Abstract
The normal pulmonary circulation is a low-pressure, high-compliance system. Pulmonary arterial compliance decreases in the presence of pulmonary hypertension because of increased extracellular matrix/collagen deposition in the pulmonary arteries. Loss of pulmonary arterial compliance has been consistently shown to be a predictor of increased mortality in patients with pulmonary hypertension, even more so than pulmonary vascular resistance in some studies. Decreased pulmonary arterial compliance causes premature reflection of waves from the distal pulmonary vasculature, leading to increased pulsatile right ventricular afterload and eventually right ventricular failure. Evidence suggests that decreased pulmonary arterial compliance is a cause rather than a consequence of distal small vessel proliferative vasculopathy. Pulmonary arterial compliance decreases early in the disease process even when pulmonary artery pressure and pulmonary vascular resistance are normal, potentially enabling early diagnosis of pulmonary vascular disease, especially in high-risk populations. With the recognition of the prognostic importance of pulmonary arterial compliance, its impact on right ventricular function, and its contributory role in the development and progression of distal small-vessel proliferative vasculopathy, pulmonary arterial compliance is an attractive target for the treatment of pulmonary hypertension.
Keywords: stiffness, impedance, resistance, right ventricle, heart failure
Arterial compliance permits passive arterial expansion during ventricular systole to accommodate much of the stroke volume and enable arterial recoil, thus continuing blood flow during diastole. Compliance is calculated as the change in cross-sectional area or volume over change in pressure at a fixed vessel length (1). Passive arterial expansion buffers ventricular contraction through arterial–ventricular coupling, the interaction between the ventricle and the arterial system, which maintains low pulse pressure and low pulsatile cardiac afterload. Loss of arterial compliance (increased arterial stiffness) increases pulse pressure, pulsatile afterload, and pulse wave velocity, causing premature wave reflections from the distal vessels to the proximal conduit arteries during late systole (1).
Systemic arterial stiffness increases with age and is strongly associated with systemic hypertension (2). Evidence indicates aortic stiffness precedes systemic hypertension (3–5). Importantly, increased systemic arterial stiffness predicts a heightened risk of myocardial infarction, stroke, and renal dysfunction (6–10). Pulmonary hypertension (PH) is also characterized by increased pulmonary arterial stiffness. There is a growing appreciation of the prognostic value of pulmonary arterial compliance in PH (11–16), and some evidence indicates pulmonary arterial compliance is a better predictor of outcomes than pulmonary vascular resistance (PVR) (17, 18). Here, we review the critical role of pulmonary arterial compliance in PH.
Right Ventricular Afterload
Right ventricular (RV) afterload is composed of steady and pulsatile components. The steady component represents opposition to forward flow, and the pulsatile component represents the energy required to overcome increased systolic pressure during ejection. In the systemic circulation, the pulsatile aortic component contributes only 10% of the total afterload, but in the pulmonary circulation the pulsatile afterload contributes approximately 23% of the workload (19), and this percentage changes minimally with pulmonary vascular disease (19). Total RV afterload is described by pulmonary input impedance, which is physiologically difficult to measure and interpret (20). A more practical model for estimating RV afterload is the three-element Windkessel model (21), which defines RV afterload in three parameters: resistance, compliance, and characteristic impedance (21).
PVR represents resistance in the Windkessel model: the mean resistive or “static” afterload. PVR is determined by the small distal pulmonary arteries based on Poiseuille’s law, which states that resistance is inversely proportional to the fourth power of the radius of the blood vessel and directly proportional to the length of the blood vessel and viscosity of the blood. PVR is defined as the ratio of the transpulmonary gradient (difference between mean pulmonary artery [PA] pressure and pulmonary capillary wedge pressure) over cardiac output. Although PVR accounts for only about 75% of the RV afterload and does not account for the pulsatile component, it is commonly used clinically to describe total RV afterload (22).
Pulmonary arterial compliance is the compliance portion of the three-element Windkessel model. It describes the pulsatile afterload that accounts for approximately one-fourth of the total RV afterload (21). Unlike the systemic circulation, where 80% of the compliance is attributable to proximal aortic elasticity, in the pulmonary circulation, owing to a large number of branching vessels, compliance is distributed throughout the pulmonary circuit (19). The main, proximal left and right pulmonary arteries together contribute only 15–20% of the total pulmonary arterial compliance (23); the distal pulmonary arterial bed contributes the major portion of both resistance and compliance in the pulmonary circulation. However, the proximal pulmonary arteries do play an important role in buffering pulsatile RV ejection and in RV–PA coupling. Table 1 compares and contrasts the elements of the Windkessel model (resistance and compliance) in the systemic and pulmonary circulation. Pulmonary arterial compliance is being increasingly used to evaluate RV afterload, along with PVR, as PVR alone inconsistently predicts outcomes in pulmonary artery hypertension (PAH).
Table 1.
Comparison of elements of Windkessel model in systemic and pulmonary circulation
| Characteristic (Ref. No.) | Pulmonary Circulation |
Systemic Circulation |
||
|---|---|---|---|---|
| Normal | Pulmonary Hypertension | Normal | Systemic Hypertension | |
| Location of vascular compliance (21) | 20% compliance in main, left and right pulmonary arteries; 80% located beyond first branches | 20% compliance in main, left and right pulmonary arteries; 80% located beyond first branches | 80% of compliance in proximal conduit vessels | 80% of compliance in proximal conduit vessels |
| Pulsatile load, % (19, 21) | 25 | 25 | 5–10 | 5–10 |
| Compliance, ml/mm Hg (15, 17, 21) | 3.8–12 | 0.4–3.8 | 2.5 | 0.8 |
| Resistance, mm Hg⋅s/ml (21, 60) | 0.11 | >0.18 | 1.0 | 1.2 |
The third element of the Windkessel model is the characteristic input impedance of the proximal pulmonary arteries, which describes the effects of blood mass on RV afterload (21). It is a relatively small component of RV afterload (21) and is not routinely used.
