Abstract
Vitronectin has been identified mainly as an adhesion protein that signals through uPAR and selected integrin receptors. In addition to its pro-adhesive properties, we identified recently vitronectin as a main chemoattractant present in diluted plasma/serum that directly stimulates migration of cancer cells. We also found that this pro-migratory activity of vitronectin can be quenched by fibrinogen. Based on this we hypothesized that this may explain preference of cancer cell to metastasize to fibrinogen-low microenvironments such as lymphatics or peritoneal cavity. Based on this, we decided to investigate a role of vitronectin in metastasis of ovarian cancer cells to peritoneal cavity. We tested migratory responsiveness of three human ovarian cancer cell lines to ascites isolated from ovarian cancer patients and characterize possible molecules involved in migration of ovarian cancer cells. The ascites samples were exposed to heat inactivation, proteinase K digested, dialyzed and charcoal stripped. We also performed cut-off filtration analysis and by employing ELISA assays to measure concentration of vitronectin in ascites fluid samples. Finally, we employed shRNA against uPAR and small molecular inhibitors of integrin receptors to assess their involvement in biological effects of vitronectin. From our studies, we found that the similarly to diluted plasma, vitronectin in absence of fibrinogen is a main chemotactic/chemokinetic protein present in ascites fluid. We also found that these pro-migratory properties of vitronectin can be quenched by addition of fibrinogen. Our studies also indicate that both uPAR and integrin receptors on ovarian cancer cells regulate migration of these cells to vitronectin gradient. In summary, we identified free soluble vitronectin as a potent direct chemoattractant for ovarian cancer cells and that its activity is suppressed after binding to fibrinogen. Since in ascites fluids vitronectin is present in free form because of a lack or low level of fibrinogen, this could explain preferences of ovarian cancer stem cells to metastasize within peritoneum. We propose that inhibitors which could sequester soluble vitronectin in similar fashion as fibrinogen, could be employed as a novel anti-metastatic drugs.
Keywords: vitronectin, ovarian cancer, urokinase plasminogen activator receptor (uPAR), integrin receptors, fibrinogen, cancer metastasis, chemotaxis, chemokinesis
INTRODUCTION
Ovarian cancer has the highest mortality rate among all gynecological malignancies. The high mortality of this tumor is mainly a result of advance stage of cancer at the time of diagnosis, in the 75% of cases, and also is a result of its high metastatic properties [1, 2]. The ovarian carcinoma metastasizes either by continuous infiltration of neighboring organs or disseminate within the abdominal cavity via peritoneal fluid [1, 2]. Metastasis of advanced ovarian carcinoma is associated with the presence of ascites which contains heterogeneous population of cells including cancer cells and cancer stem cells. Interestingly, that unlike most other cancers, ovarian tumors rarely disseminates through the blood vessels [1].
The search for potential chemotactic/chemokinetic factors involved in metastasis identified several factors enhancing migration of cancer cells such as chemokines, cytokines, growth factors, complement cascade cleavage fragments, eicosanoids, bioactive lipids, and extracellular nucleotides [3–10]. However, the chemotactic activities of most of these factors for malignant cells have been demonstrated in vitro assays when they were employed at hyperphysiological concentrations as compared to their levels in biological fluids [3–5].
Recently, we identified soluble vitronectin as a main chemoattractant present in diluted human plasma/serum and intestinal fluid [11, 12]. We also found that this pro-migratory property of vitronectin is inhibited in presence of fibrinogen which could explain the preference of most tumors cells to migrate to lymphatics and body cavities where concentration of fibrinogen is low.
Based on this observation and taking into consideration that ovarian cancer preferentially spread within peritoneal cavity and well known fact that peritoneal mesothelium secrets vitronectin into peritoneal fluid [13], we became interested to define the role of vitronectin in migration of ovarian cancer cells. Vitronectin, has been so far identified as adhesion factor for these cells and its role in cell migration had been considered rather to be indirect one [13–16].
Herein we report that soluble free vitronectin induces migration of ovarian cancer cells and presumably cancer stem cells in ligand-receptor dependent manner. Moreover, its activity is suppressed after binding to fibrinogen. Since ascites fluid is usually poor in fibrinogen, vitronectin is present in free, unbound form which may explain preference of ovarian cancer cells and cancer stem cells to disseminate within the peritoneum [1]. We propose that inhibitors which could bind soluble vitronectin in similar fashion as fibrinogen, these inhibitors could be employed as a potent anti-metastatic drugs in cancer patients.
MATERIAL AND METHODS
Cell lines
The A2780 and OVCAR-4 cancer cell lines were maintained in RPMI 1640 medium, whereas CAOV 3 was maintained in DMEM. All media were supplemented with 10% (or 20% for OVCAR4) FBS, 100 U/ml penicillin, and 10 μg/ml streptomycin. Medium for OVCAR4 was additionally supplemented with 10 μg/ml insulin. Cells were cultured in a humidified atmosphere of 5% CO2 at 37°C, and the medium was changed every 48 hours. All cell line where authenticated by STR analysis.
