Abstract
The transition from moderate to compulsive alcohol drinking is driven by increasingly dysfunctional reward and stress systems. We review behavioral and pharmacological studies of alcohol self-administration in rats that were mainly conducted within the framework of the alcohol vapor model of dependence. We discuss neurotransmitter systems that are implicated in alcohol drinking, with a focus on contrasting those neurotransmitter systems that drive behavior in the dependent vs. nondependent states. We hypothesize that the identification of systems that become increasingly dysfunctional in alcohol dependence will reveal possible targets for successful interventions to reduce the motivation that drives compulsive alcohol drinking. In our opinion, drugs that (1) normalize, rather than block, a hypofunctional reward system via restoration of the function of hypothalamic stress systems, and (2) desensitize extrahypothalamic stress systems have the potential to selectively and effectively curb compulsive alcohol drinking.
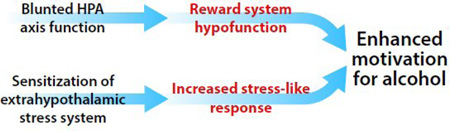
Graphical Abstract
Neuroplasticity in hypothalamic and extrahypothalamic stress systems in alcohol dependence.
“ Anxiety, yawning, rigor - wine drunk with an equal proportion of water, removes these complaints.” Hippocrates (400 B.C.E.) [1]
Introduction
Alcohol dependence (AD) is a major public health issue, the mortality and morbidity of which affects 6% of the global population [2]. Alcohol dependence has been conceptualized as a three-stage, recurring cycle that comprises binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation (“craving”) stages. These stages involve neuroplastic changes in brain reward, stress, and executive function systems that are controlled by neurocircuits that involve the basal ganglia, extended amygdala, and prefrontal cortex, respectively [3]. Although medications currently exist for the treatment of AD, they are only moderately effective [4].
In this opinion article, we argue that AD is associated with (1) disruption of hypothalamic stress systems that contribute to brain reward hypofunction and (2) sensitization of extrahypothalamic stress systems. We propose that these processes lead to the negative emotional states that characterize motivational withdrawal and drive compulsive alcohol drinking via negative reinforcement (i.e., alcohol is consumed to alleviate negative feelings).
Vapor Model of Alcohol Dependence
Multiple rodent models of alcohol drinking exist. However, a major setback in the development of valid models of AD is that rodents will rarely voluntarily self-administer alcohol to the point of dependence without the use of prohibitively lengthy experimental designs. To overcome this obstacle, passive exposure to alcohol vapors has been used for over two decades to reliably create somatic signs of dependence (e.g., tail stiffness, abnormal gait/posture, and vocalization upon touch) and motivational signs of dependence (e.g., increased anxiety- and hypohedonic-like behavior). Typically, passive daily cycles of alcohol intoxication that produce blood alcohol levels around 150–250 mg/dl and withdrawal are used in combination with voluntary, operant alcohol self-administration to measure the motivation for alcohol during alcohol withdrawal. Compared with nondependent controls that are exposed to air, vapor-exposed rodents exhibit an escalation of intake and compulsive-like drinking behavior (for review, see [5,6]).