Methods to Measure Pulmonary Arterial Compliance
Several methods have been proposed to measure total arterial compliance in the systemic and pulmonary circulations, based on the Windkessel model (24). Of these, the ratio of stroke volume to PA pulse pressure measured by right heart catheterization is the simplest and most practical method for estimating pulmonary artery compliance (15). This method overestimates compliance, as it does not account for blood flow from the pulmonary circulation into the capillary bed during systole. Nevertheless, it highly correlates with pulmonary artery compliance measured on the basis of the lumped two-element and three-element Windkessel models (25). Stroke volume and PA pressure can also be estimated noninvasively by two-dimensional and Doppler echocardiography. Mahapatra and colleagues estimated PA systolic and diastolic pressures, using the peak systolic tricuspid regurgitation velocity and the end-diastolic pulmonary regurgitation velocity, and stroke volume, by volumetric flow through the left ventricular outflow tract (14). Although indicative of compliance, this noninvasive method lacks accuracy because of the numerous estimates involved.
More recently, cardiac magnetic resonance imaging (MRI) has been used to estimate pulmonary artery compliance by combining invasive pressure measurements and cardiac MRI–derived flow data (26). Pulmonary artery compliance derived by this method strongly correlates with compliance estimated by stroke volume over PA pulse pressure (27).
Insights into pulmonary artery compliance can be gained through noninvasive measurements (28, 29). These measurements of PA stiffness (pulsatility, compliance, capacitance, distensibility, elastic modulus, and stiffness index) obtained using cardiac MRI–derived PA dimensions, in combination with right heart catheterization–derived pressure data performed on the same day, correlate with PH severity (30).
Pulmonary Arterial Compliance Is Decreased in Pulmonary Hypertension
The normal pulmonary circulation is a low-pressure, high-compliance system that can handle large increases in cardiac output that occur during exercise. As in systemic hypertension, pulmonary arterial compliance decreases in PH (15, 30), and it consistently correlates with PH severity (30). Loss of vascular compliance in PH is clearly associated with accumulation of collagen and loss of elastin in the proximal pulmonary arteries in adult-onset and neonatal PH (31–34).
Decreased Pulmonary Arterial Compliance Induces Distal Proliferative Vasculopathy
The understanding of the temporal relationship between PH and decreased pulmonary artery compliance has evolved. Initially, decreased pulmonary artery compliance in PH was thought to be a consequence of distal small-vessel proliferative vasculopathy leading to increased PVR and mean PA pressure. Certainly, increased mean PA pressure decreases compliance as a result of the nonlinear elasticity of the arteries (35). However, evidence suggests loss of pulmonary artery compliance may actually initiate PH (Figure 1) (36). Patients with exercise-induced PH and those with mild pulmonary vascular disease have reduced pulmonary artery compliance, despite a normal resting PA pressure (30, 37, 38). This indicates pulmonary artery compliance changes early, even when the resting pulmonary artery pressures are within normal limits, and thus, loss of pulmonary artery compliance could contribute to the development and progression of PH (30). It is also possible that the mild increase in PVR with exercise could potentially decrease compliance because of nonlinear elasticity of the arteries.
Figure 1.
Effect of decreased pulmonary arterial compliance in pulmonary hypertension. PA = pulmonary artery; PAC = pulmonary arterial compliance; RV = right ventricle.
In support of the hypothesis that loss of pulmonary arterial compliance causes distal proliferative vasculopathy, disruption of the internal elastic lamina occurs before the onset of pulmonary artery smooth muscle cell hypertrophy and endothelial cell proliferation (39). In the monocrotaline-induced rat PH model, fragmentation of the internal elastic lamina in the hilar pulmonary arteries occurs 2 days after monocrotaline injection and 14 days before pulmonary artery smooth muscle hypertrophy. Disruption of the internal elastic lamina is associated with increased elastolytic activity of serine elastases and matrix metalloproteinases in this animal model.
Similar findings are reported in animal models of chronic hypoxic PH (40). Inhibition of serine elastases prevents development of PH in both these animal models, suggesting that disruption of the elastic lamina is the inciting event for the pulmonary artery vasculopathy rather than a consequence (40–43). Although pulmonary artery compliance was not assessed during the early stages of PH in these animal models, loss of elastic tissue has been consistently associated with decreased vascular compliance (31, 44). Pulmonary vasoconstriction is clearly an early contributor to the development of PH in hypoxia and other models. It is likely that vasoconstriction coexists with morphological changes such as disruption of internal elastic lamina.
A mechanism by which reduced pulmonary artery compliance can induce proliferative vasculopathy in the distal small pulmonary arteries has been identified (36). In a process known as mechanotransduction, endothelial cells of the distal small pulmonary arteries sense highly pulsatile flow from decreased vascular compliance and transduce it into a signaling cascade leading to a proinflammatory response and activation of vasoactive cytokines and growth factors, characterized by increased Toll-like receptor-2 expression and NF-κB activation (45). Similar findings are observed in endothelial cells from small pulmonary arteries of animals with experimental PH and of human patients with PAH (45, 46).
