Abstract
Malignant mesothelioma is an aggressive cancer largely associated with asbestos exposure. In this review, we will discuss the significant advancements in our understanding of its genetics and molecular biology and their translational relevance. Remarkable findings included the discovery of germline and somatic mutations of BRCA1 associated protein-1 (BAP1) in patients, and the genome-wide characterization of pathways altered in mesothelioma that could be potentially exploited to design novel therapeutic approaches. Nevertheless, the clinical translation of these molecular findings has been slow and insufficient. In order to rapidly move translation from the bench to the bedside, we believe that cooperative research efforts have to be further endorsed and promoted at all levels.
Keywords: malignant mesothelioma, therapy, translation, BAP1
Clinical Features of Malignant Mesothelioma
Malignant mesothelioma (MM) is a rare and aggressive cancer arising from mesothelial cells. It has an incidence of approximately 3.200 new cases per year in the U.S. and a median survival slightly over 1 year [1, 2]. Most often, MM develops in the pleural cavity of older male adults several decades after chronic exposure to carcinogenic mineral fibers, such as asbestos (Box 1) [1]. Traditionally, the pathogenesis of MM has been linked to asbestos-induced chronic inflammation [3]. Other extrinsic factors, such as irradiation, also increase the risk of developing MM [1].
Box 1. Asbestos and Other Carcinogenic Mineral Fibers.
Asbestos is a collective name that refers to six mineral fibers commercially used for their thermal stability, and thermal and electric resistance [1, 57]. The word “asbestos” itself derives from the ancient Greek for "inextinguishable” from α, "not", and σβννυμι, "to extinguish” [58]. The Roman historian Pliny the Elder in his masterpiece Natural History describes asbestos as an incombustible linen [59]. For its proprieties, asbestos has been extensively used in the manufacturing of pipes and fireplace cement, an in pipe and ceiling insulation, among others [1]. Nowadays, unsanitized old buildings, natural deposits of asbestos, as well as old asbestos caves still constitute sources of passive exposure that will last for many decades [1]. Conclusive association between asbestos use and MM has led over the past 20–30 years to a ban in its extraction and use in several Western countries, although in rapidly industrializing countries such as China, India, and Brazil, the use of asbestos is still unrestricted [1].
Finally, it should be noted that in nature, there are ~390 unregulated and potentially carcinogenic fibrous minerals, some of them – e.g., erionite – with an even stronger carcinogenic potential than asbestos itself [57]. Moreover, some man-made fibers, such as multi-walled carbon nanotubes, show in vitro and in vivo characteristics reminiscent of asbestos [60]. Finally, other factors have been associated with MM development, alone or as co-factors with asbestos [61, 62].
Considering all these factors, the incidence of MM world-wide will likely increase in the next decades.
Malignant transformation is however relatively rare also among professionally exposed individuals –only ~5% in asbestos miners [4]. To timely identify MM patients among asbestos-exposed individuals, a number of studies investigating putative biomarkers of MM have been conducted, although none of them is routinely used in clinical settings (Box 2).
Box 2. Biomarkers of Asbestos Exposure and Malignant Mesothelioma.
Once deposited in tissues, asbestos causes chronic inflammation and DNA damage [3]. Expectedly, compared to un-exposed controls, asbestos-exposed individuals showed deregulated blood levels of several inflammatory cytokines and chemokines [63, 64], higher levels of known growth factors associated to both wound healing and cancer progression [65, 66], as well as increased levels of DNA oxidative damage marker 8-hydroxy-2’-deoxyguanosine in circulating leukocytes [65–67]. Also, levels of anti-oxidant enzymes peroxiredoxins 1 and 2 [68] and tioredoxin-1 [69] were found increased in asbestos-exposed individuals. None of these biomarkers has however moved to clinical testing, mostly due to their poor specificity.
Once cancer develops, tumor-associated antigens or tumor-specific secreted proteins can be found in the blood of patients even before the clinical detection of the disease. In the case of MM, the most studied diagnostic biomarkers in the last decades are soluble mesothelin-related peptides (SMRPs) – which include soluble variants of the protein mesothelin, and megakaryocyte potentiating factor; osteopontin, an extracellular matrix glycoprotein; and fibulin-3, the product of the epidermal growth factor containing fibulin-like extracellular matrix protein 1 (EFEMP1) gene.
