Abstract
Background
Following severe trauma, treatment of cutaneous injuries is often delayed by inadequate blood supply. The aim of the present study was to determine whether granulocyte-colony stimulating factor (G-CSF) protects endothelial cells (ECs) and enhances angiogenesis in a rat model of hemorrhagic shock (HS) combined with cutaneous injury after resuscitation.
Material/Methods
The HS rats with full-thickness defects were resuscitated and randomly divided into a G-CSF group (200 μg/kg body weight), a normal saline group, and a blank control group. Histological staining was to used estimate the recovery and apoptosis of skin. Apoptosis- and angiogenesis-related factors were analyzed by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot (WB). Scratch assay, tube formation, and WB experiments were performed to verify the functional effects of G-CSF on HUVECs in vitro.
Results
H&E staining and Masson trichrome staining showed earlier inflammation resolution and collagen synthesis in the G-CSF-treated group. Angiogenesis-related factors were elevated at mRNA and protein levels. TUNEL staining suggested fewer apoptotic cells in the G-CSF group. The apoptotic-related factors were down-regulated and anti-apoptotic factors were up-regulated in the G-CSF-treated group. Scratch assay and tube formation experiments revealed that G-CSF facilitated migration ability and angiogenic potential of HUVECs. The angiogenic and anti-apoptotic effects were also enhanced in vitro.
Conclusions
Our results suggest that G-CSF after resuscitation attenuates local apoptosis and accelerates angiogenesis. These findings hold great promise for improving therapy for cutaneous injury in severe trauma and ischemia diseases.
MeSH Keywords: Angiogenesis Modulating Agents; Apoptosis; Endothelial Cells; Receptors, Granulocyte Colony-Stimulating Factor
Background
Traumatic hemorrhagic shock (THS) is a life-threatening condition. Without proper control, it can lead to multiple organ damage syndrome (MODS) and even death. In our previous study, we demonstrated that resuscitation and G-CSF treatment accelerate wound repair in hemorrhagic shock. The present study was focused on the protective mechanisms of G-CSF after resuscitation treatment in a rat model of cutaneous injury combined with hemorrhagic shock.
Wound healing consists of well-orchestrated processes, including inflammation, proliferation and maturation, and successive remolding phases [1,2]. However, skin injuries are affected by other severe trauma in traffic accidents or war injuries, which easily develop into non-healing wounds [3,4]. Although combined injuries like hemorrhagic shock combined with cutaneous injury often occur in traffic accidents and war, little is known about wounds affected by blood loss and the wound repair process. The restriction of blood supply to skin after blood loss leads to tissue hypoxia and even tissue necrosis [5–7]. Severe blood supply inefficiency and tissue apoptosis hinder wound repair because of endothelial cell (ECs) dysfunction in injured tissues [8].
G-CSF, a hematopoietic cytokine and potent stem cell mobilization agent, has been applied in preclinical as well as clinical therapies [9–11]. G-CSF showed its angiogenesis property in endothelial cells (ECs) in vivo and in vitro [12–14], and ECs were reported to actively proliferate and form vascular-like structures in response to G-CSF [15]. Consequently, G-CSF treatment had been considered as an alternative approach for angiogenesis therapy [16,17]. Recently, increasing evidence suggested that G-CSF has tissue-protective roles through enhancing anti-apoptotic and anti-inflammatory capabilities of the host cells [18–22]. However, the effects on ECs in hemorrhagic shock combined with cutaneous injury have not been elucidated. In this study, we aimed to determine if G-CSF would benefit wound repair by accelerating angiogenesis and anti-apoptotic abilities of ECs in hemorrhagic shock.
G-CSF has been proved to promote tissue repair and regeneration in many injuries. In the present study, we applied a rat model of hemorrhagic shock combined with cutaneous injury. We found that G-CSF injection after resuscitation accelerated wound repair progression by stimulating angiogenesis and promoting early anti-apoptotic capacities. In vitro experiments showed that G-CSF increased the migration and tube formation and enhanced the anti-apoptotic abilities of HUVECs.