Chemotaxis assay
Chemotaxis assays were performed as described [6–8]. In some experiments the cells were pretreated with the inhibitors: ATN161 (100–500 μM, Medkoo Biosciences, Chapel Hill, NC) for 30 min or RGD (1 – 100 μM, ApexBio Technology, Houston, TX) for 60 min at 37°C. Inhibitors were also added to the lower chambers and were present throughout the experiment.
Treatment of ascites fluid
Molecular-weight cut off (MWCO) fractions were obtained by centrifugation of ascites fluid in a Centricon Ultracel YM (Millipore, Billerica, MA) with different MWCOs (10, 30, 50, or 100 kDa) and the flow-through fractions were used for experiments. Dialyzed ascites fluid was obtained by dialysis of plasma samples against PBS (without calcium and magnesium, HyClone, Logan, UT) in a Slide-A-Lyzer dialysis cassette with a 3,500-Da MWCO (ThermoFisher Scientific, Waltham MA) and used for experiments. Ascites fluid with enzymatically destroyed proteins was obtained by a 4-h incubation of a 10% ascites fluid sample (in PBS) at RT with proteinase K at a final concentration of 6 mAU/ml. Next, 10 μl of plasma was analyzed on a polyacrylamide gel stained with Coomassie Brilliant Blue R to confirm complete digestion of protein. The potential negative effect of proteinase K itself on cells was limited by adding 1 mM phenylmethyl sulfonyl fluoride (PMSF) into the sample before chemotaxis assay. Heat inactivation of ascites sample was by incubation of ascites fluids at different temperature for 1 h. Charcoal stripped ascites fluid was obtained by overnight incubation of ascites fluid sample with charcoal (0.1 g of activated charcoal per 0.5 ml of ascites fluid)
Adhesion to fibronectin
Cells were made quiescent for 3 hours with 0.5% BSA in RPMI 1640 medium or DMEM before incubation with plasma, ascites fluid or vitronectin for 5 min. Subsequently, cell suspensions (5 × 103/50 μl) were applied to 96-well plates covered with fibronectin (10 μg/ml) and incubated at 37°C for 10 min. The wells were coated with fibronectin overnight at 4°C and blocked with 0.5% BSA for 2 hours before the experiment. After incubation, the plates were washed three times to remove non-adherent cells, and the number of adherent cells was counted under an inverted microscope.
Phosphorylation of intracellular pathway proteins
Western blots analysis to assess phosphorylation of MAPKp42/44 and AKT was performed as described [6–8].
Flow cytometry
Cells were detached using Cell Stripper (Corning), followed by a 2-h incubation in appropriate medium with 2% FBS. Cells were stained with a mouse primary antibody against human integrin receptor α5β1 (1:25, Millipore) for 30 min at 37°C. Cells were washed and incubated with an Alexa Fluor 488 goat anti-mouse secondary antibody (1:100, Life Technologies, Carlsbad, CA). A murine phycoerythrin (PE)-conjugated anti-human CD87 antibody (1:20; clone VIM5, Biolegend, San Diego, CA) was used to analyze the uPAR receptor, and murine FITC-conjugated anti-human CD51/61 antibody (1:20; clone 2366, Biolegend) was used for staining of the human αVβ3 integrin receptor. The cells were analyzed using an LSR cell cytometer (BD Biosciences, Franklin Lakes, NJ). Analysis of the data was performed using FlowJo 7.2.5 software (FLOWJO, Ashland, OR, USA). Unstained cells and cells incubated with isotype control antibodies were used as controls.
Knockdown of uPAR with short hairpin RNA
In RNA interference (RNAi) experiments, the short hairpin RNA (shRNA)-generating plasmid pSUPER.retro.puro (Oligoengine, Seattle, WA) was used. The targeting base sequence for human uPAR was as described [12]. As a control, shRNA against Renilla was used [17]. A2780 were electroporated with 125 V for 5 ms with shRNA vector using NEPA 21 (Nepa Gene, Portsmouth, NH). Selection of pSuper.retro.puro–expressing cells was conducted by exposure of in vitro cultures to puromycin at a final concentration of 0.5 μg/mL for 6 days.
Statistical analysis
All results are presented as mean ± SD. Statistical analysis of the data was performed using Student’s t-test for unpaired samples, with p ≤ 0.05 considered significant.
RESULTS
Ascites contains factor(s) that induce chemotactic/chemokinetic response of ovarian cancer cells
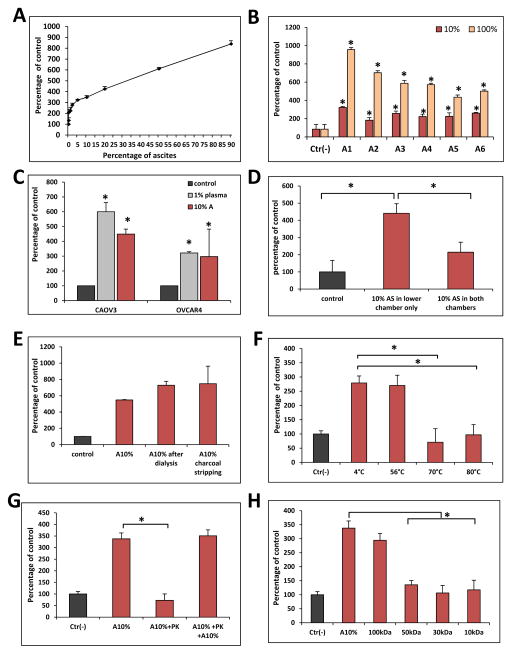
To better characterize pro-migratory properties of ovarian cancer ascites we analyzed response of A2780 cells to different dilutions of ascites and found that even highly diluted ascites fluid (x 20 diluted) stimulates migration of ovarian cancer cells in Transwell assays (Figure 1A). Moreover we observed this effect for all 6 patients’ derived ascites fluids (Figure 1B). Additionally, we also found that ascites fluid stimulates migration of not only of A2780 but also another two ovarian cancer cell lines (CAOV3, OVCAR4) (Figure 1C). Moreover, ovarian ascites possesses both chemotactic as well as chemokinetic activity since in the absence of gradient (ascites fluid added to upper and lower chamber of Transwells) inhibited only by ~50% migratory response of A2780 cells to lower chamber (Figure 1D).
