Abstract
Background
Obesity has been linked with a pro-inflammatory state and the development of inflammatory diseases, including inflammatory bowel disease (IBD). However, there is some controversy regarding whether obesity is associated with an adverse clinical course in patients with IBD. The aim of this meta-analysis was to assess the association between obesity and clinical outcomes in IBD patients.
Material/Methods
Electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) were systematically searched for studies investigating the association between obesity and clinical outcomes in patients with IBD. A meta-analysis was performed using Review Manager software.
Results
Among the 4,798 articles identified, seven met the inclusion criteria for our meta-analysis. The pooled data revealed that obese patients were significantly less likely to undergo IBD-related surgery, receive hormone therapy, and experience hospitalization compared with non-obese patients. However, no statistically significant difference was observed in perianal disease, anti-TNF use, and immunomodulator use between the two groups.
Conclusions
Our meta-analysis indicated that clinical outcomes were significantly different in obese versus non-obese patients with IBD. We found that obesity was associated with a less severe disease course of IBD. Future prospective studies are needed to confirm the relationship between obesity and the clinical course of IBD.
MeSH Keywords: Anastomosis, Surgical; Inflammatory Bowel Diseases; Obesity, Abdominal
Background
Excess body weight has emerged as a major problem affecting human beings worldwide, and obesity has been associated with a pro-inflammatory state and the development of inflammatory diseases [1]. Hence, great attention has been paid to understanding obesity. Obesity has been found to be related to the rise and severity of illnesses, including diabetes mellitus, cancer, and cardiovascular disease [2].
The two major forms of inflammatory bowel disease (IBD) are Crohn’s disease (CD) and ulcerative colitis (UC), which are both lifelong immunologically mediated disorders categorized by chronic inflammation and progressive damage to the gastrointestinal tract [3]. IBD is currently one of the most investigated human disorders, and for at least two decades it has been the focus of intense attention in basic science, translational, and clinical research. Advances in scientific knowledge of IBD pathophysiology have helped in developing novel medications to combat gut inflammation with a considerable degree of success [4].
IBD once was associated with low body weight, weight loss, and malnutrition, but recent studies have identified a growing prevalence of obesity in IBD patients [5]. In addition, some studies have explored the clinical impact of obesity on IBD. Adult CD patients with increased BMI were reported to have a predisposition for perianal disease and IBD-related surgery [6,7]. Similarly, Mendall et al. [8] described a positive connection between obesity and the development of CD. However, Flores et at. [5] found that obesity (as defined by BMI) was a marker of a less severe disease course in IBD patients. Thus, an improved understanding of the association between obesity and clinical outcomes in IBD seems to be necessary. According to the World Health Organization (WHO) guideline, BMI >30 kg/m2 is considered obese [9]. We performed this meta-analysis of observational studies to better evaluate the impact of obesity on the clinical course of IBD patients.
Material and Methods
Search strategy
This study was conducted in accordance with the guideline for Meta-analysis of Observational Studies in Epidemiology (MOOSE) group [10]. Our search strategy was performed using the databases of Medline, Embase, Web of Science, and the Cochrane Library for dates up to March 2016 to identify eligible studies. The search strategy combined free keywords with Mesh Terms as following words: (‘obesity’ or ‘obese’ or ‘body mass index’ or ‘BMI’ or ‘adiposity’) and (‘inflammatory bowel disease’ or ‘IBD’ or ‘crohn’ or ‘ulcerative colitis’).
Only English language articles were included. In addition, the reference lists of retrieved studies were also checked for additional studies that met the criteria but were not found by the electronic search.
Inclusion criteria and study selection
Studies were included if they were controlled or comparative studies that focused on the influence of obesity on clinical outcomes in IBD patients. Of note, studies focused on the pediatric IBD were excluded. The outcomes we evaluated were IBD-related surgery, perianal disease, hormone use, anti-TNF use, immunomodulator use, and hospitalization. Studies involving at least one outcome were included; review articles, expert opinions, and trials without reporting the outcome measures of interest were excluded. Obesity in selected articles was defined by BMI >30 kg/m2 according to the WHO guideline.
Quality assessment and data extraction
Two investigators (LGW, RHJ) independently assessed the studies selection and data extraction. Any disagreements were resolved by discussion and eventually determined by a senior author (WGF). Seven observational studies were included in this meta-analysis, with publication dates ranging from 2002 to 2015. The available data from the selected studies included: first author’s name, publication year, country, number of obese and non-obese patients.