Material and Methods
Experimental animals
A total of 54 male SD rats (250–300 g) were obtained from the Experimental Animal Department of the Chinese PLA General Hospital. All animal procedures in this study were conducted in accordance with the guidelines of the Institutional Animal Care Committee of the Chinese PLA General Hospital and were carried out in accordance with the guidelines of the China Council on Animal Care and Use (Approval ID: 2013022089). All surgeries and measurements were performed under sodium pentobarbital anesthesia and we tried to minimize suffering. The animals were kept under standard pathogen-free conditions, with 25±1°C room temperature, humidity 50±5%) 12-h light/dark cycles, in individual cages, and fed pellet diet and water ad libitum.
Animal model
The hemorrhagic shock combined with cutaneous injury model was established as previously described [23]. Briefly, the SD rats were anesthetized with sodium pentobarbital (40 mg/kg), and a supplement was given during the experiment. First, a full-thickness, 3-cm, circular excision wound was made after shaving the targeted dorsal fur and disinfection with iodophor. Wounds were dressed with sterile gauze held in place by elastic bandages. The dressings were wetted by normal saline and removed 3 days after injury. Controlled hemorrhagic shock was induced by withdrawing 40% of the total blood volume from the carotid artery during 1 h. All the rats received hypertonic saline solution resuscitation (4 mL/kg body weight, 7.2% NaCl/6% hydroxyethyl starch, Fresenius Company, Germany), followed by reinfusing half of the withdrawn blood. Then, all the rats were randomly divided into 3 groups: in the G-CSF group (n=18), G-CSF was subcutaneously administrated (200 μg/kg, Kyowa Hakko Kirin, Japan) for 3 consecutive days; and the control groups received an equal dose of normal saline (normal saline group, n=18) for 3 days or not (blank group, n=18), separately. All surgical procedures were performed under sterile conditions.
H&E staining and Masson Trichrome staining
At indicated timepoints after injury, the rats were killed by overdose sodium pentobarbital injection and the wounded skins were harvested for further analysis. The wound tissues were fixed with formaldehyde solution for at least 48 h and then embedded in paraffin. The sections (6-μm) were dewaxed in a graded xylene series, followed by standard H&E and Masson trichrome staining to reveal the morphology and collagen deposition during the wound recovery process. The specimens were photographed using a light microscope (Leica TCS SP2, Germany).
TUNEL assay
The tissue apoptosis was evaluated by use of an in situ TUNEL kit (Promega, USA) according to the manufacturer’s instructions. The paraffin-embedded skin tissue sections were deparaffinized and rehydrated and treated by proteinase K. Then, the sections were incubated with a nucleotide mixture containing fluorescein 12-UTP and TDT. The sections were visualized using 3′-diaminobenzidine tetrahydrochloride (DAB) solution (TIANGEN, China). The positively stained cells were counted under an inverted phase-contrast microscope (Leica, Germany).
Real-time PCR (RT-PCR)
The cDNA of each sample was synthesized by a RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) from 3 μg of total RNA. The relative mRNA expression was quantified by RT-PCR using SYBR green qPCR Mix (TOYOBO, Japan). RT-PCR was performed on an ABI PRISM 7500 device (Applied Biosystems, USA). The primers used are listed in Table 1. Each experiment was performed in triplicate. The relative gene expression was normalized by β-actin and calculated using ABI PRISM 7500 v. 2.0.6 software (Applied Biosystems, USA) with the 2−ΔΔCt method.
Table 1.
Primers used in qRT-PCR.
| Primers | Temperature | Sequences | Length |
|---|---|---|---|
| IL4 | 55°C | Forward TGATGTACCTCCGTGCTTGA | 197 bp |
| Reverse AGGACATGGAAGTGCAGGAC | |||
| CD31 | 55°C | Forward ATGGCCCAGAAATCAAGGAGC | 169 bp |
| Reverse ACCTCCAAGCAAAGCAAAGA | |||
| Col I | 55°C | Forward GACGGCTGGAGGAGAGTTC | 123 bp |
| Reverse CTGGTGAACGTGGTGCAG | |||
| α-SMA | 55°C | Forward AGGGAGTGATGGTTGGAATG | 107 bp |
| Reverse GATGATGCCGTGTTCTATCG | |||
| HGF | 55°C | Forward CATTGGTAAAGGAGGCAGCTATAAA | 253 bp |
| Reverse GGATTTCGACAGTAGTTTTCCTGTAGG | |||
| Bcl 2 | 56°C | Forward TTCGGGATGGAGTAAACTGG | 150 bp |
| Forward AAGGCTCTAGGTGGTCATTCAG | |||
| β-actin | 56°C | Forward GAGAGGGAAATCGTGCGTGAC | 580 bp |
| Reverse CATCTGCTGGAAGGTGGACA |
Col I – collagen I; HGF – hepatocyte growth factor.
