Abstract
In the past few decades, there has been explosive growth in the construction of nanoparticle-based drug delivery systems (NDDSs), namely nanomedicines, owing to their unique properties compared with traditional drug formulations. However, because of a variety of challenges, few nanomedicines are on sale in the market or undergoing clinical trial at present. Thus, it is essential to look back and re-evaluate what these NDDSs can really do in vivo, why nanomedicines are regarded as potential candidates for next-generation drugs, and what the future of nanomedicine is. Here, we focus mainly on the properties of NDDSs that extend blood circulation, enhance penetration into deep tumor tissue, enable controllable release of the payload into the cytoplasm, and overcome multi-drug resistance. We further discuss how to promote the translation of nanomedicines into reality. This review may help to identify the functions of NDDSs that are really necessary before they are designed and to reduce the gap between basic research and clinical application.
Keywords: nanoparticles, drug delivery, efficiency, biocompatilbility
Introduction
With their unique physical and chemical properties as well as their nanoscale effects, nanoparticle-based drug delivery systems (NDDSs) are currently under extensive development for applications in the treatment of diseases such as cardiovascular diseases, infectious diseases, diabetes, and cancer 1– 3. It has been reported that most cancers, as malignant diseases, can be suppressed with various NDDSs, including inorganic nanoparticles (NPs) such as metallic NPs and semiconductor nanostructures, organic NPs such as polymer carriers and carbon nanostructures, and hybrid NPs 4– 6. Disappointingly, a statistical analysis showed that only 0.7% (median) of the injected dose of the NDDS reached the tumor region in mouse models and this value has not improved in the past 10 years 7. There is no doubt that there are systemic effects on the interaction between NDDSs and organisms. From the material perspective, the circulation, biodistribution, internalization, and trafficking of NDDSs are highly dependent on the physicochemical properties of NDDSs such as size, shape, surface chemistry, and material type 8, 9. From the biological perspective, NDDSs need to cross or elude a series of complex biological barriers, including opsonization by the mononuclear phagocyte system (MPS), non-specific distribution, interstitial fluid pressure, cellular internalization, and drug efflux pumps, before exerting their therapeutic effect 7, 10. In this review, we will focus on discussing the basic functions of NDDSs for cancer therapy and present recently developed strategies for improving the efficacy of NDDSs in vivo. Finally, we propose what we need to do to accelerate the translation of nanomedicines in the future.
Extended blood circulation and accumulation with passive/active targeting
Blood circulation time is an important parameter that affects the therapeutic efficiency and outcome. The fundamental functions of NDDSs are to increase the drug concentration in targeted tissues and to reduce systemic side effects by modulating the pharmacokinetics and biodistribution of the drug payload. In order to increase the drug concentration at tumor sites, longer blood circulation time is vital because it enhances the probability of drug delivery to the tumor without sequestration by the MPS. PEGylation is a widely used approach for prolonging circulation time, and some PEGylated nanomedicines, such as Adagen, Doxil, Macugen, and Pegasys, have been approved for clinical use 11– 13. Passive and active targeting are the two alternatives for enhancing the efficiency of NDDS accumulation in tumor tissue. For passive targeting, the theoretical mechanism is the “enhanced permeability and retention” (EPR) effect, which broadly explains why NDDSs can accumulate in tumor tissue through leaky and defective blood vessels 14, 15. However, the EPR phenomenon was almost always studied in rapidly growing tumors in mouse models, and it has been suggested that the EPR effect does not work in the clinic, because of a lack of fenestrations in the tumor vessels of patients 16, 17. Therefore, the passive targeting strategy needs to be carefully evaluated and further validated in clinical trials. For active targeting, NDDSs mostly use targeting ligands such as antibodies, peptides, and aptamers, which specifically recognize overexpressed “biomarkers” in the tumor microenvironment or the surface of cancer cells after extravasation of the NDDS from blood vessels 1, 18; this approach enhances the residence time and the local drug concentration. Strikingly, the targeting ability of NDDSs may be reduced or abolished after the formation of protein corona in the biological milieu 19. Regardless of whether they possess passive or active targeting capabilities, the “foreign” NDDSs are directly exposed to the MPS once injected into the body, so the blood circulation time will be decreased.
