Abstract
Orchidectomy in rodents and lower testosterone levels in men are associated with improved cutaneous wound healing. However, due to the adverse effects on skeletal and sexual tissues, systemic androgen blockade is not a viable therapeutic intervention. Accordingly, we tested the hypothesis that topical application of an androgen antagonist would elicit accelerated wound healing without systemic androgen antagonism. Full-thickness cutaneous wounds were created on adult C57BL6/J mice. Daily topical application of androgen receptor antagonist, flutamide, resulted in improved gap closure similar to orchiectomized controls and faster than orchidectomized mice treated with topical testosterone. In vivo data showed that the effects of androgen antagonism on wound closure primarily accelerate keratinocytes migration without effecting wound contraction. Consequently, mechanisms of testosterone action on reepithelialization were investigated in vitro by scratch wounding assays in confluent keratinocytes. Testosterone inhibited keratinocyte migration and this effect was in part mediated through promotion of nuclear translocation of β-catenin and by attenuating transforming growth factor-β (TGF-β) signaling through β-catenin. The link between Wnt and TGF beta signaling was confirmed by blocking β-catenin and by following TGF-β-induced transcription of a luciferase reporter gene. Together, these data show that blockade of β-catenin can, as a potential target for novel therapeutic interventions, accelerate cutaneous wound healing.
Nonhealing wounds affect 6.5 million patients with an annual cost of 25 billion dollars in the US alone.1,2 Impaired wound healing is associated with frequent hospitalizations, and increased morbidity and mortality. Therefore, therapeutic agents that promote wound closure are desirable.
The healing of cutaneous wounds is affected by endogenous gonadal steroids, testosterone, and estrogen.3–5 Gender differences in the rates of wound healing have been attributed to the higher levels of testosterone in men than in women6 with a positive correlation between nonhealed wound area and testosterone levels.7 The skin can synthesize androgens de novo from cholesterol or by local conversion of circulating precursor steroids.8 Androgens, produced in the testes or locally in the skin, associate with androgen receptor (AR) expressed in epidermal keratinocytes, sebocytes, dermal fibroblasts,9,10 and in blood cells including macrophages, B cells, and neutrophils.11,12 Androgens inhibit cutaneous repair by affecting multiple processes in the wound healing cascade.7,13–15 Gonadectomy or systemic treatment with an AR antagonist such as flutamide promotes wound healing.5,7,16–18 However, systemic androgen deprivation is associated with adverse health effects, including sexual dysfunction, and loss of muscle and bone mass, and is not a viable therapeutic option to promote cutaneous healing.
Here we show that local inhibition of AR signaling by topical application of an AR antagonist improves wound closure without the adverse effects of systemic androgen blockade. We also demonstrate that while there is significant contraction in the wounds, topical androgen antagonism accelerates closure without effecting contraction. Because of a significant increase in wound length epithelium in flutamide-treated wounds and the lack of studies on the effects of androgens on the reepithelialization phase of wound healing, in this paper, we focused our investigation on keratinocyte that plays an important role in this critical process. For the first time, we showed that testosterone impairs keratinocyte migration in part by activating Wnt-β catenin signaling and by inhibiting transforming growth factor-β (TGF-β)/ Smad pathway through β catenin. These new mechanistic findings unveil β-catenin and other steps in the signaling cascade as potential targets for the discovery of novel molecules that promote wound healing.
METHODS AND MATERIALS
In vivo wound healing
All procedures involving animals were approved by Boston University’s animal use committee. Two full-thickness excision wounds (6 mm in diameter) were made through the skin and the panniculus carnosus muscle on the back of 8-week-old C57BL/6J mice. A subgroup of mice underwent castration (Cx) or sham operation 2 weeks before creation of cutaneous wounds. The wounds were treated topically daily with 100 μL testosterone (10 μg), flutamide (30 μg) or vehicle (petroleum jelly). A digital image of each wound with a calibration scale was recorded, and the open wound area determined using MetaMorph software (Universal Imaging, Downington, PA). For the contraction experiments, the skin adjacent to the margin of the wound, was permanently tattooed using an animal lancet (3 mm), dipped in a green tattoo ink paste (Ketchum Manufacturing Inc, Brockville, Ontario, CA). A template was used to standardize the size of each tattoo, and wounds were then placed equidistant from the 8 points of the tattoo. Wounds were photographed daily using a digital camera. A plastic template containing a 15 mm × 15 mm circular window was used to briefly hold the anesthetized mice. Digital images of the wounds were analyzed using Spot™ image analysis software (Diagnostic Instruments, Sterling Heights, MI).
Histopathological analysis
After euthanasia, the wounds were excised along with a 10 mm margin, fixed in 10% buffered formalin. Specimens were coded so that the evaluators were unaware of treatment assignment. From paraffin-embedded specimens, 4 μm sections were stained with hematoxylin and eosin. The length of the wound epithelium was calculated measuring the distances between the edges of the noninjured dermis, recognized by the last hair follicle, using sections from the middle of 6–10 wounds. The length of the wound epithelium provides an indication for the extent of keratinocyte migration in the wound tissue. Pictures were taken at 2×, 4×, and 10× magnifications.
Cell cultures
Human keratinocyte cell line, HaCaT, was grown in Dulbecco’s Modified Eagle’s Medium (DMEM, Cellgro, Manassas, VA) supplemented with 5% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 0.05 U/mL penicillin, and 0.05 μg/mL streptomycin, and incubated at 37 °C in 5.0% CO2. Twenty-four hours before intervention, the cells were switched to Dulbecco’s modified Eagle’s phenol red-free medium and 5% charcoal-stripped serum (Gemini Bio-Products, West Sacramento, CA). Cells hyperexpressing human Smad3 from the pBabe puro retrovirus (HaCaT/pBabe-Smad3) and vector control (HaCaT/pBabe) were maintained in DMEM with 5% FBS and 5 μg/mL puromycin.