Cell migration and tube formation assay
To examine the effect of G-CSF on HUVECs migration, the migration and tube formation abilities of the endothelial cells were assessed. The HUVECs were seeded on a 24-well plate. The original wounds were inflicted by dragging a sterile pipette tip across the monolayer, creating 350-mm cell-free paths. The medium was then added separately to different concentrations of G-CSF at 0.5, 5, 50, 500, and 1000 ng/mL for 12 h. The vascular tube formation assay was performed by seeding the HUVECs in a 24-well plate precoated with Matrigel™ (BD, Germany), adding different concentrations of G-CSF and incubating the plates for 12 h at 37°C. The number of capillary-like structures was quantified and visualized under an inverted phase-contrast microscope (Leica, Germany).
Western blotting
For tissue protein extraction, skin tissues were frozen and then ground in tissue lysis buffer. For cell protein extraction, the HUVECs were cultured on a 6-well plate and pretreated with G-CSF in different concentrations for 24 h and followed by H2O2 (50 μmol/L) treatment for 6 h, then the cells were lysed and total protein was harvested. The proteins were separated by 12% SDS-PAGE and then electroblotted by PVDF membranes. The blots were blocked for 1 h in 5% nonfat dry milk, and the first antibodies were added at the following proper dilutions and kept overnight at 4°C: pAKT antibody (1: 1500, CST technology, USA), AKT antibody (1: 1500, CST technology), VEGF (1: 1000, Abcam, USA), α-SMA (1: 500, BOSTER, China), collagen I (1: 500, BOSTER), Bcl 2 (1: 1000, CST technology), caspase 3 (1: 1500, CST technology), and mouse monoclonal β-actin antibody (1: 4000, Santa Cruz, USA). The blots were incubated with HRP-conjugated secondary antibody at 1: 3000 (Santa Cruz) for 50 min at room temperature using an orbital shaker. Finally, the bands were visualized using a SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific) and exposed on X-ray images. β-actin was loaded as an internal control and protein expressions were quantified by use of Image J software.
Statistical analysis
The results are depicted as mean ±SD. The statistical comparisons used the paired Wilcoxon’s test using SPSS 11.0 software (SPSS, Inc., USA). P-value <0.05 was considered to be statistically significant.
Results
G-CSF improved wound repair under hemorrhagic shock
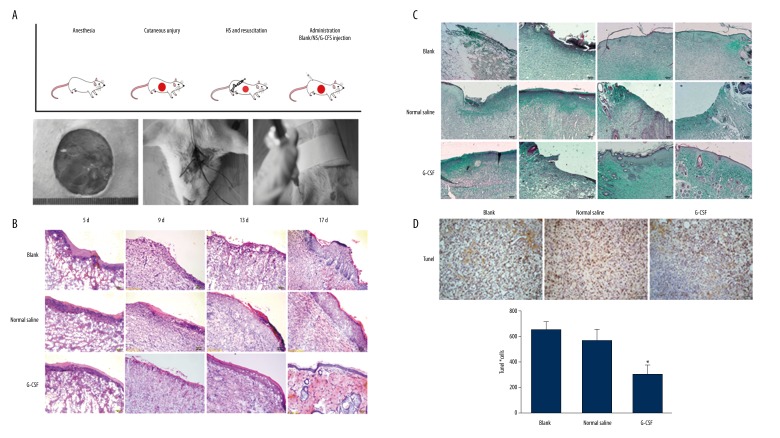
The hemorrhagic shock combined with cutaneous injury rat model was established as shown in Figure 1A. The wounded skin tissues were sectioned and stained with H&E and Masson trichrome (Figures 1B, 1C). The H&E staining results showed less inflammatory cells infiltration and earlier initiation of re-epithelialization in the G-CSF group on day 5. The G-CSF group exhibited more rapid epithelialization on day 9 and few cutaneous appendages such as follicular sebaceous units developed by day 17. Strong collagen staining with Masson trichrome suggested that the G-CSF-treated group initiated more rapid collagen synthesis and deposition after day 5, more homogeneous distribution by day 9, and continuously stable expression with complete healing by day 17. In addition, we analyzed the tissue apoptosis in the wounded skin by TUNEL staining (Figure 1D, 1E). The number of TUNEL-stained cells was significantly lower in the G-CSF group than in the normal saline group and blank group on day 9 (p<0.05). Thus, the anti-apoptotic effect on wounded skin was elevated in G-CSF compared to control groups. Collectively, these data suggest that G-CSF accelerated wound healing and ameliorated tissue apoptosis after fluid resuscitation in HS rats.