Figure 1. Ovarian ascites fluid contains protein molecule/s that stimulates migration of ovarian cancer cells.
Panel A. Dose-dependent effect of human ovarian ascites fluid on the migration of A2780 cells. Panel B. Migration of A2780 cells across Transwell membranes to various human ovarian ascites samples (A1–A6). Experiment was performed twice with similar results. *p<0.05. Panels C. Migration of CAOV3 and OVCAR4 ovarian cancer cell lines to ovarian ascites fluid (A). Experiment was performed twice with similar results. *p<0.05. Panel D. Chemotaxis (ascites in lower chamber only) vs chemokinesis (ascites fluid in upper and lower chamber) analysis of response of A2780 cells to ascites. Experiment was performed twice with similar results. *p<0.05. Panel E. The migration of A2780 cells across Transwell membranes in response to 10% ovarian ascites fluid, 10% ovarian ascites fluid dialyzed over a 3.5-kDa-MWCO membrane or 10% charcoal stripped ovarian ascites fluid. Experiment was performed twice with similar results. *p<0.05. Panel F. The temperature-dependence of 10% ascites preincubation on A2780 cell migration. *p<0.05. Experiment was performed twice with similar results. *p<0.05. Panel G. The migration of A2780 cells in response to 10% ovarian ascites fluid (A) preincubated with proteinase K (PK). To confirm the complete inhibition of PK by PMSF throughout the chemotaxis, a rescue experiment was performed in which 10% fresh was added. Experiment was performed twice with similar results. *p<0.05. Panel H. The migration of A549 cells across Transwell membranes in response to different fractions obtained by centrifugation in Centricon filters with different MWCOs. Experiment was performed twice with similar results. *p<0.05.
Evidence that the migration-enhancing factor(s) present in diluted 1% plasma is a protein with molecular mass in the range 50–100 kDa
To characterize the molecule(s) in ascites responsible for enhanced ovarian cancer cell migration, we employed several complementary approaches. First, in order to eliminate small molecules, such as extracellular nucleotides that are endowed with chemotactic properties (e.g., ATP and UTP) we dialyzed ascites fluid through 3.5-kDa-MWCO membranes. We found that dialysis did not affect the chemotactic activity of ascites fluids which suggests that molecules smaller than 3.5 kDa are not involved in this process (Figure 1E). Next we charcoal stripped ascites fluid and removed potential pro-migratory bioactive lipids [6, 8]. Figure 1E shows that charcoal stripping, similar to dialysis did not inhibit pro-migratory activity of ascites fluid, which indicates that bioactive lipids are not responsible for observed pro-migratory response of ovarian cancer cells.
Next, to test if this potential pro-migratory factor has a peptide/protein structure we exposed ascites fluid to high temperature and observed a decrease in its chemotactic activity (Figure 1F). Next, to support this better we added to ascites fluid proteinase K which also reduced the pro-migratory activity of ascites samples (Figure 1G). As demonstrated in the same Figure the response of cells could be again restored by addition of fresh ascites fluid. Finally, molecular filtration studies revealed that this chemotactic/chemokinetic factor, most likely is a protein, with molecular weight in the range 50–100 kD (Figure 1H).
Fibrinogen acts as a natural inhibitor of ascites fluid activity
Our studies with diluted 1% plasma revealed a novel role for fibrinogen that act as chaperone of vitronectin in diluted plasma and serum [11, 12]. Therefore we decided to test whether we will observe similar inhibitory effect of fibrinogen on ascites fluid-induced migration of ovarian cancer cells. In fact Figure 2A demonstrates that fibrinogen inhibits pro-migratory properties of ascites. Next we analyzed inhibitory effect of normal plasma with plasma depleted from fibrinogen on migration of A2780 cells to vitronectin. Figure 2B shows that plasma that is enriched in fibrinogen inhibits pro-migratory effect of 10% ascites whereas plasma from which fibrinogen has been chemically extracted does not possess this inhibitory activity. This confirms our previous observation that fibrinogen acts as a natural inhibitor of protein/peptide-based pro-migratory factor that is present in plasma and intestines fluids [11, 12].
Figure 2. The stimulatory effects of human ovarian ascites fluid might be quenched by fibrinogen which suggest that vitronectin is a main chemoatractan/chemokinetic factor present in ovarian ascites fluids.
