Abstract
Purpose of review:
Diabetic nephropathy (DN) is a progressive kidney disease caused by alterations in kidney architecture and function, and constitutes one of the leading causes of end-stage renal disease (ESRD). The purpose of this review is to summarize the state of the art of the DN-biomarker field with a focus on the new strategies that enhance the sensitivity of biomarkers to predict patients who will develop DN or are at risk of progressing to ESRD.
Objective:
In this review, we provide a description of the pathophysiology of DN and propose a panel of novel putative biomarkers associated with DN pathophysiology that have been increasingly investigated for diagnosis, to predict disease progression or to provide efficient personal treatment.
Methods:
We performed a review of the literature with PubMed and Google Scholar to collect baseline data about the pathophysiology of DN and biomarkers associated. We focused our research on new and emerging biomarkers of DN.
Key findings:
In this review, we summarized the critical signaling pathways and biological processes involved in DN and highlighted the pathogenic mediators of this disease. We next proposed a large review of the major advances that have been made in identifying new biomarkers which are more sensitive and reliable compared with currently used biomarkers. This includes information about emergent biomarkers such as functional noncoding RNAs, microRNAs, long noncoding RNAs, exosomes, and microparticles.
Limitations:
Despite intensive strategies and constant investigation, no current single treatment has been able to reverse or at least mitigate the progression of DN, or reduce the morbidity and mortality associated with this disease. Major difficulties probably come from the renal disease being heterogeneous among the patients.
Implications:
Expanding the proteomics screening, including oxidative stress and inflammatory markers, along with metabolomics approaches may further improve the prognostic value and help in identifying the patients with diabetes who are at high risk of developing kidney diseases.
Keywords: diabetic nephropathy, end-stage renal disease, albuminuria, tubular damage, glomerular damage, biomarkers
Abrégé
La néphropathie diabétique (ND) est une affection évolutive causée par des modifications dans la physionomie et la fonction des reins. Elle constitue l’une des principales causes d’insuffisance rénale terminale (IRT). L’objectif de cette revue est de faire état des plus récentes connaissances au sujet des biomarqueurs de la ND, en mettant l’accent sur les nouvelles stratégies qui augmentent la sensibilité des biomarqueurs à dépister les patients susceptibles de développer la ND ou qui courent le risque de voir leur état évoluer vers l’insuffisance rénale terminale.
Objectif de la revue:
Dans cette revue, nous fournissons d’abord une description de la physiopathologie de la ND. Nous proposons ensuite un panel de nouveaux biomarqueurs putatifs associés à la physiopathologie de la ND, et qui ont de plus en plus été étudiés dans le but d’établir le diagnostic de la maladie, de prédire son évolution ou de fournir un traitement individuel efficace.
Méthodologie:
Nous avons procédé à une revue de la littérature sur PubMed et Google Scholar afin de recueillir des données de base au sujet de la physiopathologie de la ND ainsi que sur les biomarqueurs qui y sont associés. Nous avons concentré nos recherches sur les biomarqueurs nouvellement identifiés ou émergents.
Principales conclusions:
La revue fait un résumé des voies de signalisation essentielles et des processus biologiques impliqués dans l’évolution de la ND, en plus de mettre en évidence les médiateurs pathogènes de la maladie. Nous faisons également état des avancées majeures réalisées dans l’identification de nouveaux biomarqueurs plus sensibles et plus fiables que ceux qui sont utilisés à l’heure actuelle. Enfin, cette revue collige des renseignements au sujet des biomarqueurs émergents tels que les ARN non codants fonctionnels, les microARN, les longs ARN non codants, les exosomes et les microparticules.
Limites de la revue:
À ce jour, malgré des stratégies de plus en plus pointues et le fait que la recherche soit en constante progression, aucun traitement unique n’a réussi à inverser ou même à freiner l’évolution de la néphropathie diabétique, ni à réduire la morbidité et la mortalité associées à cette maladie. L’hétérogénéité des maladies rénales observée dans la population des personnes atteintes contribue probablement aux difficultés rencontrées.
Conclusions:
La généralisation du dépistage par la protéomique, notamment par la mesure du stress oxydatif et des marqueurs inflammatoires, ainsi que l’approche métabolomique pourraient contribuer à améliorer la valeur pronostique et aider à cibler les patients atteints de diabète qui courent un risque élevé de développer de l’insuffisance rénale.
Why is this review important?
Recent studies are conflicting regarding the sensitivity and the specificity of biomarkers currently used in practice for DN diagnosis (eg, eGFR, albuminuria). The identification of novel biomarkers of early stages of DN and progression toward ESRD is thus mandatory to reduce the burden of chronic kidney diseases in the human population. This review focuses on presenting several potential biomarkers involved in DN pathogenesis and progression, with a hope to develop a panel of biomarkers, in particular, to classify and to broaden the therapeutic window for patients who are at different stages of DN.
What are the key messages?
When used in combination with conventional biomarkers, novel well-validated biomarkers can (1) improve understanding of the disease pathophysiology; (2) accurately stratify DN patients based on their disease stage, enabling them to have targeted personalized therapy; and (3) act as an indicator of response to treatment.
Introduction
Diabetic nephropathy (DN) is one of the leading causes of end-stage renal disease (ESRD) in developed countries and is becoming more prevalent globally due to the rise in the incidence of obesity and type 2 diabetes.1 Currently, it is estimated that more than 415 million people are diagnosed with diabetes worldwide, and this number is expected to rise to 642 million by 2040. The estimated diabetes prevalence in Canada is 44% from 2015 to 2025 (around 5 million people affected in 2025) and the consequent increase in morbidity and mortality associated with this disease (estimated diabetes statistics in Canada are generated by the Canadian Diabetes Cost Model-Canadian Diabetes Association).1,2 DN is a progressive kidney disease caused by alterations in the glomerular capillary and tubular structure and function induced by the disturbed glucose homeostasis.3 Even though major advances have been made over the past few decades in diagnosing and treating DN patients, we are still not able to significantly reduce the incidence of death among this population. Conventionally, DN severity is accessed by measuring urine albumin levels (albumin-to-creatinine ratio).4-10 Persistent microalbuminuria (between 30-300 mg/24 hr) or macroalbuminuria (levels >300 mg/24 hr) is considered a marker and predictor of DN and its progression to ESRD.4-10 However, recent studies are conflicting regarding the sensitivity and the specificity of urinary albumin.5,11,12
The pathophysiological progress of DN can be either impeded or slowed down considerably if interventions start in the early stages.9,13-15 In the long-term follow-up intervention study, STENO-2, intensive management with glucose control, renin-angiotensin system (RAS) inhibitors, cholesterol-lowering drugs, and healthy lifestyle reduced the progression of DN and its associated cardiovascular pathological conditions.16 A major obstacle for this paradigm to be efficient in the clinical scenario is a lack of biomarkers that can accurately identify diabetic patients who are at early risk of developing DN and predict the progression of the disease. Therefore, a novel well-validated group of biomarkers, when used in combination with conventional biomarkers, can improve understanding of the disease pathophysiology and can accurately stratify DN patients based on their disease stage, enabling them to have targeted personalized therapy. This review focuses on presenting several potential biomarkers involved in DN pathogenesis and progression, with a hope to develop a panel of biomarkers, in particular, to classify and to broaden the therapeutic window for patients who are at different stages of DN.
Review
DN Pathophysiology
It is well known that a collaboration of metabolic and hemodynamic alterations and inflammation are involved in the development of DN in patients with diabetes (Figure 1).17-20 However, only in the past few years, studies have provided broad insight into pathogenic mechanisms and the molecular events of DN.
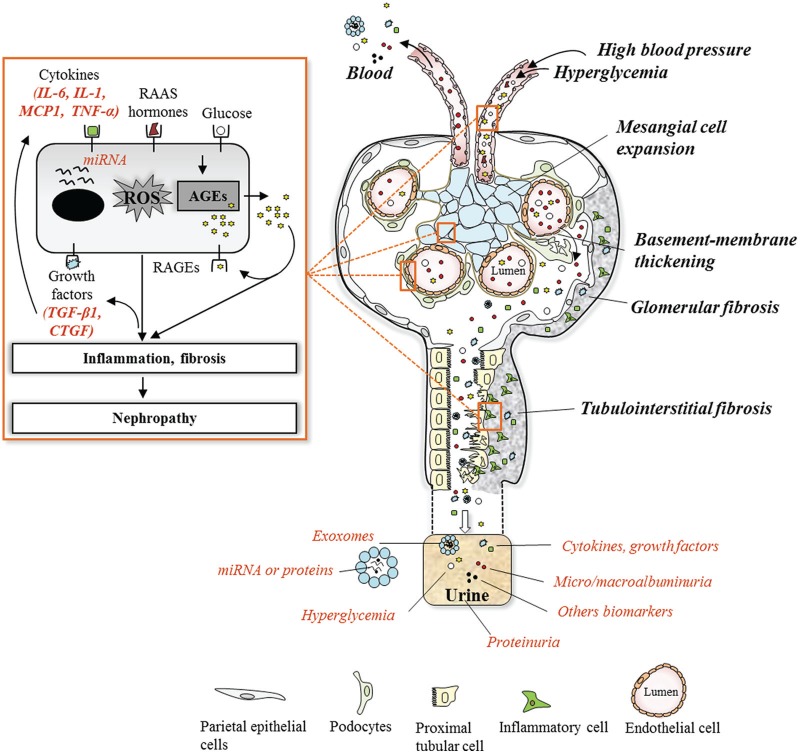
Figure 1.
Overview of DN pathogenesis and associated biomarkers.
Note. In healthy glomeruli, the fenestrated endothelial cell layer, basement membrane, and podocyte form a strong filtration barrier that is impermeable to high molecular weight proteins such as albumin. Hemodynamic and metabolic factors related to DN, such as high blood pressure, hyperglycemia, and generated AGEs, induce progressive phenotypic changes leading to the effacement of podocytes, basement membrane thickening, glomerular extracellular matrix accumulation, and tubulointerstitial fibrosis. These factors initiate pathological changes via activation of a cascade of mediators at different stages during the systematic progression of the disease, such as cytokines, growth factors, high molecular weight proteins, and exosomes. Evidence of the role of these mediators in initiation and progression of the DN can be revealed in the urine and be used as predictive biomarkers in assessing the condition of the DN. DN = diabetic nephropathy; AGEs = advanced glycation end products; IL-6 = interleukin-6; IL-1= interleukin-1; MCP1 = monocyte chemoattractant protein-1; TNF-α = tumor necrosis factor-alpha; RAAS = renin-angiotensin-aldosterone system; miRNA = microRNA; ROS = reactive oxygen species; RAGEs = receptor for AGEs; TGF-β1 = transforming growth factor-β1; CTGF = connective tissue growth factor.