Figure 1.
G-CSF improved wound healing in hemorrhagic shock. (A) The protocol of hemorrhagic shock combined with cutaneous injury model and resuscitation treatment. The rats underwent 3-cm-diameter full-thickness skin incision and 40% total blood loss for 60 min. The rats were resuscitated with a bolus of 4 mL/kg HHES infusion. After resuscitation, all the rats randomly received G-CSF, normal saline, or no treatment. H&E (B) and Masson trichrome (C) staining during wound repair. Scale bar=100 μm. (D) G-CSF attenuated tissue apoptosis in wound areas by TUNEL assay. Scale bar=100 μm. (E) The number of apoptosis cells in the wound skin. Ten pictures of each group were taken and calculated. Data are shown as mean ±SD. * P<0.05 versus blank group.
G-CSF attenuated inflammation reaction, apoptotic events, and promoted angiogenesis in wound areas
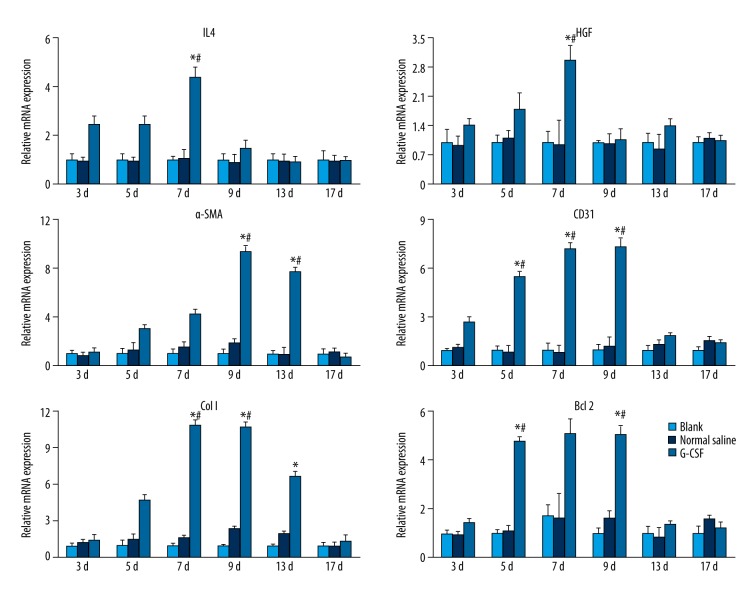
Next, we examined the anti-inflammatory factors IL4, anti-apoptotic factor Bcl 2, collagen deposition-related factors collagen I and HGF, and angiogenesis-related factors CD31 and α-SMA expression by RT-PCR (Figure 2). The quantification results implied that the anti-inflammatory factor IL 4 was significantly up-regulated since day 3. The angiogenesis-related factors, CD31 and α-SMA, were up-regulated as early as day 5 and peaked at day 9. Collagen synthesis-related genes were modulated early to initiate the repair as well as collagen deposition in the remodeling stage, and we found an enhanced expression of collagen I genes from day 7 to day 13 in the G-CSF group. HGF expression was peaked at day 7. The expression of the anti-apoptotic Bcl 2 gene was up-regulated from day 5 to day 9 compared to control groups. The up-regulation of Bcl 2 in the G-CSF group was in agreement with lower TUNEL-positive cell numbers on day 9. Nevertheless, decreased anti-apoptotic gene and anti-inflammatory gene expressions would lead to chronic non-healing ulcer in control groups.
Figure 2.
Wound healing-related mRNA modulation by G-CSF. Each result was repeated at least for 3 times. Data are shown as mean ±SD. * P<0.05 versus Blank group, # P<0.05 versus Normal saline group.