Panel A. The inhibitory effect of fibrinogen (FG) on the migration of A2780 cells in response to 10% human ovarian ascites fluid. Panel B. Migration of A2780 cell line to 10% ascites (A) is inhibited by 10% plasma (P) but not by 10% plasma without fibrinogen (P-Fb). Experiment was performed twice with similar results. *p<0.05. Panel C. ELISA analysis of vitronectin concentration in 6 ovarian ascites fluids samples. Panel D. Migration of A2780, CAOV3 and OVCAR4 ovarian cancer cell lines to vitronectin. Experiment was performed at least twice with similar results. *p<0.05 Panel E. Chemotaxis (vitronectin in lower chamber only) vs chemokinesis (vitronectin in upper and lower chamber) analysis of response of A2780 cells to vitronectin. Experiment was performed twice with similar results. *p<0.05. Panel FB. The adhesion of A2780 cells to fibronectin after stimulation with plasma, ascites and vitronectin for 5 min. Experiment was performed twice with similar results. *p<0.05.
Soluble vitronectin as a main chemoattractant/chemokinetic factor present in ascites fluids
Next, we asked if vitronectin is in fact a main chemoattractant/chemokinetic factor present in ascites. For this purpose, first we measure vitronectin concentration in 6 patients’ ascites and detected vitronectin on average ~200 μg/ml that corresponds to its concentrations in human plasma/serum (Figure 2C). In Transwell experiments, when vitronectin was added to the lower chamber in concentration corresponding to its concentration present 10% ascites (20 μg/ml) it turned out to be a chemoattractant for all three ovarian cancer cell lines employed in our studies (Figure 2D). Finally, based on results observed with ascites fluid in Figure 1D, we employed check-board assay to analyze whether vitronectin possess some chemokinetic activity, and found that vitronectin similarly as ascites fluid can stimulate migration of cells when added at the same time to the upper and lower chamber of Transwells (Figure 2E). This experiment demonstrated that vitronectin possesses both chemotactic as well chemokinetic activity. Finally, we also found that stimulation with diluted 1% plasma, ascites fluid as well as vitronectin increases adhesion of A2780 cells to fibronectin (Figure 2F).
Ascites fluid and vitronectin activate phosphorylation of p42/44 MAPK and AKT
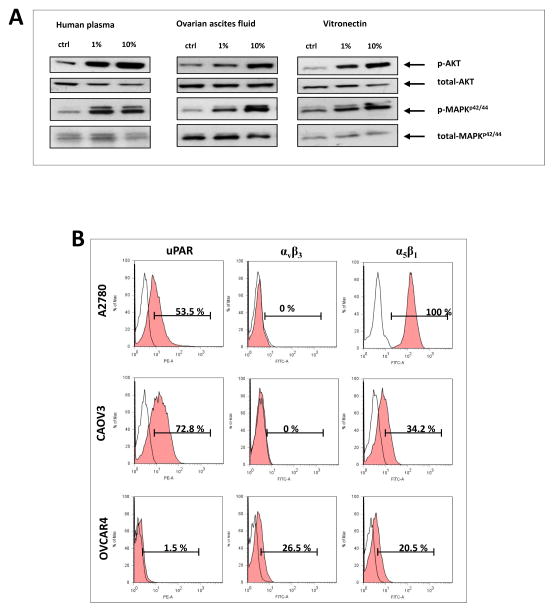
To check whether response of cells to ascites and vitronectin is receptor-dependent, we analyzed phosphorylation of p42/44 MAPK and AKT in A2780 cell line in response to stimulation by plasma, ascites fluid and fibronectin (Figure 3A). We observed an increased phosphorylation of both kinases.
Figure 3. Ovarian cancer cell lines express functional vitronectin receptor.
Panel A. Phosphorylation of p42/44 MAPK and AKT after stimulation of A2780 cells with human plasma, ovarian ascites and vitronectin. Experiment was performed twice with similar results. Panel B. Flow cytometry analysis of the expression of urokinase receptor (uPAR) and the two integrin receptors αvβ3 and α5β1 in different ovarian cancer cell lines. Flow cytometry analysis was performed at least twice for each of the cell lines.
Several receptors for vitronectin including urokinase (uPAR) and integrin α5β1 and αvβ3 have been reported [18–21]. Our flow cytometry analysis (Figure 3B) indicates that uPAR and α5β1 receptors were detectable in all tested cell lines whereas αvβ3 integrin receptor was expressed only by one out of three cell lines. Moreover, vitronectin receptors on cancer cells seem to be functional, since stimulation of cell lines with vitronectin induced p42/44 MAPK and AKT phosphorylation (Figure 3A).