Blood pressure changes within the kidney have been reported to occur early in diabetes and to be critical in the progression of DN. Impairment of glomerular microcirculation and altered intrarenal pressure lead to glomerular hypertrophy and sclerosis. Studies using in vitro model of mechanical stretch have shown that podocytes and mesangial and tubular cells release several molecules when experiencing recurrent episodes of dilatation and relaxation, similar to in vivo conditions.20,21 These molecules are responsible for the functional and structural changes in the glomeruli and include transforming growth factor-β1 (TGF-β1), glomerular capillary remodeling cytokine, capillary pressure regulators angiotensin II (Ang II), angiotensin-converting enzyme (ACE), angiotensin II receptor type 1 (AT1) and type 2 receptor (AT2), vascular endothelial growth factor (VEGF), as well as proinflammatory cytokines, such as interleukin-6 (IL-6), interleukin-18 (IL-18), and monocyte chemoattractant protein-1 (MCP-1).18 It has also been shown that these molecules induce pathogenic changes either via elevating oxidative stress through activation of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase or directly by activating cellular remodeling signaling leading to cellular morphological changes and increase synthesis of extracellular matrix (ECM) remodeling.17,19
Hyperglycemia generates advanced glycation end products (AGEs) within tissue and plasma.22-24 These are generated via nonenzymatic oxidative reaction of amino acids from proteins present in renal tissue and plasma.22-24 AGEs are known to induce renal complications by two pathways. One by remaining irreversibly bound to tissue protein such as matrix proteins (type IV collagen, laminin) and impair their degradation by matrix metalloproteinases which contribute to fibrosis via excess accumulation of ECM proteins.22,23,25-27 Second, by interacting with the receptor for AGE (RAGE) expressed by podocytes and endothelial and mesangial cells in the kidney, AGEs also induce specific cellular responses including the release of profibrotic cytokines, such as TGF-β1, connective tissue growth factor (CTGF), and the angiogenic growth factor VEGF.28,29 TGF-β1 plays an important role in the progression of DN because it promotes renal cell hypertrophy apart from stimulating ECM accumulation, the 2 hallmarks of diabetic renal disease.30 Studies considered that TGF-β1 could be useful as a biochemical marker to estimate the progression of diabetes to DN.31,32 A higher level of urinary CTGF has also been shown to correlate with progression of DN, reflecting glomerular damage and fibrosis.26,33,34 Furthermore, ligation of AGEs to RAGE also results in increased expression of NADPH oxidase and mitochondrial-dependent reactive oxygen species (ROS) generation.35,36 All these profibrotic factors and their induced oxidative stress lead to glomerular cell proliferation, expansion, or hypertrophy.
Renal inflammation also plays a significant role in DN progression. In diabetic patients, the progression of glomerular structural and functional changes leads to interstitial infiltration of inflammatory cells, particularly macrophages and lymphocytes, attracted by chemoattractant cytokines released from injured renal tissue.18,37 In turn, these inflammatory cells worsen the progression of DN via the release of proinflammatory and tissue remodeling cytokines which also promote oxidative stress through activation of NADPH oxidase subunits, such as tumor necrosis factor-alpha (TNF-α), interferon-γ,and interleukin-1 (IL-1).18,37 Further studies showed that under these conditions of stress, immune cells and renal glomerular and tubular epithelial cells also produce proinflammatory cytokines including MCP-1, IL-18, and IL-6.
Ultimately, the deposition of ECM in the tubular component of the kidney (tubulointerstitial fibrosis) is postulated to be the major determinant of the progression of renal disease in diabetes.38,39 The massive entry of proteins into the urinary space results in intense protein reabsorption activity of proximal tubular cells; this event is in turn followed by the formation of proteinaceous casts at distal points that cause tubular dilatation and obstruction.40 There is a loss of tubular basement membrane integrity, and the proteins derived from the urinary space are accumulated in an abnormal amount in the interstitium where they trigger the inflammatory reaction.41,42
Biomarkers of DN
In 1998, the National Institutes of Health Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”43 Biomarkers provide a dynamic and powerful approach to understanding the spectrum of a disease from the earliest manifestations to the terminal stage.
Current biomarkers used in practice
Significant efforts have been made to identify serum or urine biomarkers which can clinically detect early stages of DN and progressive kidney function decline in diabetic patients. In clinical practice, most commonly used markers of renal disease and progression of DN are described in Table 1, and included serum creatinine, estimated glomerular filtration rate (eGFR), blood urea and proteinuria, or albuminuria. GFR is the best index available to assess kidney function but estimations of GFR reflect late functional changes and not early structural alterations in the kidney.44 Even if novel methods such as cystatin C are explored, GFR estimation is still largely creatinine based, and there are a lot of internal factors that influence this marker compromising the estimation of GFR.45-49 Microalbuminuria has been recognized as the earliest marker of DN in clinical practice; however, a large proportion of renal impairment occurs in a nonalbuminuric state or before the onset of microalbuminuria.5,6,10,70 Indeed, several studies have shown that diabetic patients can still develop DN without any change in their urinary albumin levels, and in some instances, microalbuminuria is shown to regress back to normoalbuminuria in patients with advanced DN.5,70,71 In addition, a moderate increase in albumin excretion is associated with a variety of other conditions, including obesity, exercise, diet, smoking, infection, and inflammation.55,72-76 Together, these observations indicate that urinary albumin levels may not necessarily go in parallel with the progression of DN but rather represent an initial reversible phase of kidney damage.
Table 1.
Currently Used Biomarkers and Their Predictive Value.
| GFR | ||
| Definition | GFR measures the rate at which the glomeruli filter the plasma and remove waste products from it. If the kidney is injured, the GFR gradually declines and the glomerular function can be estimated by measuring the GFR. | 44-54 |
| Methods of measure | The normal value for GFR is 100-150 mL/min. Measurements are traditionally based on the renal clearance of a marker in plasma, expressed as the volume of plasma completely cleared of the marker per unit time. Markers used to measure GFR can be: exogenous substances continuously infused and analyzed by multiple timed urine collections (inulin, iohexol, 125Iiothalamate, 99Tc DTPA, 51Cr EDTA), but these methods are not used in clinical practice; or endogenous substances, which are freely filtered by glomerulus, such as serum creatinine or serum cystatin C. The most widely used equations by the health care community are Cockcroft-Gault and MDRD. Both equations use serum creatinine in combination with age, sex, weight, or race to estimate GFR. | |
| Advantages | GFR is a good marker for the detection of kidney disease, understanding its severity, making decisions about diagnosis, prognosis and treatment. | |
| Limitations | Measure of GFR by using exogenous substances has limitations in clinic and research purposes (labor intensive nature of these techniques, time-consuming and requirement of experienced personnel). Estimation of GFR (eGFR) that are based on serum creatinine concentration are further limited by variation in creatinine production on the basis of age, gender, race, and body composition (Cystatin C may be useful in those cases where creatinine measurement is not appropriate). Consequently, eGFR does not reflect the critical early stage of renal dysfunction because routine clinical tests do not measure the degree of GFR decline accurately. |
|
| Albuminuria | ||
| Definition | Albumin is a relatively small molecule (65 kDa) and is produced by the liver. The circulating life span is 12 to 20 days. The turnover rate is around 15 g/d. There is no storage of reserve, and it is not catabolized in starvation. A significant amount is filtered in the glomeruli, but most of it is reabsorbed by the proximal tubular cells. The resulting albuminuria in urine reflects the combined contribution of these two processes. | 5,6,9,50,51,55-60 |
| Methods of measure | Normal excreted urine contains approximately 20-mg albumin/L urine. Microalbuminuria is defined as levels of albumin ranging from 30 to 300 mg in a 24-hour urine collection and macroalbuminuria as a UAE of > 300 mg/24 h. Albuminuria can be measured in several ways: (1) measure of albumin-to-creatinine ratio in first morning spot; (2) 24-hour urine collection with measurement of creatinine to verify adequacy of the collection; (3) timed (4 hours or overnight) urine collections. |
|
| Advantages | Albuminuria is a well-known predictor of poor renal outcome in DN and remains the essential tool for monitoring DN progression and risk stratification. A change in the urine albumin excretion is one of the first asymptomatic clinical manifestations of glomerular injury and tubular impairment in diabetes. Microalbuminuria has been recognized as a predictor of progression to ESRD in type 2 and in type 1 diabetic patients. Microalbuminuria and macroalbuminuria are not only markers of nephropathy but also causes of disease progression. Consequently, an increase in albuminuria should not only be considered as a risk factor for DN, but also as evidence of early organ damage. Microalbuminuria has also been associated with an increased risk of cardiovascular events. | |
| Limitations | Recent studies have raised growing concerns about the value of microalbuminuria as a very predictable marker of progression to ESRD. Studies have reported cases of patients who have developed DN in the absence of microalbuminuria; or cases of spontaneous regression of microalbuminuria to normoalbuminuria in patient with type 1 diabetes. These data suggest that microalbuminuria may represent an initial reversible phase of kidney damage rather than the inevitability of progression to ESRD. Thus, while microalbuminuria may be an indicator of renal damage, considerable doubt has emerged that it is a predictor of ESRD in patients with diabetes. | |
| Creatinine | ||
| Definition | Creatinine is a nonenzymatic breakdown product of the phosphocreatine in muscle. Approximately 2% of the body’s creatine is converted to creatinine every day. Creatinine is transported through the bloodstream to the kidneys where most of the creatinine is filtered and released in the urine. Plasma creatinine level is produced at a relative constant rate based on age, gender, and muscle mass (0.8 to 1.4 mg/dL in adult males and 0.6 to 1.2 mg/dL in adult females). | 50,54,61-65 |