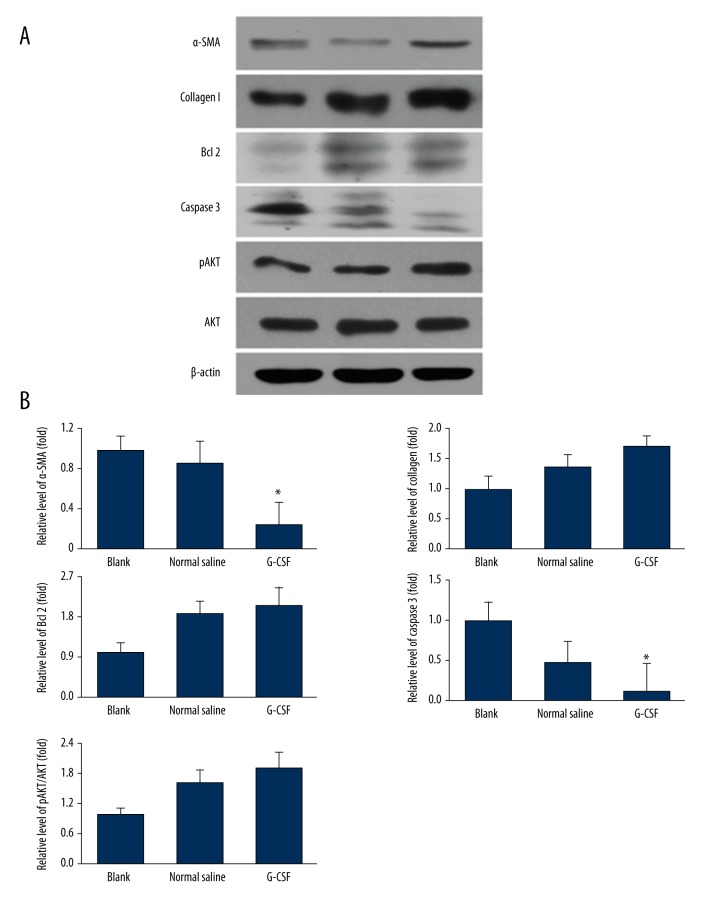
We further investigated the protein expression of α-SMA, collagen I, Bcl 2, caspase 3, AKT and pAKT post HS combined with cutaneous injury on 9 d (Figure 3A, 3B). The results indicated that G-CSF also enhanced angiogenesis, improved collagen synthesis and anti-apoptotic factors’ expression at protein level and activated AKT signaling pathway.
Figure 3.
Protective effects of G-CSF on wound healing-related protein expression. (A) α-SMA, Collagen I, Bcl 2, Caspase 3, pAKT, AKT, β-actin expressions were evaluated by WB. (B) Each protein was normalized by β-actin and showed as fold change of Blank group. Each result was repeated at least for 3 times. Data were shown as mean ±SD. * P<0.05 versus Blank group, # P<0.05 versus Normal saline group.
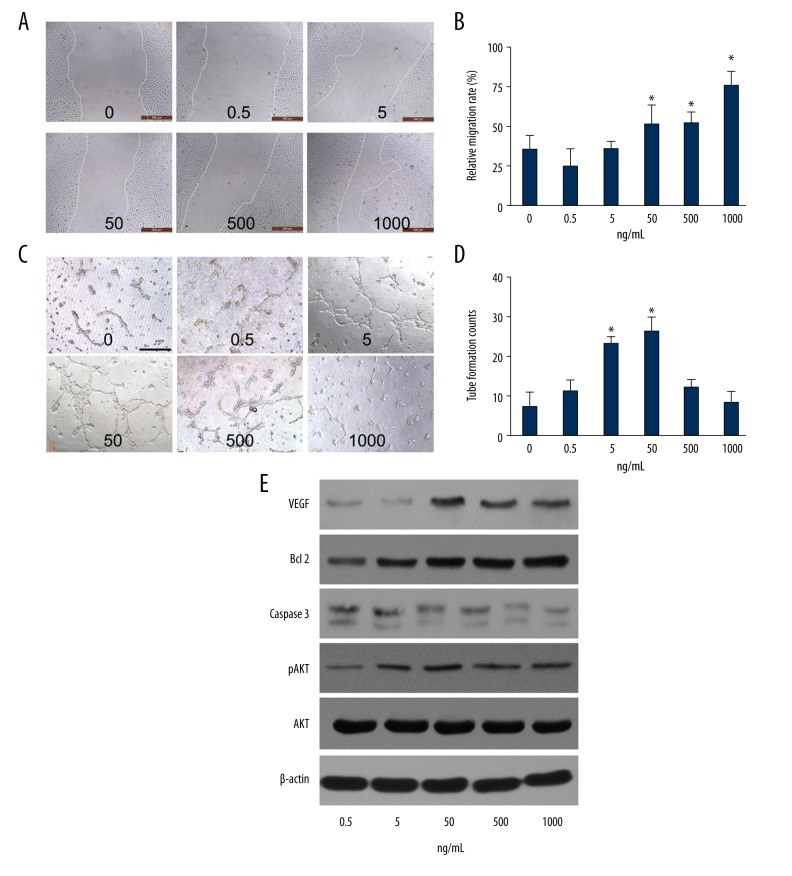
G-CSF exerted angiogenesis potential on HUVECs in vitro
To determine the effect of G-CSF treatment on the angiogenesis potential of ECs in vitro, we examined the migration and tube formation abilities of HUVECs at different concentration of G-CSF stimulation (0.5, 5, 50, 500, 1000 ng/mL) (Figure 4A, 4B). The scratch experiment showed that the migration ability of the HUVECs was enhanced significantly by G-CSF at 12 h in a concentration-dependent manner. The assay showed that tube formation ability of the HUVECs was promoted by G-CSF at 12 h and peaked at 50 ng/mL G-CSF (Figure 4C, 4D). In addition, H2O2 was added into HUVECs for 6 h to mimic the apoptotic environment in vivo. VEGF, Bcl 2, caspase 3, AKT, and pAKT were evaluated by WB (Figure 4E). VEGF was increased in the G-CSF group and peaked at 50 ng/mL. Caspase 3 was decreased while Bcl 2 was more highly expressed with the higher concentration of G-CSF. The results are in accordance with mRNA modulation in vivo, in which G-CSF stimulated proliferation, maturation, and anti-apoptotic capacities of ECs.
Figure 4.
G-CSF promoted the angiogenic potential of HUVECs in vitro. (A) Representative images depicting the effect of the G-CSF treatment at 12 h on HUVEC migration at the indicated concentrations. (B) Statistically calculated relative migration rates; Scale bar=500 μm. (C) Typical images of the tube-like structures in the presence of G-CSF cultured on Matrigel for 12 h. (D) The amounts of capillary-like structures; Scale bar=200 μm. (E) Western blot of VEGF, Bcl 2, Caspase 