uPAR and integrin receptors are involved in migration of ovarian cancer cells to vitronectin gradient
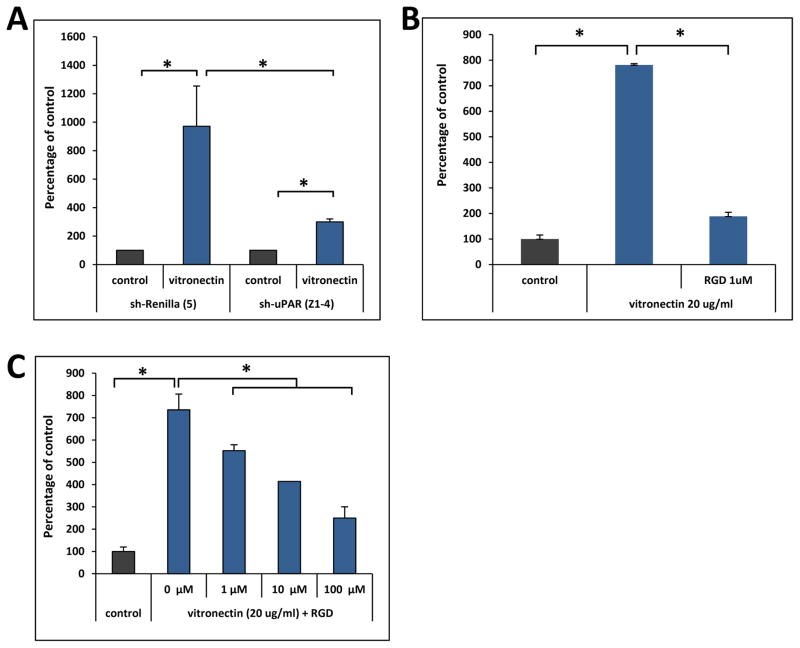
As mentioned above both uPAR and selected integrin are main receptors for vitronectin. Therefore, we tested which of the vitronectin receptor/s play a role in ovarian cancer cell migration. To address this, first we downregulated expression of uPAR receptor in A2780 cells by employing plasmid encoding shRNA and found ~70% inhibition of mRNA expression for uPAR (data not shown). This reduction somehow corresponded to ~70% inhibition in migratory response of A2780 cells to vitronectin (Figure 4A). Interestingly, we could also detect some decrease in migration of A2780 cells after pre-treatment of these cells with integrin blocking peptide RGD (Figure 4B). This suggests existence of cooperation between uPAR and integrin receptors in modulation of ovarian cancer cells responsiveness to vitronectin gradient. To support this further, as reported in Figure 4C, in CAOV3, a cell line in which we detect very low uPAR receptor but at the same time these cells expressed both analyzed integrin receptors, pre-treatment of these cells by RGD inhibited migration to vitronectin.
Figure 4. Effect of vitronectin receptor inhibition on migration of ovarian cancer cells.
Panel A. Migration of A2780 cells stable transfected cells with control (shRenilla-R5) or shRNA against uPAR (shuPAR-Z4) to vitronectin. Experiment was performed three times with similar results. *p < 0.05. Panel B. Effect of RGD pre-treatment on migration of A2780 cells to vitronectin. Experiment was performed twice with similar results. *p < 0.05. Panel C. Effect of RGD pre-treatment on migration of CAOV3 cells to vitronectin. Experiment was performed three times with similar results *p0.05.
DISCUSSION
One of the major problems in cancer therapy is ability of cancer cells to leave primary tumor and metastasize to distant tissue/organ where they establish secondary tumors [22]. In contrast to most hematogenously metastasizing tumors, ovarian cancer cells primarily disseminate within the peritoneal cavity and this process is facilitated in the presence of ovarian ascites fluid [1]. Several factors have been reported to be responsible for migration of ovarian cancer cells including chemokines [23], growth factors [24] and bioactive lipids [25]. Nevertheless, in in vitro migration assays have been employed usually at very high concentrations that are not encountered in ascites.
Therefore, we asked what factor/s present in ovarian ascites can be responsible for ovarian cancer cells migration. Based on our previous work with diluted 1% plasma which identified soluble vitronectin as main chemoattractant present in fibrinogen poor fluids [11, 12] and hypothesized that vitronectin may be a main migration-promoting factor in fibrinogen poor or even free ovarian ascites. To support this, we found that similar to diluted plasma/serum, the main chemoattractant present in ascites fluids is heat inactivation and protein K digest sensitive protein within 50–100 kDa range and that the pro-migratory activity of this protein could be inhibited by fibrinogen. Based on the molecular size and physical properties we focused on free vitronectin that is abundant in ascites from patients with ovarian cancer. It is well known that vitronectin could be produced by mesothelial cell lining peritoneal cavity [13] as well as secreted by tumor ovarian cancer cells themselves [26].
Vitronectin is a glycoprotein and is detectable at high level in peripheral blood, extracellular matrix, and bone [27]. Ovarian cancer ascites fluid was found to be enriched in vitronectin as compared to ascites fluid isolated from non-oncologic individuals which lead to the suggestion that vitronectin could serve as potential marker for ovarian cancer [28]. Our analysis indeed confirmed that in ascites fluids concentration of vitronectin is relatively high (~200 μg/ml) similar to its concentration in normal human plasma [29, 30].
Molecular analysis of the vitronectin structure revealed that it contains three structural domains: i) an N-terminal somatomedin B domain, which binds to plasminogen activator inhibitor 1 (PAI-1); ii) a central domain containing hemopexin homology; and iii) a C-terminal domain that also has hemopexin homology [31, 32]. Vitronectin also contains an RGD sequence that binds to α5β1 and αvβ3 integrin receptors and through somatomedin B domain it can bind to urokinase receptor (uPAR, also known as CD87) [33, 34]. Interestingly, despite high homology between vitronectin and hemopexin, we did not observed any stimulatory effect on ovarian carcinoma cell migration when hemopexin was added to the lower chamber (data not shown). An important question remains which of the domains of vitronectin molecule binds to fibrinogen? This interaction has been reported in the past by demonstrating that it is difficult to purify from plasma fibrinogen that is not “contaminated” by vitronectin [35].