As of today, an immunosorbent assay against soluble mesothelin (Mesomark®) is the only FDA-approved test to aid in the diagnosis of epithelioid and biphasic MM. It should be noted that approval was given under the Humanitarian Device Exemption programs, which exempts from the effectiveness requirements of a pre-market approval. In fact, recent meta-analyses of the diagnostic accuracy of Mesomark® [70] or SMRPs in general [71] showed relatively poor sensitivities and specificities and significant heterogeneities. Some discrepancies have also been found in the case of osteopontin – as shown in a recent meta-analysis [72] – as well as for fibulin-3 [73, 74].
More recently, total and especially hyper-acetylated variant of HMGB1, a prototypical damage-associated protein [75], and mesothelioma-specific variants of the ENOX2 (ecto-nicotinamide adenine dinucleotide oxidase disulfide-thiol exchanger 2) protein [76] have also been proposed. These biomarkers however have been reported in single studies and need further validation before clinical testing.
MM is resistant to all available therapies [5]. Surgery is palliative and some suggest that it may prolong survival, however there are no randomized clinical trials to support this hypothesis [6], and the only such trial failed to show any benefit of surgery [7]. Chemotherapy with cisplatin and antifolates and/or loco-regional radiotherapy has a marginal effect on survival and mostly has a palliative role as well [8–10]. In late 2015, it was shown that the addition of the anti-angiogenic drug bevacizumab (a monoclonal antibody targeting the vascular endothelial growth factor, VEGF) to pemetrexed plus cisplatin significantly improved median overall survival in MM patients not amenable to receive curative surgery, from ~16 to ~19 months [11]. This was the first report of targeted therapy in MM showing significant results in a phase III clinical trial, although the modest improvement achieved can be questioned both from a clinical and a pharmaco-economical point of view.
Given its relative rarity, it is not surprising that our knowledge of MM from a genetic and molecular perspective has grown at a much slower pace compared to other more prevalent diseases, such as breast and lung cancer. Considering however the scientific advancements of the last decade, have we finally filled the most important gaps in our understanding of MM biology? Are we close to the turning point of being able to clinically translate our new findings? Or are we just like Achilles chasing his famous tortoise?
First Translation: from Genes to Targetable Pathways
Until recently, we had not identified a single gene influencing MM risk, despite strong suggestions of a genetic risk of MM due to the existence of several familial clusters of MM, especially in some villages in Cappadocia, Turkey [12]. In 2011, the analysis of two unrelated American families with high prevalence of MM has finally led to the identification of BAP1 (BRCA1 associated protein-1) as the first gene whose germline mutations are associated to a significantly higher risk of developing MM, and the first gene that modulates gene-environment interaction in cancer [13]. This discovery started the characterization of the novel “BAP1 cancer syndrome” [14], a syndrome that now also includes cutaneous and uveal melanoma, basal cell carcinoma, clear cell renal cell carcinoma, and other malignancies [15–20]. Surprisingly, MM patients with the BAP1 cancer syndrome have a better prognosis compared to others [21].
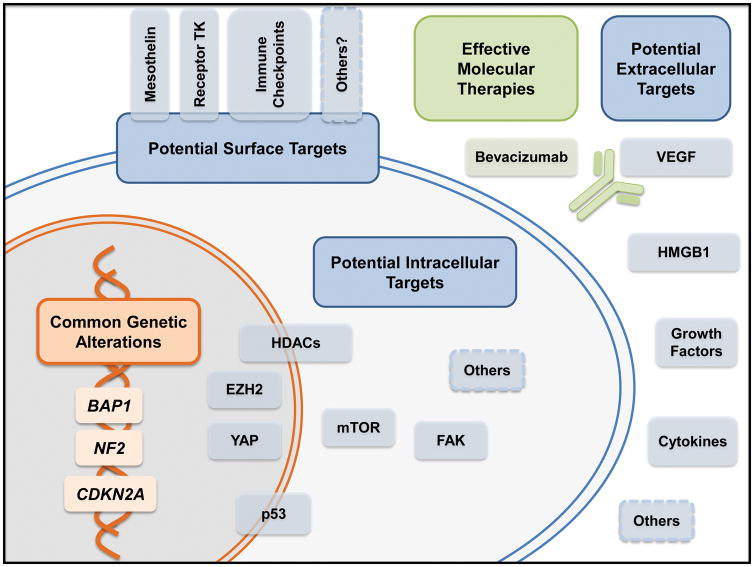
