Abstract
Despite deep brain stimulation’s positive early results in psychiatric disorders, well-designed clinical trials have yielded inconsistent clinical outcomes. One path to more reliable benefit is closed-loop therapy: stimulation that is automatically adjusted by a device or algorithm in response to changes in the patient’s electrical brain activity. These interventions may provide more precise and patient-specific treatments. In this article, we first introduce the available closed-loop neuromodulation platforms, which have shown clinical efficacy in epilepsy and strong early results in movement disorders. We discuss the strengths and limitations of these devices in the context of psychiatric illness. We then describe emerging technologies to address these limitations, including pre-clinical developments such as wireless deep neurostimulation and genetically targeted neuromodulation. Finally, we discuss ongoing challenges and limitations for closed-loop psychiatric brain stimulation development, most notably the difficulty of identifying meaningful biomarkers for titration. We consider this in the recently-released Research Domain Criteria (RDoC) framework and describe how neuromodulation and RDoC are jointly very well suited to address the problem of treatment-resistant illness.
Keywords: deep brain stimulation, neuromodulation, bioengineering, electrophysiology, transdiagnostic, biomarker
What is a closed-loop neurostimulator?
“Closed loop” refers to any system containing feedback – the ability to sense whether stimulation is having a desired effect, and to adjust stimulation in response to that sensing. By contrast, the psychiatric brain stimulation modalities tested to date in humans are “open loop” – they deliver energy, but the only readout is in the slow clinical response (or lack thereof). Most steps towards closed-loop systems for psychiatric disorders have focused on invasive electrophysiologic systems – implantable devices that record electrical activity from one or more brain region(s) and deliver electrical stimulation to the same or different brain regions (Figure 1). The detected brain signals from one or more brain regions would be processed by a controller using a real-time algorithm, and the electrical stimulation titrated based on the recorded signals. A simpler version of such a device might be “human in the loop”, where the device does not automatically self-adjust, but clinicians can observe the recorded brain signals and provide manual adjustment of the stimulation (Widge, Arulpragasam, Deckersbach, & Dougherty, 2015). Closed-loop stimulation technology has shown promise as a treatment for various diseases. For example, pain relief can be improved by sensing the patients’ body activity and position to adjust spinal cord electrical stimulation using a closed-loop system (The RestoreSensor System, Medtronic, Minneapolis, MN, USA) (Schade, Schultz, Tamayo, Iyer, & Panken, 2011; Schultz et al., 2012). In epilepsy, closed-loop responsive cortical stimulation treatment has shown >40% seizure reduction compared to baseline in a blinded clinical study (NeuroPace, Mountain View, CA, USA)(Heck et al., 2014; Morrell & Halpern, 2016). In Parkinson’s disease, closed-loop electrical stimulation has shown improvements in motor response compared to open-loop, continuous stimulation in both primates and humans (Little et al., 2013, 2014; Rosin et al., 2011). This has been demonstrated in acute settings with brief stimulation, but implanted trials are being planned.
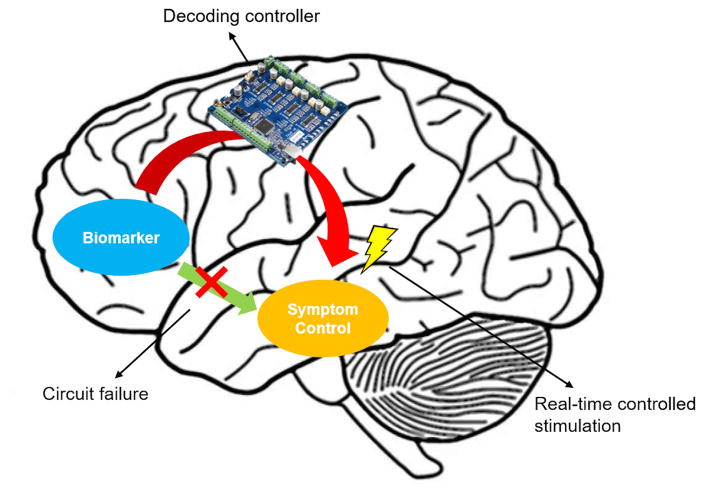
Figure 1.
Schematic of one possible closed-loop stimulator realization. The controller system senses (“decodes”) electrophysiological biomarkers from a brain region that is associated with disease symptoms. It delivers electrical stimulation to a deep brain structure based on the real-time algorithm.
The need for closed-loop systems in psychiatry
Given that most other closed-loop successes have involved implanted devices, deep brain stimulation (DBS) may be the most promising option for a closed-loop psychiatric therapy. Open-loop DBS, in open-label studies, has caused substantial improvements in patients’ symptoms (Malone et al., 2009; Mayberg et al., 2005; Nuttin, Cosyns, Demeulemeester, Gybels, & Meyerson, 1999). Two targets in particular are very well studied: the “ventral capsule/ ventral striatum” (VC/VS) (Greenberg et al., 2010; Okun et al., 2007) and subgenual cingulate gyrus (Cg25) (Lozano et al., 2008, 2012; Mayberg et al., 2005; McNeely, Mayberg, Lozano, & Kennedy, 2008). DBS was first tested for refractory obsessive-compulsive disorder (OCD) at the anterior limb of internal capsule, which evolved into VC/VS as the target itself shifted more posteriorly (Greenberg et al., 2010; Nuttin et al., 1999). Early results targeting VC/VS were promising. In an open-label clinical trial with 26 cases, the mean improvement in Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score was 38% (from 34 to 21) and clinical response was observed in 72% of the patients (Greenberg et al., 2010). Comorbid depression also improved, with a mean drop of 43% in the Montgomery-Asberg Depression Rating Scale (MADRS). 50% of patients met depression remission criteria. That study further motivated other groups to implement DBS for major depressive disorder (MDD). Open-label clinical results were positive with response and remission rates of 53% and 40% respectively (Bewernick et al., 2010; Bewernick, Kayser, Sturm, & Schlaepfer, 2012). On the other hand, Cg25 was selected as a possible target for psychiatric DBS based on years of neuro-imaging studies (Mayberg et al., 2005). The initial open-label clinical study was also positive, with a 43% remission rate after 6 years of patient follow-up (Kennedy et al., 2011; Lozano et al., 2012; Mayberg et al., 2009).
Despite promising open-label clinical evaluations, recent randomized clinical trials showed inconsistent therapeutic outcomes. A well-controlled, randomized clinical trial of DBS targeting VC/VS for MDD (RECLAIM) was terminated early after negative results from the initial cohort of 30 patients (Dougherty et al., 2015). Although not yet formally published, the BROADEN randomized trial at the Cg25 is known to have met a similar fate (Morishita, Fayad, Higuchi, Nestor, & Foote, 2014). Most recently, Bergfeld et al conducted a randomized clinical trial of DBS targeting VC/VS with 25 treatment-resistant patients (Bergfeld et al., 2016). This trial differed from BROADEN and RECLAIM in that it used an open-label, extended optimization, followed by a randomized DBS discontinuation experiment after the patients reached their stable clinical response. Patients who received active DBS during the randomized crossover phase scored significantly lower on the Hamilton Depression Rating Scale (HAM-D-17) (13.6) than patients who received sham DBS (23.1). Similarly, a study using VC/VS for OCD found separation between active and sham DBS using a blinded cross-over discontinuation design, with a median difference of 12 Y-BOCS points (Luyten, Hendrickx, Raymaekers, Gabriëls, & Nuttin, 2016). The sensitivity of trial outcomes to design highlights the difficulty of successfully programming open-loop DBS with current clinical decision rules. It also highlights the inconsistency of response -- 60% of the patients in the Bergfeld MDD trial (15/25) and 33% (8/24) in the Luyten OCD trial were non-responders. These inconsistent results across various facilities and studies raise concerns about the wider use of open-loop DBS for psychiatric disorders. Outcomes with less skilled/experienced clinicians will likely be worse. As a result, the only invasive brain stimulation treatment available today in the U.S. market is the open-loop DBS for OCD patients. The treatment holds a Humanitarian Device Exemption from the Food and Drug Administration (FDA), because very few patients meet criteria for the therapy (Garnaat et al., 2014).