Furthermore, highly pulsatile flow decreases expression of endothelial nitric oxide synthase (a potent vasodilator) and increases expression of endothelin and angiotensin-converting enzyme (potent vasoconstrictors and smooth muscle mitogens) and transforming growth factor-β1 in pulmonary artery endothelial cells (47). Collectively, the inflammatory response, vasoactive cytokines, and growth factors cause pulmonary artery smooth muscle hypertrophy with increased expression of contractile proteins, smooth muscle α-actin, and smooth muscle myosin heavy chain (47). Finally, highly pulsatile flow increases expression of mechanosensitive transient receptor potential (TRP) channels in pulmonary artery smooth muscle cells, leading to more intracellular calcium signaling and smooth muscle proliferation (48).
Suppression of cyclooxygenase (COX-2) expression is also implicated in the pathogenesis of pulmonary vascular remodeling induced by decreased pulmonary artery compliance. In vitro experiments show that the absence of COX-2 enhances stiffness-induced proliferation of human pulmonary artery smooth muscle cells and increases production of the extracellular matrix proteins collagen and fibronectin. Overexpression of COX-2 reduces stiffness-induced increases in extracellular matrix deposition. In addition, COX-2 deficiency (e.g., COX-2–deficient mice or wild-type mice in which COX-2 is pharmacologically inhibited) exacerbates hypoxia-induced PH and pulmonary artery smooth muscle cell hypertrophy (49).
Increased stiffness not only promotes vascular smooth muscle proliferation, but also activates fibroblasts in a feedback loop mechanism, leading to further extracellular matrix deposition and fibrosis. There is increased fibroblast proliferation, contraction, and matrix synthesis when fibroblasts are grown in stiff matrices but not in physiologically compliant matrices. Stiffness-induced fibroblast activation is mediated through activation of transcription factors, “Yes-associated proteins” (YAP), and transcriptional coactivator with PDZ-binding motif (TAZ) (50, 51).
There is an interesting parallel between the proliferative effect of the stiffer matrix on fibroblasts and pulmonary artery smooth muscle cells and a similar effect described in airway smooth muscle cells (52) and cancer cells (53). Work by Barman and colleagues suggests a central role for adventitial fibroblasts in the pathophysiology of pulmonary vascular remodeling in three animal models of PAH (54). These observations raise the possibility that therapy that increases pulmonary artery compliance might prevent or reverse pulmonary vascular remodeling in PH. However, this concept needs to be tested.
Effect of Decreased Pulmonary Artery Compliance on Right Ventricular Function
Decreased pulmonary artery compliance in PH increases RV pulsatile workload (22, 55), and therefore the RV must generate increased pressure to eject blood. Under normal conditions, reflected waves from the pulmonary vasculature return to the pulmonic valve during diastole, at or just before the dicrotic notch of the pulmonary artery waveform, and thus do not impact right ventricular ejection. However, with decreased pulmonary artery compliance and increased pulse wave velocity, reflected waves appear during mid or late systole, resulting in increased PA systolic pressure, pulse pressure, and RV pulsatile afterload (56). The elevated pulmonary artery systolic pressure increases RV wall stress and oxygen consumption. This over time leads to RV hypertrophy, RV dilation, and reduced cardiac output (RV–PA uncoupling) regardless of any improvement achieved in PVR, ultimately leading to right heart failure and death (Figure 1) (22, 57, 58).
It has been proposed that the RV initially adapts by compensatory hypertrophy to maintain cardiac output, but over time this compensatory method fails, leading to right heart failure and decreased cardiac output (57). However, whether there are such distinct adaptive or maladaptive RV hypertrophy programs that differentially determine the timing and degree of RV–PA uncoupling is unclear (59).
Decreased pulmonary artery compliance is independently associated with RV dysfunction, dilatation, and hypertrophy (60). The relative contribution of pulmonary artery compliance to RV stroke work index (a measure of RV contractility and workload) is 1.2- to 18-fold higher than PVR (60), further emphasizing the important contribution of pulmonary artery compliance to RV function and energetics.