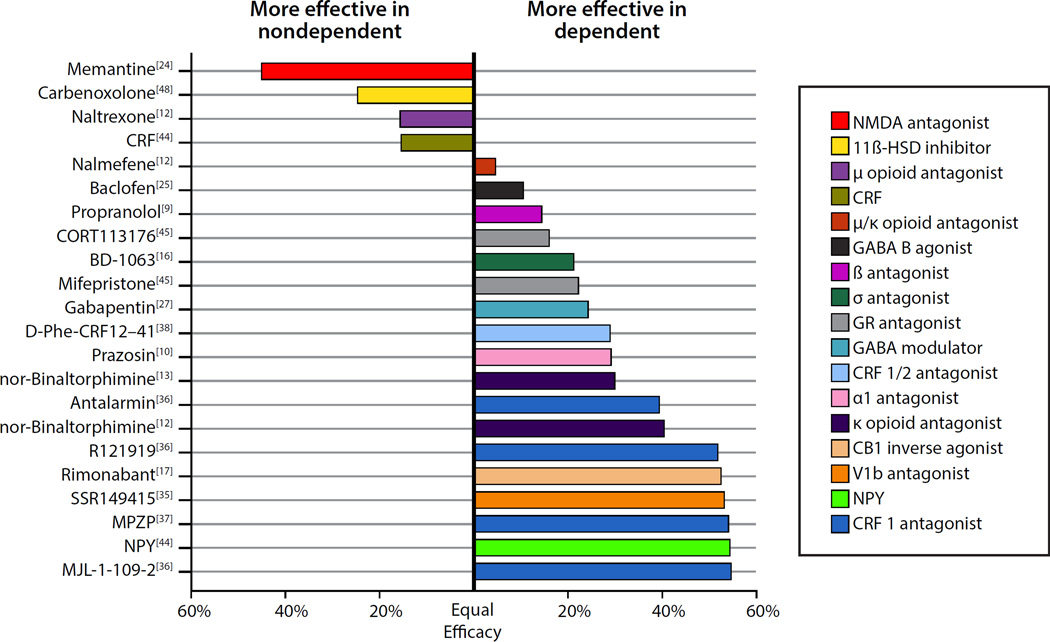
In the present article, we use the vapor model as a framework to discuss central neurotransmitter systems that are involved in normal, controlled vs. escalated, compulsive-like drinking. Most of the studies discussed herein tested the effects of acute drug treatments in rats on drinking during acute withdrawal (typically 2–8 h into withdrawal). Fig. 1 summarizes the dependent vs. nondependent specificity of the effects of systemically and intracerebroventricularly (but not site-specific brain infusions) delivered compounds that effectively reduced alcohol drinking in dependent and/or nondependent rats. We argue that compounds that preferentially reduce drinking in dependent rats may reveal targets for effective therapeutic intervention.
Fig. 1.
Effect of drugs reported to have acute effects in the alcohol-vapor dependence model of escalated alcohol drinking. To be included in this analysis, the studies had to include both dependent and nondependent rats and report a significant effect on alcohol drinking in either group. Studies were included that tested alcohol drinking during acute withdrawal (typically 2–8 h into withdrawal; memantine was tested at 24 h into withdrawal). The figure shows the difference in sensitivity between groups (i.e., percent change in drinking in the dependent group [drug relative to vehicle] minus the percent change in drinking in the nondependent group). We chose the highest dose of the drug that caused a group-specific effect. If not available (i.e., equally effective in both groups), we used the first effective dose to compare groups. Because none of the treatments caused large increases in alcohol drinking, the bars that are plotted can generally be interpreted as showing the relative effectiveness of each compound in reducing drinking in the two groups. Bars to the left of the “Equal Efficacy” line indicate compounds that are more effective in nondependent animals. Bars that are close to the “Equal Efficacy” line indicate that the treatment was similarly effective in both groups. Bars to the right of the “Equal Efficacy” line indicate compounds that are more effective in dependent animals. To collect numerical values for this analysis, figures from published reports were analyzed using a freely available tool for extracting raw data from scientific figures (Web Plot Digitizer, Version 3.9, http://arohatgi.info/WebPlotDigitizer).
Neuropsychopharmacology of Alcohol Reinforcement
Catecholamines
Dopamine has long been suggested to be a common factor that mediates alcohol and drug addiction and is implicated in incentive salience and reinforcement learning in general. Although alcohol self-administration increases extracellular dopamine levels in the nucleus accumbens [7], dopamine is not critical for the maintenance of alcohol-reinforced behavior [8]. However, norepinephrine signaling appears to play a significant role in mediating AD. Administration of the β-adrenergic receptor antagonist propranolol reduced alcohol drinking to a greater extent in dependent than in nondependent rats [9]. The α1-adrenergic receptor antagonist prazosin increased drinking in nondependent rats at a low dose, whereas it reduced drinking mainly in dependent rats when given at higher doses [10]. Intriguingly, the bidirectional modulation of drinking by noradrenergic receptor antagonists has also been observed in humans with AD. Antagonism of α-adrenergic receptors with doxazocin decreased alcohol drinking in AD patients with a family history of AD, whereas the same treatment increased drinking in AD patients without a family history of AD [11], suggesting a potential genetic component in the effects of this treatment.