Vitronectin for a long time has been considered as a factor that only in indirect manner may affect cell migration being a component of intercellular matrix that together with fibronectin plays a role in cell adhesion by interacting with integrin receptors on cell surface [18, 33]. Vitronectin also binds activator inhibitor-1 (PAI-1), extends its lifetime and through active PAI-1 controls hemostasis by inhibiting fibrinolysis and affects angiogenesis. Since this interaction blocks binding site for integrin receptors it has been postulated that PAI-1-vitronectin binding can negatively regulate adhesion of cells and thus also play a role in regulation of cell migration [36]. Binding of vitronectin to uPAR can also as postulated mediate cell adhesion to vitronectin by triggering a novel type of integrin signaling that is independent of integrin-matrix engagement [37]. This signaling is active for vitronectin mutants deficient in integrin binding site and it is also efficiently executed by integrins deficient in ligand binding [37]. Moreover it was also found that that uPAR can form complexes with α5β1 and αvβ3 integrin receptors which suggest that they cooperate in regulation of cell adhesion and migration [38, 39]. Indeed, in our studies we found that by simultaneous inhibition of uPAR as well as integrin receptors activity we can significantly reduce migration of ovarian carcinoma cells.
The first step in the metastatic process is egress of cancer cells from the primary tumor site into the interstitial fluid, which requires detachment of cells from mass and then stimulation of their migration [22]. In both these steps vitronectin plays important role as it directly stimulates cell migration and egress form the tumor. From the practical point of view, the inhibition of vitronectin-receptors on cancer cells on one hand inhibits cell migration, but on other increases detachment of cells from primary mass. Indeed, use of αvβ3 receptor inhibitors resulted in increased metastatic potential of ovarian cancer cells. Therefore, based on our data we envision that in order to decrease motility of cancer cells without impairing their adhesion and promoting detachment from primary tumor, it would be more important to develop in the future vitronectin small molecule chaperoning compounds that will mimic action of fibrinogen. For this, further studies are needed to identify fragments in vitronectin and fibrinogen that interact with each other.
In conclusion, we demonstrate herein for first time that free soluble vitronectin is a potent direct chemoattractant for ovarian cancer cells and that its activity is suppressed after binding to fibrinogen. Because of absence of fibrinogen, in ascites fluid, vitronectin is present in free and unbound form which can explain preferences of ovarian cancer cells to metastasize to peritoneum. In addition to the presence of cancer cells in ascites, existence of cancer stem cells with various markers have been reported in human ascites from patients with ovarian cancer [40–51]. These cells are reported to be chemo-resistance and cause of metastasis and recurrence of cancer [42, 52–54]. Therefore it is safe to conclude that vitronectin present in ascites that helps in migration and invasion of cancer cells is also responsible for migration and metastasis of cancer stem cells to peritoneum as well as other organs. Finally, we propose that inhibitors which would bind/chaperone soluble vitronectin in similar fashion as fibrinogen could be employed as a part of anti-metastatic treatment in patients with ovarian cancer.
Acknowledgments
We thank Dr. Buchsbaum at the University of Alabama for supplying us with human ascites samples. This work was supported by NIH grants 2R01 DK074720 and R01HL112788, the Stella and Henry Endowment, and the Harmonia NCN grant UMO-2014/14/M/NZ3/00475 to MZR.
Footnotes
Conflict of interest
The authors declare that they have no competing financial interests.