The inconsistent results cannot fully be explained by placebo effects, given the two blinded studies. Subtle misplacement of the electrodes has been suggested to play a role (Makris et al., 2015; Riva-Posse et al., 2014). One of the biggest difficulties, however, is a lack of target engagement. DBS dosages are pre-programmed by the physician and adjusted only during infrequent clinical visits, often months apart. For VC/VS and MFB, DBS is titrated by an interviewing process, based on patients’ subjective response to specific stimulation dosages. For studies targeting Cg25, practices vary, but the most experienced group does not adjust the dose or location at all once stimulation starts. In either scenario, there are no physiological measurements or feedback from the brain to verify that the alleged target circuits are being stimulated or changed (Widge & Dougherty, 2015b). This is concerning, given that acute changes in mood do not correlate well to long-term DBS outcomes (Haq et al., 2011; Widge & Dougherty, 2015a; Widge, Licon, et al., 2016).
Closed-loop systems offer a resolution for this programming challenge. Even in treatment-resistant patients with long-lasting illness episodes, the symptom level can vary dramatically within a day. Therapeutic effects might therefore be achieved by tightly matching DBS delivery to symptom changes. Biomarker-based stimulation should more directly address patients’ clinical needs, increasing their perceived quality of life. Finally, closed-loop systems could prevent “over-treating”. Overly high stimulation dosages can cause adverse side effects such as hypomania and impulsivity (Greenberg et al., 2010; Haq et al., 2010; Widge, Licon, et al., 2016). Patients have expressed a strong interest in closed-loop therapies for exactly these reasons. Klein et al. (Klein et al., 2016) interviewed participants in DBS clinical trials for depression and OCD on their perspectives towards closed-loop systems. Patients reported a general optimism about closed-loop DBS. They were very interested in the possibility of reducing the trial-and-error aspects of existing DBS, and felt that this was one of the most frustrating aspects of open-loop systems. They also recognized that closed-loop systems, with their capacity for continuous brain recording, could provide a more thorough understanding of how neuromodulation works within the brain. The main challenges now facing the field are the need for DBS devices capable of closed-loop operation and a better understanding of the biomarkers that might guide stimulation.
Available and Emerging Platforms for Closed-Loop Psychiatric DBS
There are two robust hardware platforms currently available that could support closed-loop DBS for psychiatric disorders: Activa PC+S (Medtronic, Minneapolis, MN, USA) (Stanslaski et al., 2012) and the RNS system (NeuroPace, Mountain View, CA, USA) (Morrell, 2011; Morrell et al., 2014). Activa PC+S technology is based on the Activa PC open-loop neurostimulator. The underlying PC (Primary Cell) technology has been approved by FDA and received the CE mark for treatment of Parkinson’s disease (PD) and essential tremor (ET). Activa PC+S builds on the PC technology by adding a sensing (+S) component. By default, the recording and stimulating systems are partitioned, such that closed-loop therapies require special firmware available only under an investigational license. Activa PC+S has been used to study both PD and ET in humans and macaques (Herron, Denison, & Chizeck, 2015; Houston, Blumenfeld, Quinn, Brontë-Stewart, & Chizeck, 2015; Khanna et al., 2015; Quinn et al., 2015; Ryapolova-Webb et al., 2014; Swann et al., 2016). With a laptop computer in the loop to provide ex vivo closed-loop control, it has shown some efficacy in PD tremor control (Malekmohammadi et al., 2016).
The most attractive advantage of Activa PC+S is its ability to stimulate and record on the same lead, often by delivering monopolar stimulation and recording from the “flanking” contacts (Quinn et al., 2015). PC+S is designed to address signal contamination from the stimulating artifact from the concurrent sensing and stimulating system design. The high amplitudes (5–10 Volts) of typical DBS are several orders of magnitude higher than the neural signals of interest. It is therefore difficult to extract the signals of interest, in both time and frequency domain during neurostimulation, which is exactly what most investigators need. Activa PC+S addressed the challenge systematically with its hardware and software designs (Stanslaski et al., 2009, 2012). First, the device is designed to sense differentially and symmetrically about the stimulating electrodes. The stimulation artifact can then be rejected as a common mode disturbance to minimize sensing signal contamination (Figure 2) (Stanslaski et al., 2009, 2012). Second, the system implements front-end filtering before the signals enter the active circuitry. DBS typically stimulates at high frequency (e.g. 130 Hz). Therefore, a low-pass filter further suppresses the stimulation amplitude to ensure the normal operation range of the input amplifier. Finally, the system has undergone extensive benchtop analysis by its manufacturer. For each stimulation regime, there are known sensing parameters (filtering band, chopping frequency, ADC sampling frequency, etc.) that optimize separation of the stimulation artifacts from the sensed signals or biomarkers.
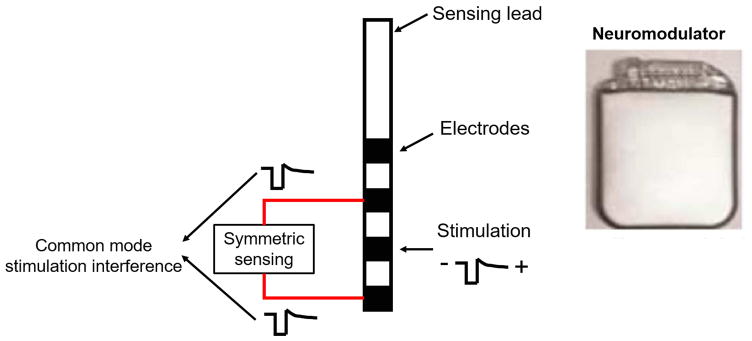
Figure 2.
The Activa PC+S electrode sensing configuration. (Left) The sensing electrodes are placed symmetrically about the stimulation electrode. The stimulation interference signals are then recorded as identical signal components (common mode signals) to the amplifier and can be rejected as a common mode disturbance. (Right) The neuromodulation stimulator case is implanted within the patient, enabling monopolar stimulation.
The PC+S system has been evaluated using a large animal (ovine) model with the lead implanted in the hippocampus and thalamus for responsive epilepsy stimulation (Stypulkowski, Stanslaski, Denison, & Giftakis, 2013). The device was able to record local field potentials (LFP) from the hippocampus during thalamic stimulation, and lasted for >1 year with consistent recording capability. This study demonstrated the feasibility, safety and durability of the closed-loop PC+S system. Similarly long recording life has been demonstrated in non-human primate, with recordings from both cortex and muscle (Ryapolova-Webb et al., 2014). Importantly, 2 years is the usual battery life of a regular Activa PC implant for psychiatric DBS, implying that PC+S can provide recording and possibly closed-loop therapy over the same duration and would not require more frequent surgery than the existing technology.
The Activa PC+S system has a CE mark (indicating safety for human use), but has no demonstrated efficacy in any medical condition at this point. It is available in the US, but only as an investigational device for physician-initiated and FDA-reviewed studies. There have been multiple implants in patients with Parkinson disease, with some early findings concerning biomarkers for closed-loop PD therapies (Gmel et al., 2015; Quinn et al., 2015; Swann et al., 2016). Anecdotally, implants of PC+S are ongoing in OCD and MDD patients at both the VC/VS and Cg25 targets. The results of these studies are not yet formally reported, but early conference presentations suggest that changes in the frequency spectrum of the local field potential at either target might be correlated with long-term mood improvement.
The NeuroPace Responsive Neurostimulation System (RNS) is approved by the FDA as a treatment to reduce seizures for patients who are over 18 years old (Sun & Morrell, 2014). The system uses electrocorticographic (EcoG) patterns as a biomarker to trigger brief bursts of brain stimulation. It includes an implantable neurostimulator in the cranium for control, a sensing lead, and a stimulation lead (Figure 3). Either lead may be a cortical strip lead or DBS-like depth lead, depending on the location of the patient’s seizure focus. The system has 4 electrodes per cortical strip lead and the depth lead. The neurostimulator continuously monitors brain activity, records signal surrounding each detection or stimulation event, and stores the data for the physician to review. The physician intermittently evaluates the device performance and adjusts the sensing and stimulation parameters if needed. Unlike PC+S, RNS comes with several built-in signal processing algorithms and is configured by default for closed-loop control.