3, phosphorylated AKT (pAKT) as well as the total AKT and β-actin expressions at different concentrations of G-CSF; the HUVECs were pre-incubated with G-CSF for 24 h then followed by H2O2 stimulation (50 μmol/L) to determine the protein activities by Western blot. Each experiment was repeated 3 times and typical pictures are shown. Data are shown as mean ±SD. * P<0.05 versus HUVECs without G-CSF stimulation.
Discussion
Wound healing is one of the most complicated biological processes [1]. In the present study, we demonstrated that G-CSF administration after resuscitation is beneficial for wound healing under conditions of hemorrhagic shock by modulating inflammation, improving collagen deposition, and enhancing function of ECs.
The potential impacts of G-CSF on angiogenesis have attracted great scientific interest. A growing number of studies indicate that G-CSF has therapeutic effects on tissue repair and regeneration, including full-thickness skin injuries, burn injuries, limb ischemia, myocardial infarction, and pulmonary hypertension, by specifically enhancing the pro-angiogenic abilities of ECs [24–27]. Angiogenesis is also of importance to the adequate healing of vessels and microvessels by providing sufficient oxygen and necessary nutrients for cell growth and development [8]. This dynamic process is regulated by interaction of diverse cytokines, growth factors, and molecular signal pathways between different cell types [1,7]. Our results of mRNA expression in the G-CSF treated group in vivo showed up-regulated expression of IL 4, CD31, α-SMA, and HGF in skin wound areas. The essential factors such as CD31 and α-SMA were augmented to played critical roles in angiogenesis at the inflammation and proliferative stage. In vitro experimentation in G-CSF-stimulated HUVECs show that the proliferative ECs and the enhanced vasculature contributed to angiogenesis and benefited wound healing. Additionally, it was previously demonstrated that EPCs are mobilized by G-CSF to damaged tissues and significantly promote angiogenesis by increasing the secretion of angiogenic cytokines in full-thickness incision, burned, and focal cerebral ischemia rats and myocardial infarcted rabbits [28–30]. Thus, G-CSF administration would be expected to play important roles in wound repair, partly by ameliorating inflammation and enhancing angiogenesis [31,32].