Independent animal models have supported the notion that BAP1 is a tumor suppressor gene whose mutations predispose to MM [22–24], as well as that low doses of asbestos might be sufficient to trigger MM in presence of a genetic predisposition [22]. However, germline BAP1 mutations are relatively rare events even among MM patients, as they are present in about 1–5% of unselected MM cases [13, 25, 26], and up to 18–20% of MM cases after careful clinical selection [27]. Therefore, germline variants in other unknown genes contributing to the individual risk of developing MM likely exists and will be hopefully identified soon. The US Department of Defense has recently funded a project with this specific goal to our group, and patients are currently being recruited in a similar study in several US institutions (NCT01590472). The pivotal role of BAP1 in the biology of MM is not only limited to its germline variants. In fact, in recent years loss of BAP1 protein has been reported in more than 50% of human MMs [13, 28–30], and somatic alterations in the gene encoding this deubiquitinating enzyme are among the most common events in MM – followed by alterations in NF2 (encoding merlin) and in CDKN2A (encoding p16INK4A and p14ARF). These results have been repeatedly confirmed and expanded upon by recent whole-exome [31, 32] and targeted next-generation sequencing studies [33]. Taking advantage of modern sequencing technologies, large-scale transcriptional profiling of MM has also been conducted [34, 35]. Based on these analyses, we now know that molecular subtypes of MM largely match their counterparts identified by classical histopathology –epithelioid, sarcomatoid, and biphasic MM – with the caveat that a subset of histologically epithelioid MMs, usually characterized by a better prognosis, transcriptionally and prognostically overlaps with the more aggressive biphasic and sarcomatoid subtypes [34, 35]. These studies and others have collectively shed light on the most frequently altered pathways in MM, paving the way to potential targeted therapies of epigenetic control via post-translational modification of histones, mTOR signaling, Hippo pathway, or the p53 pathway (Figure 1).
Figure 1 (Key Figure). Potential molecular targets in malignant mesothelioma.
The most common genetic mutations in mesothelioma are in the tumor suppressor genes BAP1, NF2, and CDKN2A. Although these molecules cannot be directly targeted, therapies can aim at surrogate intracellular targets whose activity is as a consequence increased or necessary. Promising therapies currently undergoing clinical trials include the use of monoclonal antibodies to target surface molecules specific for mesothelioma (e.g., mesothelin) and immune checkpoint (e.g. CTLA-4, PD1, PDL1). Inhibition of VEGF with the monoclonal antibody bevacizumab has been proven effective in a phase III clinical trial. Other potential soluble targets whose translational relevance is being currently investigated are also shown.
Second Translation: from Targetable Pathways to Candidate Drugs
Notably, most of the genetic alterations found in MM are loss of function of tumor suppressor genes, rather than activating events of proto-oncogenes. Contrary to what happens in other clinically relevant cases, e.g. overexpression of HER2 in breast cancer or expression of mutant ALK in lung cancer, the products of these mutations cannot therefore be directly inhibited, and surrogate targets whose activity is increased and necessary to cell survival have to be identified (Figure 1).
BAP1 is a deubiquitinating enzyme with several described biological functions, many of which could contribute to its tumor suppressive action [16]. In particular, BAP1 is directly involved in the epigenetic control of chromatin remodeling via deubiquitination of mono-ubiquitin from histone H2A lysine 119 [36]. BAP1 seems also to indirectly control other important aspects of epigenetic regulation of gene transcription, such as histone acetylation and methylation. Indeed, BAP1 might regulate the expression of histone deacetylases (HDACs), suggesting a potential window of opportunity for clinically available HDAC-inhibitors in BAP1-mutated tumors [37]. BAP1 loss is also associated to increased trimethylated histone H3 lysine 27 (H3K27me3), and elevated levels of EZH2 (enhancer of zeste homolog 2), a Polycomb repressive complex 2 subunit [38]. Preclinical evidences suggest that MM cells that lack BAP1 are selectively sensitive to pharmacologic inhibition of EZH2 [38].