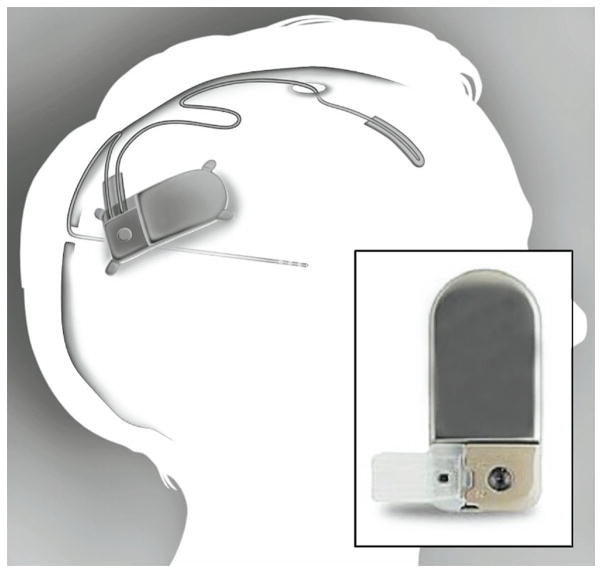
Figure 3.
The NeuroPace RNS system. The system includes a neurostimulator, a depth leads for stimulation at or near the seizure foci and a cortical strip leads for EcoG recording (Sun, Morrell, & Wharen, 2008).
Reprinted from Neurotherapeutics, Vol 5, Issue 1, Sun, Felice T, Morrell, Martha J, Wharen, Robert E. Responsive cortical stimulation for the treatment of epilepsy. Page 68–74, Copyright (2008), with permission from Springer.
The RNS system incorporates several detection tools for different ECoG patterns (Figure 4) including Half-Wave (Gotman, 1982), Line Length (Esteller, Echauz, Tcheng, Litt, & Pless, 2001), and the Area algorithm (Litt et al., 1999). The Half-Wave method detects rhythmic activity occurring in specific frequency range. The detected signals represent the amplitude and frequency component of the electrographic signals. Line Length measures changes in both amplitude and frequency of the signals. The Area algorithm identifies energy changes of the signal regardless of the signal frequency. The various detection methods provide sensitive and accurate characteristics of brain activity including oscillation changes, LFP spikes and the combination of those patterns. The RNS system has been approved by FDA as a treatment for epilepsy. It has been tested in Tourette syndrome, which is closely related to OCD, with some promising early results (Okun et al., 2013).
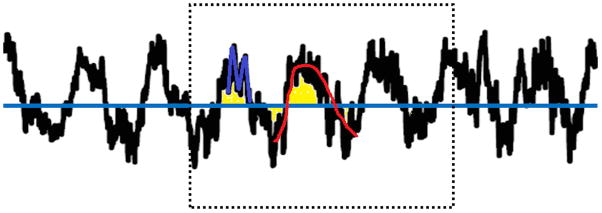
Figure 4.
The RNS detection methods. Red: Half-Wave method. The algorithm detects pre-defined segments of the signals partitioned at local and maximum values. The amplitude and duration of the half wave represent the amplitude and frequency of the signals. Blue: Line-Length method. The algorithm calculates the averaged amplitude difference between samples within a short term window. The value is compared with a long term window average and detection occurs when the short term value crosses a certain threshold pre-defined and based on the long term window average. Yellow: Area method. The algorithm calculates the averaged area underneath the curve of the signal within a short term window. Detection occurs when the short term window value crosses the threshold defined by the long term window averaged area.
Table 1 summarizes some of the advantages and disadvantages of the Activa PC+S and RNS systems (Table 1). The most distinctive difference between the two systems is the relative cost of stimulation vs. recording. Both devices are battery-powered, with no rechargeable option. The RNS system’s battery life is about 3.9 years, but this assumes very intermittent stimulation. If used to deliver stimulation for most of a day, similar to current psychiatric DBS practice, its expected life would be less than a year. Activa PC+S is estimated, with typical DBS-like use, to last about 2 years. The difference between the two systems is the mode that leads to rapid depletion. RNS is optimized for recording, and rapidly loses battery charge when stimulation is used heavily. PC+S is optimized for constant stimulation, and uses battery quickly when frequent recordings and/or telemetry are used. Each is conceivably very useful for closed-loop applications, but requires a careful consideration of design trade-offs that will limit any potential study.
Table 1.
Summary of the hardware platforms currently available that could support closed-loop DBS
| Platforms | Advantages | Disadvantages |
|---|---|---|
| Activa PC+S |
|
|
| Responsive Neurostimulation System (RNS) |
|
|
Next-Generation Technologies under Development
Improved Closed-Loop Electrophysiologic Systems
Both PC+S and RNS represented major technical advances over the prior generation of pure open-loop, non-sensing systems. However, as just described, they also have major limitations, including battery life, channel count, and limited algorithmic sophistication. A general trend in basic neuroscience is the use of increasing channel counts, into the hundreds or even thousands, for sensing and possibly for stimulation. More information can be collected and processed, which in the clinical arena might provide more precise treatments. However, there is a trade-off between the number of electrodes that can be incorporated within the system and power consumption or device packaging. More information extracted from the brain costs more power to analyze and process, and therefore decreases the battery-life. Processing also generates heat, and there are strict safety limits on how much heat a medical device can emit into the surrounding tissue. Finally, the device has to be contained within a small anatomical space, so it is challenging to package increased numbers of electrodes within such a small space. Safety and durability can be an issue if the device is too big or if very fragile connectors must be used to keep their size small. It is therefore desirable to advance the technology to provide more customizable hardware and software, better accommodating different therapeutic applications while maintaining clinical safety and feasibility. For psychiatric indications in particular, it may be essential to have leads in more than one brain location, or to have sensing leads that bring signals from both hemispheres to a single central processor. Mental illnesses are increasingly seen as network-based disorders, and controlling a network may require having multiple points of signal injection within that network.
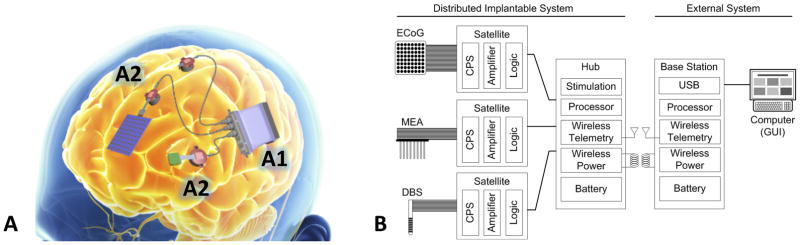
One new device currently under investigation is being developed by the TRANSFORM DBS program (Bjune et al., 2015; Wheeler et al., 2015). The system contains a central hub, multiple satellite processors for digitizing and routing neural activity, a transreceiver, and a base station. The central hub incorporates the centralized processing, power, communications and stimulus pulse generator. It can connect to up to 5 satellite systems, each with 64 channels, providing up to 320 channels for stimulation or recording. Perhaps most importantly, the central hub also has a rechargeable battery. Major surgery for battery replacement would occur only every 8–10 years, despite the massive increase in recording capability. The base station communicates with the central hub through a wireless transreceiver. This connection permits battery recharging, reprogramming the device, and download of recorded signals. It also allows the base station to serve as a companion processor, running more advanced signal processing algorithms to help identify key commands that then download to the hub. Multiple satellites can connect to the central hub, enabling access to multiple neural sites. Each satellite is implanted close to a given brain site to minimize noise for high fidelity signal recording. Each satellite has its own electrode front end, but receives power and communication from the hub. The satellite is in turn designed to connect with microelectrode arrays, grids optimized for the cortical surface, or DBS-style depth probes. This design provides the clinicians and researchers the flexibility to configure many different electrode configurations for recording or stimulation. Finally, the hub uses a rigid-flex design for compact packaging, allowing it to conform to the skull for implantation with minimal invasiveness. At present, this system is still undergoing benchtop and animal testing, but if successful, it will represent a major step forward in the platforms available for developing closed-loop psychiatric therapies. In particular, this network-scale brain access, available over time as a patient goes about his/her daily activities, should offer an unprecedented window into the biology of mental illness.