Opioids
Opioid signaling has consistently been shown to be involved in AD in a receptor-subtype specific manner. The μ-opioid antagonist naltrexone (Revia®) is an FDA-approved medication for AD that has been shown to decrease drinking in both nondependent and dependent rats, but nondependent rats were more sensitive to treatment [12]. The anti-craving effects of naltrexone may be attributable to activation of the hypothalamic-pituitary-adrenal (HPA) axis (discussed below). The mixed μ/κ-opioid receptor antagonist nalmefene suppressed alcohol drinking to a similar extent in both dependent and nondependent rats [12]. However, selective κ-opioid antagonism by nor-binaltorphimine reduced drinking only in dependent rats [12,13]. Targeted blockade of κ-opioid receptor signaling in the nucleus accumbens selectively reduced drinking in dependent rats, suggesting a role for κ-opioid receptors in reward allostasis [14]. Recent work has also implicated κ-opioid receptor signaling in the central nucleus of the amygdala (CeA) in the mediation of intensification of the motivation to drink in dependent rats, a process that is distinct from somatic withdrawal [15]. The σ receptor antagonist BD-1063 decreased drinking specifically in dependent rats [16]. Thus, μ-opioid receptors likely decrease dependent and nondependent drinking by blocking alcohol’s rewarding effects, whereas σ- and κ-opioid receptors become important during dependence.
Cannabinoids
The cannabinoid 1 (CB1) receptor inverse agonist SR141716A (rimonabant) decreased drinking in dependent rats without affecting nondependent controls [17]. Rimonabant has also shown efficacy in decreasing drinking in humans with alcohol dependence. However, the blunting of natural reward mechanisms observed in preclinical studies and the incidence of potential severe side-effects in humans (e.g., suicide) limit its clinical use [18]. Endocannabinoids, particularly in the CeA, may mediate some of the anxiety-like effects of alcohol dependence [19]. As such, the modulation of endocannabinoids with novel “neutral” antagonists or inhibitors of the degradation of endocannabinoids (which would increase CB1 signaling instead of blocking it) constitute potential targets for medication development and remain to be tested in the vapor model.
Glutamate and GABA
Acamprosate (Campral®) is an FDA-approved medication for AD. It is hypothesized to exert inhibitory effects on the modulation of N-methyl-D-aspartate (NMDA) receptors through interactions with metabotropic glutamate receptors that contribute to a hyperexcitable state in the absence of alcohol [20]. However, the precise mechanism of acamprosate's action is a subject of debate [21,22]. In rats with a history of vapor-induced alcohol dependence (i.e., protracted abstinence), acamprosate reduced escalated drinking to nondependent levels, without disrupting drinking in nondependent rats [23]. The effect of systemic acamprosate treatment in dependent rats during acute withdrawal has yet to be studied. The noncompetitive NMDA receptor antagonist memantine significantly decreased drinking in both dependent and nondependent rats but with a greater effect in nondependent rats [24].
The γ-aminobutyric acid B (GABAB) receptor agonist baclofen decreased drinking in both dependent and nondependent rats but to a greater extent in dependent rats [25]. Intra-CeA administration of the GABAA receptor agonist muscimol reduced drinking in dependent rats post-vapor, without affecting drinking in nondependent rats [26]. The indirect GABA modulator gabapentin, via actions on voltage-gated calcium channels, significantly reduced drinking in dependent rats, with no effects in nondependent rats [27]. A 2014 randomized clinical trial reported evidence of the safety and efficacy of gabapentin for the treatment of AD [28]. These findings provide translational evidence of the role of GABA transmission in AD. Altogether, these studies highlight the preferential effects of GABA modulators on drinking in dependent rats and suggest a potential clinical target.