Merlin (encoded by NF2) is another important tumor suppressor mutated in about 50% of MMs [33]. It normally interacts with a number of other molecules, and its loss results in up-regulation of at least three important cellular pathways: mammalian target of rapamycin (mTOR), focal adhesion kinase (FAK), and Hippo signaling pathways [39]. As for BAP1, targeting these surrogate activated molecules might hold the key to improve treatment of MM. Indeed, merlin-negative MM cells are more sensitive in vitro to the mTOR inhibitor rapamycin, compared to merlin-positive cells [40]. Similarly, FAK inhibitors also appear to be particularly active against merlin-negative cells [41, 42], and particularly against MM cancer stem cells [41]. Over-stimulation of the Hippo pathway in merlin-negative cells might be counteracted via inhibition of the transcriptional co-activator Yes-associated protein (YAP), which is constitutively activated in cells mutated for NF2 [43, 44]. A note of caution about the potential role of NF2 mutations in MM comes from the observation of Lo Iacono et al. that although 50% of the MMs tested contained NF2 mutations, 97% had normal NF2 expression by immunostaining [33]. It is still unclear what percentage of the detected mutations is therefore of minor biological significance and what percentage represents mutations that might deregulate NF2 without affecting protein expression and stability.
Finally, mutations in the CDKN2A locus, found in about 27–50% of MMs [31–33] result in alterations in the p53 and retinoblastoma pathways. Since TP53 is itself only rarely mutated in MM (about 8% of MMs) [34], the use of nutlin-3 (a drug that increases p53 stability) has been proposed to counterbalance the effects of p14ARF loss [45].
Besides targeting intracellular molecules whose expression levels and activity are selectively increased in the presence of particular mutations, other potential therapeutic approaches in MM include1) targeting soluble factors that promote MM growth or their cellular receptors; and 2) targeting tumor-associated surface antigens and stimulating the immune system to autonomously eliminate MM cells.
Bevacizumab is the most important example of the former option, and so far the only drug showing clinical activity [11]. Promiscuous inhibition of multiple receptor tyrosine kinases has failed in multiple phase II clinical trials [5]. Inhibition of the alarmin HMGB1 (High-mobility Group Box Protein 1) with salicylates or specific antagonists has also shown efficacy at a preclinical stage and is moving toward clinical testing [46].
The latter option is being explored in a number of ways. For example, the surface molecule mesothelin, which is over-expressed in epithelioid MM, has been targeted with antibodies and their conjugated versions, with vaccines, and with chimeric recombinant T cells [5]. Among these, amatuximab, a chimeric anti-mesothelin antibody [47], is being evaluated in the important phase II clinical trial ARTEMIS (ihttps://clinicaltrials.gov/ct2/show/NCT02357147). Other immune-therapeutic approaches in different phases of development include immune check-point inhibitors; vaccines against survivin, another tumor-associated antigen [48]; and oncolytic viruses, e.g. the trial NCT01503177 that is currently recruiting patients to test the efficacy of intrapleural injections of a modified strain of measles virus [49] (iihttps://clinicaltrials.gov/ct2/show/NCT01503177).
Third Translation: from Candidate Drugs to Effective Molecular Therapies
Candidate drugs with promising effects in preclinical model not always succeed when translated into human trials. Indeed, as of 2010, ~50% of all candidate drugs either failed during the phase III trial or were rejected by the national regulatory agency [50]. As previously mentioned, bevacizumab was the only targeted therapy that showed significant activity in a phase III clinical trial with advanced MM patients [11]. Given the amount of progress made at the “bench”, our failure at the “bedside” is surprisingly dismal.
Several drugs have been stopped early during clinical development due to lack of activity against MM, including tyrosine kinase inhibitors that effectively slowed growth of other tumor types, like sunitinib, dasatinib, and erlotinib [5]. These negative results seem to be constantly present in MM research, across different drug typologies and targets. The HDAC inhibitor vorinostat failed to improve overall survival as a second-line or third-line therapy in possibly the largest MM trial ever conducted (VANTAGE-014) [51], weakening preclinical findings on the activity of HDAC inhibitors in BAP1-mutated MM cells [37]. Moreover, in February of this year, the check-point inhibitor tremelimumab, which seemed to have clinical and immunological activity in advanced MM patients [52], was revealed by the developing company to be unable to increase overall survival as monoteraphy in MM patients in a phase IIb trial [53].
Concluding Remarks
The incidence of MM is expected to peak in the next 1–2 decades in many Western countries which have banned asbestos use, and will substantially increase also in developing countries which are still currently extracting and/or using asbestos [1]. What can we do to finally translate our remarkable advancements in understanding of MM genetics and molecular biology into clinical successes? This is one of few fundamental points in the mesothelioma research field that will have to be tackled as soon as possible (see Outstanding Questions).