New Physical Modalities of Brain Stimulation
Atop the currently available closed-loop devices, researchers have been developing next-generation technologies that do not specifically rely on electricity. Optogenetics has attracted attention in recent years as a new neuromodulation approach (Aston-Jones & Deisseroth, 2013; Grosenick, Marshel, & Deisseroth, 2015; Steinberg, Christoffel, Deisseroth, & Malenka, 2015). It is a biological technique to provide precise control of brain and behavior through light. The biggest advantage of optogenetics is its precision. Electrical brain stimulation is non-specific, activating many different cell types within a single nucleus. Optogenetics uses genetic tools to introduce non-mammalian ion channels, making specific cells or pathways of interest sensitive to light. Pulses of laser light can then excite or inhibit specifically those cells. This has caused profound behavior changes in animal models, which might be very powerful if translatable to humans. Creed et al. (Creed et al., 2015) showed an example of a translational approach, using both DBS and optogenetics to reverse cocaine-evoked behavior in mice, through “optogenetically inspired DBS”. First, they identified a key sub-set of cells that, when genetically targeted and altered by light, abolished drug-seeking behavior. Second, they combined non-specific electrical stimulation with local blockade of metabotropic glutamate receptors through drug infusion. This caused electrical stimulation to acquire specificity, because it altered local excitability of a key neural population. There remain many translational barriers, and the physics of light scattering are very different in primate brain (Bentley, Chestek, Stacey, & Patil, 2013), but optogenetic techniques continue to revolutionize neuroscience and will almost certainly have an impact on how we develop future DBS systems As an example of a step towards building optogenetics into a closed-loop technology, Canales et al. (Canales et al., 2015) developed a fiber that allows simultaneous optical stimulation, neural recording and drug delivery channels. These fibers were fabricated from a thermal drawing process that incorporates optical fiber, electrical wires and microfluidic channels into a single physically integrated neural probe. The system has been validated in mice, and could be scaled up to the dimensions of a human DBS probe with relative ease. This would allow, for example, electrical recording with optical stimulation, completely eliminating the artifact problems that limit existing closed-loop approaches.
Another emerging technology is wireless brain stimulation. This is particularly attractive because it does not require external connections, which may minimize surgical complications or infective risk. It has been theorized that closed-loop DBS might be used to target neuroplasticity, where a relatively short course of treatment could normalize brain circuits by strengthening or weakening target synapses (Widge, Dougherty, & Moritz, 2014). Wireless stimulators would be an excellent option for such treatments, since patients could come to the office for brief treatment courses and otherwise avoid the complications of batteries and wires. This might be a path to achieve the power of DBS with the relative convenience of technologies such as transcranial magnetic stimulation (TMS).
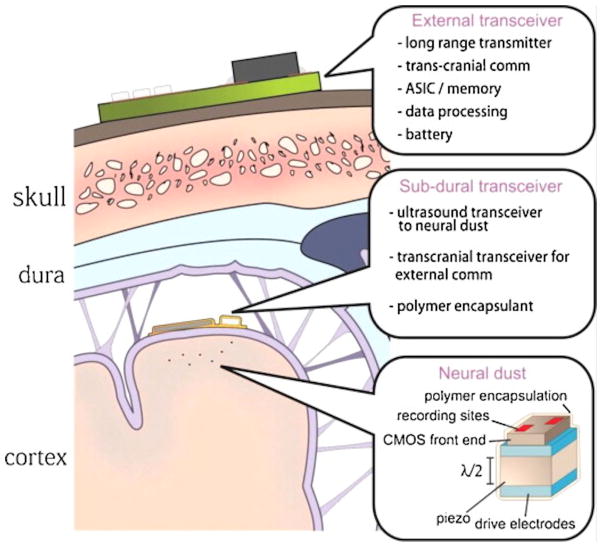
One example is wireless magnetothermal deep brain stimulation. This is a minimally invasive and remote neural stimulation technology that activates heat-sensitive neurons with magnetic particles. When exposed to a rapidly alternating field, the nanoparticles heat up, opening ion channels and causing neurons to fire. This may be a very useful general mechanism, as temperature-sensitive ion channels are found throughout the nervous system (Moran, Xu, & Clapham, 2004). Multiple groups have demonstrated the feasibility of the system using rodent models (Chen, Romero, Christiansen, Mohr, & Anikeeva, 2015; Huang, Delikanli, Zeng, Ferkey, & Pralle, 2010). However, the technology currently lacks a good sensing component and therefore requires more investigation before it can be a viable closed-loop neurostimulator. Another example of an emerging wireless technology is neural dust (Figure 6) (Seo, Carmena, Rabaey, Alon, & Maharbiz, 2013). This system enables recording and stimulation with two technologies: 1) thousands of 10–100 μm free-floating and independent sensor nodes that can detect and report electrophysiological data through ultrasonic backscattering and 2) and a sub-cranial ultrasonic transreceiver to power and communicate with the neural dust. Each dust “mote” is also a piezoelectric antenna that can be made to deliver electrical stimulation when pulsed with a different ultrasonic regime. The system is promising not only because it is wireless, but because it can in theory access thousands of independent channels. There are substantial questions of heat, power, and feasibility to solve, but the massive amount of data obtainable through neural dust could be very beneficial to help decipher the signals of mental illness.
Figure 6.
The neural dust system. Dust “motes” (small recording or stimulating units, about 100x smaller than existing electrodes) are implanted within the cortex. A sub-cranial transreceiver is implanted below the dura mater and powered by another external transreceiver through radio frequency (RF) power transfer. The sub-cranial transreceiver couples ultrasound energy into tissue to interrogate each sensing node and deliver stimulation through targeted activating nodes (Seo, Carmena, Rabaey, Maharbiz, & Alon, 2015). In other variants of this system concept, the implanted transreceiver is eliminated and the dust motes are activated/interrogated entirely from outside the skull, for a true minimally invasive system.
Reprinted from Journal of Neuroscience Methods, Vol 244, Seo, Dongjin, Carmena, Jose M., Rabaey, Jan M. Maharbiz, Michel M. Alon, Elad. Model validation of untethered, ultrasonic neural dust motes for cortical recording. Page 114–122, Copyright (2015), with permission from Elsevier.
Ongoing challenges and limitations of closed-loop approaches to psychiatric disorders
Despite the available and emerging closed-loop devices for psychiatric disorders, many challenges still remain. The biggest of these is biomarker selection. As previously described, closed-loop technology provides clinicians and researchers the ability to modulate treatment in real time in response to changes in an electrical disease biomarker. However, in psychiatric disorders, there are currently no electrophysiological signals identified that can reliably track patients’ overall condition or specific symptoms (Widge, Deckersbach, et al., 2016). It is not yet clear how a pathological state can be differentiated from a healthy state. Progress has been made in “affective decoding” to generally classify emotions, but there are reasons to believe that this healthy-volunteer research will not work well in clinical populations (Widge et al., 2014). Furthermore, psychiatric diagnoses are highly heterogeneous and likely contain multiple neurological entities. The same clinical phenotype might arise from very different brain pathologies (Cuthbert & Insel, 2013; Insel & Wang, 2010). As a result, while methods such as quantitative electroencephalography have identified candidate markers of treatment response, these have generally not been successfully replicated by independent teams (Mcloughlin, Makeig, & Tsuang, 2014; Widge, Avery, & Zarkowski, 2013; Widge, Zorowitz, et al., 2015). Much work will be needed to identify suitable biomarkers that can be sensed and controlled.