Oxytocin, hypocretin, nociception, and substance P
Unpublished data from our laboratory indicated that oxytocin (Tunstall et al., unpublished data) and hypocretin receptor 2 (Schmeichel et al., unpublished data) are involved in dependent alcohol drinking. Based on the literature, we hypothesize that nociceptin and substance P may also be dysregulated in alcohol dependence [3,29]. The latter two systems remain to be tested in the vapor model.
Dysregulation of the HPA Axis and Extrahypothalamic Systems in Alcohol Dependence
Positive reinforcement and reward associated with alcohol involve the activity of multiple neurotransmitter systems (e.g., dopamine, μ-opioid, and CB1). These neurotransmitter systems also mediate the rewarding effects of nondrug reinforcers. Therefore, blocking these systems is expected to have limited effectiveness in AD where reward processing is already compromised [30]. Conversely, correcting/boosting the reward system rather than blocking it may be more beneficial in AD, with the aim of improving mood and moderating compulsive drinking via an anti-dysphoria/anhedonia effect.
Similar to stress, acute alcohol intoxication activates the HPA axis in both humans and rodents. In rats, adrenalectomy abolished alcohol drinking in nondependent rats, which could be recovered by corticosterone replacement [31], indicating that glucocorticoids facilitate alcohol reinforcement. The bidirectional relationship between stress and alcohol reward is complicated. Low glucocorticoid levels or high glucocorticoid levels may have similar effects in disrupting alcohol self-administration. Within the functional range of HPA axis activity, increasing the level of stress has been shown to facilitate the self-administration of many drugs of abuse, including alcohol [32].
Chronic alcohol use, however, disrupts the HPA axis. Although some individuals with AD exhibit an altered stress response that is characterized by high glucocorticoid release (with symptomatology that mimics Cushing’s syndrome), excessive HPA axis activation more commonly results in blunted HPA axis activity (for review, see [33]). Dysregulation of the HPA axis is thought to contribute to deficits in reward function that contribute to anhedonia/dysphoria and craving in AD (see graphical abstract and [34]). Thus, withdrawal is associated with opponent process-like rebound effects that occur during intoxication (i.e., excessive reward system activation), resulting in a hypohedonic-like emotional state. In this review, we focus on the role of blunted HPA axis activity in the persistence of reward hypofunction and stress sensitization.
A remarkable consequence of intense/frequent HPA axis activation (e.g., frequent excessive drinking) is opposing glucocorticoid receptor (GR)-mediated regulation of corticotropin-releasing factor (CRF) in the paraventricular nucleus (blunted activation) and extrahypothalamic stress systems (enhanced activation; for review, see [33]). Increases in extrahypothalamic CRF signaling, together with an increase in vasopressin (a co-regulator of the HPA axis that potentiates CRF’s effects [35]), drive compulsive-like drinking. The CRF1 receptor antagonists antalarmin, MJL-1-109-2, R121919, and MPZP selectively decreased alcohol drinking in dependent rats [36,37]. Selective blockade of alcohol drinking in dependent rats was also reported using intracerebroventricular [38] or direct injections of the CRF1/2 receptor antagonist D-Phe-CRF12–41 in the CeA [39]. Here, intra-CeA but not intra-nucleus accumbens or intra-bed nucleus of the stria terminalis injections of CRF antagonists decreased escalated alcohol drinking in dependent but not nondependent rats [39]. Additionally, CRF2 receptor agonism in the CeA [40], similar to CRF1 antagonism, decreased alcohol drinking specifically in dependent rats. These findings suggest that CRF receptors are dysregulated in alcohol dependence, and the blockade of CRF1 or activation of CRF2 receptors selectively attenuates withdrawal-induced escalated alcohol drinking. Two recent laboratory human studies did not support the efficacy of CRF1 antagonists in reducing alcohol craving in humans with AD [41,42]. These studies were conducted in treatment-seeking, detoxified subjects with alcoholism. In addition to differences in drinking patterns, treatment-seeking individuals, compared with non-treatment-seekers, present with more impulsivity, anxiety and mood disorders. However, both populations exhibit craving, compulsive seeking, and excessive drinking of alcohol, as well as the emergence of negative emotional states during withdrawal [43]. The effects of CRF1 antagonism remain to be tested in non-treatment-seeking subjects with alcoholism who are currently drinking.