| Methods of measure | Creatinine clearance requires a 24-h urine collection. A blood sample is performed at some point during the 24-h period, and creatinine clearance is calculated to estimate the rate of filtration by kidneys. Serum creatinine is commonly measured by alkaline picrate, enzymatic, and high-performance liquid chromatography methods. Currently, there are about 47 different prediction equations in adults based on serum creatinine concentration that were currently available for estimating GFR. The 2 most common in use are the Cockcroft-Gault and the MDRD. These equations include variables such as serum or plasma creatinine, age in years, gender, and weight. | |
| Advantages | Creatinine has been found to be a fairly reliable indicator of kidney function because a high creatinine level in the blood is associated with poor clearance of creatinine by the kidneys. | |
| Limitations | The use of serum creatinine as an indirect filtration marker is limited by its biological variability because several factors influence serum creatinine level other than renal factors, including age, race, gender, pregnancy, muscle mass, drug metabolism, protein intake, hydration medications (corticosteroids), drugs. These intraindividual variabilities compromise the generalizability of the eGFR equations. However, the estimation of GFR by serum creatinine differs between healthy people and patients with CKD because of differences in GFR range and creatinine production between these 2 populations. As a result of these confounding factors, there is a risk to over or underestimation of the true GFR, and the magnitude of the over/underestimation is not predictable. Finally, its sensitivity is poor in the early stages of renal impairment, as by the time an increase in serum level is detectable, a significant decline in GFR has already taken place. GFR may deteriorate by more than 50% prior to a significant rise in serum creatinine. Consequently, serum creatinine concentration is not a good biomarker for detecting mild-to-moderate kidney failure. | |
| Cystatin C | ||
| Definition | Cystatin C is a low-molecular weight (13 kDa) protease inhibitor produced by all nucleated cells. Cystatin C is less physiologically variable than creatinine, is synthesized at constant rate, is not affected by muscle mass, is not secreted or reabsorbed in tubules, and is completely filtered by the kidney glomerulus and metabolized by proximal renal tubular cells (no detectable Cystatin C in urine). | 46,50,66-69 |
| Methods of measure | Cystatin levels are measured by different methodologies using latex particle-enhanced immunoturbidimetry or immunonephelometric assays. | |
| advantages | Elevated Cystatin C is a better early predictor compared with serum creatinine-based formulae, even when measured enzymatically. It is useful to detect early and mild DN in both type 1 and type 2 diabetes, even before development of microalbuminuria. However, serum creatinine was as efficient as serum cystatin C to detect advanced DN. Numerous studies have validated Cystatin C as a marker of renal function. Its levels are well correlated with GFR and, unlike serum creatinine, are unaffected by muscle mass. In addition, Cystatin C levels not only correlate with progression of nephropathy but also show a more sensitive marker of early DN when eGFR remains > 60 mL/min. | |
| Limitations | The test to detect Cystatin C levels is currently not widely available (higher cost of the immunoassay), and not all assays have been universally calibrated. Both these factors limit its use in clinical practice at present. Some factors can also influence Cystatin C levels, such as alterations in thyroid function. Consequently, Cystatin C should not be considered for evaluation of GFR without assessing thyroid function tests. They are also liable to change in patients with CKD receiving glucocorticoids. | |
| BUN | ||
| Definition | Urea is a waste product formed in the liver when protein is metabolized. Urea is released into the blood and is filtered through healthy kidneys and excreted in the urine. There is usually a small but stable amount of urea nitrogen in the blood. BUN is a blood test that gives an indication of the kidney function. | 50,65 |
| Methods of measure | A BUN test measures the amount of urea nitrogen in the blood or plasma. Multiple methods for analysis of BUN have evolved over the years. Most of those in current use are automated and give clinically reliable and reproducible results. The BUN is interpreted in conjunction with the creatinine test. The BUN-to-creatinine ratio is a good measurement of kidney function. The normal level of BUN is 7-20 mg/dL. | |
| Advantages | A high BUN level could be an indicator of kidney damage and dysfunction because this product is not correctly excreted by the kidney and accumulates in the blood. This test is useful for the initial diagnosis of acute or chronic kidney injury. The BUN-to-creatinine ratio generally provides more precise information about kidney function and its possible underlying cause compared with creatinine level alone. | |
| Limitations | Several factors that influence blood volume and renal blood flow may impact on BUN levels: febrile illness, high protein diet, alimentary tube feeding, gastrointestinal bleeding, dehydrated patients, and drugs. Because the synthesis of urea depends on the liver, severe liver disease can cause a decreased BUN. | |
Note. CKD = chronic kidney disease; DTPA = diethylenetriaminepentaacetic acid; EDTA = ethylenediaminetetraacetic acid; GFR = glomerular filtration rate; HPLC = high-performance liquid chromatography; MDRD = modification of diet in renal disease; eGFR = estimated glomerular filtration rate; UAE = urinary albumin excretion; DN = diabetic nephropathy; ESRD = end-stage renal disease; BUN = blood urea nitrogen.
The research community is now focusing on a different strategy to enhance the sensitivity of biomarkers to predict patients who will develop DN or are at risk of progressing to ESRD. Part of the difficulty in finding biomarkers is the complex pathogenesis of DN; as mentioned above, DN is clearly multifactorial and involves multiple genes, proteins, metabolic pathways, and environmental factors. Increasing knowledge of the early molecular events in DN will spur the development of new alternative drugs and, combined with methods already used in practice, may prevent the onset of disease entirely. The next section links important aspects of DN pathogenesis, including the processes of oxidative stress, tubular damage, and renal inflammation with some of the promising new biomarkers in serum and urine (Table 2) which are directly or indirectly related to these pathogenic mechanisms.
Table 2.
Biomarkers of Diabetic Nephropathy Pathophysiology.
| Class | Biomarkers | Clinical importance | Method of detection | Ref. |
|---|---|---|---|---|
| Oxidative stress | Pentosidine | Predictor of progression; influenced by glycemic levels and renal function; biomarker of microvascular complications and diabetic cardiovascular risk. | Serum/urine | 77,78 |
| 8-OHdG | Predictor of advanced stage; related to the severity of DN, associated with macroalbuminuria. | Urine | 79,80 | |
| Uric acid | Predictor of progression; associated with various stages of DN, onset and progression; potential target for therapeutic intervention in diabetes. | Serum | 81,82 | |
| Fibrosis | TGF-β1 | Predictor of advanced stage DN; positively correlates with micro- and macroalbuminuria | Serum/urine | 31 |
| CTGF | Predictor of ESRD; correlates with the rate of decline in GFR. | Serum/urine | 83,84 | |
| VEGF | Predictor of progression; increased during the earlier stage of DN and shown to significantly correlate with urinary albumin excretion. | Serum/urine | 85,86 | |
| Glomerular damage | Transferrin | Predictor of early stage; increased before development of microalbuminuria. | Urine | 87 |
| Type IV collagen | Predictor of advanced stage of DN; associated with a faster decline in eGFR. | Urine | 88,89 | |
| Cystatin C | Predictor of early stage DN; raised early in DN and pre-DN; increased in patients with microalbuminuria without any other urinary abnormality. | Serum/urine | 90,91 | |
| Tubular damage | L-FABP | Predictor of early stage and progression of DN; increased from the microalbuminuric stage; elevated in patients with reduced eGFR. | Urine | 92,93 |
| NGAL | Predictor of early stage and progression of DN; found in diabetic patients without early signs of glomerular damage (normoalbuminuric). | Urine | 94,95 | |
| KIM-1 | Predictor of early stage DN; increased even before the onset of albuminuria and proteinuria. | Serum/urine | 96,97 | |
| ACE2 | Biomarker of increased metabolism of Ang II in DN; its downregulation or excretion in urine predicts tubular injury and reduced renal function. | Serum/urine | 98,99 | |
| Angiotensinogen | Predictor of early and development of kidney injury; levels correlated with albuminuria, biomarker of the intrarenal RAAS. | Urine | 100,101 | |
| NAG | Predictor of early stage DN; associated with normoalbuminuric and microalbuminuric stages; increased in parallel with the severity of disease. | Urine | 102,103 | |
| α1-microglobulin | Predictor of early stage DN; directly correlates with albuminuria and severity of the disease. | Urine | 104,105 | |
| FGF23 | Predictor of DN progression to ESRD; associated with macroalbuminuria and risk of mortality. | Serum | 106,107 | |
| Inflammation | TNF-α;TNFR1/2 | Predictor of DN progression to ESRD and GFR loss; associated with the presence and severity of microalbuminuric stage. | Serum/urine | 108,109 |
| MCP-1 | Predictor of progressive renal disease; correlated significantly with albuminuria levels; accelerate nephropathy by increasing inflammation and fibrosis; potential for therapeutic target for treating DN. | Urine | 110,111 | |
| IL-18, IL-1, IL-6, IL-8 | Predictor of DN progression; strongly associated with future risk of early progressive renal decline. | Serum/urine | 18,112-114 |
Note. 8-OHdG = 8-hydroxy-2′-deoxyguanosine; DN = diabetic nephropathy; TGF-β1 = transforming growth factor-β1; CTGF = connective tissue growth factor; ESRD = end-stage renal disease; GFR = glomerular filtration rate; VEGF = vascular endothelial growth factor; eGFR = estimated glomerular filtration rate; L-FABP = liver-type fatty acid-binding protein; NGAL = neutrophil gelatinase-associated lipocalin; KIM-1 = kidney injury molecule-1; ACE2 = angiotensin-converting enzyme-2; Ang II = angiotensin II; RAAS = renin-angiotensin-aldosterone system; NAG = N-acetyl-beta-d-glucosaminidase; FGF23 = fibroblast growth factor 23; TNF-α = tumor necrosis factor-α; TNFR1/2 = tumor necrosis factor receptor 1/2; MCP-1 = monocyte chemoattractant protein-1; IL = interleukin.