In vitro scratch/wound assay
The keratinocyte migration was measured using a scratch migration assay. HaCaT cells (2 × 105/well) were seeded in 24-well plates and grown until confluent. Each monolayer was scratched using a sterile pipette tip to generate a cross-shaped cell-free zone (0.8–1 mm). After washing with DMEM, the cells were incubated for 24 hours with testosterone (100 nM) and/or flutamide (1 μM). Every experiment had its own internal control, and to evaluate migration, the cells were imaged immediately after wounding using a Nikon Eclipse TE 2000U phase-contrast microscope (Nikon Instruments, Inc., Melville, NY); the same field was imaged after 16 or 24 hours. The extent of migration was analyzed using Spot™ image analysis software.
Cell proliferation
Cell suspensions (2,000 cells/well) were plated in growth medium alone or medium containing testosterone and/or flutamide for 48 hours. After a 4-hour incubation in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL in phosphate buffered saline solution [PBS]) at 37 °C, formazan was dissolved in 150 μL dimethylsulfoxide, and absorbance was measured at 550 nm (λref = 630 nm), using SAFIRE plate reader (Tecan, Durham, NC). In the CyQUANT® Cell Proliferation Assay (Invitrogen, Carlsbad, CA), fluorescence measurements were made with excitation at 485 nm and emission detection at 530 nm using SAFIRE plate reader. Cell number was calculated from a calibration curve established in the same assay.
Silencing of RNA (siRNA) and viral vectors
Pretested duo-pack RNAi oligonucleotides for β-catenin and nonspecific control oligonucleotide were purchased from Invitrogen (Carlsbad, CA) and transfected into HaCaT cells using the Lipofectamine protocol (Invitrogen). Type 5 adenovirus encoding β-catenin with eGPF tag was obtained from VectorBiolab (Philadelphia, PA). Confluent HaCaT cells were washed twice with PBS, and growth medium was replaced with DMEM containing 1% FBS. Adenoviral particles (4 × 109) were diluted in 100 μL PBS containing 0.4 mM polyethyleneimine (PEI) and incubated for 20 minutes at room temperature. The viral-PEI complex was added to HaCaT cells for 18 hours. After infection, cells were washed twice with PBS, scratched and incubated in growth medium. β-catenin nuclear translocation was visualized using fluorescence microscopy (Nikon Eclipse TE 2000U). A counterstain 4′,6-diamidino-2-phenylindole (DAPI) was used to localize the nuclei.
The number of cells with β-catenin nuclear staining was expressed as a percent of the total number of cells counted in corresponding phase-contrast images.
Retroviral human Smad3 construct (pBabe-Smad3) was purchased from Addgene Inc (Cambridge, MA). Parental vector pBabe and retroviral packaging helper vector gap and ENV were a gift from Dr. Zhijun Luo (Boston University). pBabe-Smad3 and pBabe were each cotransfected into HEK293T with helper vectors gap and ENV. After selection with puromycin, HEK293 cells stably expressing Smad3 or empty vector were incubated in serum-free medium overnight. The retroviral stocks thus generated were mixed with polybene (8 μg/mL final concentration) and added to exponentially growing HaCaT cells for 4 hours. The cells were washed and replenished with growth medium. Transfected HaCaT cells were selected by puromycin. Ectopic expression of Smad3 was confirmed by Western analysis (not shown).
Antibodies
The antibodies for p-Smad3, β-tubulin, and β-catenin were obtained from Calbiochem, Santa Cruz Biotechnology (Santa Cruz, CA), and Invitrogen (Carlsbad, CA), respectively.
Western analysis
HaCaT total cell and nuclear lysates were prepared in radio-immunoprecipitation assay buffer, containing protease and phosphatase inhibitors (Sigma, St. Louis, MO). Protein content was measured by the Bradford method, and equal amounts of protein were loaded for electrophoresis on a 4–20% gradient gel. The protein expression was detected by chemiluminescence, quantified by densitometry, and expressed as fold-change vs. control.
Luciferase reporter assays
Transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad CA). HaCaT cells were transfected in 24-well plates using a 3TP-lux construct (Dr. Y. Mochida, Boston University). The experiment was conducted in triplicates and performed with two independent transfections.
Data analysis
Data are shown as mean ± SEM. The data were analyzed using analysis of variance (ANOVA; for multiple groups) or Student’s t test (for two independent samples). If ANOVA revealed an overall effect, then intergroup differences were analyzed using Newman–Keuls test. Statistical significance was set at 0.05.
RESULTS
Topical application of an AR antagonist accelerates cutaneous wound healing without systemic androgen deprivation
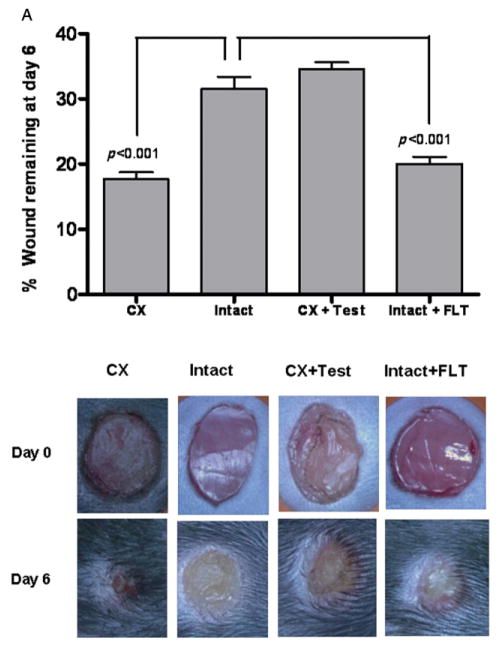
Eight-week old male mice were assigned to one of four groups: sham-operated, intact mice treated with vehicle; sham-operated, intact mice treated with topical flutamide (30 μg/day) ointment; orchiectomized mice treated with vehicle; and orchiectomized mice treated with testosterone (10 μg) ointment. Six-mm punch wounds were created on the dorsal surface of the trunk under isoflurane anesthesia. The wounds were imaged daily, and the wound area was digitized and quantitated using Metamorph™ morphometry program. The wound area was significantly smaller in vehicle-treated, orchiectomized mice than in intact controls (p < 0.001) or in orchiectomized mice treated with topical testosterone (p < 0.001) for 6 days (Figure 1A). Intact mice treated with topical flutamide, an AR antagonist, also had smaller wound area than intact mice treated with vehicle (p < 0.001) or orchiectomized mice treated with testosterone (p < 0.001).