To overcome the first biological barrier, namely opsonization and sequestration by the MPS, some “invisibility cloak” NDDSs have recently been developed on the basis of biomimetic strategies 20– 24. For instance, Zhang et al. have reported platelet membrane-cloaked NPs, with biodegradable polymeric NPs inside and immunomodulatory and adhesion antigens on the surface 23. The results showed that the cloaked NPs were less efficiently recognized by macrophage-like cells and did not induce complement activation. Also, the cloaked NPs have enhanced adhesion to damaged vasculature and improved therapeutic efficacy compared with uncloaked NPs. The lesson in these cases is that we need to rethink what the body really needs—maybe it is better to work with the body’s natural processes than against them.
Enhanced penetration depth in tumor tissue
Weak penetration of drug into the deep tissue of solid tumors dramatically attenuates treatment efficacy. Although NDDSs with long circulation periods have the advantage of increased accumulation in the tumor microenvironment after extravasation from blood vessels, penetration into deep tissue remains a challenge because of the high interstitial fluid pressures and poor lymphatic drainage in tumors 25. In early studies, thanks to the controllable physicochemical parameters of NDDSs, strategies focused mostly on how to optimize single physicochemical characteristics of NDDSs, such as size, shape, and surface chemistry, to obtain excellent penetration and therapeutic effects 9, 26– 28. Recently, a few smart “multistage” NDDSs have been reported to “adapt” biological barriers and improve penetration 10, 29, 30. Shen et al. have reported an injectable micrometer-sized generator loaded with a pH-sensitive polymeric drug (iNPG-pDox) 31. Once released from iNPG after iNPG-pDoxs accumulates at the tumor site, the pDox self-assembly forms NPs inside the tumors and then the pDox NPs are internalized and transported to the perinuclear region. Kohane et al. have developed a photoswitchable spiropyran-based NDDS 32. Upon irradiation at 365 nm, the size decreased from 103 to 49 nm and the shrinkage enhanced penetration into deep tissue and drug release.
Logically, from a biological perspective, the tumor’s pathophysiological characteristics should be clearly understood so that the NDDS can be designed to effectively penetrate into deep tissue. Tumors are highly heterogeneous and the tumor characteristics depend on the cancer type, pathological state, location, and individual factors such as age, lifestyle, and genetics 33. Subpopulations of cancer cells with unique genomes exist in different times and places in one tumor 34. This heterogeneity is challenging for clinical diagnosis and causes the heterogeneous distribution of free drug or NDDS in tumor tissue 35. Thus, tumor heterogeneity should not be ignored when NDDSs are designed to increase the targeting ability and enhance the penetration. For instance, Kataoka et al. have discovered “dynamic vents” which undergo spontaneous and transient opening and closure within tumor microvessels 36. The eruption process can increase accumulation and retention of large NPs in sparsely vascularized tumors. By exploiting this natural phenomenon, investigators can develop new strategies to efficiently deliver and retain large NDDSs in deep tumor tissue. In addition, Chan et al. have reported that tumor pathophysiology and volume can significantly influence the targeting of NPs 37. As the Chinese proverb says, “Know yourself and know your enemy, you will win every war”. The more we know about the NDDS and the tumor characteristics, the better the therapeutic effect.
Controllable release of the payload into the cytoplasm
Targeting and controllable release are the basic characteristics of NDDSs. Controllable release, which means that the loaded drugs are liberated in the right place and time, can significantly reduce administration times and avoid toxicity to other organs. In general, controllable release is facilitated mainly by external and internal stimulus-response strategies 38, 39. Internal stimulus-responsive NDDSs are sensitive to factors such as pH, redox status, and enzyme levels, which are often abnormal in tumor cells. Thus, the response efficiency is highly dependent on the specific biological conditions within the treated mouse model. Among the aforementioned strategies, enzyme-sensitive NDDSs have been enthusiastically explored because of their high selectivity and specificity 40, 41. However, the enzyme-triggered activation of NDDSs should be properly understood and the sensitivity of NDDSs in vivo should be measured because of the possibility of steric hindrance at the active site in the interior of NDDS. In contrast, external stimulus-responsive NDDSs are sensitive to external physical factors, including temperature, magnetic fields, ultrasound, light, and electrical fields. External stimulation offers more precise control profiles compared with internal stimulation, but the choice of “therapeutic window” is directly related to the outcome. It is still unclear whether these external physical factors can facilitate tumor metastasis and cause damage to the normal tissues. Meanwhile, the need for large external equipment to apply the stimulus also increases the difficulty of translation and application.