From pathogenic pathways to DN biomarkers
Biomarkers of oxidative stress
Oxidative stress plays a pivotal role in cellular injury from hyperglycemia. A prolonged oxidative stress renders endothelial cell dysfunction, enhances influx of inflammatory cells, and increases ECM synthesis and cellular proliferation. In the diabetic state, the presence of high levels of markers for oxidative DNA damage, such as 8-hydroxydeoxy-guanosine (8-OHdG), and protein oxidation, such as pentosidine, has been reported.77,115-117
Several studies showed that urinary 8-OHdG is increased in the urine of diabetic patients with nephropathy and tends to increase with the severity of the glomerular lesions.79,118 Naito et al demonstrated that the urinary albumin levels increased in db/db (diabetic) mice in parallel with the increase in urinary 8-OHdG levels.119 Because the increased oxidative stress has a primary role in the pathogenesis of DN, the 8-OHdG in urine could be a useful clinical marker to predict the development of DN in patients. However, when the performance of this biomarker is compared with urinary albumin, urinary 8-OHdG is demonstrated to be not a useful clinical marker for early detection or to predict the development of DN in diabetic patients as compared with urine albumin-to-creatinine ratio.120 Altogether, these studies suggest that 8-OHdG is most likely a predictor of DN severity.
The intracellular formation of AGEs is a stress caused by hyperglycemia.22 The rate of accumulation of glycoxidation products is accelerated in diabetes. Among AGEs, N(6)-carboxymethyllysine and pentosidine correlate with the severity of complications in diabetic patients.121,122 Pentosidine is one of the best chemically characterized AGE compounds. Studies have reported elevated urine and serum concentrations of pentosidine in type 2 DN patients with microalbuminuria and renal dysfunction compared with control patients or type 2 DN patients without microalbuminuria.123,124 Plasma pentosidine level was significantly influenced by the quality of glycemic control and renal function.78,125 Pentosidine level was also correlated with hypertension and ischemic heart disease, suggesting that it is a reliable biomarker of DN and its related cardiovascular risk.77
Recent studies suggest that uric acid (UA), a molecule involved in oxidative stress, also plays a role in the progression of DN and could be a predictor of the disease.81 UA is the final enzymatic product in the degradation of purine nucleosides and free bases in humans. Produced by the liver, UA enters the bloodstream and is then filtered by glomeruli into the renal tubule. Most of filtered UA (about 90%) is reabsorbed by proximal tubule, and approximately 10% of filtered UA is excreted in urine.81 There is a paradox concerning UA function in chronic kidney diseases.126 Studies have shown that UA is a powerful scavenger of singlet oxygen and radicals and is one of the major antioxidants of the plasma.127,128 On the contrary, it has also been documented that once UA enters the cell, it can induce oxidative stress, endothelial dysfunction, stimulate vascular RAS, initiate inflammatory cascades, and profibrotic cytokine activation.129-134 Accordingly, some studies have documented that an elevated serum UA level independently predicts the development of DN. For example, in type 1 diabetic patients, baseline serum UA levels were predictive of persistent macroalbuminuria, and there is a significant association between UA levels (within the normal range) and early GFR loss.135-137 There is evidence that UA is involved in various stages of DN onset and progression.81 Randomized controlled trials have demonstrated that chronic kidney disease progression can be decreased by lowering serum UA levels in diabetic patients.82,138-140 A clinical trial studying the effect of the UA lowering drug allopurinol in preventing early loss of kidney function among patients with type 1 diabetes is currently undergoing.141
Biomarker of glomerular damage
A considerable number of studies in animal models and human have pointed the potentialities of the glomerular podocyte protein nephrin as a noninvasive early urinary marker of DN.142 Nephrin is an 180-kDa transmembrane protein associated with an autosomal recessively inherited disorder called the Congenital Nephrotic Syndrome of the Finnish type (NPHS1), which leads to massive proteinuria and death in neonates. Besides this critical role, changes in nephrin excretion are also linked to podocyte injury. Indeed, elevated nephrin urinary levels have been found in several disease conditions including DN, glomerulonephritis, and preeclampsia.142 Importantly, studies using animal models of type 1 diabetes mellitus and DN either streptozotocin (STZ) rats or Akita mice (the last bear a point mutation in the insulin2 gene) have shown that the peak of nephrinuria precedes changes in albuminuria. This suggests that measurement of nephrin in urine can be useful in early detection of DN.96,143 In accordance with these encouraging works, analysis of nephrin levels in urine from cohorts of DN patients, by analyzing either messenger RNA (mRNA) by reverse transcription polymerase chain reaction (RT-PCR) or protein levels using western blot or enzyme-linked immunosorbent assay, corroborates studies in animal models. This work shows that nephrinuria is higher in DN patients versus control and that levels correlate with the albumin/creatinine ratio as well as eGFR.144-146 Interestingly, a recent study analyzed renal nephrin mRNA and urinary nephrin-to-creatinine ratio in different animal models with podocyte dysfunction and the effect of some candidate drugs for the treatment of podocyte dysfunction. The authors proposed that the urinary nephrin-to-creatinine ratio is a reliable marker for predicting the effectiveness of the treatment.147 In spite of these encouraging data, there is still few clinical assays validating the efficacy of nephrinuria and no studies performed so far in the population of children or adolescents affected by DN.
Biomarkers of tubular damage
The tubulointerstitium plays a pivotal role in the pathogenesis of various kidney diseases, and the degree of tubulointerstitial damage is strongly associated with renal prognosis.148 Thus, tubular markers of kidney injury may be capable of reflecting the degree of sustained renal damage in diabetic patients.
Urinary liver-type fatty acid-binding protein (L-FABP),92,149-151 urinary neutrophil gelatinase-associated lipocalin (NGAL),152,153 and urinary kidney injury molecule-1 (KIM-1)154 are newly established tubular biomarkers that have been reported as early detectors of acute kidney injury. The renal proximal tubular cells abundantly express L-FABP, and urinary excretion of this molecule is elevated in patients even before the development of glomerular damage or albuminuria.92,149,150 NGAL is also considered to be a sensitive and more accurate early predictor of acute renal damage. Higher urinary NGAL has been associated with the decline in GFR in type 2 diabetic patients with micro- or macroalbuminuria.94,155 KIM-1 is a membrane protein expressed on the apical membrane of renal proximal tubule cells and reflects tubular damage in the most advanced stages of the renal disease in diabetic patients.97,156 Urinary levels of these tubular markers during DN progression reflect not only the severity of the kidney injury but also the degree of tubulointerstitial fibrosis. These urinary markers may be useful for detecting patients with higher risk of progression to CKD in clinical practice. Moreover, studies have shown that treatment with RAS blocking agents reduced urinary KIM-1 excretion in parallel with a reduction in blood pressure and urine albumin excretion.157,158
In contrast to the aforementioned markers, angiotensin-converting enzyme-2 (ACE2), a homolog of ACE, seems to plays a protective role in the diabetic kidney and an important determinant of DN.98 This monocarboxypeptidase enzyme regulates the levels of Ang II by cleaving it to the vasodilatory and antiproliferative Ang 1-7.159 As such, ACE2 serves as an endogenous negative regulator of the RAS. ACE2 is localized on the brush border of proximal tubules and to a lesser extent in the glomerular cells and the renal vasculature.159,160 ACE2 activity has been shown to be altered in diabetic kidney disease.159-161 Pharmacological inhibition of ACE2 in STZ-induced diabetes in mice causes increased albuminuria and glomerular matrix expansion,99,162 whereas overexpression of human ACE2 attenuates the development of nephropathy.163,164 Studies demonstrated that ACE2 downregulation may cause excessive Ang II accumulation, particularly in the glomerular region, leading to increased albuminuria and glomerular damage.159 Serum and urinary ACE2 activity were increased in a type 2 diabetic mice model and in STZ-induced type 1 diabetic mice.165 Conversely, in humans, studies have suggested that downregulated ACE2 expression is seen at both the glomerular and tubular levels in biopsy samples collected from patients with type 2 diabetes with established DN.166,167 The difference of ACE2 expression in DN between humans and mice may reflect a different stage of the disease and the relative sparing of tubulointerstitial damage in the diabetic mice model. However, the role of ACE2 in diabetes and renal disease is currently in the early phases of exploration and human data are emerging. A recent study has demonstrated that in patients with type 1 diabetes, urinary ACE2 protein and activity levels are increased, even before abnormal albuminuria occurs.168 Altogether, these studies suggest that measuring renal protective mediators such as ACE2 along with urine levels of L-FABP, NGAL, and KIM-1 might provide prognostic information in DN. Future work is required to validate these encouraging findings in larger groups of human subjects.
Biomarkers of renal inflammation
In the course of DN, renal inflammation and an influx of inflammatory cells cause the release of interleukins and cytokines, such as TNF-α, MCP-1, TGF-β1, IL-1β, IL-6, and IL-8, creating a proinflammatory microenvironment that amplifies tissue injury.18,169-171 Several studies have investigated their potential clinical use in studying DN.
TNF-α is an important cytokine produced under high glucose conditions by macrophages, renal tubular cells, and glomerular mesangial cells. Apart from being a major participant in promoting inflammation, TNF-α is known to induce apoptosis and accumulation of ECM in glomerular and tubular regions leading to alteration of glomerular filtration, tubular permeability, and reabsorption. Moreover, aberrant levels of this cytokine correlate with increased expression of tubular cell injury markers such as NADPH oxidase and ROS.172-174 These actions are mediated via binding to TNF-α receptors.175,176 Clinical studies have reported higher serum and urinary levels of TNF-α in diabetic patients with renal dysfunction, which further increase with progression of the disease.174,177,178 In addition, circulating levels of TNF-α receptors 1 and 2 are elevated in diabetic patients independent of albuminuria status,179-181 suggesting that assessing TNF-α or their receptor levels in the serum and urine could predict the progression of DN to ESRD in the presence or absence of proteinuria.