Figure 1.
Effect of topical blockade of androgen receptor on wound healing. (A) Mean ± SEM unhealed wound area after 6 days in vehicle-treated castrated (Cx), sham-operated (Intact), testosterone-treated castrated (CX + Test), and flutamide-treated sham-operated (Intact + FLT). n = 12/group. Illustrative photographs of the wounds on Days 0 and 6 from each treatment group are shown in the lower panel. (B) Histological analysis of wounds. Pictures were taken at 2×, 4×, and 10× magnifications. Intact: Scale crust (C) overlying the ulcer bed (between arrows). The base of the ulcer shows fibrin (F) and necrotic debris. A large epithelial gap is apparent with substantially less reepithelialization than in the flutamide-treated wound, underlying granulation tissue (G). Flutamide: Scale crust overlying the ulcer bed (between arrows). The base of the ulcer shows minimal fibrinous exudate (F) and marked granulation tissue. (C) Morphometric analysis of the length of the wound epithelium.
Additional experiments were performed to confirm if in castrated mice local testosterone synthesis elicited an additional inhibitory effect on wound healing. Castrated mice treated with flutamide did not show any additional improvement in wound healing (data not shown).
Histological examination of sections obtained from wounds harvested on Day 6 confirmed the acceleration of wound healing after topical flutamide application. Flutamide-treated wounds showed significantly accelerated reepithelialization compared with intact controls, as indicated by the reduced epithelial gap (Figure 1B). To prove that keratinocyte migration is involved in the accelerated healing after topical blockade of AR, we conducted morphometric analysis of the length of the wound epithelium. As shown in Figure 1C, the length of the wound epithelium was increased in the wounds treated with flutamide compared with the ones treated with the only vehicle.
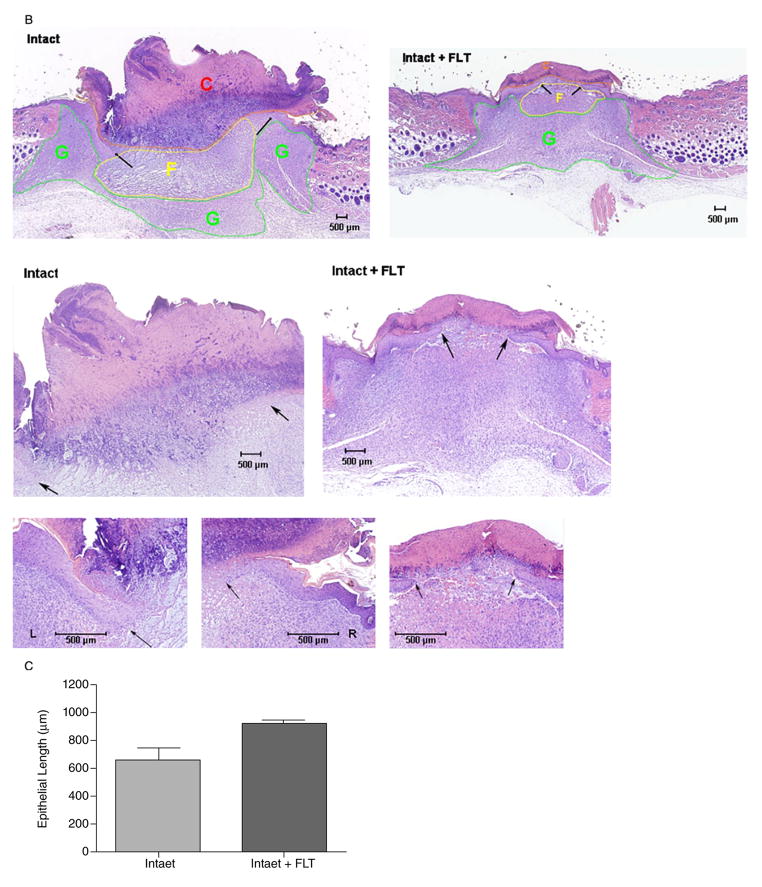
To determine whether topical flutamide had systemic effects, we treated intact sham-operated mice with two doses of flutamide (30 μg: FLT1 and 300 μg: FLT2) for 6 days and compared the wet weights of androgen-sensitive organs—levator ani, seminal vesicles, and kidneys (Figure 2A) with those in orchiectomized or sham-operated, vehicle-treated mice. As expected, the levator ani muscle, seminal vesicle and kidney weights in the orchiectomized mice were significantly lower than those in the sham-operated mice (p < 0.001). The weights of these androgen-sensitive organs in mice treated with topical flutamide did not differ significantly from those in sham-operated, intact controls. Thus, topical flutamide improved wound healing without systemic antiandrogen effects.
Figure 2.