Several strategies have been proposed towards this aim. First, leaders in the US National Institutes of Health (NIH) have proposed a new framework to study not disorders but cross-diagnostic “Research Doman Criteria” (RDoC) (Cuthbert & Insel, 2013; Insel & Wang, 2010). RDoC seeks to classify an individual patient’s mental illness based on functional problems that give rise to those symptoms instead of the symptom clusters (Regier et al., 2013). Researchers and clinicians are expected to develop assessment for those functional domains, and could then apply neuromodulation to domain-specific symptoms and circuits. This domain-oriented approach might addresses the diagnostic overlap issue and further help biomarker identification. For example, both posttraumatic stress disorder (PTSD) and MDD patients have a common deficit in emotion regulation, at the intersection of the Negative Valence and Cognitive Control RDoC constructs (“RDoC Matrix,” 2009). That regulation is linked to brain connectivity between prefrontal cortex and the amygdala (Milad et al., 2009; Rive et al., 2013; Rougemont-Bücking et al., 2011; Widge, Ellard, et al., 2016). DBS that targets this circuit could relieve emotional dysregulation across disorders. Applying this using sensing technologies (such as PC+S, RNS, or TRANSFORM) might help identify human electrophysiological biomarkers in this specific functional domain. Furthermore, this approach also links between human and the animal research communities. It is difficult to screen psychiatric treatments in animals, as commonly used animal behavioral tests are only partly analogous to human emotion (Widge, Arulpragasam, et al., 2015). RDoC constructs and similar domains are designed to align better to those common laboratory assays. A transdiagnostic approach might therefore focus on neurological entities that can be measured in both animals and humans, which might yield new ways to screen for biomarkers.
Another strategy to address the biomarker issue is to explore the possibility of a closed-loop DBS that does not need an explicit biomarker. Our group recently demonstrated platform technology for an alternate approach, where patients directly control the stimulator by thinking about what they want it to do (Widge et al., 2014; Widge & Moritz, 2014). A patient would evaluate whether the current simulation parameters (particularly the intensity of stimulation) match his/her needs, and could then volitionally modulate the stimulation parameters. The proposal is a direct combination of DBS with brain computer interfacing (BCI), a technology where neural activity is used as a volitional control signal for prosthetic devices. In this scenario, there is no need to identify a biomarker for each disorder or transdiagnostic construct, although we would still need stimulation target(s) for each. The device would utilize patients’ fundamental desire to relieve their symptoms. The patient would only receive treatment when they explicitly will it, making this a more personalized treatment compared to more traditional concepts of closed-loop stimulation. In rodent models, we have shown that animals can use this type of intentional control to trigger brain stimulation to the medial forebrain bundle (MFB), a reward center that is also a DBS target for MDD (Widge & Moritz, 2014). There would be much work to translate such a device to the clinic, including identifying a DBS target and stimulation regime that caused noticeable and immediate symptom relief. The technology nevertheless offers a different approach to the challenge of biomarker selection.
The final challenge is data volume, especially from next-generation devices. As closed-loop concepts become more popular, many groups have been developing (Ahrens, Orger, Robson, Li, & Keller, 2013; Bjune et al., 2015; Seo et al., 2013; Wheeler et al., 2015). Using benchtop laboratory systems, terabytes to petabytes of detailed data can already be extracted from the brain. The advance allows both clinicians and neuroscientists to study neural activities from single-neuron level to population level (Cunningham & Yu, 2014). However, challenges arise for the analysis of this large-scale neural activity. “Big data” require massive computational power and very carefully designed algorithms to extract neuronal signatures for a given hypothesis. Traditional data analysis approaches, which average neural features across trials and smooth over time, often yield results that are difficult to interpret. Information can still be buried within the data or even damped out due to the averaging. On the other hand, high channel counts can raise the risk of false-positive findings if not carefully statistically controlled. They also create power considerations for implantable devices, where this kind of massively parallel processing may not be feasible.
Dimensionality reduction can help address the challenge. These methods extract low-dimensional representations of the high-dimensional data, where certain features are preserved or highlighted (Cunningham & Yu, 2014; Wang, Olson, Ojemann, Rao, & Brunton, 2015; Yuxiao Yang & Shanechi, 2015). The widely used dimensionality reduction methods are principal component analysis (PCA) and factor analysis (FA) (Cunningham & Yu, 2014). PCA converts a set of data using orthogonal transformation to capture the greatest variance in the data and FA preserves variance shared across neurons in low-dimensional space (Churchland et al., 2010). However, PCA is a static model that does not account for temporal dynamics of time-series data, whereas the low-dimensional structure of neural data is likely not constant over time. Brunton et al. (Brunton, Johnson, Ojemann, & Kutz, 2016) recently proposed a new approach, dynamic mode decomposition (DMD), to analyze spatial-temporal patterns of large-scale neural recordings. DMD combines power spectral analysis and PCA, enabling rotation of the low-dimensional PCA such that each vector has its own temporal resolution. Other dimensionality reduction methods with likely applications in neural time series are hidden Markov models (Jones, Fontanini, Sadacca, Miller, & Katz, 2007; Ponce-Alvarez, Nacher, Luna, Riehle, & Romo, 2012; Seidemann, Meilijson, Abeles, Bergman, & Vaadia, 1996), Gaussian process factor analysis (GPFA) (Luttinen & Ilin, 2009; Yu et al., 2009) and latent linear dynamical systems (LDS) (Paninski et al., 2009; Pfau, Pnevmatikakis, & Paninski, 2013; Smith & Brown, 2003; Yousefi et al., 2015). Those methods each assume that there are underlying unobservable variables that explain the population activity, and apply principled statistical methods to extract the most likely values of those variables over time. Finally, neural data can be nonlinear in the high-dimensional space, and linear methods might result in loss of features or skewed data interpretation. Therefore, there also exist nonlinear dimensional reduction models, such as locally linear embedding (Broome, Jayaraman, & Laurent, 2006; Brown, Joseph, & Stopfer, 2005; Saha et al., 2013) or Isomap (Jenkins & Matarić, 2004; Tenenbaum, de Silva, & Langford, 2000), etc.). Each closed-loop problem will require careful consideration of whether one of these methods is necessary or appropriate, but the clinical neuroscientist’s toolkit is continuing to grow.
Conclusion
Deep brain stimulation for psychiatric disorders has great promise. Well-designed clinical trials have not yielded a strong enough or consistent enough response rate to justify dissemination of open-loop technologies. Closed-loop devices may be a solution, based on lessons learned from our open-loop experience. In the interim, there is increasing use of open-loop DBS devices with sensing capability, which should enable more precise and patient-specific treatment. Patients are strongly interested in any technology that can reduce the trial-and-error nature of DBS or the amount of time between device implant and their first experience of symptom relief. This helps build a case with funding agencies to move the technology forward. Furthermore, closed-loop approaches will help tremendously in understanding the underlying biology of psychiatric disorders. Every closed-loop device is also a tool for neuroscience, as it creates and stores detailed snapshots of the patient’s brain at resolutions not reachable with any other technology. Still, as described in this article, much work remains before closed-loop devices reach clinical psychiatric use: device design, ethical concerns, biomarker selection, and eventually evidence of clinical effectiveness. That work is actively ongoing and even accelerating, on both clinical and basic engineering fronts. The past five years alone have seen the debut of two new devices for chronic implantable human use, evidence that this aspect of neuromodulation is prone for growth. We are already well on our way towards more reliable and effective neuromodulation treatments for psychiatric disorders.
Figure 5.
TRANSFORM DBS proposed system. (A), schematic of cranially mounted device, including the central Hub signal processor and aggregator (A1) and the Satellite systems for signal digitization and stimulation routing (A2). (B), block diagram of system processing, illustrating partitioning of key components to different aspects of the end-to-end closed-loop therapy. (Figure courtesy of Draper Laboratory)
Footnotes
Declaration of Interest
Preparation of this work was supported in part by grants from the Brain & Behavior Research Foundation, Harvard Brain Initiative, Defense Advanced Research Projects Agency (cooperative agreement W911NF-14-2-0045, issued by the Army Research Office contracting office in support of DARPA’s SUBNETS program), and National Institute of Mental Health (MH109722-01). The views, opinions, and/or findings expressed are those of the author(s) and should not be interpreted as representing the official views or policies of the Department of Defense, the U.S. Government. ASW has consulted for and receives device donations from Medtronic, which manufactures devices discussed in the article. ASW and ML are named inventors on patent applications related to closed-loop neurostimulation.