References
- 1.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;25:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, Houghton P, Janowska-Wieczorek A, Ratajczak MZ. Both hepatocyte growth factor (HGF) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only HGF enhances their resistance to radiochemotherapy. Cancer Res. 2003;63:7926–35. [PubMed] [Google Scholar]
- 4.Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597–06. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 5.Grymula K, Tarnowski M, Wysoczynski M, Drukala J, Barr FG, Ratajczak J, Kucia M, Ratajczak MZ. Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas. Int J Cancer. 2010;127(11):2554–68. doi: 10.1002/ijc.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider G, Bryndza E, Abdel-Latif A, Ratajczak J, Maj M, Tarnowski M, Klyachkin YM, Houghton P, Morris AJ, Vater A, Klussmann S, Kucia M, Ratajczak MZ. Bioactive lipids S1P and C1P are prometastatic factors in human rhabdomyosarcoma, and their tissue levels increase in response to radio/chemotherapy. Mol Cancer Res. 2013;11:793–07. doi: 10.1158/1541-7786.MCR-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider G, Glaser T, Lameu C, Abdelbaset-Ismail A, Sellers ZP, Moniuszko M, Ulrich H, Ratajczak MZ. Extracellular nucleotides as novel, underappreciated pro-metastatic factors that stimulate purinergic signaling in human lung cancer cells. Mol Cancer. 2015;14:201. doi: 10.1186/s12943-015-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider G, Sellers ZP, Abdel-Latif A, Morris AJ, Ratajczak MZ. Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy. Mol Cancer Res. 2014;12:1560–73. doi: 10.1158/1541-7786.MCR-14-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182(6):3827–36. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi H, Aharonovitz O, Alexander RT, Touret N, Furuya W, Orlowski J, Grinstein S. Na+/H+ exchange and pH regulation in the control of neutrophil chemokinesis and chemotaxis. Am J Physiol Cell Physiol. 2008;294:C526–34. doi: 10.1152/ajpcell.00219.2007. [DOI] [PubMed] [Google Scholar]
- 11.Schneider G, Garbett NC, Bryndza E, Poniewierska-Baran A, Merchant ML, Serwin K, Sellers ZP, Dolegowska B, Ratajczak MZ. Abstract 1698: Novel evidence that blood plasma vitronectin is a major chemoattractant for cancer cells and its pro-migratory activity is suppressed/chaperoned after binding to fibrinogen Proceedings. AACR 107th Annual Meeting 2016; April 16–20, 2016; New Orleans, LA. [Google Scholar]
- 12.Schneider G, Bryndza E, Poniewierska-Baran A, Serwin K, Suszynska M, Sellers ZP, Merchant ML, Kaliappan A, Ratajczak J, Kucia M, Garbett NC, Ratajczak MZ. Evidence that vitronectin is a potent migration-enhancing factor for cancer cells chaperoned by fibrinogen—a novel view of the metastasis of cancer cells to low-fibrinogen lymphatics and body cavities; submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyman L, Leroy-Dudal J, Fernandes J, Seyer D, Dutoit S, Carreiras F. Mesothelial vitronectin stimulates migration of ovarian cancer cells. Cell Biol Int. 2010;34:493–02. doi: 10.1042/CBI20090331. [DOI] [PubMed] [Google Scholar]
- 14.Carduner L, Agniel R, Kellouche S, Picot CR, Blanc-Fournier C, Leroy-Dudal J, Carreiras F. Ovarian cancer ascites-derived vitronectin and fibronectin: combined purification, molecular features and effects on cell response. Biochim Biophys Acta. 2013;1830:4885–97. doi: 10.1016/j.bbagen.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Heyman L, Kellouche S, Fernandes J, Dutoit S, Poulain L, Carreiras F. Vitronectin and its receptors partly mediate adhesion of ovarian cancer cells to peritoneal mesothelium in vitro. Tumour Biol. 2008;29:231–44. doi: 10.1159/000152941. [DOI] [PubMed] [Google Scholar]
- 15.Leroy-Dudal J, Heyman L, Gauduchon P, Carreiras F. Adhesion of human ovarian adenocarcinoma IGROV1 cells to endothelial cells is partly mediated by the alphav integrins-vitronectin adhesive system and induces an alteration of endothelial integrity. Cell Biol Int. 2005;29:482–88. doi: 10.1016/j.cellbi.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Gomes C, Smith SC, Youssef MN, Zheng JJ, Hagg T, Hetman M. RNA polymerase 1-driven transcription as a mediator of BDNF-induced neurite outgrowth. J Biol Chem. 2011;286:4357–63. doi: 10.1074/jbc.M110.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heyman L, Kellouche S, Fernandes J, Dutoit S, Poulain L, Carreiras F. Vitronectin and its receptors partly mediate adhesion of ovarian cancer cells to peritoneal mesothelium in vitro. Tumour Biol. 2008;29:231–44. doi: 10.1159/000152941. [DOI] [PubMed] [Google Scholar]
- 18.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–25. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 20.Pijuan-Thompson V, Gladson CL. Ligation of integrin alpha5beta1 is required for internalization of vitronectin by integrin alphavbeta3. J Biol Chem. 1997;272(5):2736–43. doi: 10.1074/jbc.272.5.2736. [DOI] [PubMed] [Google Scholar]
- 21.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signaling. Eur J Cell Biol. 2008;87:617–29. doi: 10.1016/j.ejcb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. Madame Curie Bioscience Database [Internet] Austin (TX): Landes Bioscience; 2000–2013. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. [Google Scholar]