Quality assessment of the observational studies included in this meta-analysis was assessed by the Newcastle Ottawa Scale (NOS) [11] as recommended by the Cochrane non-randomized studies methods working group, which consisted of population selection, comparability of exposed (obese) and unexposed (non-obese) and adequate assessment of clinical outcomes. Uncertainty or discrepancy was discussed to reach consensus. Ratings for the level of evidence for each study were assigned using criteria established by Wright et al. [12].
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to estimate the quality of the evidence for every clinical course. Although the evidence of observational research was considered as low-quality using the GRADE approach, several criteria helped to elevate the quality level: a large effect, a dose-response gradient, and if all plausible confounding factors would decrease an obvious treatment effect or, in case of no effect, would create a spurious effect [13]. The evidence quality for each outcome was generally moderate (Table 1).
Table 1.
Results of the meta-analysis.
| Outcomes | Number of studies [references] | Number of patients | RR (95% CI) | Heterogeneity | GRADE evidence | |
|---|---|---|---|---|---|---|
| Obese | Non-obese | |||||
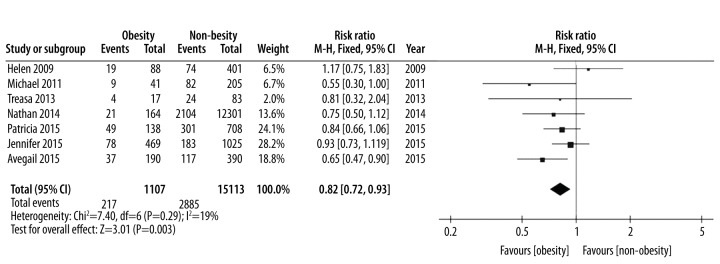
| IBD-related surgery | 7 [5,8,15–19] | 1107 | 15113 | 0.82 (0.72, 0.93) | I2=19%, P=0.29 | Moderate |
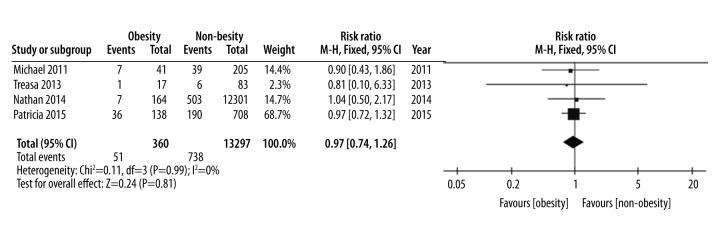
| Perianal disease | 4 [8,16–18] | 360 | 13297 | 0.97 (0.74, 1.26) | I2=0%, P=0.81 | Moderate |
| Medical treatment | ||||||
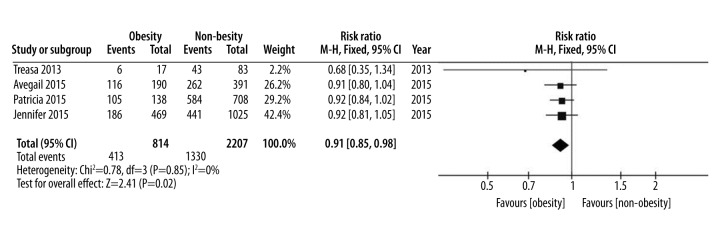
| Hormone use | 4 [5,16,17,19] | 814 | 2207 | 0.91 (0.85, 0.98) | I2=0%, P=0.85 | Moderate |
| Anti-TNF use | 3 [5,16,19] | 287 | 906 | 0.89 (0.72, 1.09) | I2=71%, P=0.03 | Low |
| Immunomodulator use | 3 [5,16,19] | 659 | 1415 | 0.96 (0.88,1.06) | I2=0%, P=0.43 | Moderate |
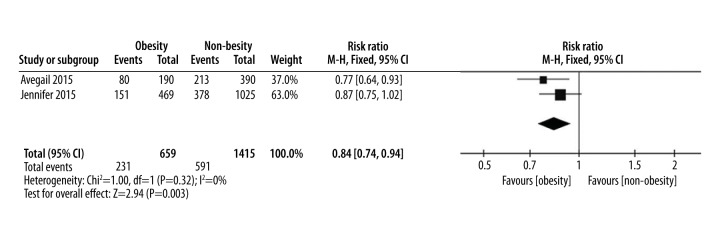
| Hospitalization | 2 [5,19] | 659 | 1415 | 0.84 (0.74, 0.94) | I2=0%, P=0.32 | Moderate |
GRADE Working Group grades of evidence: High quality – further research is very unlikely to change our confidence in the estimate of effect; Moderate quality – further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality – further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; RR – risk ratio; GRADE – Grading of Recommendations Assessment, Development and Evaluation; TNF – tumor necrosis factor.