Outstanding Questions.
Have we reached an understanding of mesothelioma biology sufficient to develop better therapies?
What will be the role of BAP1, NF2, and CDKN2A mutations in personalized therapy?
Will immunotherapy be a useful therapy in mesothelioma?
How can we build collaborative efforts to foster basic and translational research in the field of mesothelioma?
In the era of targeted therapies and selection of specific patients’ sub-populations based on biomarker expression, statistical restrictions and good sense impose to the scientific community that is studying MM, to collaborate to a much further extent. The VANTAGE-014 trial, other international collaborative trials, and finally the National Mesothelioma Virtual Bank – a virtual MM biospecimen registry (iiihttp://mesotissue.org/) – have taught us that joining forces is the only way to accelerate the clinical translation of our preclinical findings.
Instead, when groups do not work together, research is slowed down. For example, research in MM biomarker has not led yet to a clinically useful and widely accepted and validated biomarker. Validation has been often hampered by lack of reproducibility, in part due to differences in study population, sample collection and storage, and technical factors. As a community, we should support and encourage the standardization of the study of biomarkers in MM research, as elsewhere already proposed [54].
Finally, to succeed in increasing the overall survival of MM patients, we should also consider that MM is a polyclonal tumor [55], and that different clones may harbor different mutations that are susceptible to different drugs. Moreover, micro-environmental factors, such as hypoxia, have to be considered as factors modulating response to therapies and potential targets themselves [56]. Trials with molecular therapies should therefore always follow or at least be simultaneously performed with testing of predictive biomarkers. When multiple potential targets are identified, tailored combination therapies that simultaneously tackle different clones will have to be tested and only those will most likely provide a prolonged clinical benefit. As simultaneous administration of different molecular therapies might be toxic – a problem that so far has hampered several clinical trials in modern oncology – ideal combinations will have to be identified first.
Trends Box.
Malignant mesothelioma is a rare and poorly understood cancer with very limited therapeutic options
The last decade of research provided a better understanding of the molecular biology and genetics of mesothelioma
Translational applications findings have been so far limited and not effective
Acknowledgments
GRANT SUPPORT
This study was supported by the NCI-R01 CA198138, by the University of Hawai’i Foundation, which received an unrestricted gift to support MM research from Honeywell International Inc., and from the United-4-A-Cure Riviera Foundation to M.C.
Footnotes
CONFLICT OF INTEREST
MC has pending patent applications on BAP1 and provides consultation for MM expertise and diagnosis. AN declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carbone M, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol. 2012;227(1):44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henley SJ, et al. Mesothelioma incidence in 50 states and the District of Columbia, United States, 2003– 2008. Int J Occup Environ Health. 2013;19(1):1–10. doi: 10.1179/2049396712Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res. 2012;18(3):598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sluis-Cremer GK, et al. The mortality of amphibole miners in South Africa, 1946–80. Br J Ind Med. 1992;49(8):566–75. doi: 10.1136/oem.49.8.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bononi A, et al. Latest developments in our understanding of the pathogenesis of mesothelioma and the design of targeted therapies. Expert Rev Respir Med. 2015;9(5):633–54. doi: 10.1586/17476348.2015.1081066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone M, et al. Consensus Report of the 2015 Weinman International Conference on Mesothelioma. J Thorac Oncol. 2016 doi: 10.1016/j.jtho.2016.04.028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treasure T, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12(8):763–72. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelzang NJ, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 9.Stahel RA, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol. 2015;16(16):1651–8. doi: 10.1016/S1470-2045(15)00208-9. [DOI] [PubMed] [Google Scholar]
- 10.van Meerbeeck JP, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23(28):6881–9. doi: 10.1200/JCO.20005.14.589. [DOI] [PubMed] [Google Scholar]
- 11.Zalcman G, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10026):1405–14. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 12.Roushdy-Hammady I, et al. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357(9254):444–5. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- 13.Testa JR, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43(10):1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone M, et al. BAP1 and cancer. Nat Rev Cancer. 2013;13(3):153–9. doi: 10.1038/nrc3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiesner T, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011;43(10):1018–21. doi: 10.1038/ng.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbone M, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179. doi: 10.1186/1479-5876-10-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Fouchardiere A, et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet. 2015;88(3):273–7. doi: 10.1111/cge.12472. [DOI] [PubMed] [Google Scholar]
- 18.Wadt KA, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet. 2015;88(3):267–72. doi: 10.1111/cge.12501. [DOI] [PubMed] [Google Scholar]