The therapeutic effect also has a close relationship with the controlled-release mechanism, which affects how the payload drug is transported from the interior of the NDDS to the cytoplasm. Thanks to mathematical models, some drug release mechanisms have been simulated. For example, the process of protein release from an NDDS has been accurately simulated with an established mathematical model by Shoichet et al. 42. Importantly, the simulated release process has also been confirmed by experiments with two proteins. The main controlled-release mechanisms that have been discovered so far include diffusion, osmosis, erosion, and dissolution 43, 44. Thus, computer simulation provides a powerful tool for us to better design NDDSs for controllable release and to understand the release process.
Circumvention of tumor drug resistance
Drug resistance, including intrinsic resistance and acquired resistance, is the main reason for the failure of chemotherapy 45. The major mechanisms of multi-drug resistance (MDR) are decreased drug uptake, increased efflux of drugs, and changes in cell behavior 46. Among these, increased efflux of drugs mediated by ATP-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp) or ABCB1, has a close relationship with MDR 47. New approaches have also been developed to tackle MDR in tumors. For instance, NPs mimicking Salmonella have been engineered by McCormick et al. 48. The Salmonella mimics were constructed from gold NPs coated with the S. typhimurium type III secreted effector protein SipA, which reduces the function of P-gp. The bacterial mimics efficiently reduced the P-gp level and increased the tumor sensitivity to doxorubicin. Meanwhile, Artzi et al. have developed an implantable hydrogel-embedded ON/OFF molecular nanoswitch probe to sense and overcome cancer MDR 49. Although NDDSs have successfully overcome MDR in some cases, the question remains whether NDDS-induced resistance will occur after administration of multiple doses. Also, as discussed above, tumor heterogeneity makes this problem more complex because it is unclear which subpopulations of cancer cells are actually responsible for the resistance. Nevertheless, NDDSs are a promising choice for reversing drug resistance.
Nanoparticle-based drug delivery systems as a drug-like modulator for therapy
NDDSs have always been used as a vehicle to deliver therapeutic drugs or imaging agents. Their physicochemical characteristics are carefully optimized to meet the demand of delivery and targeting. Interestingly, the fact that their physicochemical properties can be modified means that NDDSs can also be applied as drug-like modulators, without any loaded cargo, to treat disease. For example, Overholtzer et al. have reported that ultrasmall poly(ethylene glycol)-coated silica NPs can inhibit tumor growth by inducing ferroptosis in starved cancer cells and in sensitive tumors 50. At the same time, a study by Daldrup-Link et al. reported that iron oxide NPs can induce pro-inflammatory macrophage polarization in tumor tissues to suppress tumor growth 51. The outcome was based only on the intrinsic therapeutic effect of pure NPs or material rather than loaded drug, indicating that the pure NPs also have therapeutic potential. The intrinsic therapeutic effects of other nanomaterials should be explored next. It will be worth investigating the modulatory effects of NDDSs on growth inhibition, metastasis, and recurrence through regulation of tumor metabolism, signal transduction, ion transport, and other biological processes that are common in tumors. However, it is important to note that the potential toxicity of drug-like modulators to healthy organs should not be ignored, even though they may have a therapeutic effect on abnormal tissues.
What do we need to do in the future?