Topical application of androgen antagonist is not associated with systemic androgen blockade and its effects are confined to the site of application. (A) Topical application of flutamide does not induce a state of systemic androgen antagonism. Two doses of flutamide (Intact + FLT1: 30 μg; Intact + FLT2: 300 μg) were applied topically on the wounds of intact animals. The weights of the androgen-sensitive tissues (seminal vesicles, kidneys, and levator ani muscle) were similar among intact mice treated with the vehicle and those treated with either of the two doses of flutamide. (B) Two wounds were created on the back of intact mice. One side was treated with placebo (Intact = CNT) and the other with flutamide (FLT, 30 μg). Topical flutamide significantly accelerates wound healing. Data are mean ± SEM; n = 8/group. CX, castrated.
To verify that the action of topical flutamide was confined only to the application site, we created two excision wounds on the dorsal surface of androgen-replete intact mice. The wound on one side was treated with flutamide ointment and the wound on the other side with vehicle. The wounds treated with topical flutamide healed faster than those treated with vehicle alone, confirming that the effect of androgen blockade did not extend to other regions of the skin remote from the application site (Figure 2B).
Topical flutamide application does not increase wound contraction
In rodents, because of the presence of the panniculus carnosus muscle in the subcutaneous tissue, contraction plays a major role in wound closure.19,20 In order to establish if wound contraction was involved in the acceleration of cutaneous healing during local blockage of AR, we performed a series of in vivo experiments after tattooing the skin at the periphery of the wound. A template was used to standardize the size of each tattoo, and wounds were then placed equidistant from the 8 points of the tattoo. A plastic template (T) containing a 15 mm × 15 mm circular window was used to briefly hold the mice (Figure 3A). On the 6th day, hair was removed by Nair cream (Church & Dwight Co, Inc. Princetown, NJ) to visualize the tattoos before euthanasia (Figure 3B). Digital images of the wounds were analyzed, and daily measurements of the size of the wounded area (W) and the area between the tattoos (C) were recorded as degree of wound reepithelialization and contraction, respectively. To account for animal to animal variability, ratios of wound size and contraction for treated and untreated wounds were calculated. As shown in Figure 3C, local application of the AR antagonist flutamide, increased wound healing mainly increasing reepithelialization, without affecting wound contraction.
Figure 3.
Topical application of androgen antagonist is not associated with wound contraction. (A) Schematic of the Contraction area (C) and Wound area (W). A plastic Template (T) containing a circular window was used to briefly hold the mice. (B) Representative pictures of the wounds on Days 0 and 6 of control and flutamide (FLT)-treated intact animals (n = 6/group). (C) Effect of topical flutamide on wound healing and wound contraction. Data were expressed as a ratio between treated and not-treated wounds (n = 6/group).
AR activation impairs keratinocyte migration
The closure of epidermal wounds involves migration and proliferation of keratinocytes for reepithelialization. To determine whether flutamide affects keratinocyte migration or proliferation, we used a scratch assay in confluent HaCaT cells, which represent human immortalized keratinocytes. These cells display a migration index similar to that of primary human keratinocytes and have been used widely as an in vitro model of keratinocyte-mediated gap “wound” closure.21–23
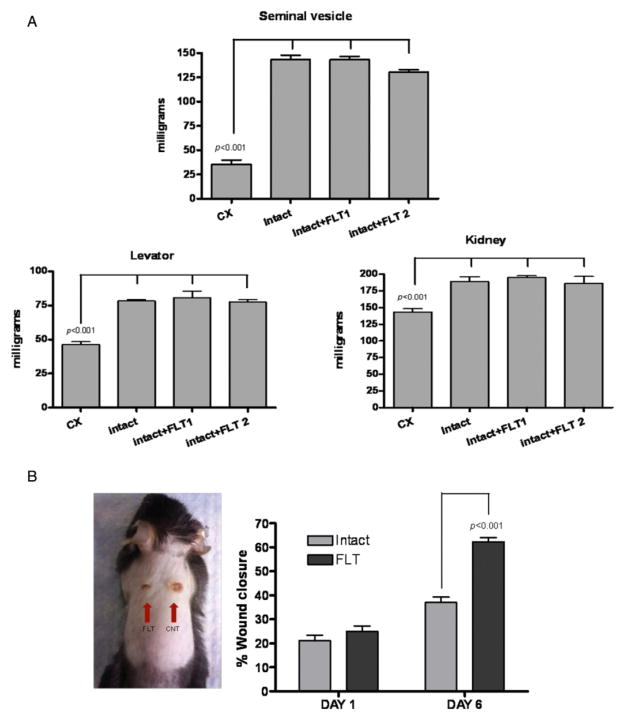
HaCaT cell cultures were grown to confluence and incubated for 2 hours with mitomycin C to inhibit proliferation. A cross-shaped wound was created and the cells were treated with 100 nM testosterone with and without 1 μM flutamide. This testosterone concentration was selected because in dose-response studies, 100 nM testosterone induced submaximal suppression of gap closure in HaCaT cell scratch assay (Figure 4A), and because this is within the range of concentrations that have been used in studies of androgen effects in vitro.24–26 Keratinocytes migration was significantly decreased after 24 hours of incubation with testosterone relative to incubation with medium alone (p < 0.001; Figure 4B). The inhibitory effect of testosterone on gap closure in this model was blocked by flutamide. Flutamide alone had no significant effect on gap closure.
Figure 4.
Effects of testosterone in an in vitro scratch assay. (A) HaCaT monolayers were scratch-wounded and treated with increasing concentrations of testosterone (10 nM–10 μM); gap closure was measured after 24 hours. (B) Flutamide (FLT) blocks the effects of testosterone in the in vitro scratch-wound assay. Testosterone treatment (100 nM) delayed gap closure compared with control. Co-incubation with flutamide (1 μM) blocked the inhibitory effects of testosterone. The right panel shows illustrative photographs of the HaCaT cell monolayers at baseline, and after 24 hours of incubation in respective treatments. (C, D) Testosterone does not affect HaCaT cell proliferation. HaCaT cells were treated for 48 hours with testosterone (100 nM) with or without flutamide (1 μM), and cell growth was determined using the MTT (C) or CyQUANT® Cell Proliferation Assay (D).