- 19.Farley MN, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res. 2013;11(9):1061–71. doi: 10.1158/1541-7786.MCR-13-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popova T, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013;92(6):974–80. doi: 10.1016/j.ajhg.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann F, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36(1):76–81. doi: 10.1093/carcin/bgu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitano A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene. 2016;35(15):1996–2002. doi: 10.1038/onc.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014;74(16):4388–97. doi: 10.1158/0008-5472.CAN-14-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadariya Y, et al. Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sneddon S, et al. Absence of germline mutations in BAP1 in sporadic cases of malignant mesothelioma. Gene. 2015;563(1):103–5. doi: 10.1016/j.gene.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Rusch A, et al. Prevalence of BRCA-1 associated protein 1 germline mutation in sporadic malignant pleural mesothelioma cases. Lung Cancer. 2015;87(1):77–9. doi: 10.1016/j.lungcan.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Carbone M, et al. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015;11(12):e1005633. doi: 10.1371/journal.pgen.1005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bott M, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43(7):668–72. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasu M, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10(4):565–76. doi: 10.1097/JTO.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshikawa Y, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci. 2012;103(5):868–74. doi: 10.1111/j.1349-7006.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo G, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75(2):264–9. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 32.Ugurluer G, et al. Genome-based Mutational Analysis by Next Generation Sequencing in Patients with Malignant Pleural and Peritoneal Mesothelioma. Anticancer Res. 2016;36(5):2331–8. [PubMed] [Google Scholar]
- 33.Lo Iacono M, et al. Targeted next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol. 2015;10(3):492–9. doi: 10.1097/JTO.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 34.Bueno R, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48(4):407–16. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 35.de Reynies A, et al. Molecular classification of malignant pleural mesothelioma: identification of a poor prognosis subgroup linked to the epithelial-to-mesenchymal transition. Clin Cancer Res. 2014;20(5):1323–34. doi: 10.1158/1078-0432.CCR-13-2429. [DOI] [PubMed] [Google Scholar]
- 36.Scheuermann JC, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465(7295):243–7. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacco JJ, et al. Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors. Oncotarget. 2015;6(15):13757–71. doi: 10.18632/oncotarget.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaFave LM, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med. 2015;21(11):1344–9. doi: 10.1038/nm.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrilli AM, Fernandez-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene. 2016;35(5):537–48. doi: 10.1038/onc.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Lago MA, et al. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29(15):4235–49. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro IM, et al. Merlin deficiency predicts FAK inhibitor sensitivity: a synthetic lethal relationship. Sci Transl Med. 2014;6(237):237ra68. doi: 10.1126/scitranslmed.3008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poulikakos PI, et al. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25(44):5960–8. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 43.Li W, et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26(1):48–60. doi: 10.1016/j.ccr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Striedinger K, et al. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10(11):1204–12. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammoun S, et al. The p53/mouse double minute 2 homolog complex deregulation in merlin-deficient tumours. Mol Oncol. 2015;9(1):236–48. doi: 10.1016/j.molonc.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis. 2015;6:e1786. doi: 10.1038/cddis.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan R, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res. 2014;20(23):5927–36. doi: 10.1158/1078-0432.CCR-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann PR, et al. Preclinical development of HIvax: Human survivin highly immunogenic vaccines. Hum Vaccin Immunother. 2015;11(7):1585–95. doi: 10.1080/21645515.2015.1050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, et al. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17(8):550–8. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arrowsmith J. Trial watch: phase III and submission failures: 2007–2010. Nat Rev Drug Discov. 2011;10(2):87. doi: 10.1038/nrd3375. [DOI] [PubMed] [Google Scholar]
- 51.Krug LM, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE- 014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015;16(4):447–56. doi: 10.1016/S1470-2045(15)70056-2. [DOI] [PubMed] [Google Scholar]
- 52.Calabro L, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3(4):301–9. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 53.AstraZeneca. AstraZeneca reports top-line result of tremelimumab monotherapy trial in mesothelioma. 2016 Available from: iv https://www.astrazeneca.com/media-centre/press-releases/2016/astrazeneca-reports-top-line-result-of-tremelimumab-monotherapy-trial-in-mesothelioma-29022016.html.