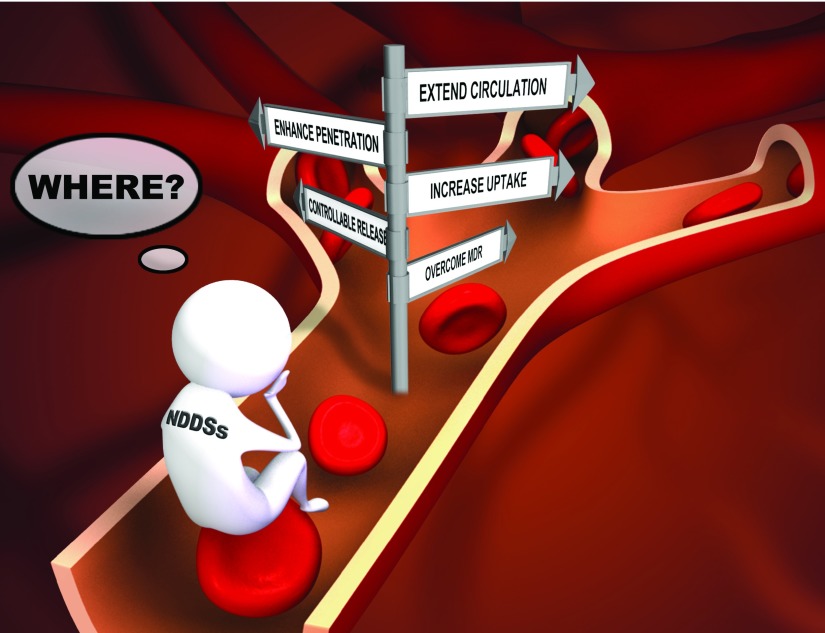
NDDSs have been designed to kill cancer cells by using the strategies discussed above, and their in vivo behavior has been partly investigated in mouse models. In fact, an organism is a complex system and NDDSs encounter different biological milieus in different parts of the body. Therefore, the properties of NDDS will change dynamically during the delivery journey in vivo. Unfortunately, NDDSs may lose the ability to execute the tactics designed by the “coach” (scientist). Although scientists have developed many smart and complex NDDSs, these NDDSs may be too complicated for clinical trials or therapeutic use. Vigilance is necessary to ensure that a “multi-functional” NDDS is not a “non-functional” NDDS in which the overall effectiveness is severely compromised by the weakest function in the system. Thus, further work is still needed to answer the question “What can NDDSs really do in vivo?” ( Figure 1) and improve the translation of nanomedicines.
Figure 1. Nanoparticle-based drug delivery systems: What can they really do and what should they do in vivo?
This problem may be solved in the following ways. Firstly, the fate of NDDSs, such as their integrity, surface characteristics, pharmacokinetics, biodistribution, and immunological effects, needs detailed tracing and analysis 52– 54. Advanced technologies and methods are essential for this challenging exploration. Secondly, there should be a normative evaluation framework to assess the efficiency of NDDSs, and rational animal models, such as organs-on-a-chip systems, should be established instead of just relying on the tumor size or survival curves in mouse models 55. The 5R framework (AstraZeneca’s 5R principle: right target/efficacy, right tissue/exposure, right safety, right patient, and right commercial potential) may be of beneficial guidance 56. It is equally important to combine the skills of chemists, mathematicians, biologists, and medical scientists to design clinically valuable NDDSs. Understanding the heterogeneity and biological nature of the tumor will really help us create NDDSs which may meet the expected treatment efficiency. In addition, we should pay more attention to structurally simple and reproducibly synthesized NDDSs because these have the greatest potential to reach the patient. Finally, it is important for us to keep in mind that we should constantly rethink what we are doing now and what we need to do in the future.
Conclusions
NDDSs provide a flexible and versatile platform for tumor therapy. Given their longevity and targeting, NDDSs can efficiently penetrate tissue and controllably release their payload into the cytoplasm. Moreover, NDDSs can be used to overcome tumor drug resistance. In particular, the intrinsic therapeutic effects of pure NPs can be regarded as a new therapeutic strategy. However, our understanding of what NDDSs can really do is still limited in vivo, and translation of NDDSs is challenging. In summary, NDDSs are a promising choice for tumor therapy but many questions still need to be answered for their effective clinical translation.