References
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nature Methods. 2013;10(5):413–420. doi: 10.1038/nmeth.2434. http://doi.org/10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Deisseroth K. Recent advances in optogenetics and pharmacogenetics. Brain Research. 2013 doi: 10.1016/j.brainres.2013.01.026. http://doi.org/10.1016/j.brainres.2013.01.026. [DOI] [PMC free article] [PubMed]
- Bentley JN, Chestek C, Stacey WC, Patil PG. Optogenetics in epilepsy. Neurosurgical Focus. 2013;34(June):E4. doi: 10.3171/2013.3.FOCUS1364. http://doi.org/10.3171/2013.3.FOCUS1364. [DOI] [PubMed] [Google Scholar]
- Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, Van Laarhoven J, … Denys D. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456–64. doi: 10.1001/jamapsychiatry.2016.0152. http://doi.org/10.1001/jamapsychiatry.2016.0152. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, … Schlaepfer TE. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological Psychiatry. 2010;67(2):110–6. doi: 10.1016/j.biopsych.2009.09.013. http://doi.org/10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2012;37(9):1975–85. doi: 10.1038/npp.2012.44. http://doi.org/10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune CK, Marinis TF, Sriram TS, Brady JM, Moran J, Parks PD, … Eskandar EN. Packaging architecture for an implanted system that monitors brain activity and applies therapeutic stimulation. International Symposium on MicroelectronicsInternational Symposium on Microelectronics. 2015;2015(1):548–554. Retrieved from http://imapsource.org/doi/10.4071/isom-2015-THA13. [Google Scholar]
- Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51(4):467–482. doi: 10.1016/j.neuron.2006.07.018. http://doi.org/10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Brown SL, Joseph J, Stopfer M. Encoding a temporally structured stimulus with a temporally structured neural representation. Nature Neuroscience. 2005;8(11):1568–76. doi: 10.1038/nn1559. http://doi.org/10.1038/nn1559. [DOI] [PubMed] [Google Scholar]
- Brunton BW, Johnson LA, Ojemann JG, Kutz JN. Extracting spatial-temporal coherent patterns in large-scale neural recordings using dynamic mode decomposition. Journal of Neuroscience Methods. 2016;258:1–15. doi: 10.1016/j.jneumeth.2015.10.010. http://doi.org/10.1016/j.jneumeth.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, … Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nature Biotechnology. 2015;33(3):277–284. doi: 10.1038/nbt.3093. http://doi.org/10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science (New York, NY) 2015;347(6229):1477–80. doi: 10.1126/science.1261821. http://doi.org/10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, … Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nature Neuroscience. 2010;13(3):369–378. doi: 10.1038/nn.2501. http://doi.org/10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Pascoli VJ, Lüscher C, Benabid AL, Pollak P, Gervason C, … Nobrega JN. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science (New York, NY) 2015;347(6222):659–64. doi: 10.1126/science.1260776. http://doi.org/10.1126/science.1260776. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, Yu BM. Dimensionality reduction for large-scale neural recordings. Nature Neuroscience. 2014;17(11):1500–1509. doi: 10.1038/nn.3776. http://doi.org/10.1038/nn.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11(1):126. doi: 10.1186/1741-7015-11-126. http://doi.org/10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, … Malone DA., Jr A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biological Psychiatry. 2015;78(4):240–248. doi: 10.1016/j.biopsych.2014.11.023. http://doi.org/10.1016/j.biopsych.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Esteller R, Echauz J, Tcheng T, Litt B, Pless B. Line length: an efficient feature for seizure onset detection. 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2001. pp. 1707–1710. http://doi.org/10.1109/IEMBS.2001.1020545. [Google Scholar]
- Garnaat SL, Greenberg BD, Sibrava NJ, Goodman WK, Mancebo MC, Eisen JL, Rasmussen SA. Who qualifies for deep brain stimulation for OCD? Data from a naturalistic clinical sample. The Journal of Neuropsychiatry and Clinical Neurosciences. 2014;26(1):81–6. doi: 10.1176/appi.neuropsych.12090226. http://doi.org/10.1176/appi.neuropsych.12090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmel GE, Hamilton TJ, Obradovic M, Gorman RB, Single PS, Chenery HJ, … AJOM A new biomarker for subthalamic deep brain stimulation for patients with advanced parkinson’s disease—a pilot study. Journal of Neural Engineering. 2015;12(6):066013. doi: 10.1088/1741-2560/12/6/066013. http://doi.org/10.1088/1741-2560/12/6/066013. [DOI] [PubMed] [Google Scholar]
- Gotman J. Automatic recognition of epileptic seizures in the EEG. Electroencephalography and Clinical Neurophysiology. 1982;54(5):530–540. doi: 10.1016/0013-4694(82)90038-4. http://doi.org/10.1016/0013-4694(82)90038-4. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Rezai AR, Friehs GM, Okun MS, … Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Molecular Psychiatry. 2010;15(1):64–79. doi: 10.1038/mp.2008.55. http://doi.org/10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015 doi: 10.1016/j.neuron.2015.03.034. http://doi.org/10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed]
- Haq IU, Foote KD, Goodman WG, Wu SS, Sudhyadhom A, Ricciuti N, … Okun MS. Smile and laughter induction and intraoperative predictors of response to deep brain stimulation for obsessive-compulsive disorder. NeuroImage. 2011;54(SUPPL 1) doi: 10.1016/j.neuroimage.2010.03.009. http://doi.org/10.1016/j.neuroimage.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq IU, Foote KD, Goodman WK, Ricciuti N, Ward H, Sudhyadhom A, … Okun MS. A case of mania following deep brain stimulation for obsessive compulsive disorder. Stereotactic and Functional Neurosurgery. 2010;88(5):322–328. doi: 10.1159/000319960. http://doi.org/10.1159/000319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, … Morrell MJ. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–41. doi: 10.1111/epi.12534. http://doi.org/10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J, Denison T, Chizeck HJ. Closed-loop DBS with movement intention. 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER); IEEE; 2015. pp. 844–847. http://doi.org/10.1109/NER.2015.7146755. [Google Scholar]
- Houston B, Blumenfeld Z, Quinn E, Brontë-Stewart H, Chizeck H. Long-term detection of parkinsonian tremor activity from subthalamic nucleus local field potentials. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE; 2015. pp. 3427–3431. http://doi.org/10.1109/EMBC.2015.7319129. [DOI] [PubMed] [Google Scholar]
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nature Nanotechnology. 2010;5(8):602–606. doi: 10.1038/nnano.2010.125. http://doi.org/10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wang PS. Rethinking mental illness. Journal of the American Medical Association. 2010;303(19):1970–1971. doi: 10.1001/jama.2010.555. http://doi.org/10.1001/jama.2010.555. [DOI] [PubMed] [Google Scholar]
- Jenkins OC, Matarić MJ. A spatio-temporal extension to Isomap nonlinear dimension reduction. Proceedings of the 21st International Conference on Machine Learning; 2004. p. 56. http://doi.org/10.1145/1015330.1015357. [Google Scholar]
- Jones LM, Fontanini A, Sadacca BF, Miller P, Katz DB. Natural stimuli evoke dynamic sequences of states in sensory cortical ensembles. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18772–18777. doi: 10.1073/pnas.0705546104. http://doi.org/10.1073/pnas.0705546104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. The American Journal of Psychiatry. 2011;168(5):502–10. doi: 10.1176/appi.ajp.2010.10081187. http://doi.org/10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Khanna P, Stanslaski S, Xiao Y, Ahrens T, Bourget D, Swann N, … Denison T. Enabling closed-loop neurostimulation research with downloadable firmware upgrades. IEEE Biomedical Circuits and Systems Conference: Engineering for Healthy Minds and Able Bodies, BioCAS 2015 - Proceedings; IEEE; 2015. pp. 1–6. http://doi.org/10.1109/BioCAS.2015.7348348. [Google Scholar]
- Klein E, Goering S, Gagne J, Shea CV, Franklin R, Zorowitz S, … Widge AS. Brain-computer interface-based control of closed-loop brain stimulation: attitudes and ethical considerations. Brain-Computer Interfaces. 2016;3(3):140–148. http://doi.org/10.1080/2326263X.2016.1207497. [Google Scholar]
- Litt B, Esteller R, D’Alessandro M, Echuaz J, Shor R, Bowen C, Vachstevanos G. Evolution of accumulated energy predicts seizures in mesial temporal lobe epilepsy. Proceedings of the First Joint BMES/EMBS Conference: Serving Humanity, Advancing Technology; 1999. p. 440. http://doi.org/10.1109/IEMBS.1999.802518. [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, … Brown P. Adaptive deep brain stimulation in advanced parkinson disease. Annals of Neurology. 2013;74(3):449–457. doi: 10.1002/ana.23951. http://doi.org/10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zrinzo L, Hariz M, Foltynie T, … Brown P. Controlling parkinson’s disease with adaptive deep brain stimulation. Journal of Visualized Experiments: JoVE. 2014;(89):1–5. doi: 10.3791/51403. http://doi.org/10.3791/51403. [DOI] [PMC free article] [PubMed]
- Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, … Mayberg HS. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. Journal of Neurosurgery. 2012;116(2):315–322. doi: 10.3171/2011.10.JNS102122. http://doi.org/10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. http://doi.org/10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Luttinen J, Ilin A. Variational gaussian-process factor analysis for modeling spatio-temporal data. Processing. 2009:1–9. [Google Scholar]
- Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Molecular Psychiatry. 2016;21(9):1272–1280. doi: 10.1038/mp.2015.124. http://doi.org/10.1038/mp.2015.124. [DOI] [PubMed] [Google Scholar]
- Makris N, Rathi Y, Mouradian P, Bonmassar G, Papadimitriou G, Ing WI, … Dougherty DD. Variability and anatomical specificity of the orbitofrontothalamic fibers of passage in the ventral capsule/ventral striatum (VC/VS): precision care for patient-specific tractography-guided targeting of deep brain stimulation (DBS) in obsessive compulsive. Brain Imaging and Behavior. 2015:1–14. doi: 10.1007/s11682-015-9462-9. http://doi.org/10.1007/s11682-015-9462-9. [DOI] [PMC free article] [PubMed]
- Malekmohammadi M, Herron J, Velisar A, Blumenfeld Z, Trager MH, Chizeck HJ, Brontë-Stewart H. Kinematic adaptive deep brain stimulation for resting tremor in parkinson’s disease. Movement Disorders. 2016 doi: 10.1002/mds.26482. http://doi.org/10.1002/mds.26482. [DOI] [PubMed]
- Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, … Greenberg BD. Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Treatment-Resistant Depression. Biological Psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. http://doi.org/10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, … Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. http://doi.org/10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, McKenna MT, Michaud CM, Murray CJ, Marks JS, Warden D, … Eidelberg D. Targeted electrode-based modulation of neural circuits for depression. Journal of Clinical Investigation. 2009;119(4):717–725. doi: 10.1172/JCI38454. http://doi.org/10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcloughlin G, Makeig S, Tsuang MT. In search of biomarkers in psychiatry: EEG-based measures of brain function. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2014;165(2):111–121. doi: 10.1002/ajmg.b.32208. http://doi.org/10.1002/ajmg.b.32208. [DOI] [PubMed] [Google Scholar]
- McNeely HE, Mayberg HS, Lozano AM, Kennedy SH. Neuropsychological impact of Cg25 deep brain stimulation for treatment-resistant depression. The Journal of Nervous and Mental Disease. 2008;196(5):405–410. doi: 10.1097/NMD.0b013e3181710927. http://doi.org/10.1097/NMD.0b013e3181710927. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. http://doi.org/10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Current Opinion in Neurobiology. 2004 doi: 10.1016/j.conb.2004.05.003. http://doi.org/10.1016/j.conb.2004.05.003. [DOI] [PubMed]
- Morishita T, Fayad SM, Higuchi M, Nestor KA, Foote KD. Deep brain stimulation for treatment-resistant depression: dystematic review of clinical outcomes. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2014;11:475–84. doi: 10.1007/s13311-014-0282-1. http://doi.org/10.1007/s13311-014-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. http://doi.org/10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Halpern C. Responsive direct brain stimulation for epilepsy. Neurosurgery Clinics of North America. 2016;27(1):111–121. doi: 10.1016/j.nec.2015.08.012. http://doi.org/10.1016/j.nec.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Halpern C, Heck CN, King-Stephens D, Massey AD, Nair DR, … Morrell MJ. Responsive direct brain stimulation for epilepsy. Neurosurgery Clinics of North America. 2014;55(3):111–121. doi: 10.1016/j.nec.2015.08.012. http://doi.org/10.1111/epi.12534. [DOI] [PubMed] [Google Scholar]
- Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. The Lancet. 1999;354(9189):1526. doi: 10.1016/S0140-6736(99)02376-4. http://doi.org/10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, … Sanchez JC. A trial of scheduled deep brain stimulation for Tourette syndrome: moving away from continuous deep brain stimulation paradigms. JAMA Neurology. 2013;70(1):85. doi: 10.1001/jamaneurol.2013.580. http://doi.org/10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, … Goodman WK. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(3):310–4. doi: 10.1136/jnnp.2006.095315. http://doi.org/10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paninski L, Ahmadian Y, Ferreira DG, Koyama S, Rahnama Rad K, Vidne M, … Wu W. A new look at state-space models for neural data. Journal of Computational Neuroscience. 2009;29(1–2):107–126. doi: 10.1007/s10827-009-0179-x. http://doi.org/10.1007/s10827-009-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau D, Pnevmatikakis EA, Paninski L. Robust learning of low-dimensional dynamics from large neural ensembles. Advances in Neural Information Processing Systems. 2013:2391–2399. Retrieved from http://papers.nips.cc/paper/4995-robust-learning-of-low-dimensional-dynamics-from-large-neural-ensembles.
- Ponce-Alvarez A, Nacher V, Luna R, Riehle A, Romo R. Dynamics of cortical neuronal ensembles transit from decision making to storage for later report. J Neurosci. 2012;32(35):11956–11969. doi: 10.1523/JNEUROSCI.6176-11.2012. http://doi.org/32/35/11956 [pii] 10.1523/JNEUROSCI.6176-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn EJ, Blumenfeld Z, Velisar A, Koop MM, Shreve LA, Trager MH, … Brontë-Stewart H. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Movement Disorders. 2015;30(13):1750–1758. doi: 10.1002/mds.26376. http://doi.org/10.1002/mds.26376. [DOI] [PubMed] [Google Scholar]
- RDoC Matrix. 2009 Retrieved September 11, 2016, from http://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml.
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, Kupfer DJ. DSM-5 field trials in the United States and Canada, part II: Test-retest reliability of selected categorical diagnoses. American Journal of Psychiatry. 2013;170(1):59–70. doi: 10.1176/appi.ajp.2012.12070999. http://doi.org/10.1176/appi.ajp.2012.12070999. [DOI] [PubMed] [Google Scholar]
- Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, … Mayberg HS. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2014;76(12):963–969. doi: 10.1016/j.biopsych.2014.03.029. http://doi.org/10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive MM, Van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37(10 Pt 2):2529–53. doi: 10.1016/j.neubiorev.2013.07.018. http://doi.org/10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, … Bergman H. Closed-loop deep brain stimulation is superior in ameliorating Parkinsonism. Neuron. 2011;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. http://doi.org/10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, … Milad MR. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neuroscience and Therapeutics. 2011;17(4):227–236. doi: 10.1111/j.1755-5949.2010.00152.x. http://doi.org/10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryapolova-Webb E, Afshar P, Stanslaski S, Denison T, de Hemptinne C, Bankiewicz K, Starr PA. Chronic cortical and electromyographic recordings from a fully implantable device: preclinical experience in a nonhuman primate. Journal of Neural Engineering. 2014;11(1):016009. doi: 10.1088/1741-2560/11/1/016009. http://doi.org/10.1088/1741-2560/11/1/016009. [DOI] [PubMed] [Google Scholar]
- Saha D, Leong K, Li C, Peterson S, Siegel G, Raman B. A spatiotemporal coding mechanism for background-invariant odor recognition. Nature Neuroscience. 2013;16(12):1830–9. doi: 10.1038/nn.3570. http://doi.org/10.1038/nn.3570. [DOI] [PubMed] [Google Scholar]
- Schade CM, Schultz DM, Tamayo N, Iyer S, Panken E. Automatic adaptation of neurostimulation therapy in response to changes in patient position: results of the posture responsive spinal cord stimulation (PRS) research study. Pain Physician. 2011;14(5):407–17. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/21927044. [PubMed] [Google Scholar]
- Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician. 2012;15(1):1–12. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22270733. [PubMed] [Google Scholar]
- Seidemann E, Meilijson I, Abeles M, Bergman H, Vaadia E. Simultaneously recorded single units in the frontal cortex go through sequences of discrete and stable states in monkeys performing a delayed localization task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16(2):752–68. doi: 10.1523/JNEUROSCI.16-02-00752.1996. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8551358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Carmena JM, Rabaey JM, Alon E, Maharbiz MM. Neural dust: an ultrasonic, low powersSolution for chronic brain-machine interfaces. Cornell University Library. 2013 Apr;:1–11. Retrieved from http://arxiv.org/abs/1307.2196.