The Resistance–Compliance Relationship in the Pulmonary Circulation
Resistance and compliance in the pulmonary circulation exhibit an inverse hyperbolic relationship. The product of resistance and compliance (RC time) is mostly constant (61, 62) in both healthy and diseased states, except for a few exceptions discussed below (61, 62).
RC Time Is Constant
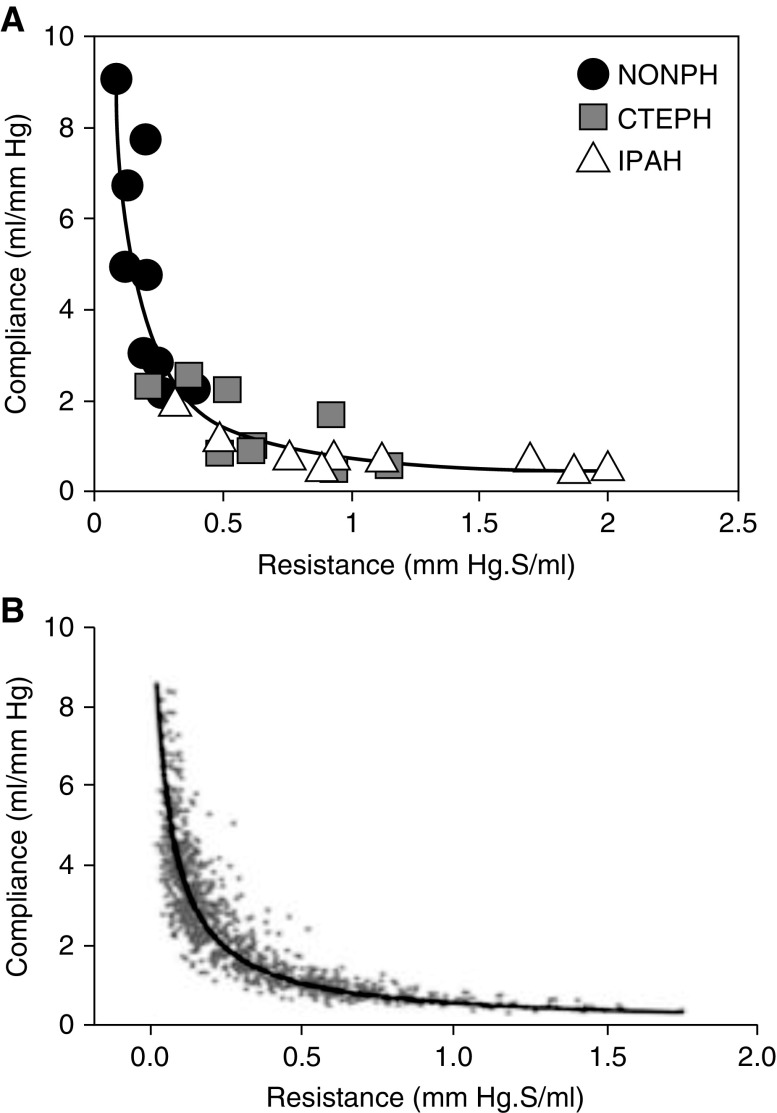
Lankhaar and colleagues quantified RV afterload, using the three-element Windkessel model in patients with idiopathic PAH, patients with chronic thromboembolic PH, and healthy control subjects (62). They showed that PVR and pulmonary artery compliance exhibited an inverse-hyperbolic relationship and that the RC time was constant (0.48 ± 0.17 s), regardless of the underlying disease state (63) (Figure 2A). Similarly, Tedford and colleagues demonstrated an inverse-hyperbolic relationship between PVR and pulmonary artery compliance with a constant RC time (0.48 ± 0.17 s) in 1,009 patients with suspected or confirmed PH and normal pulmonary capillary wedge pressure (Figure 2B). This relationship was unaffected by interstitial lung disease and was minimally affected by age (61). Similarly, in the study by Lankhaar and colleagues of 52 patients with PAH and 10 patients with distal chronic thromboembolic disease, mentioned previously, RC time did not change with PAH-specific therapy (62).
Figure 2.
Inverse hyperbolic relationship between resistance and compliance in the pulmonary circulation as depicted by (A) Lankhaar and colleagues (adapted by permission from Reference 62) and (B) Tedford and colleagues (adapted by permission from Reference 61). CTEPH = chronic thromboembolic pulmonary hypertension; IPAH = idiopathic pulmonary artery hypertension; NONPH = healthy control subjects.
Determinants of Resistance–Compliance Relationship
Two reasons have been proposed for the constant relationship between PVR and pulmonary artery compliance. First, increased resistance raises intravascular pressure, which decreases compliance because of the nonlinear elasticity of the arteries (35). The second reason is based on the uniform distribution of compliance throughout the pulmonary circulation. The small distal vessels account for most of the resistance and compliance in the pulmonary circulation. This is due to the 10 times greater number of distal small arterioles in the pulmonary compared with the systemic circulation (21, 23). In patients with PAH and an acute vasodilator response, after inhalation of nitric oxide, pulmonary arterial compliance decreases immediately with a proportional decrease in PVR (64). Because inhaled nitric oxide is believed to act locally adjacent to the alveolar space, this would suggest that the distal pulmonary arteries are the major determinant of both pulmonary arterial compliance and PVR.
Clinical Implications of a Constant Resistance–Compliance Relationship
The constant relationship between PVR and pulmonary artery compliance has important clinical applications. The hyperbolic shape of the pulmonary artery compliance–PVR curve suggests that substantial declines in pulmonary artery compliance occur before increases in PVR (61, 62), as suggested earlier. Thus, assessment of pulmonary artery compliance may allow for diagnosis of pulmonary vascular disease before PVR elevations.
Factors Influencing the Resistance–Compliance Relationship in the Pulmonary Circulation
Elevated Left-Heart Filling Pressures
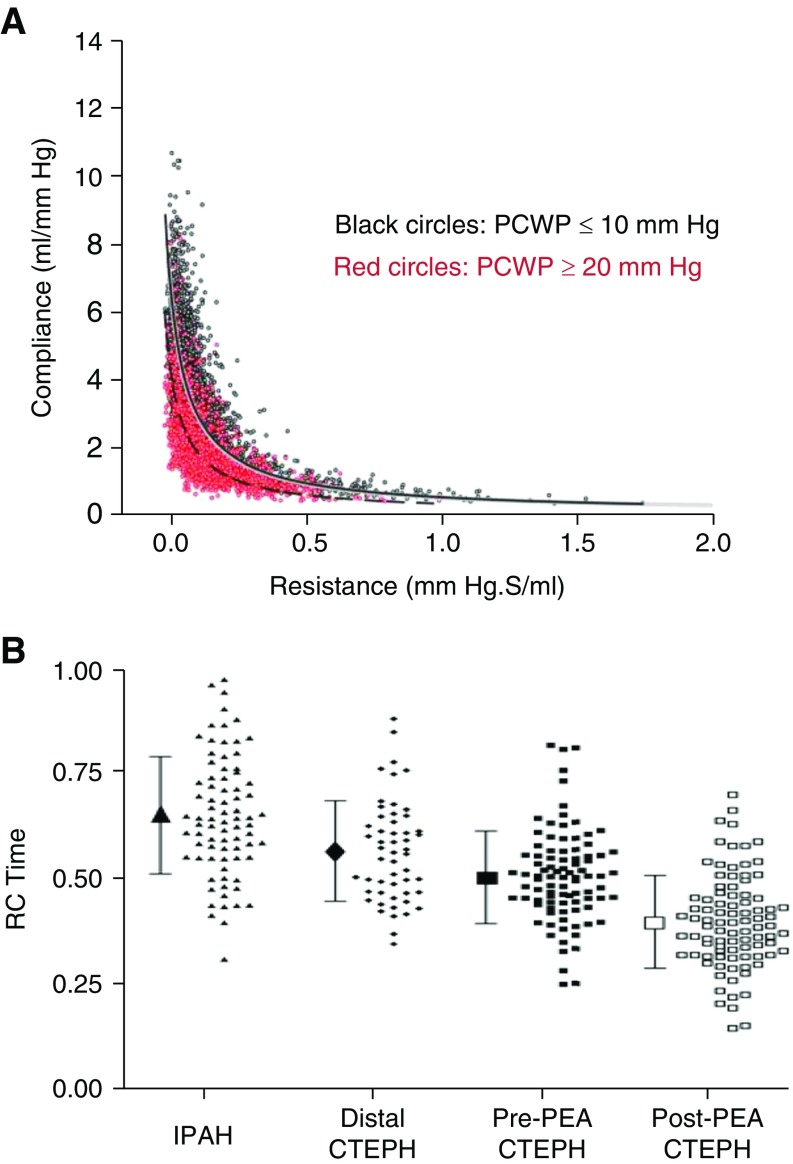
Elevated left-sided filling pressures affect the PVR and pulmonary artery compliance relationship by shifting the curve leftward and downward (Figure 3A). Thus, for any given PVR, the pulmonary artery compliance is lower, with a lower RC time, when pulmonary capillary wedge pressure is elevated (61). Reduced RC time in the setting of elevated left-sided filling pressures increases the pulsatile afterload of the RV (61). Hence, patients with PH due to left heart disease are more prone to develop right heart failure due to lower pulmonary artery compliance for any given PVR compared with those who have normal pulmonary capillary wedge pressures. This may explain why RV function is a leading outcome determinant in patients with impaired systolic and/or diastolic left ventricular function (65, 66).