Abbreviations
ABC, ATP-binding cassette; EPR, enhanced permeability and retention; MDR, multi-drug resistance; MPS, mononuclear phagocyte system; NDDS, nanoparticle-based drug delivery system; NP, nanoparticle; P-gp, P-glycoprotein.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Xiaoyuan (Shawn) Chen, Laboratory of Molecular Imaging and Nanomedicine (LOMIN), National Institute of Biomedical Imaging and Bioengineering (NIBIB) and National Institutes of Health (NIH), Bethesda, Maryland, USA
Zhen Gu, Department of Biomedical Engineering, UNC Eshelman Pharmacy School, University of North Carolina at Chapel Hill, NC, USA
Kenneth A. Dawson, Centre for BioNano Interactions, School of Chemistry and Chemical Biology, University College Dublin, Dublin, Ireland
Funding Statement
This work was supported by the Natural Science Foundation key project (31630027 and 31430031) and a National Distinguished Young Scholars grant (31225009). The authors appreciate the support from the “Strategic Priority Research Program” of the Chinese Academy of Sciences (grant XDA09030301) and support from the external cooperation program of Bureau of International Co-operation, Chinese Academy of Sciences (grant 121D11KYSB20130006).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Torchilin VP: Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–27. 10.1038/nrd4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta A, Landis RF, Rotello VM: Nanoparticle-Based Antimicrobials: Surface Functionality is Critical [version 1; referees: 2 approved]. F1000Res. 2016;5: pii: F1000 Faculty Rev-364. 10.12688/f1000research.7595.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adiseshaiah PP, Crist RM, Hook SS, et al. : Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat Rev Clin Oncol. 2016;13(12):750–65. 10.1038/nrclinonc.2016.119 [DOI] [PubMed] [Google Scholar]
- 4. Biju V: Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744–64. 10.1039/c3cs60273g [DOI] [PubMed] [Google Scholar]
- 5. Singh R, Lillard JW, Jr: Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86(3):215–23. 10.1016/j.yexmp.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen G, Roy I, Yang C, et al. : Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem Rev. 2016;116(5):2826–85. 10.1021/acs.chemrev.5b00148 [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm S, Tavares AJ, Dai Q, et al. : Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1: 16014. 10.1038/natrevmats.2016.14 [DOI] [Google Scholar]
- 8. Duan X, Li Y: Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small. 2013;9(9–10):1521–32. 10.1002/smll.201201390 [DOI] [PubMed] [Google Scholar]
- 9. Albanese A, Tang PS, Chan WC: The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:1–16. 10.1146/annurev-bioeng-071811-150124 [DOI] [PubMed] [Google Scholar]
- 10. Blanco E, Shen H, Ferrari M: Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. 10.1038/nbt.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suk JS, Xu Q, Kim N, et al. : PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. 10.1016/j.addr.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Couvreur P: Nanoparticles in drug delivery: past, present and future. Adv Drug Deliv Rev. 2013;65(1):21–3. 10.1016/j.addr.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 13. Wang AZ, Langer R, Farokhzad OC: Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98. 10.1146/annurev-med-040210-162544 [DOI] [PubMed] [Google Scholar]
- 14. Maeda H: The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. 10.1016/S0065-2571(00)00013-3 [DOI] [PubMed] [Google Scholar]
- 15. Iyer AK, Khaled G, Fang J, et al. : Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–8. 10.1016/j.drudis.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 16. Danhier F: To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A):108–21. 10.1016/j.jconrel.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 17. Nichols JW, Bae YH: EPR: Evidence and fallacy. J Control Release. 2014;190:451–64. 10.1016/j.jconrel.2014.03.057 [DOI] [PubMed] [Google Scholar]
- 18. Byrne JD, Betancourt T, Brannon-Peppas L: Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–26. 10.1016/j.addr.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 19. Salvati A, Pitek AS, Monopoli MP, et al. : Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8(2):137–43. 10.1038/nnano.2012.237 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Parodi A, Quattrocchi N, van de Ven AL, et al. : Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–8. 10.1038/nnano.2012.212 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Luk BT, Fang RH, Hu CM, et al. : Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics. 2016;6(7):1004–11. 10.7150/thno.14471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dehaini D, Fang RH, Zhang L: Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med. 2016;1(1):30–46. 10.1002/btm2.10004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu CM, Fang RH, Wang K, et al. : Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–21. 10.1038/nature15373 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Hu Q, Sun W, Qian C, et al. : Anticancer Platelet-Mimicking Nanovehicles. Adv Mater. 2015;27(44):7043–50. 10.1002/adma.201503323 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Heldin CH, Rubin K, Pietras K, et al. : High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–13. 10.1038/nrc1456 [DOI] [PubMed] [Google Scholar]
