Abstract
Background
Scoliosis is prominent in Rett syndrome (RTT). Following the prior report from the US Natural History Study (NHS), the onset and progression of severe scoliosis (≥40° Cobb angle) and surgery were examined regarding functional capabilities and specific genotypes, addressing the hypothesis that abnormal muscle tone, poor oral feeding, puberty, and delays or absence of sitting balance, ambulation, may be responsible for greater risk in RTT.
Methods
The multicenter RTT Natural History study (NHS) gathered longitudinal data for classic RTT including mutation type, scoliosis, muscle tone, sitting, ambulation, hand function, and feeding. Cox regression models were used to examine the association between scoliosis and functional characteristics. All analyses utilized SAS 9.4; two-sided p-values of <0.05 were considered significant.
Results
913 females with classic RTT were included. Scoliosis frequency and severity increased with age. Severe scoliosis was found in 251 participants (27%), 113 of whom developed severe scoliosis during the follow-up assessments. 168 (18%) had surgical correction. Severe MECP2 mutations (R106W, R168X, R255X, R270X, and large deletions) showed higher proportion of scoliosis. Individuals developing severe scoliosis or requiring surgery were less likely to sit, ambulate, or use their hands and were more likely to have begun puberty. Significant differences were absent for epilepsy rates, sleep problems, or constipation.
Discussion
Scoliosis requires vigilance regarding the risk factors noted, particularly specific mutations and the role of puberty and motor abilities. Bracing is recommended for moderate curves and surgery for severe curves in accordance with published guidelines for scoliosis management.
Keywords: Rett syndrome, scoliosis, MECP2 mutations, surgery, puberty
Background
Rett syndrome (RTT; MIM: 312750) is a rare, X-linked dominant disorder associated with mutations in the methyl-CpG-binding protein 2 (MECP2) gene, encoded at Xq28. The disorder was first described 50 years ago by Andreas Rett1 in Vienna, Austria, but only came to world-wide attention as RTT with the paper of Hagberg et al.2 in 1983. Since that time, much has been learned about this unique neurodevelopmental disorder. Occurring almost exclusively in females, the disorder follows a typical pattern of apparently normal early development followed by cognitive impairment, communication dysfunction, stereotypic hand movements, and pervasive failure of growth. The last feature is first evident as abnormal deceleration in the rate of head growth as early as 1.5 months of age followed by abnormal deceleration in the rate of weight and linear growth3. In addition to epilepsy, periodic breathing, and gastrointestinal dysfunction, progressive scoliosis has long been recognized as a significant problem in RTT.
Worsening scoliosis was noted by Hagberg et al.2, and other reports occurred shortly thereafter4–8. In the next several years, a number of papers described scoliosis in 48–94%9–14. In 2003, Kerr et al. reported that surgical correction provided improved overall outcome in 42 of 50 (84%) individuals with classic RTT with specific benefits in independent ambulation, sitting posture, and feeding15, findings supported by subsequent studies16,17. In 2010, results from the US Natural History study revealed that scoliosis was present in >85% of participants by age 16. Of this group, 13% had spinal instrumentation18. The report of Riise et al. confirmed the high frequency of scoliosis19.
Although scoliosis is evident as early as age four years, what remains to be determined is how scoliosis progresses over time, clinical characteristics of RTT related to the development and progression of scoliosis, and specifically, how is this progression related to pubertal development in RTT? We have recently shown that pubertal development in RTT differs from typically developing females20. More than 25% may demonstrate premature onset of puberty, the time between the onset of puberty and the onset of menarche is significantly longer than normal (3.9 years in RTT versus 3.0 years in typical females), and the age of menarche is later in RTT (13.0 years) than in typical females (12.5 years). In addition, while synchronous development of puberty occurs in 52%, similar to typical females, in RTT 32% begin with adrenarche (pubic) and only 15% with thelarche (breast), the opposite of the typical pattern.
In order to address the questions raised, we examined factors that could be predictors of the onset and progression of scoliosis such as muscle tone, sitting, ambulation, feeding, genotype, and puberty. We hypothesized that those with abnormal muscle tone and delays or absence of sitting balance, ambulation, puberty, and specific mutations as well as poor feeding by mouth would be at greater risk for developing severe scoliosis and require surgery for spinal stabilization.