Figure 3.
Alteration in resistance–compliance relationship in pulmonary hypertension. (A) Elevated pulmonary capillary wedge pressure shifts the PVR–PAC curve to the left as depicted by Tedford and colleagues (adapted by permission from Reference 61). (B) RC time is reduced in CTEPH patients both pre- and postendarterectomy as depicted by MacKenzie and colleagues (adapted by permission from Reference 67). CTEPH = chronic thromboembolic pulmonary hypertension; IPAH = idiopathic pulmonary artery hypertension; PCWP = pulmonary capillary wedge pressure; PEA = pulmonary endarterectomy; RC = resistance and compliance.
Influence of Proximal Chronic Thromboembolic Pulmonary Hypertension and Pulmonary Endarterectomy
Patients with proximal chronic thromboembolic pulmonary vascular disease also have reduced RC time. Ross and colleagues demonstrated lower RC time in patients with proximal chronic thromboembolic PH compared with idiopathic PAH or distal chronic thromboembolic pulmonary hypertension (CTEPH) (Figure 3B) (67). This is caused by increased wave reflection from proximal obstructions. Similar findings were observed when Pagnamenta and colleagues compared RC time in experimental animal models of proximal and distal CTEPH. Compared with dogs with distal CTEPH induced by microembolization, dogs with proximal CTEPH induced by pulmonary artery ensnarement had a lower RC time and increased RV pulsatile afterload (68).
Finally, age affects the relationship between resistance and compliance in the pulmonary circulation. Increased age is associated with a slightly lower RC time, with a 20% reduction in pulmonary artery compliance in those at least 70 years old (61). There is also a sex-based difference in proximal aortic stiffness and ventriculo–vascular interaction. Proximal aortic stiffness is greater in women than men, predisposing them to increased pulsatile afterload of the left ventricle (69, 70). Whether sex affects RC time in the pulmonary circulation is not known.
Prognostic Significance of Pulmonary Artery Compliance
Decreased pulmonary artery compliance increases the risk of mortality in patients with PAH (13–16). In a landmark study of 109 patients with PAH, pulmonary artery compliance was the strongest predictor of mortality. Every 1-unit (ml/mm Hg) decrease in pulmonary artery compliance resulted in a 17-fold increased risk of death. Patients with the lowest quartile pulmonary artery compliance (<0.81 ml/mm Hg) had less than 40% survival at 4 years, but patients with the highest quartile pulmonary artery compliance (>2.00 ml/mm Hg) had 100% survival at 4 years (15). In this study, PVR was not associated with increased mortality, suggesting pulmonary artery compliance is a better predictor of outcomes. Decreased pulmonary artery compliance has also been associated with increased mortality in children with PAH (11).
Furthermore, increased pulmonary artery stiffness measured by cardiac MRI is associated with increased mortality in patients with PAH (16). When compared with control subjects, patients with PAH have a smaller relative area change of the right pulmonary artery. As in pulmonary artery compliance, relative area change has a hyperbolic relationship with mean pulmonary artery pressure. Relative area change less than 16% is associated with poor survival (16).
Decreased pulmonary artery compliance is also associated with increased mortality in patients with PH due to left heart failure (World Health Organization group II) (12, 17, 18, 71). In 463 ambulatory patients with heart failure and reduced ejection fraction, pulmonary artery compliance not exceeding 2.0 ml/mm Hg had a 3.5-fold increased risk of death compared with those with pulmonary artery compliance greater than 5.0 ml/mm Hg (71). Moreover, Pellegrini and colleagues examined 306 patients with similar characteristics and found decreased pulmonary artery compliance (<2.15 ml/mm Hg) increased risk of mortality (17). Importantly, reduced pulmonary artery compliance (<2.15 ml/mm Hg) predicted increased risk of death, even in patients with normal PVR (17). More recently, pulmonary artery compliance was found to be a better predictor of mortality than PVR in patients with PH due to heart failure with preserved ejection fraction (18), reinforcing the importance of pulmonary artery compliance in clinical practice.