- 26. Huang K, Ma H, Liu J, et al. : Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6(5):4483–93. 10.1021/nn301282m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chauhan VP, Popović Z, Chen O, et al. : Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed Engl. 2011;50(48):11417–20. 10.1002/anie.201104449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cabral H, Matsumoto Y, Mizuno K, et al. : Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–23. 10.1038/nnano.2011.166 [DOI] [PubMed] [Google Scholar]
- 29. Tasciotti E, Liu X, Bhavane R, et al. : Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3(3):151–7. 10.1038/nnano.2008.34 [DOI] [PubMed] [Google Scholar]
- 30. Wong C, Stylianopoulos T, Cui J, et al. : Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–31. 10.1073/pnas.1018382108 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Xu R, Zhang G, Mai J, et al. : An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat Biotechnol. 2016;34(4):414–8. 10.1038/nbt.3506 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Tong R, Chiang HH, Kohane DS: Photoswitchable nanoparticles for in vivo cancer chemotherapy. Proc Natl Acad Sci U S A. 2013;110(47):19048–53. 10.1073/pnas.1315336110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. von Roemeling C, Jiang W, Chan CK, et al. : Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017;35(2):159–71. 10.1016/j.tibtech.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 34. Bedard PL, Hansen AR, Ratain MJ, et al. : Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64. 10.1038/nature12627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bae YH, Park K: Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153(3):198–205. 10.1016/j.jconrel.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsumoto Y, Nichols JW, Toh K, et al. : Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat Nanotechnol. 2016;11(6):533–8. 10.1038/nnano.2015.342 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Sykes EA, Dai Q, Sarsons CD, et al. : Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc Natl Acad Sci U S A. 2016;113(9):E1142–51. 10.1038/nmat3776 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Mura S, Nicolas J, Couvreur P: Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. 10.1038/nmat3776 [DOI] [PubMed] [Google Scholar]
- 39. Jhaveri A, Deshpande P, Torchilin V: Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release. 2014;190:352–70. 10.1016/j.jconrel.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 40. Hu J, Zhang G, Liu S: Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem Soc Rev. 2012;41(18):5933–49. 10.1039/c2cs35103j [DOI] [PubMed] [Google Scholar]
- 41. Kuang T, Liu Y, Gong T, et al. : Enzyme-responsive Nanoparticles for Anticancer Drug Delivery. Curr Nanosci. 2016;12(1):38–46. 10.2174/1573413711666150624170518 [DOI] [Google Scholar]
- 42. Vulic K, Pakulska MM, Sonthalia R, et al. : Mathematical model accurately predicts protein release from an affinity-based delivery system. J Control Release. 2015;197:69–77. 10.1016/j.jconrel.2014.10.032 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kamaly N, Yameen B, Wu J, et al. : Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev. 2016;116(4):2602–63. 10.1021/acs.chemrev.5b00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peppas NA, Narasimhan B: Mathematical models in drug delivery: how modeling has shaped the way we design new drug delivery systems. J Control Release. 2014;190:75–81. 10.1016/j.jconrel.2014.06.041 [DOI] [PubMed] [Google Scholar]
- 45. Gottesman MM: Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. 10.1146/annurev.med.53.082901.103929 [DOI] [PubMed] [Google Scholar]
- 46. Szakács G, Paterson JK, Ludwig JA, et al. : Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–34. 10.1038/nrd1984 [DOI] [PubMed] [Google Scholar]
- 47. Gottesman MM, Lavi O, Hall MD, et al. : Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu Rev Pharmacol Toxicol. 2016;56:85–102. 10.1146/annurev-pharmtox-010715-103111 [DOI] [PubMed] [Google Scholar]
- 48. Mercado-Lubo R, Zhang Y, Zhao L, et al. : A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat Commun. 2016;7: 12225. 10.1038/ncomms12225 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Conde J, Oliva N, Artzi N: Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc Natl Acad Sci U S A. 2015;112(11):E1278–87. 10.1073/pnas.1421229112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Kim SE, Zhang L, Ma K, et al. : Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11(11):977–85. 10.1038/nnano.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Zanganeh S, Hutter G, Spitler R, et al. : Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–94. 10.1038/nnano.2016.168 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Kreyling WG, Abdelmonem AM, Ali Z, et al. : In vivo integrity of polymer-coated gold nanoparticles. Nat Nanotechnol. 2015;10(7):619–23. 10.1038/nnano.2015.111 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Chen F, Wang G, Griffin JI, et al. : Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12(4):387–393. 10.1038/nnano.2016.269 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Sartain F, Greco F, Hill K, et al. : Emerging nanomedicine applications and manufacturing: progress and challenges. Nanomedicine (Lond). 2016;11(6):577–80. 10.2217/nnm.16.17 [DOI] [PubMed] [Google Scholar]
- 55. Jang HL, Zhang YS, Khademhosseini A: Boosting clinical translation of nanomedicine. Nanomedicine (Lond). 2016;11(12):1495–7. 10.2217/nnm-2016-0133 [DOI] [PubMed] [Google Scholar]
- 56. Hare JI, Lammers T, Ashford MB, et al. : Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25–38. 10.1016/j.addr.2016.04.025 [DOI] [PubMed] [Google Scholar]