- Seo D, Carmena JM, Rabaey JM, Maharbiz MM, Alon E. Model validation of untethered, ultrasonic neural dust motes for cortical recording. Journal of Neuroscience Methods. 2015;244:114–122. doi: 10.1016/j.jneumeth.2014.07.025. http://doi.org/10.1016/j.jneumeth.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Smith AC, Brown EN. Estimating a State-Space Model from Point Process Observations. Neural Comput. 2003;15(5):965–991. doi: 10.1162/089976603765202622. http://doi.org/10.1162/089976603765202622. [DOI] [PubMed] [Google Scholar]
- Stanslaski S, Afshar P, Cong P, Giftakis J, Stypulkowski P, Carlson D, … Denison T. Design and validation of a fully implantable, chronic, closed-loop neuromodulation device with concurrent sensing and stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2012;20(4):410–421. doi: 10.1109/TNSRE.2012.2183617. http://doi.org/10.1109/TNSRE.2012.2183617. [DOI] [PubMed] [Google Scholar]
- Stanslaski S, Cong P, Carlson D, Santa W, Jensen R, Molnar G, … Denison T. An implantable bi-directional brain-machine interface system for chronic neuroprosthesis research. Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, EMBC 2009; 2009. pp. 5494–5497. http://doi.org/10.1109/IEMBS.2009.5334562. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Current Opinion in Neurobiology. 2015 doi: 10.1016/j.conb.2014.08.004. http://doi.org/10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed]
- Stypulkowski PH, Stanslaski SR, Denison TJ, Giftakis JE. Chronic evaluation of a clinical system for deep brain stimulation and recording of neural network activity. Stereotactic and Functional Neurosurgery. 2013;91(4):220–232. doi: 10.1159/000345493. http://doi.org/10.1159/000345493. [DOI] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ. Closed-loop neurostimulation: the clinical experience. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2014;11(3):553–63. doi: 10.1007/s13311-014-0280-3. http://doi.org/10.1007/s13311-014-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Morrell MJ, Wharen RE. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics. 2008;5(1):68–74. doi: 10.1016/j.nurt.2007.10.069. http://doi.org/10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Miocinovic S, Qasim S, Wang SS, Ziman N, … Starr PA. Gamma oscillations in the hyperkinetic state detected with chronic human brain recordings in parkinson’s disease. Journal of Neuroscience. 2016;36(24):6445–6458. doi: 10.1523/JNEUROSCI.1128-16.2016. http://doi.org/10.1523/JNEUROSCI.1128-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum JB, de Silva V, Langford JC. A global geometric framework for nonlinear dimensionality reduction. Science (New York, NY) 2000;290(5500):2319–23. doi: 10.1126/science.290.5500.2319. http://doi.org/10.1126/science.290.5500.2319. [DOI] [PubMed] [Google Scholar]
- Wang N, Olson JD, Ojemann JG, Rao RPN, Brunton BW. Unsupervised decoding of long-term, naturalistic human neural recordings with automated video and audio annotations. arXiv. 2015:1–14. doi: 10.3389/fnhum.2016.00165. http://doi.org/10.3389/fnhum.2016.00165. [DOI] [PMC free article] [PubMed]
- Wheeler JJ, Baldwin K, Kindle A, Guyon D, Nugent B, Segura C, … Eskandar EN. An implantable 64-channel neural interface with reconfigurable recording and stimulation. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS; 2015. pp. 7837–7840. 2015-Novem. http://doi.org/10.1109/EMBC.2015.7320208. [DOI] [PubMed] [Google Scholar]
- Widge AS, Arulpragasam AR, Deckersbach T, Dougherty DD. Emerging Trends in the Social and Behavioral Sciences. John Wiley & Sons, Inc; 2015. Deep brain stimulation for psychiatric disorders. [Google Scholar]
- Widge AS, Avery DH, Zarkowski P. Baseline and treatment-emergent EEG biomarkers of antidepressant medication response do not predict response to repetitive transcranial magnetic stimulation. Brain Stimulation. 2013;6(6):929–931. doi: 10.1016/j.brs.2013.05.001. http://doi.org/10.1016/j.brs.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Deckersbach T, Eskandar EN, Dougherty DD, Nuttin B, Cosyns P, … Dougherty DD. Deep brain stimulation for treatment-resistant psychiatric illnesses: What has gone wrong and what should we do next? Biological Psychiatry. 2016;79(4):e9–e10. doi: 10.1016/j.biopsych.2015.06.005. http://doi.org/10.1016/j.biopsych.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Widge AS, Dougherty DD. Deep brain stimulation for treatment-refractory mood and obsessive-compulsive disorders. Current Behavioral Neuroscience Reports. 2015a;2(4):187–197. doi: 10.1007/s40473-015-0036-3. http://doi.org/10.1007/s40473-015-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Dougherty DD. Managing patients with psychiatric disorders with deep brain stimulation. In: Marks WJ Jr, editor. Deep Brain Stimulation Management. 2. Cambridge: New York: Cambridge University Press; 2015b. [Google Scholar]
- Widge AS, Dougherty DD, Moritz CT. Affective brain-computer interfaces as enabling technology for responsive psychiatric stimulation. Brain Computer Interfaces (Abingdon, England) 2014;1(2):126–136. doi: 10.1080/2326263X.2014.912885. http://doi.org/10.1080/2326263X.2014.912885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Ellard KK, Paulk AC, Basu I, Yousefi A, Zorowitz S, … Eskandar EN. Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Experimental Neurology. 2016;287(4):361–472. doi: 10.1016/j.expneurol.2016.07.021. http://doi.org/10.1016/j.expneurol.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Widge AS, Licon E, Zorowitz S, Corse A, Arulpragasam AR, Camprodon JA, … Dougherty DD. Predictors of hypomania during ventral capsule/ventral striatum deep brain stimulation. The Journal of Neuropsychiatry and Clinical Neurosciences. 2016;28(1):38–44. doi: 10.1176/appi.neuropsych.15040089. http://doi.org/10.1176/appi.neuropsych.15040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Moritz CT. Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface. Journal of Neural Engineering. 2014;11(2):024001. doi: 10.1088/1741-2560/11/2/024001. http://doi.org/10.1088/1741-2560/11/2/024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge AS, Zorowitz S, Link K, Miller EK, Deckersbach T, Eskandar EN, … Postle BR. Ventral capsule/ventral striatum deep brain stimulation does not consistently diminish occipital cross-frequency coupling. Biological Psychiatry. 2015;80(7):e59–e60. doi: 10.1016/j.biopsych.2015.10.029. http://doi.org/10.1016/j.biopsych.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi A, Paulk AC, Deckersbach T, Dougherty DD, Eskandar EN, Widge AS, Eden UT. Cognitive state prediction using an EM algorithm applied to Gamma distributed data. Conference Proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference; 2015. pp. 7819–24. http://doi.org/10.1109/EMBC.2015.7320205. [DOI] [PubMed] [Google Scholar]
- Yu BM, Cunningham JP, Santhanam G, Ryu SI, Shenoy KV, Sahani M. Gaussian-process factor analysis for low-dimensional single-trial analysis of neural population activity. Journal of Neurophysiology. 2009;102:614–35. doi: 10.1152/jn.90941.2008. 0022-3077 (Print) http://doi.org/10.1152/jn.90941.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuxiao Yang Y, Shanechi MM. A framework for identification of brain network dynamics using a novel binary noise modulated electrical stimulation pattern. 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE; 2015. pp. 2087–2090. http://doi.org/10.1109/EMBC.2015.7318799. [DOI] [PubMed] [Google Scholar]