Could Assessment of Pulmonary Artery Compliance Promote Early Diagnosis of Pulmonary Hypertension?
At present, there is an unmet need for earlier diagnosis of PAH. Although survival in PAH has improved in the last two decades, it remains a fatal disease with a 1-year mortality of approximately 15–20% (72, 73). In the contemporary PAH registries, mean PVR at diagnosis ranges between 8 and 10 Wood units, which is associated with reduced pulmonary artery compliance (74–76), suggesting the disease is well established at diagnosis. Unfortunately, none of the currently available pulmonary vasodilator therapies, both mono- and combination therapies, effectively or consistently decrease PVR to a level on the RC curve with higher compliance (61). Sequential combination therapy with phosphodiesterase-5 inhibitors and endothelin receptor antagonists in the ATHENA (Add-on Ambrisentan Therapy to Background Phosphodiesterase Type-5 Inhibitor Therapy in Pulmonary Arterial Hypertension) study increased pulmonary artery compliance in some but not in all patients (77).
Assessment of pulmonary artery compliance can be a tool for the early diagnosis of PAH. In the early stages of pulmonary vascular disease, pulmonary artery compliance drops considerably while PVR increases minimally. Hence, serial assessment of pulmonary artery compliance can promote early diagnosis of pulmonary vascular disease even when PVR is within normal limits. This strategy might be useful especially in screening “high risk” patients (patients with connective tissue disease, portal hypertension, HIV, lung disease, left-sided heart disease, or a strong family history of PAH), as noninvasive measures of pulmonary artery compliance become more widely implemented. Serial echocardiography or cardiac MRI can potentially detect the onset of pulmonary vascular disease earlier when pulmonary arterial compliance decreases despite normal pulmonary artery pressures and PVR. Early diagnosis may potentially improve outcomes in PAH via early initiation of therapy; however, this has not yet been studied.
Pulmonary Artery Compliance as a Novel Therapeutic Target in Pulmonary Hypertension
With the recognition of the prognostic importance of pulmonary artery compliance, its role in the development and progression of distal small-vessel proliferative vasculopathy, and its impact on RV function, pulmonary artery compliance is an attractive target for the treatment of PH. However, whether a significant improvement in pulmonary arterial compliance will lead to an improvement in hard clinical outcomes, such as time to clinical worsening or more importantly survival, remains to be tested.
None of the currently available PAH-specific therapies, except parenteral prostacyclin, improve pulmonary arterial compliance (78). In a small single-center study, parenteral prostacyclin therapy improved pulmonary arterial compliance minimally, and this was associated with improvement in 6-minute-walk distance (78). A pooled analysis of four randomized controlled trials in PAH showed a small (0.2 ml/mm Hg), but significant, improvement in pulmonary artery compliance with pulmonary vasodilator therapy (79). However, change in compliance was not associated with a reduction in short-term (12 wk) clinical outcomes, likely due to the small magnitude of change (79). Thus, there is an unmet need for novel approaches to increase pulmonary artery compliance significantly and thereby hopefully improve RV function, the major determinant of long-term outcomes in PAH.
In conclusion, PH is associated with an early and progressive decrease in pulmonary artery compliance. Loss of vascular compliance increases the pulsatile afterload of the RV by causing premature reflection of waves from the distal pulmonary circulation (Figure 1). Furthermore, evidence suggests that highly pulsatile flow, resulting from decreased pulmonary artery compliance, plays an important role in the development and progression of distal small pulmonary artery vasculopathy (Figure 1). Loss of pulmonary artery compliance occurs early in the course of the disease process, even when PVR is normal, and is an independent and consistent predictor of mortality (Table 2 summarizes these key points). Going forward, pulmonary artery compliance may be an attractive target for treatment and an early screening tool for at-risk populations in PH.
Table 2.
Summary of key points
| Key Points |
|---|
| 1. Pulmonary arterial compliance represents pulsatile afterload of the right ventricle, which contributes to approximately one-fourth of the total right ventricular afterload. Unlike in the systemic circulation, it is distributed uniformly across the entire pulmonary circuit |
| 2. Pulmonary arterial compliance is a strong and independent predictor of mortality in patients with pulmonary hypertension, better than pulmonary vascular resistance |
| 3. Growing evidence suggests that decreased pulmonary arterial compliance is a cause rather than a consequence of distal small-vessel proliferative vasculopathy |
| 4. Pulmonary arterial compliance decreases early in the disease process even when pulmonary artery pressure and pulmonary vascular resistance are normal, potentially enabling early diagnosis and treatment of pulmonary vascular disease, especially in high-risk populations |
Footnotes
Supported by American Heart Association Scientist Development Award 15SDG25560048 (T.T.) and National Institutes of Health F32 Grant HL129554 (K.W.P.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 3.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, et al. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension. 2013;62:1105–1110. doi: 10.1161/HYPERTENSIONAHA.113.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, Hennigs JK, Salomons F, Eken S, Emrich FC, et al. Transcription factor runx2 promotes aortic fibrosis and stiffness in type 2 diabetes mellitus. Circ Res. 2015;117:513–524. doi: 10.1161/CIRCRESAHA.115.306341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–846. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- 7.Baulmann J, Homsi R, Uen S, Düsing R, Fimmers R, Vetter H, Mengden T. Pulse wave velocity is increased in patients with transient myocardial ischemia. J Hypertens. 2006;24:2085–2090. doi: 10.1097/01.hjh.0000244959.92856.7e. [DOI] [PubMed] [Google Scholar]