Even more intriguing, CRF and neuropeptide Y (NPY) have been found to have opposing behavioral effects. Central administration of CRF peptide (which is anxiogenic) decreased alcohol drinking in dependent and nondependent rats, whereas NPY (which is anxiolytic) reduced drinking only in dependent rats [44]. Thus, CRF appears to disrupt behavior in general, whereas NPY effectively reduces drinking via an opposing effect but only in dependent rats (i.e., rats that are highly “anxious”). This study also found that CRF and NPY co-administration had a clear interaction, leaving drinking in both groups unchanged compared with the control condition.
We hypothesize that a reduction of GR signaling may block the sensitization of extrahypothalamic stress systems and, as a result, compulsive drinking. Glucocorticoid receptor blockade with either mifepristone (a GR and progesterone receptor antagonist) or CORT113176 (a selective GR antagonist) decreased alcohol drinking mainly in dependent rats, an effect that may involve the sensitization of GR signaling in the CeA [45]. Evidence also indicates a functional role for GR in escalated drinking in rats with a history of vapor-induced AD during protracted abstinence [46,47].
Carbenoxolone, a nonselective 11β-hydroxysteroid dehydrogenase (HSD) inhibitor, decreased drinking in both dependent and nondependent rats [48]. The inhibition of 11β-HSD1 might be expected to selectively reduce drinking in dependent animals because it leads to a reduction of GR signaling in the brain. However, 11β-HSD2 is expressed primarily in a subpopulation of neurons in the nucleus tractus solitarii that projects to both reward- and stress-related brain regions. Thus, the nonspecific inhibition of 11β-HSD isozymes may decrease alcohol reinforcement in general. Future experiments should evaluate the effects of inhibitors that are selective for 11β-HSD isozyme subtypes.
Continuous GR antagonism abolished the escalation of intake in rats that were exposed to alcohol vapor [47], suggesting that both genomic and non-genomic GR actions may be engaged in the chronic and acute effects of GR antagonism in escalated alcohol drinking. In addition to GR-mediated gene transcription, GR-mediated fast-acting CRF release has been reported [49]. Recently, a double-blind clinical and laboratory-based study demonstrated that individuals with AD who received 1-week treatment with mifepristone reported a reduction of alcohol drinking and craving for alcohol compared with placebo-treated subjects. Treated individuals also exhibited improvements in markers of liver function compared with placebo-treated subjects [45]. Mifepristone may exert therapeutic effects by restoring negative feedback along the HPA axis, which rescues reward function, and desensitizing extrahypothalamic stress systems (see Graphical Abstract).
Altogether, these findings suggest that initial HPA axis activation contributes to early drug use within the binge/intoxication stage of the addiction cycle, but chronic HPA axis activation sensitizes extrahypothalamic stress systems that characterize the withdrawal/negative-affect stage.
Summary
Alcohol dependence is associated with a decrease in reward function and sensitization of stress systems. These allostatic changes provide powerful incentives for compulsive alcohol drinking via negative reinforcement. Neurotransmitter systems, such as CRF, vasopressin, GABA, norepinephrine, glucocorticoids, NPY, and dynorphin (the endogenous ligand for κ-opioid receptors), create stress-like states that drive compulsive-like alcohol drinking. Supporting the predictive validity of the vapor model, initial studies in humans who suffer from AD indicated the potential of GABA (gabapentin), norepinephrine (doxazocin), and glucocorticoid (mifepristone) systems in the treatment of AD. Drugs that reset the function of the HPA axis and reward systems and desensitize extrahypothalamic stress systems may have the potential to treat AD. Future studies will be designed to test the efficacy of such compounds. Glucocorticoid receptor antagonists are an example of promising candidates for this dual approach.
Highlights.