MCP-1 is a cytokine secreted by mononuclear leukocytes, cortical tubular epithelial cells, and podocytes and is implicated in renal inflammation, glomerular damage, tubular atrophy, and fibrosis.182 MCP-1 is synthesized via nuclear factor-kappa B.110 A number of studies indicated that urinary detection of MCP-1 is a reliable early marker of DN over other conventional markers.111,183 It has been reported that high urinary levels of MCP-1 correlated with an early drop of GFR in patients with type 1 diabete.184,185 More recently, Fufaa et al have shown that urinary MCP-1 levels also correlated with early changes in cortical interstitial expansion and development of DN in normotensive normoalbuminuric individuals with type 1 diabetes, before the onset of clinical signs of DN.186 Patients with type 2 diabetes excrete high levels of MCP-1 in the urine, which correlates with albuminuria, and macroalbuminuric patients with diabetes had higher MCP-1 levels compared with micro or normoalbuminuric patients.182,187-189 Together, these studies indicate that urinary MCP-1 may also be useful as a marker for evaluating the degree of renal injury and early prediction of DN.111,183
Elevated serum levels of IL-6 have been associated with the progression of DN in type 2 diabetic patients.112,177 Sekizuka et al first reported that levels of IL-6 were significantly higher in the serum of type 2 diabetic patients with nephropathy compared with diabetic patients without nephropathy.190 Furthermore, it was shown that IL-6 mRNA is expressed in glomerular cells in kidney biopsies from patients with DN and levels change with progression of the disease.191 In an STZ-induced diabetes model of DN, IL-6 mRNA was expressed at high levels in renal tissue, and IL-6 urinary excretion correlated with urinary albumin excretion.192 Mechanistic studies have shown that IL-6 induced renal injury via alteration of endothelial cell permeability, mesangial cell proliferation, ECM accumulation, and basement membrane thickening.193,194 Supporting these studies, a strong correlation has been reported between elevated serum IL-6 levels and glomerular basement membrane thickening in proteinuric patients with type 2 diabetes.195 Evaluating urinary levels of IL-6 along with other proinflammatory cytokines may assist in determining the extent of glomerular injury in patients at different stages of DN.
Taken together, these data stress that fundamental and preclinical DN research is a growing field. Despite the significant number of candidate biomarkers (Table 2), there is a need for further large-scale validation in prospective clinical studies to determine whether they can make the transition from bench to bedside. Also, a further understanding of their function and molecular mechanisms underlying the pathogenesis of DN is mandatory to further improve management of this disease. Of particular interest are emerging mechanisms and their role in the development of DN involving functional noncoding RNAs such as microRNAs (miRNAs) and long noncoding RNAs, microparticles (MPs), and exosomes. We next discuss the potentialities of these emerging biomarkers as diagnostic biomarkers or novel therapeutic targets for DN.
Emerging biomarkers
MicroRNA
MicroRNAs (miRNAs) are new attractive diagnostic biomarkers of DN. They represent small noncoding endogenous RNAs (20-30 nucleotides) that regulate the expression of genes by binding to the 3′ untranslated regions (3′ UTR) of specific mRNAs, inducing their degradation or translational repression.196,197 Consequently, miRNAs have been implicated in the posttranscriptional regulation of many gene expressions and control of different processes such as apoptosis, DNA repair, oxidative stress response, cancer, and cellular development. A number of recent studies using either in vitro or in vivo models of DN have shown that miRNAs target genes associated with inflammation, fibrosis, and oxidative stress.198 A deregulated level of several miRNAs has been found in serum or urine of human diabetic patients.198,199 Studies using mouse models of DN have shown that miRNAs act downstream of TGF-β/Smad signaling in the cascade of events leading to DN. Particularly, studies in STZ-induced type 1 diabetic mice and type 2 diabetic db/db mice have shown that TGF-β1 signaling upregulated expression of miR-192, miR-200b/c, miR216a, and miR-217 in mesangial cells and glomeruli.200 Interestingly, others have reported that miR-192 upregulated ECM and fibrotic effector genes, Col1a2 and Col4a1, as well as the expression of other miRNAs, which subsequently amplify the TGF-β signaling pathway and its fibrotic response.201 In accordance with these animal studies, Pezzolesi and colleagues showed a deregulation of circulating plasma levels of several TGF-β-regulated miRNAs in early type 1 diabetic patients who are at risk for rapid progression to ESRD.198 In particular, they found high circulating levels of miRNAs let-7b-5p, miR-21-5p, miR-29a-3p and let-7c-5p.198 These studies showed the potential application of assessing circulating miRNAs levels as predictors of progression from DN to ESRD. However, more studies are needed to investigate the precise role of miRNA in DN, for example, to determine whether the observed deregulation of miRNA is a consequence of DN progression or a cause or whether expression of these miRNA is specifically deregulated in the kidney, and if so, the mechanism through which these miRNAs contribute to the progression of the DN.
Long noncoding RNA
Long noncoding RNA (lncRNA) is noncoding transcripts of variable size (extending from 200 nucleotides up to 100 kbp) without protein-coding functions.202 LncRNA expression has been found to correlate with miRNA expression in models of DN.202 Several studies have implicated a role for plasmacytoma variant translocation 1 (PVT1) lncRNA in DN pathogenesis. PVT1 has been identified as a candidate gene for ESRD in type 2 diabetes.203 It is located in a potential locus for ESRD and maps with 5 miRNAs (miR-1204, miR-1205, miR-1206, miR-1207, and miR-1208) whose expressions are increased in human mesangial cells treated with high glucose.204,205 PVT1 is shown to promote ECM accumulation by mediating TGF-β signaling in mesangial cells.202 LncRNA are highly stable in biofluids and can be easily detected, which make these molecules useful predictive biomarkers of DN, as well as interesting therapeutic targets. In spite of these promising properties, very few studies have been published on this subject, and the potential of lncRNA as biomarkers still remains to be determined.
Urinary exosomes
Urinary exosomes are small vesicles (40-100 nm) released by most types of renal cells. They harbor various types of cytosolic, membrane, and transport proteins, as well as nucleic acids.206 Exosomes reflect the pathophysiological status of their host cells and have emerged as promising noninvasive source of biomarkers for DN and indicator of disease stage and progression.207 Since their first description by Pisitkun et al in healthy urinary samples, the interest in urinary exosome research has significantly increased concomitantly with the advance of novel technologies to isolate, purify, and characterize their molecular composition.207,208 A significant number of exosomal proteins has been identified.207,209 An interesting candidate biomarker of DN is the enzyme dipeptidyl peptidase which is important for T-cell activation. Levels of dipeptidyl peptidase are increased in plasma and urinary exosome samples from diabetic patients.210 More recent proteomic approaches have enlarged the spectrum of novel urinary exosomes-associated proteins. Using a liquid chromatography (LC)-mass spectrometry (LC-MS/MS) method, Raimondo et al compared the protein profile of isolated urinary exosomes from Zucker diabetic fatty (ZDF) rats, a model of type 2 diabetes, and reported a differential expression of several proteins including the Xaa-Pro dipeptidase and major urinary protein 1.211 Similarly, using LC-MS/MS method followed by selected reaction monitoring validation, Zubiri and colleagues identified 352 different proteins in human urinary exosomes. Among these proteins, levels of α-microglobulin/bikunin precursor (AMBP), histone-lysine N-methyltransferase (MLL3), and voltage-dependent anion-selective channel protein 1 (VDAC1) were found differentially changed (AMBP and MLL3 increased, VDAC1 decreased) in samples from patients with DN versus control individuals.212 More recently, the same group reported on another protein “regucalcin” also known as senescence marker protein-30 (SMP30). Using animal models as well as a pilot study in humans, Zubiri and colleagues showed that expression of regucalcin is downregulated in DN kidneys and that these changes can be detected in human urinary exosomes.213
Urinary exosomes are also carriers and source of miRNAs. A study from Barutta and colleagues evaluated the miRNA expression in urinary exosomes from diabetic patients with DN and found a differential expression of 22 exosomal miRNAs including miR-145, miR-130a, miR155, and miR-424 in urinary exosomes from patients with type 1 diabetes compared with control individuals.214 Mechanistic studies in the STZ-induced DN mouse model and cultured mesangial cells indicated that mesangial-derived exosome miR-145 levels increased upon high glucose conditions, thus suggesting that urinary exosomes screening may be used as an early diagnostic tool for detecting the development of DN.214
Microparticles
Microparticles are extracellular vesicles released from the surface of cells under stress or injury. MPs are larger in size with respect to exosomes (0.1-1 µm) and exhibit a particular molecular composition, ie, they expose phosphatidylserine at the surface.215 MPs are released from renal cells under glycemic conditions and can be detected in the plasma and urine before the onset of DN. These properties, together with their facility of isolation from fluids via noninvasive methods, have attracted their attention as potential biomarkers for predicting the progression of the DN. A recent study from Burger and colleagues showed the potential of podocyte-derived MPs in urine as early markers of glomerular injury in DN.216 By using 3 different mouse type 1 diabetes models (OVE26, STZ-treated, Akita), the type II diabetes db/db mice, as well as different diabetes-inducing stress conditions, they showed that high glucose and mechanical stretch-induced podocyte MP release into the urine during the earliest stages of diabetic renal injury, which preceded changes in albuminuria.216 These interesting findings have to be confirmed in clinical settings for considering the use of urinary podocyte MPs as biomarkers of human DN.
Conclusion
Over the past few years, a better understanding of DN pathogenesis has revolutionized and improved the approaches used for treating the patients with diabetes and its associated renal complications. Aggressive blockade of the renin-angiotensin-aldosterone system with either high-dose of ACE inhibitors and angiotensin receptor blockers (ARBs), or therapy with ACE inhibitor-ARB combinations and with personalized dose regime to control blood glycemic levels, has been shown to reduce the further decline in the kidney function in patients with diabetes and DN. However, many patients still progress to ESRD and remain at high risk for fatal events. One scenario through which pathophysiological progress of DN can be either delayed or slowed down considerably is by starting these interventions during early stages of DN. The identification of biomarkers of early stages of DN, and progression toward ESRD, is thus of critical importance. In this review, we have summarized the novel biomarker based on the pathogenesis of the DN and presented a list of several putative prognostic biomarkers. Despite this wealth of information, the scientific community has just started to translate assessing these biomarkers into routine clinical practice to provide personalized treatment to patients. A urinary proteomics-based approach, namely CKD 273 classifier, was developed to predict CKD progression and has been able to discriminate CKD patients according to disease severity.217,218 Expanding this proteomics screening, including oxidative stress and inflammatory markers, along with metabolomics approach may further improve the prognostic value and help in identifying the patients with diabetes who are at high risk of developing kidney diseases more specifically with high efficiency.
Acknowledgments
We thank Dr Adeera Levin, Dr Kevin Burns, and the team of the KRESCENT program for critical reading of the manuscript.