- 8.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 10.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douwes JM, Roofthooft MT, Bartelds B, Talsma MD, Hillege HL, Berger RM. Pulsatile haemodynamic parameters are predictors of survival in paediatric pulmonary arterial hypertension. Int J Cardiol. 2013;168:1370–1377. doi: 10.1016/j.ijcard.2012.12.080. [DOI] [PubMed] [Google Scholar]
- 12.Dragu R, Rispler S, Habib M, Sholy H, Hammerman H, Galie N, Aronson D. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur J Heart Fail. 2015;17:74–80. doi: 10.1002/ejhf.192. [DOI] [PubMed] [Google Scholar]
- 13.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:1045–1050. doi: 10.1016/j.echo.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 16.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;132:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, et al. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest. 2014;145:1064–1070. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 18.Al-Naamani N, Preston IR, Paulus JK, Hill NS, Roberts KE. Pulmonary arterial capacitance is an important predictor of mortality in heart failure with a preserved ejection fraction. JACC Heart Fail. 2015;3:467–474. doi: 10.1016/j.jchf.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saouti N, Westerhof N, Helderman F, Marcus JT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right ventricular oscillatory power is a constant fraction of total power irrespective of pulmonary artery pressure. Am J Respir Crit Care Med. 2010;182:1315–1320. doi: 10.1164/rccm.200910-1643OC. [DOI] [PubMed] [Google Scholar]
- 20.Chesler NC, Roldan A, Vanderpool RR, Naeije R. How to measure pulmonary vascular and right ventricular function. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:177–180. doi: 10.1109/IEMBS.2009.5333835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saouti N, Westerhof N, Postmus PE, Vonk-Noordegraaf A. The arterial load in pulmonary hypertension. Eur Respir Rev. 2010;19:197–203. doi: 10.1183/09059180.00002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62(25) Suppl:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H2154–H2160. doi: 10.1152/ajpheart.00694.2009. [DOI] [PubMed] [Google Scholar]
- 24.Stergiopulos N, Meister JJ, Westerhof N. Evaluation of methods for estimation of total arterial compliance. Am J Physiol. 1995;268:H1540–H1548. doi: 10.1152/ajpheart.1995.268.4.H1540. [DOI] [PubMed] [Google Scholar]
- 25.Chemla D, Hébert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume–to–aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 26.Schiebler ML, Bhalla S, Runo J, Jarjour N, Roldan A, Chesler N, François CJ. Magnetic resonance and computed tomography imaging of the structural and functional changes of pulmonary arterial hypertension. J Thorac Imaging. 2013;28:178–193. doi: 10.1097/RTI.0b013e31828d5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthurangu V, Atkinson D, Sermesant M, Miquel ME, Hegde S, Johnson R, Andriantsimiavona R, Taylor AM, Baker E, Tulloh R, et al. Measurement of total pulmonary arterial compliance using invasive pressure monitoring and MR flow quantification during MR-guided cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;289:H1301–H1306. doi: 10.1152/ajpheart.00957.2004. [DOI] [PubMed] [Google Scholar]
- 28.Tian L, Chesler NC. In vivo and in vitro measurements of pulmonary arterial stiffness: a brief review. Pulm Circ. 2012;2:505–517. doi: 10.4103/2045-8932.105040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger RM, Cromme-Dijkhuis AH, Hop WC, Kruit MN, Hess J. Pulmonary arterial wall distensibility assessed by intravascular ultrasound in children with congenital heart disease: an indicator for pulmonary vascular disease? Chest. 2002;122:549–557. doi: 10.1378/chest.122.2.549. [DOI] [PubMed] [Google Scholar]
- 30.Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Dodson RB, Morgan MR, Galambos C, Hunter KS, Abman SH. Chronic intrauterine pulmonary hypertension increases main pulmonary artery stiffness and adventitial remodeling in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2014;307:L822–L828. doi: 10.1152/ajplung.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ooi CY, Wang Z, Tabima DM, Eickhoff JC, Chesler NC. The role of collagen in extralobar pulmonary artery stiffening in response to hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1823–H1831. doi: 10.1152/ajpheart.00493.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure–function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol. 2008;295:H1451–H1459. doi: 10.1152/ajpheart.00127.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC. Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H1209–H1217. doi: 10.1152/ajpheart.01129.2003. [DOI] [PubMed] [Google Scholar]
- 35.Reuben SR. Compliance of the human pulmonary arterial system in disease. Circ Res. 1971;29:40–50. doi: 10.1161/01.res.29.1.40. [DOI] [PubMed] [Google Scholar]
- 36.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? [2013 Grover Conference series] Pulm Circ. 2014;4:560–580. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau E, Chemla D, Godinas L, Zhu K, Sitbon O, Savale L, Jais X, Montani D, Simonneau G, Humbert M, et al. Reduced distensibility of the pulmonary circulation during exercise in early pulmonary vascular disease [abstract] Am J Respir Crit Care Med. 2015;191:A4787. [Google Scholar]
- 38.Mullin CJTR, Damico RL, Kolb TM, Kass DA, Hassoun PM, Mathai SC. Resting compliance and stroke volume is associated with abnormal pulmonary vascular response to exercise in systemic sclerosis [abstract] Am J Respir Crit Care Med. 2015;191:A4790. [Google Scholar]
- 39.Todorovich-Hunter L, Dodo H, Ye C, McCready L, Keeley FW, Rabinovitch M. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis. 1992;146:213–223. doi: 10.1164/ajrccm/146.1.213. [DOI] [PubMed] [Google Scholar]
- 40.Maruyama K, Ye CL, Woo M, Venkatacharya H, Lines LD, Silver MM, Rabinovitch M. Chronic hypoxic pulmonary hypertension in rats and increased elastolytic activity. Am J Physiol. 1991;261:H1716–H1726. doi: 10.1152/ajpheart.1991.261.6.H1716. [DOI] [PubMed] [Google Scholar]