G-CSF was reported to protect tissue by strengthening anti-apoptotic effects of host cells under adverse conditions. In central nervous system (CNS) disease, G-CSF was suggested to inhibit neurocyte apoptosis and attenuated glutamate-induced cell death [24]. G-CSF displayed strong anti-apoptotic activity in mature nerve cells by activating multiple survival signaling mechanisms [33,34]. Several studies reported that G-CSF increased anti-inflammatory activities and facilitated neuron protective and angiogenesis effects [33,35,36]. Bcl-2 plays a vital role in the apoptosis signaling pathway. Our in vivo and in vitro experiments showed up-regulated Bcl 2 expression in the presence of G-CSF, which suggests the anti-apoptosis capacity was enhanced under injury circumstances. Initiation of apoptosis is one of the natural processes during wound healing. Among the effective approaches for cutaneous therapeutics, enhancing the survival of host cells promotes wound healing quality. In addition, it will be interesting to determine the interconnection between anti-apoptotic treatments and wound healing quality or prognosis, which may provide complementary information about wound repair combined with severe trauma. Future studies will attempt to better resolve the mechanisms by which G-CSF provokes the anti-apoptotic effect in tissue repair and regeneration.
Conclusions
To summarize, we provided evidence for a cytoprotective and beneficial effect of G-CSF administration after resuscitation in a hemorrhagic shock combined with cutaneous injury rat model. Tissue damage was ameliorated and angiogenesis was significantly elevated to support tissue repair and regeneration by G-CSF administration. These findings uphold the suggestion that G-CSF administration would be beneficial for patients in cutaneous therapy, even in hemorrhagic shock.
Footnotes
Conflict of interests
The authors have no conflict of interest to declare.
Source of support: This study was supported by the National Basic Science and Development Program [2012CB518103], the 863 Projects of Ministry of Science and Technology of China [2013AA020105 and 2012AA020502]
References
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: Wound mechanotransduction in repair and regeneration. J Invest Dermatol. 2011;131:2186–96. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- 3.Broughton G, 2nd, Janis JE, Attinger CE. Wound healing: An overview. Plast Reconstruct Surg. 2006;117:1e-S–32e-S. doi: 10.1097/01.prs.0000222562.60260.f9. [DOI] [PubMed] [Google Scholar]
- 4.McGinn FP. Proceedings: Effect of haemorrhage on wound healing in the rat. Br J Surg. 1976;63:163. [PubMed] [Google Scholar]
- 5.Bjerkvig CK, Strandenes G, Eliassen HS, et al. “Blood failure” time to view blood as an organ: how oxygen debt contributes to blood failure and its implications for remote damage control resuscitation. Transfusion. 2016;56(Suppl 2):S182–89. doi: 10.1111/trf.13500. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Eckle T. Ischemia and reperfusion – from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Layman H, Sacasa M, Murphy AE, et al. Co-delivery of FGF-2 and G-CSF from gelatin-based hydrogels as angiogenic therapy in a murine critical limb ischemic model. Acta Biomater. 2009;5:230–39. doi: 10.1016/j.actbio.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Adachi Y, Iwasaki M, et al. G-CSF and/or M-CSF accelerate differentiation of bone marrow cells into endothelial progenitor cells in vitro. Oncol Rep. 2006;15:1523–27. [PubMed] [Google Scholar]
- 11.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–38. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 12.Bussolino F, Wang JM, Defilippi P, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989;337:471–73. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Aoki M, Kondo T, et al. Therapeutic angiogenesis with intramuscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler Thromb Vasc Biol. 2005;25:2535–41. doi: 10.1161/01.ATV.0000190609.28293.17. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Sereti KI, Wu BM, Ardehali R. Translational aspects of cardiac cell therapy. J Cell Mol Med. 2015;19:1757–72. doi: 10.1111/jcmm.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Q, Hodara V, Butler SD, et al. Differential bone marrow stem cell mobilization by G-CSF injection or arterial ligation in baboons. J Cell Mol Med. 2009;13:1896–906. doi: 10.1111/j.1582-4934.2008.