Statistical analysis
Revman 5.3 software (The Nordic Cochrane Centre, Copenhagen, Denmark) was used to pool data. Relative risk (RR) with 95% CI was calculated to assess the association between obesity and IBD-related surgery, perianal disease, hormone use, immunomodulator use, and anti-TNF use. Z test was used to evaluate the statistical significance of the pooled estimates. A value of p<0.05 was considered to be statistically significant. The I2 statistic and Cochrane’s Q were used to explore heterogeneity across studies [14]. In absence of significant heterogeneity (Cochrane’s Q p>0.10 and I2 <5 0%), the data was pooled using a fixed-effect model. Otherwise, the random-effect model was used.
Results
Characteristics of included studies
We identified seven studies published from 2002 to 2015 that met our inclusion criteria for meta-analysis [5,8,15–19]. The detailed procedures of our literature research are shown in Figure 1. Among the included studies, four were performed in USA, two in the UK and one in Ireland. The quality rating of the included studies ranged from six to eight stars using the NOS. More comprehensive information about the study characteristics is presented in Table 2. Not all of the studies contain the defined interest of outcomes: seven studies for IBD-related surgery; four studies for perianal disease; four studies for hormone use; three studies for anti-TNF use; three studies for immunomodulator use; and two studies for hospitalization.
Figure 1.
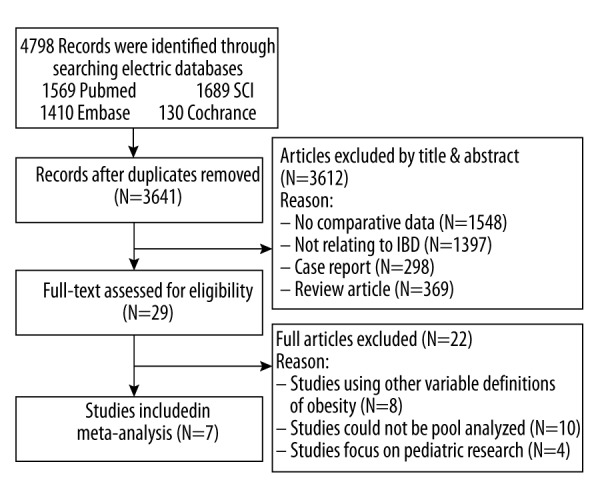
The flow chart shows the article selection process we performed for this meta-analysis. SCI – Web of Science; IBD – inflammatory bowel disease.
Table 2.
Characteristics of selected studies.
| Study | Setting | Enrolment time | Study design | Diagnosis | Number of patients | Mean age (years) | NOS score | LOE* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Obese | Non-obese | Obese | Non-obese | |||||||
| Avegail, 2015 [5] | USA | 2000–2012 | Retrospectively | IBD | 190 | 391 | NR | NR | 8 | II |
| Michael, 2011 [8] | UK | 2001–2008 | Retrospectively | CD | 41 | 205 | NR | NR | 6 | II |
| Helen, 2009 [15] | UK | Preceding 12 months | Retrospectively | IBD | 88 | 401 | NR | NR | 6 | II |
| Patricia, 2015 [16] | USA | 2004–2015 | Retrospectively | IBD | 138 | 708 | 38 | 45 | 6 | II |
| Treasa, 2013 [17] | Ireland | NR | Prospectively | CD | 17 | 83 | 35 | 41 | 7 | I |
| Nathan, 2014 [18] | USA | 2009 | Retrospectively | IBD | 164 | 12301 | 16 | 16 | 7 | II |
| Jennifer, 2015 [19] | USA | 2009–2011 | Prospective | IBD | 469 | 1025 | 43 | 48 | 7 | I |
NOS – Newcastle Ottawa Ottawa; LOE – levels of evidence; CD – Crohn’s disease; IBD – inflammatory bowel disease; NR – no reported.
A levels of evidence based on Wright et al. [11].