Keratinocyte proliferation is not affected by AR activation
The observed effects of testosterone on gap closure in the scratch assay could be due to inhibition of either keratinocyte migration or keratinocyte proliferation. Besides using mitomycin C in our scratch assay, we also determined the effects of testosterone and flutamide on HaCaT cell proliferation, using two complementary quantitative assays, the tetrazodium dye (MTT) assay and the Cyquant assay. Keratinocyte proliferation was not affected by either testosterone or flutamide (Figure 4C and D), suggesting that the effects of testosterone on gap closure in the scratch assay were not due to its effects on cell growth. Therefore, we infer that testosterone impairs gap closure in the scratch assay primarily by inhibiting keratinocyte migration.
AR activation impairs keratinocyte migration by increasing β-catenin nuclear translocation
To determine the mechanism by which AR activation inhibits keratinocyte migration, we focused on two signaling pathways that are known to play a role in keratinocyte migration: β-catenin/Wnt pathway and TGF-β/Smad pathway. Previous studies have shown that β-catenin activation is an important inhibitor of keratinocyte migration.27,28 Accordingly, nuclear lysates, collected from the scratched plates at different time points after treatment with testosterone and/or flutamide, were subjected to Western blot analyses using anti-β-catenin antibody. Testosterone increased the level of nuclear β-catenin after 1 hour (Figure 5A); this effect was blocked by flutamide. Flutamide alone had no significant effect on nuclear β-catenin expression. The expression level of phospho-GSK3b was not altered by the presence or the absence of testosterone or flutamide (data not shown).
Figure 5.
Testosterone increases nuclear β-catenin translocation. (A) HaCaT cells were treated for 1 hour with testosterone (100 nM) with or without flutamide (1 μM), and analyzed for β-catenin expression. Densitometric analysis shows that cells incubated with testosterone had higher level of nuclear β-catenin. (B) HaCaT cell infected with type 5 adenovirus encoding β-catenin with eGPF were scratch-wounded and treated with medium, testosterone (100 nM), or lithium chloride LiCl (2 mM). Extent of β-catenin nuclear translocation was monitored after 1 hour; 4′,6-diamidino-2-phenylindole (DAPI) counterstain was used to localize the nuclei. Three independent experiments were performed, and representative photographs are shown. (C) β-catenin small interfering RNA siRNA decreases the expression levels of β-catenin. HaCaT cells were transiently transfected with specific β-catenin siRNA (βcat-siRNA) and representative densitometric data of one of multiple independent experiments are shown. (D) βcat-siRNA attenuates testosterone’s effect on gap closure. HaCaT cells, transfected with either nonspecific oligonucleotide (c-siRNA) or specific βcat-siRNA were incubated with or without testosterone (100 nM).
To confirm the role of β-catenin in mediating the inhibitory effect of testosterone on keratinocytes migration, we evaluated the effects of testosterone on β-catenin nuclear translocation, a hallmark of canonical Wnt activation, using a β-catenin-green fluorescent protein (GFP) fusion construct. HaCaT cells were infected with an adenovirus vector carrying a recombinant β-catenin-GFP construct. After 24 hours, the cell monolayer was scratched to create a cross-shaped wound and treated either with regular growth medium, medium containing 100 nM testosterone, or medium containing 2 mM lithium chloride (LiCl) as a positive control (Figure 5B). As expected, LiCl induced the nuclear translocation of β-catenin. Testosterone also induced β-catenin translocation in HaCaT cells that were close to the scratch, confirming that testosterone activates canonical Wnt/β-catenin pathway.
Subsequently, we knocked down β-catenin using small interfering RNA (siRNA) that targeted β-catenin (Figure 5C). To minimize the possibility of nonspecific, off-target effects, we used siRNA oligonucleotides targeting two different segments of β-catenin mRNA. A random siRNA was used as a control. The knockdown of β-catenin blocked testosterone’s effects on keratinocyte migration (Figure 5D), confirming an important role of β-catenin in mediating testosterone’s effects on keratinocyte migration.
AR activation impairs keratinocyte migration by blocking TGF-β action
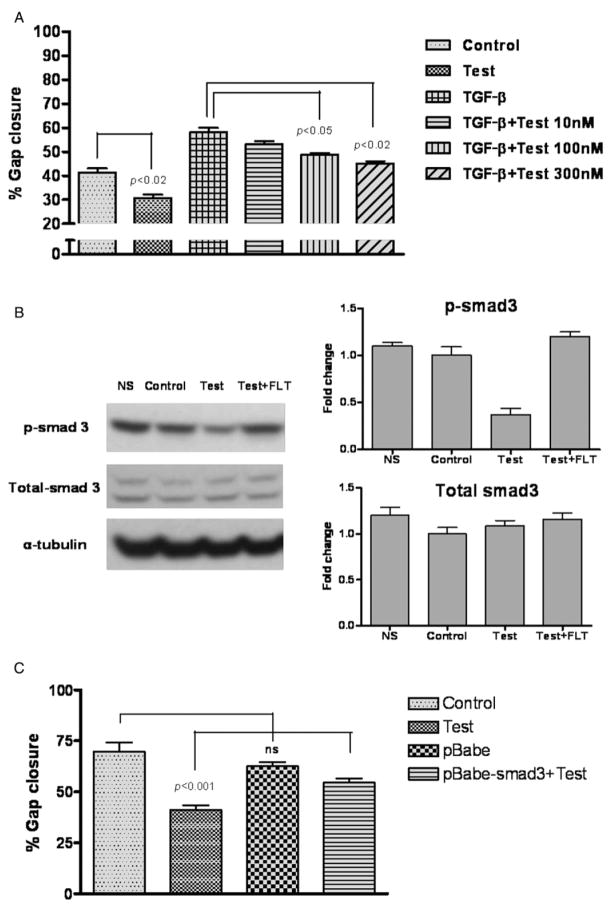
TGF-β plays an important role in wound healing through its effects on a number of processes including reepithelialization.29–32 To investigate the possible interaction between testosterone and TGF-β signaling during keratinocyte migration, we performed the scratch assay after treating HaCaT cell with 10 ng TGF-β and ascending doses of testosterone (30, 100, or 300 nM). Co-incubation with testosterone significantly attenuated the effects of TGF-β on keratinocyte migration in a dose-dependent manner (Figure 6A).