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 17.Moazzami K, Moazzami B, Roohi A, et al. Local intramuscular transplantation of autologous mononuclear cells for critical lower limb ischaemia. Cochrane Database Syst Rev. 2014;(12):CD008347. doi: 10.1002/14651858.CD008347.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schabitz WR, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–51. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama H, Iohara K, Hayashi Y, et al. Enhanced regeneration potential of mobilized dental pulp stem cells from immature teeth. Oral Dis. 2016 doi: 10.1111/odi.12619. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Yang S, Wang H, et al. G-CSF/SCF exert beneficial effects via anti-apoptosis in rabbits with steroid-associated osteonecrosis. Exp Mol Pathol. 2013;94:247–54. doi: 10.1016/j.yexmp.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Lan X, Qu H, Yao W, Zhang C. Granulocyte-colony stimulating factor inhibits neuronal apoptosis in a rat model of diabetic cerebral ischemia. Tohoku J Exp Med. 2008;216:117–26. doi: 10.1620/tjem.216.117. [DOI] [PubMed] [Google Scholar]
- 22.Pitzer C, Klussmann S, Kruger C, et al. The hematopoietic factor granulocyte-colony stimulating factor improves outcome in experimental spinal cord injury. J Neurochem. 2010;113:930–42. doi: 10.1111/j.1471-4159.2010.06659.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Liu J, Hao H, et al. G-CSF Administration after the intraosseous infusion of hypertonic hydroxyethyl starches accelerating wound healing combined with hemorrhagic shock. BioMed Res Int. 2016;2016:5317630. doi: 10.1155/2016/5317630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strecker JK, Sevimli S, Schilling M, et al. Effects of G-CSF treatment on neutrophil mobilization and neurological outcome after transient focal ischemia. Exp Neurol. 2010;222:108–13. doi: 10.1016/j.expneurol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka R, Masuda H, Kato S, et al. Autologous G-CSF-mobilized peripheral blood CD34+ cell therapy for diabetic patients with chronic nonhealing ulcer. Cell Transpl. 2014;23:167–79. doi: 10.3727/096368912X658007. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Yun JY, Hur J, et al. Erythropoietin priming improves the vasculogenic potential of G-CSF mobilized human peripheral blood mononuclear cells. Cardiovasc Res. 2014;104:171–82. doi: 10.1093/cvr/cvu180. [DOI] [PubMed] [Google Scholar]
- 27.Liu JF, Du ZD, Chen Z, et al. Granulocyte colony-stimulating factor attenuates monocrotaline-induced pulmonary hypertension by upregulating endothelial progenitor cells via the nitric oxide system. Exp Ther Med. 2013;6:1402–8. doi: 10.3892/etm.2013.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fox A, Smythe J, Fisher N, et al. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95:244–51. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 29.Minatoguchi S, Takemura G, Chen XH, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–80. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 30.Lee ST, Chu K, Jung KH, et al. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005;1058:120–28. doi: 10.1016/j.brainres.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 31.Agalar F, Iskit AB, Agalar C, et al. The effects of G-CSF treatment and starvation on bacterial translocation in hemorrhagic shock. J Surg Res. 1998;78:143–47. doi: 10.1006/jsre.1998.5386. [DOI] [PubMed] [Google Scholar]
- 32.Ghannam S, Bouffi C, Djouad F, et al. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider A, Kruger C, Steigleder T, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komine-Kobayashi M, Zhang N, Liu M, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26(3):402–13. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 35.Sehara Y, Hayashi T, Deguchi K, et al. Decreased focal inflammatory response by G-CSF may improve stroke outcome after transient middle cerebral artery occlusion in rats. J Neurosci Res. 2007;85:2167–74. doi: 10.1002/jnr.21341. [DOI] [PubMed] [Google Scholar]
- 36.Solaroglu I, Cahill J, Tsubokawa T, et al. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009;31:167–72. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]