Footnotes
Ethics Approval and Consent to Participate: Not applicable
Consent for Publication: All authors read and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable
Authors’ Note: Carole G. Campion and Oraly Sanchez-Ferras contributed equally to this work.
Author Contributions: CGC, OSF, and SNB all conceived of and contributed to the research and writing of this article. CGC developed and conceived the figures, tables, and their legends. All authors read and approved this final article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Carole G. Campion, Dr Oraly Sanchez-Ferras, and Dr Sri N. Batchu are supported by a KRESCENT Post-Doctoral Fellowship. Dr Sri N. Batchu is also supported by Heart and Stroke/Richard Lewar Center of Excellence Fellowship Award. Dr. Oraly Sanchez-Ferras is also supported by McGill Integrated Cancer Research Training Program (MICRTP) fellowship.
References
- 1. International Diabetes Federation. IDF Diabetes Atlas 7th edition. idf.org. Published 2015. Accessed April 11, 2017.
- 2. World Health Organization. Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 3. Berkman J, Rifkin H. Unilateral nodular diabetic glomerulosclerosis (Kimmelstiel-Wilson): report of a case. Metabolism. 1973;22:715-722. [DOI] [PubMed] [Google Scholar]
- 4. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225-232. [DOI] [PubMed] [Google Scholar]
- 5. Perkins BA, Ficociello LH, Roshan B, Warram JH, Krolewski AS. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 2010;77:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Viberti GC, Jarrett RJ, Mahmud U, Hill RD, Argyropoulos A, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;319:1430-1432. [DOI] [PubMed] [Google Scholar]
- 7. Araki S, Haneda M, Koya D, et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes. 2007;56:1727-1730. [DOI] [PubMed] [Google Scholar]
- 8. Cerasola G, Cottone S, Mulè G. The progressive pathway of microalbuminuria: from early marker of renal damage to strong cardiovascular risk predictor. J Hypertens. 2010;28:2357-2369. [DOI] [PubMed] [Google Scholar]
- 9. De Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309-2320. [DOI] [PubMed] [Google Scholar]
- 10. Palmer BF. Proteinuria as a therapeutic target in patients with chronic kidney disease. Am J Nephrol. 2007;27:287-293. [DOI] [PubMed] [Google Scholar]
- 11. Fioretto P, Steffes MW, Mauer M. Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes. 1994;43:1358-1364. [DOI] [PubMed] [Google Scholar]
- 12. Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285-2293. [DOI] [PubMed] [Google Scholar]
- 13. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869. [DOI] [PubMed] [Google Scholar]
- 14. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941-1951. [DOI] [PubMed] [Google Scholar]
- 15. Haller H, Ito S, Izzo JL, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907-917. [DOI] [PubMed] [Google Scholar]
- 16. Gaede P, Pedersen O. Intensive integrated therapy of type 2 diabetes: implications for long-term prognosis. Diabetes. 2004;53(suppl 3):S39-S47. [DOI] [PubMed] [Google Scholar]
- 17. Cao Z, Cooper ME. Pathogenesis of diabetic nephropathy. J Diabetes Investig. 2011;2:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433-442. [DOI] [PubMed] [Google Scholar]
- 19. Schena FP. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16:S30-S33. [DOI] [PubMed] [Google Scholar]
- 20. Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69-84. [DOI] [PubMed] [Google Scholar]
- 21. Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol. 2010;31:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bucala R, Vlassara H. Advanced glycosylation end products in diabetic renal and vascular disease. Am J Kidney Dis. 1995;26:875-888. [DOI] [PubMed] [Google Scholar]
- 23. Daroux M, Prévost G, Maillard-Lefebvre H, et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010;36:1-10. [DOI] [PubMed] [Google Scholar]
- 24. Weiss MF, Erhard P, Kader-Attia FA, et al. Mechanisms for the formation of glycoxidation products in end-stage renal disease. Kidney Int. 2000;57:2571-2585. [DOI] [PubMed] [Google Scholar]
- 25. Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:31S-38S. [DOI] [PubMed] [Google Scholar]
- 26. Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 2004;165:2033-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742-755. [DOI] [PubMed] [Google Scholar]
- 28. D’Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol. 2010;6:352-360. [DOI] [PubMed] [Google Scholar]
- 29. Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005;289:F645-F659. [DOI] [PubMed] [Google Scholar]
- 30. Castro NE, Kato M, Park JT, Natarajan R. Transforming growth factor β1 (TGF-β1) enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem. 2014;289:29001-29013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaker YM, Soliman HA, Ezzat E, et al. Serum and urinary transforming growth factor beta 1 as biochemical markers in diabetic nephropathy patients. Beni-Suef Univ J Basic Appl Sci. 2014;3:16-23. [Google Scholar]
- 32. Goldfarb S, Ziyadeh FN. TGF-beta: a crucial component of the pathogenesis of diabetic nephropathy. Trans Am Clin Climatol Assoc. 2001;112:27-32. [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Denichilo M, Brubaker C, Hirschberg R. Connective tissue growth factor in tubulointerstitial injury of diabetic nephropathy. Kidney Int. 2001;60:96-105. [DOI] [PubMed] [Google Scholar]
- 34. Roestenberg P, van Nieuwenhoven FA, Wieten L, et al. Connective tissue growth factor is increased in plasma of type 1 diabetic patients with nephropathy. Diabetes Care. 2004;27:1164-1170. [DOI] [PubMed] [Google Scholar]
- 35. Tan ALY, Forbes JM, Cooper ME. AGE, RAGE, and ROS in diabetic nephropathy. Semin Nephrol. 2007;27:130-143. [DOI] [PubMed] [Google Scholar]
- 36. Coughlan MT, Mibus AL, Forbes JM. Oxidative stress and advanced glycation in diabetic nephropathy. Ann N Y Acad Sci. 2008;1126:190-193. [DOI] [PubMed] [Google Scholar]
- 37. Navarro-González JF, Mora-Fernández C, Muros de, Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [DOI] [PubMed] [Google Scholar]
- 38. Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819-1834. [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005;68:82-86. [DOI] [PubMed] [Google Scholar]
- 40. Remuzzi G, Bertani T. Is glomerulosclerosis a consequence of altered glomerular permeability to macromolecules? Kidney Int. 1990;38:384-394. [DOI] [PubMed] [Google Scholar]
- 41. Abbate M, Zoja C, Corna D, Capitanio M, Bertani T, Remuzzi G. In progressive nephropathies, overload of tubular cells with filtered proteins translates glomerular permeability dysfunction into cellular signals of interstitial inflammation. J Am Soc Nephrol. 1998;9:1213-1224. [DOI] [PubMed] [Google Scholar]
- 42. Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Atkinson AJJ, Colburn WA, DeGruttola VG, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95. [DOI] [PubMed] [Google Scholar]
- 44. Currie G, McKay G, Delles C. Biomarkers in diabetic nephropathy: present and future. World J Diabetes. 2014;5:763-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933-1953. [PubMed] [Google Scholar]
- 46. Lee BW, Ihm SH, Choi MG, Yoo HJ. The comparison of cystatin C and creatinine as an accurate serum marker in the prediction of type 2 diabetic nephropathy. Diabetes Res Clin Pract. 2007;78:428-434. [DOI] [PubMed] [Google Scholar]
- 47. Rule AD, Rodeheffer RJ, Larson TS, et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc. 2006;81:1427-1434. [DOI] [PubMed] [Google Scholar]
- 48. Botev R, Mallie J-P, Wetzels JFM, Couchoud C, Schuck O. The clinician and estimation of glomerular filtration rate by creatinine-based formulas: current limitations and quo vadis. Clin J Am Soc Nephrol. 2011;6:937-950. [DOI] [PubMed] [Google Scholar]
- 49. Rostoker G, Andrivet P, Pham I, Griuncelli M, Adnot S. Accuracy and limitations of equations for predicting the glomerular filtration rate during follow-up of patients with non-diabetic nephropathies. BMC Nephrol. 2009;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dabla PK. Renal function in diabetic nephropathy. World J Diabetes. 2010;1:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bruno G, Merletti F, Bargero G, et al. Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia. 2007;50:941-948. [DOI] [PubMed] [Google Scholar]
- 52. Camargo EG, Soares AA, Detanico AB, et al. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is less accurate in patients with type 2 diabetes when compared with healthy individuals. Diabet Med. 2011;28:90-95. [DOI] [PubMed] [Google Scholar]
- 53. Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55:622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461-470. [DOI] [PubMed] [Google Scholar]
- 55. Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria? Kidney Int. 2006;70:1214-1222. [DOI] [PubMed] [Google Scholar]
- 56. Moresco RN, Sangoi MB, De Carvalho JAM, Tatsch E, Bochi GV. Diabetic nephropathy: traditional to proteomic markers. Clin Chim Acta. 2013;421:17-30. [DOI] [PubMed] [Google Scholar]
- 57. Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int. 2006;69:440-449. [DOI] [PubMed] [Google Scholar]
- 58. Gross JL, De Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176. [DOI] [PubMed] [Google Scholar]
- 59. Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matheson A, Willcox MDP, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev. 2010;26:150-171. [DOI] [PubMed] [Google Scholar]
- 61. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [DOI] [PubMed] [Google Scholar]
- 62. Donadio C, Lucchesi A, Tramonti G, Bianchi C. Creatinine clearance predicted from body cell mass is a good indicator of renal function. Kidney Int Suppl. 1997;63:S166-S168. [PubMed] [Google Scholar]
- 63. Walser M. Assessing renal function from creatinine measurements in adults with chronic renal failure. Am J Kidney Dis. 1998;32:23-31. [DOI] [PubMed] [Google Scholar]
- 64. Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14:1000-1005. [DOI] [PubMed] [Google Scholar]
- 65. Hosten AO. BUN and creatinine. In: Walker HK, Hall WD, Hurst JW. eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:874-878. [PubMed] [Google Scholar]
- 66. McNamara NV, Chen R, Janu MR, Bwititi P, Car G, Seibel M. Early renal failure detection by cystatin C in type 2 diabetes mellitus: varying patterns of renal analyte expression. Pathology. 2009;41:269-275. [DOI] [PubMed] [Google Scholar]
- 67. Ogawa Y, Goto T, Tamasawa N, et al. Serum cystatin C in diabetic patients. Not only an indicator for renal dysfunction in patients with overt nephropathy but also a predictor for cardiovascular events in patients without nephropathy. Diabetes Res Clin Pract. 2008;79:357-361. [DOI] [PubMed] [Google Scholar]
- 68. Kyhse-Andersen J, Schmidt C, Nordin G, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921-1926. [PubMed] [Google Scholar]