Disruption of HPA axis leads to reward system hypofunction in alcohol dependence
Extrahypothalamic stress systems are sensitized in alcohol dependence
Loss of reward and stress sensitization drive compulsive alcohol drinking
Acknowledgments
The National Institute on Drug Abuse (NIDA) Intramural Research Program supported this work. The authors thank Michael Arends for editorial assistance and NIDA media services for assistance in figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
* of special interest
** of outstanding interest
- 1.Adams F. Hippocrates. The Genuine Works of Hippocrates: Translated from the Greek with a Preliminary Discourse and Annotations. Vol. II. New York: W. Wood; 1886. [Google Scholar]
- 2.World Health Organization. Global status report on alcohol and health. 2014 [Google Scholar]
- 3.Koob GF, Mason BJ. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu Rev Pharmacol Toxicol. 2016;56:299–322. doi: 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- 4.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- 5.Becker HC, Lopez MF. An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. Int Rev Neurobiol. 2016;126:157–177. doi: 10.1016/bs.irn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: Focus on the vapor model. Alcohol. 2014;48:277–286. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 8.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 9.Gilpin NW, Koob GF. Effects of β-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker BM, Rasmussen DD, Raskind MA, Koob GF. Alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, et al. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2015 doi: 10.1111/adb.12275. *The authors reported that the α1-adrenergic receptor blocker doxazosin significantly reduced alcohol drinking in alcohol-dependent individuals who sought outpatient treatment with a high “family history of density of alcoholism,” but increased drinking in patients with a low “family history of density of alcoholism” Interestingly, using the vapor model of alcohol dependence, Walker et al. [10]. reported that the α1-adrenergic blocker prazocin reduced alcohol drinking in dependent rats, whereas low doses of prazocin increased drinking in nondependent rats.
- 12.Walker BM, Koob G. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacol. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker BM, Zorrilla EP, Koob GF. Systemic κ-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. κ-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kissler JL, Walker BM. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala κ-Opioid Receptors. Neuropsychopharmacol. 2016;41:560–567. doi: 10.1038/npp.2015.183. *The authors reported a pharmacological dissociation between motivational and somatic withdrawal in alcohol-dependent rats. Administration of the κ-opioid receptor antagonist nor-binaltorphimine in the central nucleus of the amygdala decreased escalated alcohol drinking in dependent rats during both acute withdrawal and protracted abstinence. However, the same treatment did not affect somatic withdrawal scores at either time point
- 16.Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Steardo L, et al. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacol. 2009;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–1114. [PubMed] [Google Scholar]
- 18.Henderson-Redmond AN, Guindon J, Morgan DJ. Roles for the endocannabinoid system in ethanol-motivated behavior. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:330–339. doi: 10.1016/j.pnpbp.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littleton JM. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J Addict Med. 2007;1:115–125. doi: 10.1097/ADM.0b013e318156c26f. [DOI] [PubMed] [Google Scholar]
- 21.Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, et al. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacol. 2014;39:783–791. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann K, Hoffmann S, Pawlak CR. Does Acamprosate Really Produce its Anti-Relapse Effects via Calcium? No Support from the PREDICT Study in Human Alcoholics. Neuropsychopharmacol. 2016;41:659–660. doi: 10.1038/npp.2015.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J Off Publ Fed Am Soc Exp Biol. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 24.Alaux-Cantin S, Buttolo R, Houchi H, Jeanblanc J, Naassila M. Memantine reduces alcohol drinking but not relapse in alcohol-dependent rats. Addict Biol. 2015;20:890–901. doi: 10.1111/adb.12177. [DOI] [PubMed] [Google Scholar]
- 25.Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts AJ, Cole M, Koob GF. Intra-amygdala Muscimol Decreases Operant Ethanol Self-administration in Dependent Rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 27.Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci Off J Soc Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:70–77. doi: 10.1001/jamainternmed.2013.11950. ** The authors reported that gabapentin was safe and effective in treating alcohol dependence. Treatment also improved indices of mood, craving, and sleep. This study provided further evidence of the predictive validity of the vapor model of alcohol dependence, given that gabapentin decreased alcohol drinking in dependent but not nondependent rats [27]