Meta-analysis of obesity and clinical course in IBD patients
Seven articles reported IBD-related surgery. To be specific, the interested data of this outcome included the number of patients with at least one surgery. A fixed-effect model was employed in pooling the data about IBD-related surgery owing to low heterogeneity (p=0.29, I2=19%). The calculated results demonstrated that obese patients were significantly less likely to undergo surgery than non-obese patients (RR: 0.82, 95% CI 0.72–0.93, p=0.003) (Table 1, Figure 2). The funnel plot of IBD-related surgery was employed to evaluate publication bias. The funnel plot showed symmetrical which suggested no publication bias for surgery meta-analysis (Figure 3).
Figure 2.
IBD-related surgery, forest plot.
Figure 3.
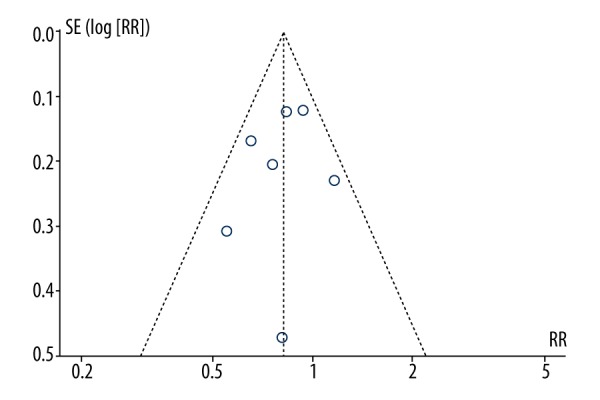
The funnel plot of the IBD-related surgery.
Four studies involving 13,297 patients examined the association between obesity and perianal disease in patients with IBD, and a fix-effect model was used because of low heterogeneity (p=0.99, I2=0%). The pooled analysis of data suggested that there was no significant difference in perianal disease between obese and non-obese groups (RR=0.97, 95% CI 0.74–1.26, p=0.81) (Table 1, Figure 4).
Figure 4.
Perianal disease, forest plot.
As for medical treatment, the data for hormone use, anti-TNF use, and immunomodulator use were pooled. Four studies were included for hormone use, three studies for anti-TNF use, and only two for immunomodulator use. A fixed-effect model was employed for hormone use, and the results demonstrated that no heterogeneity between the groups (p=0.61, I2=0%) and less hormone use was observed in the obese patient group than in the non-obese patient group (RR=0.80, 95% CI 0.68–0.96, p=0.01) (Figure 5). However, according to the overall pooled data for anti-TNF use and immunomodulator use, no differences were observed between the obese patient group and non-obese patient group (RR=0.89, 95% CI 0.72–1.09, p=0.26 and RR=0.96, 95% CI 0.88–1.06, p=0.43, respectively) (Supplementary Figures 1, 2).
Figure 5.
Hormone use, forest plot.
Only two studies were eligible for data extraction for hospitalization outcomes. The overall pooled results showed a significant difference between the two groups (RR=0.84, 95% CI 0.74–0.94, p=0.003). Obese IBD patients would be less likely to experience hospitalization than non-obese IBD patients. There was no heterogeneity among the studies with an I2 of 0% (Figure 6).
Figure 6.
Hospitalization, forest plot.
Discussion
According to the WHO, overweight and obesity are linked to more deaths worldwide; in addition, 44% of diabetes cases, 23% of ischemic heart disease cases and 7–41% of certain cancer cases are attributable to overweight and obesity [20]. Obesity has been reported to become increasingly more common among IBD patients, although IBD once was considered a disorder associated with low body weight [21]. However, results of the association between obesity and the clinical outcomes of IBD patients remain a subject for debate. The aim of our meta-analysis was to evaluate IBD-related surgery, perianal disease, medical treatment (hormone use, anti-TNF use, and immunomodulator use), and hospitalization in obese IBD and non-obese IBD patients.
The results of our meta-analysis showed that obese IBD patients were significantly less likely to receive hormone treatment, undergo surgery, or experience a hospitalization than non-obese IBD patients and no significant differences were observed in perianal disease, anti-TNF use, and immunomodulator use. In spite of the plausible mechanisms whereby obesity might exacerbate IBD progression, we have found that obesity (as defined by BMI) was a reflection of a less severe disease course or represented “wellness” in obese patients compared to non-obese patients in IBD.