Figure 6.
(A) Testosterone inhibits the effects of transforming growth factor-β TGF-β on keratinocyte migration in a dose-dependent manner. HaCaT cell monolayers were scratch-wounded and treated with TGF-β (10 ng/mL) with our without testosterone (10 nM to 300 nM). Gap closure was measured 16 hours after treatment. No significant difference was observed between control and cells treated with TGF-β + 300 nM of testosterone. (B) The effects of testosterone on p-Smad3 expression in HaCaT cell monolayer. HaCaT cells were treated with testosterone (100 nM) with or without flutamide (1 μM) for 1 hour after which the cells were analyzed for p-Smad3 and total-Smad3 expression. NS = nonscratched control. Respective densitometric analyses are shown in the right panels. (C) Smad3 hyperexpression partially blocks the effect of testosterone on keratinocyte migration in HaCaT cell scratch assay.
Many effects of TGF-β are mediated by canonical signaling through Smad3. Accordingly, we determined the effects of testosterone on Smad3 phosphorylation. Western blot analysis revealed that testosterone reduced the levels of p-Smad3 (Figure 6B); testosterone’s effect was blocked by co-incubation with flutamide. Total Smad-3 was not affected.
In separate experiments, we performed the scratch assay after hyperexpression of wild type Smad3. The inhibitory effects of testosterone on HaCaT cell migration were significantly attenuated by constitutive Smad3 expression (Figure 6C).
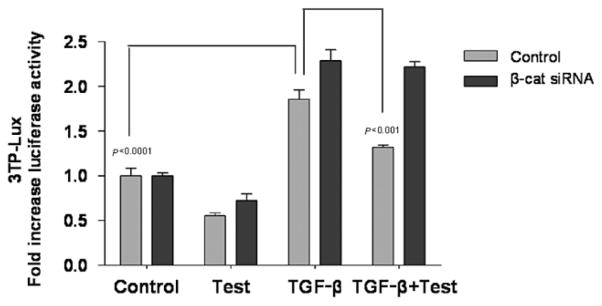
We determined the effect of testosterone on TGF-β-induced transcriptional activity by using a TGF-β-responsive luciferase reporter construct, 3TP-lux,33 in the presence or absence of β-catenin siRNA. Incubation of HaCaT cells with 10 ng/mL TGF-β significantly increased luciferase reporter (3TP-lux) activity compared with control (p < 0.0001; Figure 7). Co-treatment with testosterone (100 nM) significantly attenuated the induction of luciferase transcriptional activity by TGF-β (p < 0.001), reducing it by nearly 50%. The inhibitory effect of testosterone on TGF-β-induced luciferase activity was not observed when β-catenin was knocked down (Figure 7). These data provide evidence of the cross-communication among AR, β-catenin, and TGF-β signaling pathways in mediating the inhibitory effect of testosterone on keratinocyte migration.
Figure 7.
β-catenin small interfering RNA siRNA attenuates the effects of testosterone on transforming growth factor-β TGF-β-induced luciferase reporter activity HaCaT cell were co-transfected with 3TP-Lux and β-catenin siRNA. After 48 hours of β-catenin silencing, cells were scratched and incubated with testosterone (100 nM), TGF-β (10 ng/mL), or both. Mean ± SD luciferase activity from two separate experiments conducted in triplicates is shown.
DISCUSSION
Our data provide several novel insights into the mechanisms by which AR antagonists promote cutaneous wound healing. We show that AR antagonists facilitate reepithelialization of the wound by promoting keratinocyte migration, without affecting keratinocyte proliferation. Androgen effects on keratinocyte migration are mediated activation of the Wnt-β-catenin pathway and cross-communication of the signal to TGF-β/Smad pathway. These mechanistic insights have important therapeutic implications.
Previous studies in humans and animal models have reported an important role of gonadal hormones in cutaneous wound healing; the estrogens exert a positive effect on wound healing, while androgens inhibit wound healing.14,32,34 Systemic androgen deprivation has been shown to improve wound healing in male animals. However, the adverse effects associated with systemic androgen deprivation, including sexual dysfunction, loss of muscle and bone mass, increase in fat mass, and insulin resistance, render this approach untenable as a therapeutic strategy. Our studies show that by topical application of an AR antagonist, it is possible to promote cutaneous wound healing by selective blockade of androgen signaling locally without inducing systemic androgen deprivation.
β-catenin and TGF-β signaling pathways play a pivotal role in molecular events that result in reepithelialization of cutaneous wounds. In human skin wounds grown as organ culture, β-catenin impairs keratinocyte motility by altering the expression of the cytoskeletal keratins K6 and K16,28 while TGF-β increases motility by inducing the expression of integrins.35,36
Our data show that β-catenin plays an essential role in mediating androgen effects on keratinocyte migration. Testosterone induces nuclear translocation of β-catenin in keratinocytes and knockdown of β-catenin blocks testosterone’s effects on keratinocyte migration and wound closure. In other organ systems, such as the skeletal muscle and adipogenic cells, agonist-bound AR has been shown to associate with β-catenin, promoting its nuclear translocation and activation of downstream Wnt target genes.37 Our data are consistent with the findings of Gilliver et al.13 that β-catenin is down-regulated in wounds of rats treated with a steroid 5-alpha-reductase inhibitor (MK-434); however, in that report the mechanisms of the 5α-dihydrotestosterone (DHT)-mediated regulation of β-catenin were not clear. The authors demonstrated that global blockade of DHT biosynthesis markedly accelerated reepithelialization of incisional and excisional wounds and reduced local expression of β-catenin. They showed that the effect of DHT was partially rescued by the co-treatment with an AKT inhibitor, which reduced active β-catenin levels, but they do not explain any mechanisms of how androgens and β-catenin interact together. However, the authors suggested that the effects of DHT on β-catenin may be indirect because DHT did not influence keratinocyte expression of active β-catenin in vitro.