- 69. Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699-707. [PubMed] [Google Scholar]
- 70. Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353-1361. [DOI] [PubMed] [Google Scholar]
- 71. Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens. 2011;20:246-257. [DOI] [PubMed] [Google Scholar]
- 72. American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27:S79-S83. [DOI] [PubMed] [Google Scholar]
- 73. Heathcote KL, Wilson MP, Quest DW, Wilson TW. Prevalence and duration of exercise induced albuminuria in healthy people. Clin Invest Med. 2009;32:E261-E265. [DOI] [PubMed] [Google Scholar]
- 74. Hogan SL, Vupputuri S, Guo X, et al. Association of cigarette smoking with albuminuria in the United States: the third National Health and Nutrition Examination Survey. Ren Fail. 2007;29:133-142. [DOI] [PubMed] [Google Scholar]
- 75. O-charoen P, Gangcuangco LMA, Chow DC, Ndhlovu LC, Barbour JD, Shikuma CM. Inflammation and albuminuria in HIV-infected patients receiving combination antiretroviral therapy. Hawaii J Med Public Health. 2014;73:37. [Google Scholar]
- 76. Sharma K. The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int. 2009;76:145-148. [DOI] [PubMed] [Google Scholar]
- 77. Kerkeni M, Saïdi A, Bouzidi H, et al. Pentosidine as a biomarker for microvascular complications in type 2 diabetic patients. Diab Vasc Dis Res. 2013;10:239-245. [DOI] [PubMed] [Google Scholar]
- 78. Sugiyama S, Miyata T, Ueda Y, et al. Plasma levels of pentosidine in diabetic patients: an advanced glycation end product. J Am Soc Nephrol. 1998;9:1681-1688. [DOI] [PubMed] [Google Scholar]
- 79. Xu GW, Yao QH, Weng QF, Su BL, Zhang X, Xiong JH. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal. 2004;36:101-104. [DOI] [PubMed] [Google Scholar]
- 80. Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120-139. [DOI] [PubMed] [Google Scholar]
- 81. Jalal DI, Maahs DM, Hovind P, Nakagawa T. Uric acid as a mediator of diabetic nephropathy. Semin Nephrol. 2011;31:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nguyen TQ, Tarnow L, Andersen S, et al. Urinary connective tissue growth factor excretion correlates with clinical markers of renal disease in a large population of type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:83-88. [DOI] [PubMed] [Google Scholar]
- 84. Nguyen TQ, Tarnow L, Jorsal A, et al. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care. 2008;31:1177-1182. [DOI] [PubMed] [Google Scholar]
- 85. Kim NH, Kim KB, Kim DL, et al. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitus. Diabet Med. 2004;21:545-551. [DOI] [PubMed] [Google Scholar]
- 86. Cha DR, Kang YS, Han SY, et al. Vascular endothelial growth factor is increased during early stage of diabetic nephropathy in type II diabetic rats. J Endocrinol. 2004;183:183-194. [DOI] [PubMed] [Google Scholar]
- 87. Cohen-Bucay A, Viswanathan G. Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephrol. 2012;2012:146987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Araki SI, Haneda M, Koya D, et al. Association between urinary type IV collagen level and deterioration of renal function in type 2 diabetic patients without overt proteinuria. Diabetes Care. 2010;33:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Watanabe H, Sanada H, Shigetomi S, Katoh T, Watanabe T. Urinary excretion of type IV collagen as a specific indicator of the progression of diabetic nephropathy. Nephron. 2000;86:27-35. [DOI] [PubMed] [Google Scholar]
- 90. Assal HS, Tawfeek S, Rasheed EA, El-Lebedy D, Thabet EH. Serum cystatin C and tubular urinary enzymes as biomarkers of renal dysfunction in type 2 diabetes mellitus. Clin Med Insights Endocrinol Diabetes. 2013;6:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Uslu S, Efe B, Alataş Ö, et al. Serum cystatin C and urinary enzymes as screening markers of renal dysfunction in diabetic patients. J Nephrol. 2005;18:559-567. [PubMed] [Google Scholar]
- 92. Panduru NM, Forsblom C, Saraheimo M, et al. Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2013;36:2077-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kamijo-Ikemori A, Sugaya T, Yasuda T, et al. Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care. 2011;34:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595-605. [DOI] [PubMed] [Google Scholar]
- 95. Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin as an early biomarker of nephropathy in diabetic patients. Kidney Blood Press Res. 2009;32:91-98. [DOI] [PubMed] [Google Scholar]
- 96. Alter ML, Kretschmer A, Von Websky K, et al. Early urinary and plasma biomarkers for experimental diabetic nephropathy. Clin Lab. 2012;58:659-671. [PubMed] [Google Scholar]
- 97. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237-244. [DOI] [PubMed] [Google Scholar]
- 98. Burns KD. The emerging role of angiotensin-converting enzyme-2 in the kidney. Curr Opin Nephrol Hypertens. 2007;16:116-121. [DOI] [PubMed] [Google Scholar]
- 99. Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 2007;72:614-623. [DOI] [PubMed] [Google Scholar]
- 100. Satirapoj B, Siritaweesuk N, Supasyndh O. Urinary angiotensinogen as a potential biomarker of diabetic nephropathy. Clin Kidney J. 2014;7:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fiseha T. Urinary biomarkers for early diabetic nephropathy in type 2 diabetic patients. Biomark Res. 2015;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sheira G, Noreldin N, Tamer A, Saad M. Urinary biomarker N-acetyl-β-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J Diabetes Metab Disord. 2015;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ambade V, Singh P, Somani BL, Basannar D. Urinary N-acetyl beta glucosaminidase and gamma glutamyl transferase as early markers of diabetic nephropathy. Indian J Clin Biochem. 2006;21:142-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hong CY, Hughes K, Chia KS, Ng V, Ling SL. Urinary alpha1-microglobulin as a marker of nephropathy in type 2 diabetic Asian subjects in Singapore. Diabetes Care. 2003;26:338-342. [DOI] [PubMed] [Google Scholar]
- 105. Petrica L, Vlad A, Gluhovschi G, et al. Proximal tubule dysfunction is associated with podocyte damage biomarkers nephrin and vascular endothelial growth factor in type 2 diabetes mellitus patients: a cross-sectional study. PLoS ONE. 2014;9:e112538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Titan SM, Zatz R, Graciolli FG, et al. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol. 2011;6:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gohda T, Tomino Y. Novel biomarkers for the progression of diabetic nephropathy: soluble TNF receptors. Curr Diab Rep. 2013;13:560-566. [DOI] [PubMed] [Google Scholar]
- 109. Lampropoulou I, Stangou M, Papagianni A, Didangelos T, Iliadis F, Efstratiadis G. TNF-α and microalbuminuria in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:394206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Viedt C. MCP-1 induces inflammatory activation of human tubular epithelial cells: involvement of the transcription factors, nuclear factor-kappaB and activating protein-1. J Am Soc Nephrol. 2002;13:1534-1547. [DOI] [PubMed] [Google Scholar]
- 111. Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697-F701. [DOI] [PubMed] [Google Scholar]
- 112. Nechemia-Arbely Y, Barkan D, Pizov G, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2:72-79. [PubMed] [Google Scholar]
- 114. Nakamura A, Shikata K, Hiramatsu M, et al. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care. 2005;28:2890-2895. [DOI] [PubMed] [Google Scholar]
- 115. Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43:550-557. [DOI] [PubMed] [Google Scholar]
- 116. Horie K, Miyata T, Maeda K, et al. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J Clin Invest. 1997;100:2995-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tanji N, Markowitz GS, Fu C, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656-1666. [DOI] [PubMed] [Google Scholar]
- 118. Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron. 2002;91:327-329. [DOI] [PubMed] [Google Scholar]
- 119. Naito Y, Uchiyama K, Aoi W, et al. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors. 2004;20:49-59. [DOI] [PubMed] [Google Scholar]
- 120. Serdar M, Sertoglu E, Uyanik M, et al. Comparison of 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels using mass spectrometer and urine albumin creatinine ratio as a predictor of development of diabetic nephropathy. Free Radic Res. 2012;46:1291-1295. [DOI] [PubMed] [Google Scholar]
- 121. Hirata K, Kubo K. Relationship between blood levels of N-carboxymethyl-lysine and pentosidine and the severity of microangiopathy in type 2 diabetes. Endocr J. 2004;51:537-544. [DOI] [PubMed] [Google Scholar]
- 122. Miura J, Yamagishi S, Uchigata Y, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with type 1 diabetes. J Diabetes Complications. 2003;17:16-21. [DOI] [PubMed] [Google Scholar]
- 123. Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Piarulli F, Sartore G, Ceriello A, et al. Relationship between glyco-oxidation, antioxidant status and microalbuminuria in type 2 diabetic patients. Diabetologia. 2009;52:1419-1425. [DOI] [PubMed] [Google Scholar]
- 125. Sell DR, Nagaraj RH, Grandhee SK, et al. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev. 1991;7:239-251. [DOI] [PubMed] [Google Scholar]
- 126. Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Becker BF. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14:615-631. [DOI] [PubMed] [Google Scholar]
- 129. Roncal CA, Mu W, Croker B, et al. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;292:F116-F122. [DOI] [PubMed] [Google Scholar]
- 130. Kang D-H, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888-2897. [DOI] [PubMed] [Google Scholar]
- 131. Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577-582. [PubMed] [Google Scholar]
- 132. Talaat KM, El-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27:435-440. [DOI] [PubMed] [Google Scholar]
- 133. Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26:269-275. [DOI] [PubMed] [Google Scholar]
- 134. Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287-1293. [DOI] [PubMed] [Google Scholar]
- 135. Jalal DI, Rivard CJ, Johnson RJ, et al. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25:1865-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving H-H. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58:1668-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128-132. [PubMed] [Google Scholar]
- 140. Miao Y, Ottenbros SA, Laverman GD, et al. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2-7. [DOI] [PubMed] [Google Scholar]