- 23.Guo Q, Gao BL, Zhang XJ, Liu GC, Xu F, Fan QY, Zhang SJ, Yang B, Wu XH. CXCL12-CXCR4 Axis Promotes Proliferation, Migration, Invasion, and Metastasis of Ovarian Cancer. Oncol Res. 2014;22:247–58. doi: 10.3727/096504015X14343704124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henic E, Sixt M, Hansson S, Høyer-Hansen G, Casslén B. EGF-stimulated migration in ovarian cancer cells is associated with decreased internalization, increased surface expression, and increased shedding of the urokinase plasminogen activator receptor. Gynecol Oncol. 2006;101:28–39. doi: 10.1016/j.ygyno.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Park KS, Kim MK, Lee HY, Kim SD, Lee SY, Kim JM, Ryu SH, Bae YS. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356:239–44. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 26.Carreiras F, Cruet S, Staedel C, Sichel F, Gauduchon P. Human ovarian adenocarcinoma cells synthesize vitronectin and use It to organize their adhesion. Gynecol Oncol. 1999;72:312–22. doi: 10.1006/gyno.1998.5262. [DOI] [PubMed] [Google Scholar]
- 27.Boron WF, Boulpaep EL. Medical Physiology. Updated Edition Grune & Stratton Inc; 2012. [Google Scholar]
- 28.Bery A, Leung F, Smith CR, Diamandis EP, Kulasingam V. Deciphering the ovarian cancer ascites fluid peptidome. Clin Proteomics. 2014;11:13. doi: 10.1186/1559-0275-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamada S, Suou T, Kawasaki H. Plasma vitronectin concentrations in patients with chronic hepatitis C treated with interferon alpha. Clin Chim Acta. 1997;261:81–90. doi: 10.1016/s0009-8981(96)06503-5. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi J, Yamada S, Kawasaki H. Distribution of vitronectin in plasma and liver tissue: relationship to chronic liver disease. Hepatology. 1994;20:1412–17. doi: 10.1002/hep.1840200606. [DOI] [PubMed] [Google Scholar]
- 31.Zhou A. Functional structure of the somatomedin B domain of vitronectin. Protein Sci. 2007;16:1502–08. doi: 10.1110/ps.072819107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanley KK. Homology with hemopexin suggests a possible scavenging function for S-protein/vitronectin. FEBS Lett. 1986;199:249–53. doi: 10.1016/0014-5793(86)80489-6. [DOI] [PubMed] [Google Scholar]
- 33.Ständker L, Enger A, Schulz-Knappe P, Wohn KD, Germer M, Raida M, Forssmann WG, Preissner KT. Structural and functional characterization of vitronectin-derived RGD-containing peptides from human hemofiltrate. Eur J Biochem. 1996;241(2):557–63. doi: 10.1111/j.1432-1033.1996.00557.x. [DOI] [PubMed] [Google Scholar]
- 34.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol. 2007;177:927–39. doi: 10.1083/jcb.200612058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podor TJ, Campbell S, Chindemi P, Foulon DM, Farrell DH, Walton PD, Weitz JI, Peterson CB. Incorporation of vitronectin into fibrin clots. Evidence for a binding interaction between vitronectin and gamma A/gamma’ fibrinogen. J Biol Chem. 2002;277:7520–28. doi: 10.1074/jbc.M109677200. [DOI] [PubMed] [Google Scholar]
- 36.Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10(7):541–4. doi: 10.1038/nsb943. [DOI] [PubMed] [Google Scholar]
- 37.Ferraris GM, Schulte C, Buttiglione V, De Lorenzi V, Piontini A, Galluzzi M, Podestà A, Madsen CD, Sidenius N. The interaction between uPAR and vitronectin triggers ligand-independent adhesion signaling by integrins. EMBO J. 2014;33:2458–72. doi: 10.15252/embj.201387611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276:3983–90. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- 39.Tarui T, Andronicos N, Czekay RP, Mazar AP, Bdeir K, Parry GC, Kuo A, Loskutoff DJ, Cines DB, Takada Y. Critical role of integrin alpha 5 beta 1 in urokinase (uPA)/urokinase receptor (uPAR, CD87) signaling. J Biol Chem. 2003;278:29863–72. doi: 10.1074/jbc.M304694200. [DOI] [PubMed] [Google Scholar]
- 40.Tomao F, Papa A, Strudel M, Rossi L, Lo Russo G, Benedetti Panici P, Ciabatta FR, Tomao S. Investigating Molecular Profiles of Ovarian Cancer: An Update on Cancer Stem Cells. J Cancer. 2014;5:301–10. doi: 10.7150/jca.8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed N, Abubaker K, Findlay J, Quinn M. Epithelial mesenchymal transition and cancer stem cell-like phenotypes facilitate chemoresistance in recurrent ovarian cancer. Curr Cancer Drug Targets. 2010;10:268–78. doi: 10.2174/156800910791190175. [DOI] [PubMed] [Google Scholar]
- 42.Latifi A1, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA, Findlay JK, Ahmed N. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–29. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 44.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramdass B, Duggal R, Minev B, Chowdhary A, Koka P. Functional role of solid tumor stem cells in disease etiology and susceptibility to therapeutic interventions. J Stem Cells. 2013;8:189–31. [PubMed] [Google Scholar]
- 46.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;7:2405–13. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–80. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landen CN, Jr, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Jr, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther 2010. 2010;9:3186–99. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X, Xiong L, Sun T, Peng R, Zou L, Zhu H, Zhang J, Li H, Zhao J. Expression features of SOX9 associate with tumor progression and poor prognosis of hepatocellular carcinoma. Diagn Pathol. 2012;7:44. doi: 10.1186/1746-1596-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surowiak P, Materna V, Maciejczyk A, Kaplenko I, Spaczynski M, Dietel M, Lage H, Zabel M. CD46 expression is indicative of shorter revival-free survival for ovarian cancer patients. Anticancer Res 2006. 2006;26:4943–48. [PubMed] [Google Scholar]
- 52.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18:869–81. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103(30):11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vathipadiekal V1, Saxena D, Mok SC, Hauschka PV, Ozbun L, Birrer MJ. Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One. 2012;7:e29079. doi: 10.1371/journal.pone.0029079. [DOI] [PMC free article] [PubMed] [Google Scholar]