- 54.Blyth KG. Inconsistent results or inconsistent methods? A plea for standardisation of biomarker sampling in mesothelioma studies. Thorax. 2015;70(4):374. doi: 10.1136/thoraxjnl-2014-206464. [DOI] [PubMed] [Google Scholar]
- 55.Comertpay S, et al. Evaluation of clonal origin of malignant mesothelioma. J Transl Med. 2014;12:301. doi: 10.1186/s12967-014-0301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paolicchi E, et al. Targeting hypoxic response for cancer therapy. Oncotarget. 2016;7(12):13464–78. doi: 10.18632/oncotarget.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol. 2013;14(7):576–8. doi: 10.1016/S1470-2045(13)70257-2. [DOI] [PubMed] [Google Scholar]
- 58.Shorter Oxford English Dictionary. 5. O.U. Press; 2002. [Google Scholar]
- 59.Pliny the Elder. Natural History. AD 77;Chapters IV and XXXVI [Google Scholar]
- 60.Nagai H, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci U S A. 2011;108(49):E1330–8. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carbone M, Rizzo P, Pass H. Simian virus 40: the link with human malignant mesothelioma is well established. Anticancer Res. 2000;20(2A):875–7. [PubMed] [Google Scholar]
- 62.Gazdar AF, Carbone M. Molecular pathogenesis of malignant mesothelioma and its relationship to simian virus 40. Clin Lung Cancer. 2003;5(3):177–81. doi: 10.3816/CLC.2003.n.031. [DOI] [PubMed] [Google Scholar]
- 63.Comar M, et al. Increased levels of C-C chemokine RANTES in asbestos exposed workers and in malignant mesothelioma patients from an hyperendemic area. PLoS One. 2014;9(8):e104848. doi: 10.1371/journal.pone.0104848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, et al. Chemokine (C-C motif) ligand 3 detection in the serum of persons exposed to asbestos: A patient-based study. Cancer Sci. 2015;106(7):825–32. doi: 10.1111/cas.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amati M, et al. Profiling tumor-associated markers for early detection of malignant mesothelioma: an epidemiologic study. Cancer Epidemiol Biomarkers Prev. 2008;17(1):163–70. doi: 10.1158/1055-9965.EPI-07-0607. [DOI] [PubMed] [Google Scholar]
- 66.Amati M, et al. Assessment of biomarkers in asbestos-exposed workers as indicators of cancer risk. Mutat Res. 2008;655(1–2):52–8. doi: 10.1016/j.mrgentox.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Marczynski B, et al. Levels of 8-hydroxy-2'-deoxyguanosine in DNA of white blood cells from workers highly exposed to asbestos in Germany. Mutat Res. 2000;468(2):195–202. doi: 10.1016/s1383-5718(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 68.Rostila A, et al. Peroxiredoxins and tropomyosins as plasma biomarkers for lung cancer and asbestos exposure. Lung Cancer. 2012;77(2):450–9. doi: 10.1016/j.lungcan.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Demir M, et al. Evaluation of New Biomarkers in the Prediction of Malignant Mesothelioma in Subjects with Environmental Asbestos Exposure. Lung. 2016 doi: 10.1007/s00408-016-9868-1. [DOI] [PubMed] [Google Scholar]
- 70.Hollevoet K, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol. 2012;30(13):1541–9. doi: 10.1200/JCO.2011.39.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui A, et al. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open. 2014;4(2):e004145. doi: 10.1136/bmjopen-2013-004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin H, et al. Performance of osteopontin in the diagnosis of malignant pleural mesothelioma: a meta-analysis. Int J Clin Exp Med. 2014;7(5):1289–96. [PMC free article] [PubMed] [Google Scholar]
- 73.Pass HI, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N Engl J Med. 2012;367(15):1417–27. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Creaney J, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax. 2014;69(10):895–902. doi: 10.1136/thoraxjnl-2014-205205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Napolitano A, et al. HMGB1 and Its Hyperacetylated Isoform are Sensitive and Specific Serum Biomarkers to Detect Asbestos Exposure and to Identify Mesothelioma Patients. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morre DJ, et al. ENOX2-based early detection (ONCOblot) of asbestos-induced malignant mesothelioma 4–10 years in advance of clinical symptoms. Clin Proteomics. 2016;13:2. doi: 10.1186/s12014-016-9103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]