IBD patients were previously considered to be malnourished. One of earliest studies found that 3.6% of 2,065 French patients with CD suffered from obesity [6]. More recent studies found relatively high rates of obesity in both adult and pediatric IBD patients [15,17,22,23]. The rise in the prevalence of IBD in Western countries has not been as tremendous as the overall rise in the prevalence of obesity. This suggests that obesity may not contribute to the pathogenesis of IBD and that the increase in the frequency of obesity in IBD patients merely mirrors the rising frequency of obesity in the general population. A recent European epidemiologic study, in support of this contention, found no relationship between high BMI and the development of IBD [1].
Although we did not find that obesity exacerbated IBD, there are plausible biological mechanisms for how obesity may affect the clinical outcomes of IBD patients. Excess adipose tissue would probably contribute to these plausible mechanisms, which could lead to a hyper-inflammatory state [24]. In addition, BMI has been associated with C-reactive protein (CRP, an inflammatory marker) in healthy people [25]. Owing to inflammatory cytokines and adipokines produced by adipose tissue, excess adiposity conceivably might contribute to inflammation in IBD [26]. However, unlike other studies, the results of our meta-analysis did not find an association between obesity and the exacerbation of IBD. We considered whether this could be a result of a poor linear relationship between BMI and total body fat. For instance, if BMI as a measure of body fat is inaccurate, it can lead to bias in measuring the effects of obesity on health outcomes, thus any calculation of risk ratios, risk differences, or attributable proportions will reflect the error inherent in BMI as a measure of obesity and potentially be biased [27]. For example, waist-to-hip ratio (WHR), one of the alternative measures of adiposity, can properly measures different aspects of body composition and fat distribution. Although WHR is a better marker of central adiposity and visceral fat, it does not differentiate between the different visceral fat compartments effectively [28]. Furthermore, recently studies have suggested that mesenteric fat is the significant response factor in CD, as opposed to subcutaneous adipose tissue, and obesity per se [28,29]. In CD, for some patients, mesenteric fat overexpresses CRP compared with subcutaneous adipose fat, and it may be that mesenteric fat is involved in the adverse clinical course of IBD. In addition, a prospective population-based study found that the high inflammatory response caused by mesenteric fat correlated with poor clinical outcomes leading to surgery [30]. Another study performed in healthy Chinese persons has associated central obesity from waist circumference (i.e., >90 cm in men and >85 cm in women) with mesenteric fat thickness based on ultrasonic examination [31]. These study results suggest that other measures of adiposity and fat compartments are required to better explain the relationship between increased BMI and clinical outcomes in IBD patients.
To our surprise, our meta-analysis found that the clinical course of the non-obese IBD patient group was more severe than for the obese IBD patient group. One of possible reason to explain this phenomenon is that a lower BMI accelerates IBD activity while a higher BMI protects against it. However, it is far more likely that a lower BMI is just the result of inflammatory progression rather than the cause of IBD activity and that obesity is merely a reflection of less aggressive or less severe IBD. One of the included studies in our meta-analysis by Treasa et al. [17] found significantly lower Crohn’s disease activity index and white cell count levels in overweight and obese CD patients compared with healthy weight CD patients, and they suggested that systemic fat mass does not appear to have a negative impact on CD severity, which supports our results. Though our results suggest that obesity is not a risk factor for IBD severity, potential biological mechanisms for the association between obesity and disease progression in IBD needs to be clarified.
There are some limitations in this meta-analysis. Most of the studies included were retrospective case-control studies, which are more prone to have selection, detection, and performance biases. Our literature review only searched English language reports, which may have missed studies. In addition, our study was based on only on the results sections from published reports; therefore, some articles with high quality data could not be entered into the pooled analysis for lack of primary data. In addition, half of the studies were from the USA (n=4); the results may or may not generalize well to other populations. As a result, future studies conducted in other countries are needed.
Conclusions
We have shown in our meta-analysis that there were significance differences in clinical outcomes between obese and non-obese patients with IBD, and obese IBD patients were less likely to undergo surgery, receive hormone therapy, and experience hospitalization. Despite the plausible biological mechanisms whereby obesity might exacerbate IBD or other measurements of adiposity correlated with the adverse outcomes in IBD, we have discovered that obesity defined by BMI was a reflection of a less severe course in IBD.
Supplementary Figures
Anti-TNF use, forest plot.