- 141. Maahs DM, Caramori L, Cherney DZI, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kandasamy Y, Smith R, Lumbers ER, Rudd D. Nephrin—a biomarker of early glomerular injury. Biomark Res. 2014;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chang JH, Paik SY, Mao L, et al. Diabetic kidney disease in FVB/NJ akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS ONE. 2012;7:e33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ng DPK, Tai BC, Tan E, et al. Nephrinuria associates with multiple renal traits in type 2 diabetes. Nephrol Dial Transplant. 2011;26:2508-2514. [DOI] [PubMed] [Google Scholar]
- 145. do Nascimento JF, Canani LH, Gerchman F, et al. Messenger RNA levels of podocyte-associated proteins in subjects with different degrees of glucose tolerance with or without nephropathy. BMC Nephrol. 2013;14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang G, Lai FMM, Tam LS, et al. Messenger RNA expression of podocyte-associated molecules in urinary sediment of patients with lupus nephritis. J Rheumatol. 2007;34:2358-2364. [PubMed] [Google Scholar]
- 147. Wada Y, Abe M, Moritani H, et al. Potential of urinary nephrin as a biomarker reflecting podocyte dysfunction in various kidney disease models. Exp Biol Med (Maywood). 2016;241:1865-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26:1765-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McMahon BA, Murray PT. Urinary liver fatty acid-binding protein: another novel biomarker of acute kidney injury. Kidney Int. 2010;77:657-659. [DOI] [PubMed] [Google Scholar]
- 150. Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Matsui K, Kamijo-Ikemori A, Sugaya T, Yasuda T, Kimura K. Usefulness of urinary biomarkers in early detection of acute kidney injury after cardiac surgery in adults. Circ J. 2012;76:213-220. [DOI] [PubMed] [Google Scholar]
- 152. Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231-1238. [DOI] [PubMed] [Google Scholar]
- 154. Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS ONE. 2014;9:e84131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nauta FL, Boertien WE, Bakker SJL, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care. 2011;34:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJL, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209-217. [DOI] [PubMed] [Google Scholar]
- 157. Nielsen SE, Rossing K, Hess G, et al. The effect of RAAS blockade on markers of renal tubular damage in diabetic nephropathy: u-NGAL, u-KIM1 and u-LFABP. Scand J Clin Lab Invest. 2012;72:137-142. [DOI] [PubMed] [Google Scholar]
- 158. Nielsen SE, Sugaya T, Tarnow L, et al. Tubular and glomerular injury in diabetes and the impact of ACE inhibition. Diabetes Care. 2009;32:1684-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067-3075. [DOI] [PubMed] [Google Scholar]
- 160. Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004;204:587-593. [DOI] [PubMed] [Google Scholar]
- 161. Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132-2139. [DOI] [PubMed] [Google Scholar]
- 162. Wong DW, Oudit GY, Reich H, et al. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liu CX, Hu Q, Wang Y, et al. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med. 2011;17:59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nadarajah R, Milagres R, Dilauro M, et al. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 2012;82:292-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wysocki J, Garcia-Halpin L, Ye M, et al. Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol. 2013;305:F600-F611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610-1616. [DOI] [PubMed] [Google Scholar]
- 167. Mizuiri S, Hemmi H, Arita M, et al. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613-623. [DOI] [PubMed] [Google Scholar]
- 168. Cherney DZI, Xiao F, Zimpelmann J, et al. Urinary ACE2 in healthy adults and patients with uncomplicated type 1 diabetes. Can J Physiol Pharmacol. 2014;92:703-706. [DOI] [PubMed] [Google Scholar]
- 169. Lim AKH, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124:139-152. [DOI] [PubMed] [Google Scholar]
- 171. Verhave JC, Bouchard J, Goupil R, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101:333-340. [DOI] [PubMed] [Google Scholar]
- 172. Donate-Correa J, Martín-Núñez E, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF. Inflammatory cytokines in diabetic nephropathy. J Diabetes Res. 2015;2015:948417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Koike N, Takamura T, Kaneko S. Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF-alpha stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 2007;80:1721-1728. [DOI] [PubMed] [Google Scholar]
- 174. Navarro JF, Mora C, Muros M, García J. Urinary tumour necrosis factor-alpha excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial Transplant. 2006;21:3428-3434. [DOI] [PubMed] [Google Scholar]
- 175. Navarro JF, Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17:441-450. [DOI] [PubMed] [Google Scholar]
- 176. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4-7. [DOI] [PubMed] [Google Scholar]
- 177. Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291-300. [DOI] [PubMed] [Google Scholar]
- 178. Wu CC, Chen JS, Lu KC, et al. Aberrant cytokines/chemokines production correlate with proteinuria in patients with overt diabetic nephropathy. Clin Chim Acta. 2010;411:700-704. [DOI] [PubMed] [Google Scholar]
- 179. Niewczas MA, Ficociello LH, Johnson AC, et al. Serum concentrations of markers of TNFα and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2009;4:62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492-1499. [DOI] [PubMed] [Google Scholar]
- 183. Giunti S, Barutta F, Perin PC, Gruden G. Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr Vasc Pharmacol. 2010;8:849-860. [DOI] [PubMed] [Google Scholar]
- 184. Wada T, Yokoyama H, Furuichi K, et al. Intervention of crescentic glomerulonephritis by antibodies to monocyte chemotactic and activating factor (MCAF/MCP-1). FASEB J. 1996;10:1418-1425. [PubMed] [Google Scholar]
- 185. Yokoyama H, Wada T, Furuichi K, et al. Urinary levels of chemokines (MCAF/MCP-1, IL-8) reflect distinct disease activities and phases of human IgA nephropathy. J Leukoc Biol. 1998;63:493-499. [DOI] [PubMed] [Google Scholar]
- 186. Fufaa GD, Weil EJ, Nelson RG, et al. Urinary monocyte chemoattractant protein-1 and hepcidin and early diabetic nephropathy lesions in type 1 diabetes mellitus. Nephrol Dial Transplant. 2015;30:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yokoyama H, Wada T, Furuichi K, et al. Urinary levels of chemokines (MCAF/MCP-1, IL-8) reflect distinct disease activities and phases of human IgA nephropathy. J Leukoc Biol. 1998;63:493-499. [DOI] [PubMed] [Google Scholar]
- 188. Morii T, Fujita H, Narita T, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11-15. [DOI] [PubMed] [Google Scholar]
- 189. Camilla R, Brachemi S, Pichette V, et al. Urinary monocyte chemotactic protein 1: marker of renal function decline in diabetic and nondiabetic proteinuric renal disease. J Nephrol. 2011;24:60-67. [DOI] [PubMed] [Google Scholar]
- 190. Sekizuka K, Tomino Y, Sei C, et al. Detection of serum IL-6 in patients with diabetic nephropathy. Nephron. 1994;68:284-285. [DOI] [PubMed] [Google Scholar]
- 191. Suzuki D, Miyazaki M, Naka R, et al. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes. 1995;44:1233-1238. [DOI] [PubMed] [Google Scholar]
- 192. Navarro JF, Milena FJ, Mora C, León C, García J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562-570. [DOI] [PubMed] [Google Scholar]
- 193. Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443-449. [DOI] [PubMed] [Google Scholar]
- 194. Ruef C, Budde K, Lacy J, et al. Interleukin 6 is an autocrine growth factor for mesangial cells. Kidney Int. 1990;38:249-257. [DOI] [PubMed] [Google Scholar]
- 195. Dalla Vestra M. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16:S78-S82. [DOI] [PubMed] [Google Scholar]
- 196. Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126-139. [DOI] [PubMed] [Google Scholar]
- 197. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509-524. [DOI] [PubMed] [Google Scholar]
- 198. Pezzolesi MG, Satake E, McDonnell KP, Major M, Smiles AM, Krolewski AS. Circulating TGF-β1-regulated miRNAs and the risk of rapid progression to ESRD in type 1 diabetes. Diabetes. 2015;64:3285-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Badal SS, Danesh FR. Diabetic nephropathy: emerging biomarkers for risk assessment. Diabetes. 2015;64:3063-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Kato M, Natarajan R. MicroRNA circuits in transforming growth factor-β actions and diabetic nephropathy. Semin Nephrol. 2012;32:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kato M, Natarajan R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Hanson RL, Craig DW, Millis MP, et al. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes. 2007;56:975-983. [DOI] [PubMed] [Google Scholar]
- 204. Huppi K, Volfovsky N, Runfola T, et al. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. 2008;6:212-221. [DOI] [PubMed] [Google Scholar]
- 205. Alvarez ML, Khosroheidari M, Eddy E, Kiefer J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS ONE. 2013;8:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46:824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne). 2014;5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368-13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gámez-Valero A, Lozano-Ramos SI, Bancu I, Lauzurica-Valdemoros R, Borràs FE. Urinary extracellular vesicles as source of biomarkers in kidney diseases. Front Immunol. 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Sun A-L, Deng J-T, Guan G-J, et al. Dipeptidyl peptidase-IV is a potential molecular biomarker in diabetic kidney disease. Diab Vasc Dis Res. 2012;9:301-308. [DOI] [PubMed] [Google Scholar]
- 211. Raimondo F, Corbetta S, Morosi L, et al. Urinary exosomes and diabetic nephropathy: a proteomic approach. Mol Biosyst. 2013;9:1139-1146. [DOI] [PubMed] [Google Scholar]
- 212. Zubiri I, Posada-Ayala M, Sanz-Maroto A, et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics. 2014;96:92-102. [DOI] [PubMed] [Google Scholar]
- 213. Zubiri I, Posada-Ayala M, Benito-Martin A, et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl Res. 2015;166:474-484.e4. [DOI] [PubMed] [Google Scholar]
- 214. Barutta F, Tricarico M, Corbelli A, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE. 2013;8:e73798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013;124:423-441. [DOI] [PubMed] [Google Scholar]
- 216. Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CRJ. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol. 2014;25:1401-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pontillo C, Mischak H. Urinary biomarkers to predict CKD: is the future in multi-marker panels? Nephrol Dial Transplant. 2016;31:1373-1375. [DOI] [PubMed] [Google Scholar]
- 218. Argilés À, Siwy J, Duranton F, et al. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS ONE. 2013;8:e62837. [DOI] [PMC free article] [PubMed] [Google Scholar]