In the current study, we clearly establish that it is the direct interaction of AR and β-catenin which regulates dynamics of keratinocyte migration in wound closure assays. We demonstrate that even without an alteration in β-catenin, flutamide treatment accelerates wound healing by disrupting AR-mediated nuclear translocation of β-catenin. These novel findings provide mechanistic insights into the androgenic modulation of wound healing.
TGF-β promotes wound closure by regulating many aspects of wound repair including reepithelialization, inflammation, chemotaxis, and deposition of extracellular matrix.38–41 TGF-β receptors are expressed in normal skin and during wound healing.38 We provide several lines of evidence that testosterone blocks TGF-β signaling in keratinocytes. First, testosterone blocks the effects of TGF-β on keratinocyte migration in a dose-dependent manner. Second, testosterone down regulates the expression of p-Smad3. Third, Smad3 hyperexpression antagonizes the inhibitory effects of testosterone on wound closure in vitro. Finally, testosterone blocks TGF-β-stimulated transcription of a TGF-β-inducible luciferase reporter construct.
Our data show that AR, Wnt/β-catenin, and TGF-β pathways are interconnected and cross-communicate to regulate cutaneous wound healing in response to androgenic stimulus. Testosterone binding to AR promotes nuclear translocation of β-catenin and down regulation of TGF-β-induced transcription. Knockdown of β-catenin blocks testosterone’s inhibitory effects on TGF-β-induced transcription, suggesting that testosterone’s effects on TGF-β signaling are mediated through β-catenin, upstream from Smad3. The pivotal role of β-catenin in mediating the effects of testosterone on keratinocyte migration renders it an attractive therapeutic target for the development of drugs for promoting wound healing.
Our investigation was focused on the effects of androgen signaling on keratinocyte migration, which plays an important role in reepithelialization of cutaneous wounds and which was stimulated by flutamide; our data do not exclude additional actions of the androgen antagonist on inflammation, angiogenesis, and extracellular matrix deposition. We recognize that no in vitro or in vivo experimental model can replicate fully the complexity of the human disease state; the combined application of in vitro and in vivo approaches, as we have done in this investigation, can enhance the applicability of findings to human disease. We used excision wounds as a model to study wound healing; chronic ulcers may differ from acute excision wounds in their path physiology. Also, it should be recognized that Smad3 null mice exhibit enhanced rates of epithelialization.16 The improved wound healing in Smad3 null mice seems paradoxical in light of the large body of data showing that TGF-β promotes cutaneous wound healing. The mechanistic basis of this apparent discrepancy between the positive effects of TGF-β on wound healing and the enhanced wound healing reported in Smad3 null mice is unknown. One explanation could be that gene inactivation during embryonic life may be associated with adaptations that may not be observed after gene inactivation in adult life, but most importantly, SMAD3 knockout animals have castrate levels of testosterone.16 Therefore use of SMAD3 knockout model in examining androgen action can be confounded by the inherent hypogonadal state. Additional experiments should be performed with SMAD3 knockout animals, in which testosterone levels are replete for an extended period to reach a sustained steady state before wounds are created.
In summary, our findings indicate that testosterone inhibits keratinocyte migration by facilitating β-catenin nuclear translocation and by cross-communication of the signal to the TGF-β pathway, which is blocked by testosterone. Topical application of an androgen antagonist promotes cutaneous wound closure without the systemic adverse consequences of androgen deprivation. Multiple steps in the androgen signaling cascade uncovered by this investigation provide logical targets for drug discovery and merit additional validation for facilitating drug discovery; their topical application is an attractive, broadly applicable approach for increasing the benefit-to-risk ratio. Future work will be directed to further explore the role of the local modulation of androgen action in model of pathological wound healing.
Acknowledgments
We thank Dr. Y. Mochida (Boston University School of Dental Medicine) for the gift of 3TP-lux and Michelle Beady and Lan Jiang for technical assistance. This work was supported by Evans Research Foundation Award.