- 41.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation. 2002;105:516–521. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 42.Ilkiw R, Todorovich-Hunter L, Maruyama K, Shin J, Rabinovitch M. SC-39026, a serine elastase inhibitor, prevents muscularization of peripheral arteries, suggesting a mechanism of monocrotaline-induced pulmonary hypertension in rats. Circ Res. 1989;64:814–825. doi: 10.1161/01.res.64.4.814. [DOI] [PubMed] [Google Scholar]
- 43.Ye CL, Rabinovitch M. Inhibition of elastolysis by SC-37698 reduces development and progression of monocrotaline pulmonary hypertension. Am J Physiol. 1991;261:H1255–H1267. doi: 10.1152/ajpheart.1991.261.4.H1255. [DOI] [PubMed] [Google Scholar]
- 44.Le VP, Stoka KV, Yanagisawa H, Wagenseil JE. Fibulin-5 null mice with decreased arterial compliance maintain normal systolic left ventricular function, but not diastolic function during maturation. Physiol Rep. 2014;2:e00257. doi: 10.1002/phy2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan Y, Tseng PO, Wang D, Zhang H, Hunter K, Hertzberg J, Stenmark KR, Tan W. Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-κB pathway. PLoS One. 2014;9:e102195. doi: 10.1371/journal.pone.0102195. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Li M, Tan Y, Stenmark KR, Tan W. High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-κB. Cardiovasc Eng Technol. 2013;4:26–38. doi: 10.1007/s13239-012-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol. 2013;304:L70–L81. doi: 10.1152/ajplung.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Song S, Yamamura A, Yamamura H, Ayon RJ, Smith KA, Tang H, Makino A, Yuan JX. Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307:C373–C383. doi: 10.1152/ajpcell.00115.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, et al. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation. 2008;117:2114–2122. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shkumatov A, Thompson M, Choi KM, Sicard D, Baek K, Kim DH, Tschumperlin DJ, Prakash YS, Kong H. Matrix stiffness–modulated proliferation and secretory function of the airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1125–L1135. doi: 10.1152/ajplung.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tilghman RW, Blais EM, Cowan CR, Sherman NE, Grigera PR, Jeffery ED, Fox JW, Blackman BR, Tschumperlin DJ, Papin JA, et al. Matrix rigidity regulates cancer cell growth by modulating cellular metabolism and protein synthesis. PLoS One. 2012;7:e37231. doi: 10.1371/journal.pone.0037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, et al. NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arterioscler Thromb Vasc Biol. 2014;34:1704–1715. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Z, Chesler NC. Pulmonary vascular wall stiffness: an important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm Circ. 2011;1:212–223. doi: 10.4103/2045-8932.83453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 2001;37:1085–1092. doi: 10.1016/s0735-1097(00)01212-2. [DOI] [PubMed] [Google Scholar]
- 57.Rich S. Right ventricular adaptation and maladaptation in chronic pulmonary arterial hypertension. Cardiol Clin. 2012;30:257–269. doi: 10.1016/j.ccl.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 58.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 59.Sutendra G1, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013;91:1315–1327. doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 60.Stevens GR, Garcia-Alvarez A, Sahni S, Garcia MJ, Fuster V, Sanz J. RV dysfunction in pulmonary hypertension is independently related to pulmonary artery stiffness. JACC Cardiovasc Imaging. 2012;5:378–387. doi: 10.1016/j.jcmg.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 63.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 64.Newman JH, Brittain EL, Robbins IM, Hemnes AR. Effect of acute arteriolar vasodilation on capacitance and resistance in pulmonary arterial hypertension. Chest. 2015;147:1080–1085. doi: 10.1378/chest.14-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 66.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacKenzie Ross RV, Toshner MR, Soon E, Naeije R, Pepke-Zaba J. Decreased time constant of the pulmonary circulation in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H259–H264. doi: 10.1152/ajpheart.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pagnamenta A, Vanderpool R, Brimioulle S, Naeije R. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol (1985) 2013;114:1586–1592. doi: 10.1152/japplphysiol.00033.2013. [DOI] [PubMed] [Google Scholar]
- 69.Nethononda RM, Lewandowski AJ, Stewart R, Kylinterias I, Whitworth P, Francis J, Leeson P, Watkins H, Neubauer S, Rider OJ. Gender specific patterns of age-related decline in aortic stiffness: a cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson. 2015;17:20. doi: 10.1186/s12968-015-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular–arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1:290–299. doi: 10.1016/j.jchf.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 72.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 74.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J. 2007;30:1103–1110. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 75.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 76.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 77.Patarroyo MSA, Gillies HC, Blair C, Shao Y, Pritzker M. Two drug therapy with PDE V inhibition and ambrisentan improves pulmonary artery compliance [abstract] Am J Respir Crit Care Med. 2013;187:A3286. [Google Scholar]
- 78.Brittain EL, Pugh ME, Wheeler LA, Robbins IM, Loyd JE, Newman JH, Austin ED, Hemnes AR. Prostanoids but not oral therapies improve right ventricular function in pulmonary arterial hypertension. JACC Heart Fail. 2013;1:300–307. doi: 10.1016/j.jchf.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ventetuolo CE, Gabler NB, Fritz JS, Smith KA, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Are hemodynamics surrogate end points in pulmonary arterial hypertension? Circulation. 2014;130:768–775. doi: 10.1161/CIRCULATIONAHA.114.009690. [DOI] [PMC free article] [PubMed] [Google Scholar]