Immunomodulator use, forest plot.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
Source of support: This study was supported by grants from National Natural Science Foundation of China (81270478)
References
- 1.Chan SS, Luben R, Olsen A, et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: Data from a European Prospective Cohort Study (The IBD in EPIC Study) Am J Gastroenterol. 2013;108:575–82. doi: 10.1038/ajg.2012.453. [DOI] [PubMed] [Google Scholar]
- 2.Tobias DK, Pan A, Jackson CL, et al. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233–44. doi: 10.1056/NEJMoa1304501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lankarani KB, Sivandzadeh GR, Hassanpour S. Oral manifestation in inflammatory bowel disease: A review. World J Gastroenterol. 2013;19:8571–79. doi: 10.3748/wjg.v19.i46.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiocchi C. Inflammatory bowel disease pathogenesis: Where are we? J Gastroenterol Hepatol. 2015;30:12–18. doi: 10.1111/jgh.12751. [DOI] [PubMed] [Google Scholar]
- 5.Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: A marker of less severe disease. Dig Dis Sci. 2015;60:2436–45. doi: 10.1007/s10620-015-3629-5. [DOI] [PubMed] [Google Scholar]
- 6.Blain A, Cattan S, Beaugerie L, et al. Crohn’s disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 7.Hass DJ, Brensinger CM, Lewis JD, Lichtenstein GR. The impact of increased body mass index on the clinical course of Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:482–88. doi: 10.1016/j.cgh.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011;56:837–44. doi: 10.1007/s10620-010-1541-6. [DOI] [PubMed] [Google Scholar]
- 9.Consultation WHO, who and who. Obesity: Preventing and Managing the Global Epidemic: Report of a Who Consultation. 2000. Obesity: Preventing and managing the global epidemic - Introduction; pp. 1–253. [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology – A proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Wright JG, Swiontkowski MF, Heckman JD. Introducing levels of evidence to the Journal. J Bone Joint Surg Am. 2003;85-A(1):1–3. [PubMed] [Google Scholar]
- 13.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–94. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steed H, Walsh S, Reynolds N. A brief report of the epidemiology of obesity in the inflammatory bowel disease population of Tayside, Scotland. Obes Facts. 2009;2:370–72. doi: 10.1159/000262276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pringle PL, Stewart KO, Peloquin JM, et al. Body mass index, genetic susceptibility, and risk of complications among individuals with Crohn’s disease. Inflamm Bowel Dis. 2015;21(10):2304–10. doi: 10.1097/MIB.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nic Suibhne T, Raftery TC, McMahon O, et al. High prevalence of overweight and obesity in adults with Crohn’s disease: Associations with disease and lifestyle factors. J Crohns Colitis. 2013;7(7):e241–48. doi: 10.1016/j.crohns.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Zwintscher NP, Horton JD, Steele SR. Obesity has minimal impact on clinical outcomes in children with inflammatory bowel disease. J Pediatr Surg. 2014;49:265–68. doi: 10.1016/j.jpedsurg.2013.11.033. discussion 268. [DOI] [PubMed] [Google Scholar]
- 19.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2857–63. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Obesity and Overweight. [accessed June 2014]. 2006. URL http://www.mclveganway.org.uk/Publications/WHO_Obesity_and_overweight.pdf.
- 21.Ferguson A, Sedgwick DM. Juvenile-onset inflammatory bowel-disease – height and body-mass index in adult life. BMJ. 1994;308:1259–63. doi: 10.1136/bmj.308.6939.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease 12. Inflamm Bowel Dis. 2011;17:2162–68. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kugathasan S, Nebel J, Skelton JA, et al. Body mass index in children with newly diagnosed inflammatory bowel disease: Observations from two multicenter North American inception cohorts. J Pediatr. 2007;151:523–27. doi: 10.1016/j.jpeds.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield JR, Samaras K, Jenkins AB, et al. Obesity is an important determinant of baseline serum C-reactive protein concentration in monozygotic twins, independent of genetic influences. Circulation. 2004;109:3022–28. doi: 10.1161/01.CIR.0000130640.77501.79. [DOI] [PubMed] [Google Scholar]
- 26.Karmiris K, Koutroubakis IE, Kouroumalis EA. The emerging role of adipocytokines as inflammatory mediators in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:847–55. doi: 10.1097/01.mib.0000178915.54264.8f. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32(Suppl 3):S56–59. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 28.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec family study. Am J Clin Nutr. 2001;74:315–21. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 29.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: A predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–23. doi: 10.1136/gut.2007.146357. [DOI] [PubMed] [Google Scholar]
- 31.Zulian A, Cancello R, Micheletto G, et al. Visceral adipocytes: Old actors in obesity and new protagonists in Crohn’s disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anti-TNF use, forest plot.
Immunomodulator use, forest plot.