Glossary
- AR
Androgen receptor
- Cx
Orchidectomized
- FLT
Flutamide
- LiCl
Lithium chloride
- lux
Luciferase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- p-Smad 3
phospho-Smad3
- siRNA
Small interfering RNA
- Test
Testosterone
- TGF-β
Transforming growth factor-β
References
- 1.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–80. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Rep Regen. 2009;17:763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat Med. 1997;3:1209–15. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–18. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119 (Pt 4):722–32. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RJ. Mouseyes revisited: upgrading a computer program that aids wound measurement. J Wound Care. 2002;11:213–6. doi: 10.12968/jowc.2002.11.6.26404. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J Clin Invest. 2002;110:615–24. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont M, Luu-The V, de Launoit Y, Labrie F. Expression of human 17 beta-hydroxysteroid dehydrogenase in mammalian cells. J Steroid Biochem Mol Biol. 1992;41:605–8. doi: 10.1016/0960-0760(92)90391-u. [DOI] [PubMed] [Google Scholar]
- 9.Zouboulis CC, Degitz K. Androgen action on human skin—from basic research to clinical significance. Exp Dermatol. 2004;13 (Suppl 4):5–10. doi: 10.1111/j.1600-0625.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 10.Liang T, Hoyer S, Yu R, Soltani K, Lorincz AL, Hiipakka RA, Liao S. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol. 1993;100:663–6. doi: 10.1111/1523-1747.ep12472330. [DOI] [PubMed] [Google Scholar]
- 11.Khetawat G, Faraday N, Nealen ML, Vijayan KV, Bolton E, Noga SJ, Bray PF. Human megakaryocytes and platelets contain the estrogen receptor beta and androgen receptor (AR): testosterone regulates AR expression. Blood. 2000;95:2289–96. [PubMed] [Google Scholar]
- 12.Mantalaris A, Panoskaltsis N, Sakai Y, Bourne P, Chang C, Messing EM, Wu JH. Localization of androgen receptor expression in human bone marrow. J Pathol. 2001;193:361–6. doi: 10.1002/1096-9896(0000)9999:9999<::AID-PATH803>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 13.Gilliver SC, Ruckshanthi JP, Hardman MJ, Zeef LA, Ashcroft GS. 5alpha-dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. 2009;217:73–82. doi: 10.1002/path.2444. [DOI] [PubMed] [Google Scholar]
- 14.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fimmel S, Zouboulis CC. Influence of physiological androgen levels on wound healing and immune status in men. Aging Male. 2005;8:166–74. doi: 10.1080/13685530500233847. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft GS, Mills SJ, Flanders KC, Lyakh LA, Anzano MA, Gilliver SC, Roberts AB. Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Rep Regen. 2003;11:468–73. doi: 10.1046/j.1524-475x.2003.11614.x. [DOI] [PubMed] [Google Scholar]
- 17.Gilliver SC, Ruckshanthi JP, Atkinson SJ, Ashcroft GS. Androgens influence expression of matrix proteins and proteolytic factors during cutaneous wound healing. Lab Invest. 2007;87:871–81. doi: 10.1038/labinvest.3700627. [DOI] [PubMed] [Google Scholar]
- 18.Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149:5747–57. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- 19.Chan RK, Liu PH, Pietramaggiori G, Ibrahim SI, Hechtman HB, Orgill DP. Effect of recombinant platelet-derived growth factor (Regranex) on wound closure in genetically diabetic mice. J Burn Care Res. 2006;27:202–5. doi: 10.1097/01.BCR.0000202898.11277.58. [DOI] [PubMed] [Google Scholar]
- 20.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–47. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buth H, Luigi Buttigieg P, Ostafe R, Rehders M, Dannenmann SR, Schaschke N, Stark HJ, Boukamp P, Brix K. Cathepsin B is essential for regeneration of scratch-wounded normal human epidermal keratinocytes. Eur J Cell Biol. 2007;86:747–61. doi: 10.1016/j.ejcb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Kioka N, Ito T, Yamashita H, Uekawa N, Umemoto T, Motoyoshi S, Stark HJ, Boukamp P, Brix K. Crucial role of vinexin for keratinocyte migration in vitro and epidermal wound healing in vivo. Exp Cell Res. 2010;316:1728–38. doi: 10.1016/j.yexcr.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Tochio T, Tanaka H, Nakata S, Hosoya H. Fructose-1,6-bisphosphate aldolase A is involved in HaCaT cell migration by inducing lamellipodia formation. J Dermatol Sci. 2010;58:123–9. doi: 10.1016/j.jdermsci.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Altenburger R, Kissel T. The human keratinocyte cell line HaCaT: an in vitro cell culture model for keratinocyte testosterone metabolism. Pharm Res. 1999;16:766–71. doi: 10.1023/a:1011945212831. [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–68. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serra C, Bhasin S, Tangherlini F, Barton ER, Ganno M, Zhang A, Shansky J, Vandenburgh HH, Travison TG, Jasuja R, Morris C. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152:193–206. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- 28.Stojadinovic O, Brem H, Vouthounis C, Lee B, Fallon J, Stallcup M, Merchant A, Galiano RD, Tomic-Canic M. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopecki Z, Luchetti MM, Adams DH, Strudwick X, Mantamadiotis T, Stoppacciaro A, Gabrielli A, Ramsay RG, Cowin AJ. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J Pathol. 2007;211:351–61. doi: 10.1002/path.2113. [DOI] [PubMed] [Google Scholar]
- 30.Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–73. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 31.Joo CK, Seomun Y. Matrix metalloproteinase (MMP) and TGF beta 1-stimulated cell migration in skin and cornea wound healing. Cell Adh Migr. 2008;2:252–3. doi: 10.4161/cam.2.4.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, Cross B, Nye E, Hart IR, Dipersio CM, Hodivala-Dilke KM. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118:965–74. doi: 10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–14. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 35.Gailit J, Welch MP, Clark RA. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994;103:221–7. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- 36.Jeong HW, Kim IS. TGF-beta1 enhances betaig-h3-mediated keratinocyte cell migration through the alpha3beta1 integrin and PI3K. J Cell Biochem. 2004;92:770–80. doi: 10.1002/jcb.20110. [DOI] [PubMed] [Google Scholar]
- 37.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–54. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank S, Madlener M, Werner S. Transforming growth factors beta1, beta2, and beta3 and their receptors are differentially regulated during normal and impaired wound healing. J Biol Chem. 1996;271:10188–93. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- 39.Sporn MB, Roberts AB, Shull JH, Smith JM, Ward JM, Sodek J. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983;219:1329–31. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- 40.Beck LS, Deguzman L, Lee WP, Xu Y, McFatridge LA, Amento EP. TGF-beta 1 accelerates wound healing: reversal of steroid-impaired healing in rats and rabbits. Growth Factors. 1991;5:295–304. doi: 10.3109/08977199109000293. [DOI] [PubMed] [Google Scholar]
- 41.Beck LS, DeGuzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92:2841–9. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]