Furthermore, an increased staining rate as invasion depth increased demonstrated that RNase 5 may contribute to the progression of colon adenocarcinomas.
Conclusions
Necl 4 may have prognostic value in colon adenocarcinomas. However, more work is required to determine its role in gastric adenocarcinomas. Interestingly, RNase 5 was compared to normal gastric and colonic mucosa and demonstrated increased nuclear expression in stomach and colon adenocarcinomas. This is important for drawing attention to the nuclear translocation in neoplastic cells, which suggests that RNase 5 may contribute to tumorigenesis of colon and gastric adenocarcinomas.
Footnotes
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Fukami T, Satoh H, Williams YN, et al. Isolation of the mouse Tsll1 and Tsll2 genes, orthologues of the human TSLC1-like genes 1 and 2 (TSLL1 and TSLL2) Gene. 2003;323:11–18. doi: 10.1016/j.gene.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 5.Nilsson UW, Abrahamsson A, Dabrosin C. Angiogenin regulation by estradiol in breast tissue: tamoxifen inhibits angiogenin nuclear translocation and antiangiogenin therapy reduces breast cancer growth in vivo. Clin Cancer Res. 2010;16(14):3659–69. doi: 10.1158/1078-0432.CCR-10-0501. [DOI] [PubMed] [Google Scholar]
- 6.Abtin A, Eckhart L, Mildner M, et al. Degradation by stratum corneum proteases prevents endogenous RNase inhibitor from blocking antimicrobial activities of RNase 5 and RNase7. J Invest Dermatol. 2009;129(9):2193–201. doi: 10.1038/jid.2009.35. [DOI] [PubMed] [Google Scholar]
- 7.Fett JW, Strydom DJ, Lobb RR, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24(20):5480–86. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Monti DM, Hu G. Angiogenin activates human umbilical artery smooth muscle cells. Biochem Biophys Res Commun. 2001;285(4):909–14. doi: 10.1006/bbrc.2001.5255. [DOI] [PubMed] [Google Scholar]
- 9.Frenette PS, Denisa D, Wagner DD. Adhesion molecules. N Engl J Med. 1996;334:1527–29. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- 10.Evans RD, Perkins VC, Henry A, et al. A tumor associated β1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol. 2003;169:589–96. doi: 10.1083/jcb.200209016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz, Martin A, Michael D, et al. Integrins: Emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–99. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 12.Raveh S, Gavert N, Spiegel I, et al. The cell adhesion nectin-like molecules (Necl) 1 and 4 suppress the growth and tumorigenic ability of colon cancer cells. J Cell Biochem. 2009;108:326–36. doi: 10.1002/jcb.22258. [DOI] [PubMed] [Google Scholar]
- 13.Jang SM, Han H, Jun Y, et al. Clinicopathological significance of CADM4 expression, and its correlation with expression of E-cadherin and Ki-67 in colorectal adenocarcinomas. J Clin Pathol. 2012;65(10):902–6. doi: 10.1136/jclinpath-2012-200730. [DOI] [PubMed] [Google Scholar]
- 14.Zhu B, Chen H, Zhan W, et al. (–)- Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J Gastroenterol. 2011;17(18):2315–25. doi: 10.3748/wjg.v17.i18.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie MM, Fang GE, Wang XH, et al. [Anti-tumor effect of gene-viral therapeutic system CNHK300-murine endostatin on nude mouse gastric cancer]. Chinese Journal of Gastrointestinal Surgery. 2007;10(6):565–69. [in Chinese] [PubMed] [Google Scholar]
- 16.Nasir O, Wang K, Föller M, et al. Downregulation of angiogenin transcript levels and inhibition of colonic carcinoma by Gum Arabic (Acacia senegal) Nutr Cancer. 2010;62(6):802–10. doi: 10.1080/01635581003605920. [DOI] [PubMed] [Google Scholar]
- 17.Satoh Y, Goi T, Nakazawa T, et al. Polysaccharide K suppresses angiogenesis in colon cancer cells. Exp Ther Med. 2012;4(3):370–74. doi: 10.3892/etm.2012.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 19.Li WW, Hutnik M, Gehr G. Antiangiogenesis in haematological malignancies. Br J Haematol. 2008;143(5):622–31. doi: 10.1111/j.1365-2141.2008.07372.x. [DOI] [PubMed] [Google Scholar]
- 20.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6(7):395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 22.Isik A, Okan I, Firat D, et al. A new prognostic strategy for gastric carcinoma: Albumin level and metastatic lymph node ratio. Minerva Chir. 2014;69(3):147–53. [PubMed] [Google Scholar]
- 23.Peker K, Sayar I, Gelincik I, et al. The diagnostic importance of matrix metalloproteinase-7 and nestin in gastrointestinal stromal tumors. Med Sci Monit. 2014;20:674–80. doi: 10.12659/MSM.890303. [DOI] [PMC free article] [PubMed] [Google Scholar]