- 29.George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- 30.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahlke C, Hård E, Eriksson CJ, Engel JA, Hansen S. Consequence of long-term exposure to corticosterone or dexamethasone on ethanol consumption in the adrenalectomized rat, and the effect of type I and type II corticosteroid receptor antagonists. Psychopharmacology (Berl) 1995;117:216–224. doi: 10.1007/BF02245190. [DOI] [PubMed] [Google Scholar]
- 32.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 33.Edwards S, Little HJ, Richardson HN, Vendruscolo LF. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol Fayettev N. 2015;49:811–816. doi: 10.1016/j.alcohol.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res Curr Rev. 2012;34:468–483. [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF. Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats: V1bR in ethanol dependence. Addict Biol. 2012;17:76–85. doi: 10.1111/j.1369-1600.2010.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Funk CK, Zorrilla EP, Lee MJ, Rice KC, & Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biological psychiatry. 2007;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased Ethanol Self-Administration and Anxiety-Like Behavior During Acute Ethanol Withdrawal and Protracted Abstinence: Regulation by Corticotropin-Releasing Factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 39.Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci Off J Soc Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2015;40:1053–1063. doi: 10.1038/npp.2014.306. * The authors reported negative results on the effect of the CRF1 receptor antagonist pexacerfont in suppressing stress-induced alcohol craving in treatment-seeking alcohol-dependent patients. Non-treatment-seeking individuals with AD were not included in the study. This is an example of translation failure, and understanding the factors that contributed to these negative findings is of great interest
- 42. Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. The CRF1 Antagonist Verucerfont in Anxious Alcohol Dependent Women: Translation of Neuroendocrine, but not of Anti-Craving Effects. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2016 doi: 10.1038/npp.2016.61. * This study by the same group as Kwako et al. [41]. reported that another CRF1 receptor antagonist, verucerfont, failed to reduce stress-induced craving in anxious, alcohol-dependent humans. The patients in this study completed detoxification before treatment. Remaining to be determined are the effects of CRF1 receptor antagonists in individuals with AD that are currently drinkers and in alcohol-dependent patients during acute withdrawal
- 43.Rohn MCH, Lee MR, Kleuter SB, Schwandt ML, Falk DE, Leggio L. Phenotypic Differences between Treatment-Seeking and Nontreatment-Seeking Alcohol Dependent Research Participants: an Exploratory Analysis. Alcohol. 2016 doi: 10.1111/acer.13304. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorsell A, Slawecki CJ, Ehlers CL. Effects of neuropeptide Y and corticotropin-releasing factor on ethanol intake in Wistar rats: interaction with chronic ethanol exposure. Behav Brain Res. 2005;161:133–40. doi: 10.1016/j.bbr.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 45. Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. ** This preclinical and clinical study provided clear evidence of the potential of glucocorticoid receptor antagonists in the treatment of alcoholism. The study found that systemic and intra-central nucleus of the amygdala administration of the glucocorticoid receptor antagonist mifepristone reduced alcohol intake in alcohol-dependent rats but not in nondependent animals. In humans with alcohol dependence, individuals who received mifepristone exhibited a substantial reduction of alcohol-cued craving in the laboratory and reduced alcohol consumption during the 1-week treatment phase and 1-week post-treatment phase relative to placebo. This study is an example of translational success.
- 46.Repunte-Canonigo V, Shin W, Vendruscolo LF, Lefebvre C, van der Stap L, Kawamura T, et al. Identifying candidate drivers of alcohol dependence-induced excessive drinking by assembly and interrogation of brain-specific regulatory networks. Genome Biol. 2015;16:68. doi: 10.1186/s13059-015-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci Off J Soc Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanna PP, Kawamura T, Chen J, Koob GF, Roberts AJ, Vendruscolo LF, et al. 11β-hydroxysteroid dehydrogenase inhibition as a new potential therapeutic target for alcohol abuse. Transl Psychiatry. 2016;6:e760. doi: 10.1038/tp.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiol Behav. 2002;75:455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]