Methods
Study population
The multicenter RTT Natural History study (RNHS) database was assessed. Individuals with classic RTT were recruited from 2006 through 2015 and evaluated as described previously21. A RNHS neurologist or geneticist (D.G.G., J.L.N., A.K.P., S.A.S., and W.E.K.) confirmed the diagnosis of classic RTT based upon the consensus diagnostic criteria22,23. Only female participants were included in this analysis. All participants had MECP2 testing by a qualified laboratory, >95% of those with classic RTT having a mutation. To evaluate overall severity of RTT, the clinical severity scale (CSS) and motor behavioral analysis (MBA), were utilized3. Institutional review board approval was obtained for each participating institution. Participants’ families granted informed assent. The RNHS is registered as Clinical Trial NCT00296764.
Data Coding
Data were coded in order to provide specific ages for scoliosis. Information on scoliosis was provided by the parents based on radiographic reports determining Cobb angle from prior orthopedic assessments. If no prior assessment had occurred, the degree of curvature was estimated by clinical inspection. If a curve were detected that exceeded 10°, orthopedic assessment was requested and the resultant Cobb angle recorded in the database. Developmental history was determined as previously reported24 and re-evaluated at each visit along with standard medical assessments including growth and nutrition, neurological history and assessment, and general examination including Tanner stages B2, PH2, and menarche. Mutation data were recorded for all participants.
Statistical Analysis
Descriptive statistics were obtained for given characteristics. Categorization of MECP2 mutation based on phenotypic similarities25,26 was as follows: mild mutations included R133C, R294X, R306C, and 3′ truncations; moderate included T158M, Exon1, Insertion/deletion, Splice site, and other mutations; and severe included R106W, R168X, R255X, R270X, and large deletion. Mutation negative participants were excluded from mutation analysis. To examine the association between MECP2 mutation and scoliosis in all subjects, a logistic regression model was used after adjusting for age at enrollment. ANOVA (Analysis of variance) was used to compare the mean age between mutation groups. In order to examine factors that could be predictors of the onset and progression of scoliosis prospectively, we used a Cox regression model for severe scoliosis identified during the follow-up. Time to event was age at the study visit for those who developed severe scoliosis during the follow-up, and time of censoring was age at the last study visit for those who had not yet developed. Age at enrollment was incorporated in the Cox regression analysis, in order to take into account only those in the study at the age of consideration. Additionally, having puberty during the follow up was analyzed as time dependent covariate by flagging whether puberty was reported prior to the onset of severe scoliosis.
All analyses were performed using SAS, Version 9.4 (SAS Institute Inc. Cary, NC, USA). All reported p-values are two-sided and p-value<0.05 was considered statistically significant.
Results
The US Natural History database was assessed as of January 31, 2016. At that time, 913 participants had been enrolled and 909 reported their scoliosis status at the baseline visit. Mean age at enrollment was 10 years (standard deviation, SD=9, min=1 and max=66) and mean follow-up time was 4 years (SD=3). Of this group, 138 reported severe scoliosis (≥40° Cobb angle or surgery) at their baseline visit and 113 had progressed to severe scoliosis during the follow-up assessments. The overall rate of severe scoliosis was 251/913 (27.5%), and of the total, 168/913 (18%) reported surgical correction. At baseline, most participants were younger than 13 years old (665/913; 72.8%), while most with severe scoliosis were age ≥13 years (109/138; 79.0%). Of those 665 girls, severe scoliosis was identified at a follow-up assessment in 103 (103/113; 91.2%), and only 19 of them (18%) were ≥ age 13 years at the visit. Table 1 shows the prevalence of scoliotic curves across all age groups. Among 4 year olds, 28% had measurable scoliosis; by age 13 and above, ≥79% had measurable scoliosis. The proportion of severe scoliosis also increased with age from 13% (31/246) in 9 year olds to 38% (63/165) of 12 year olds and remained stable above 40% through age 19 years. Table 1 also shows that not all with a severe curve receive surgery at first awareness. For example, at age 9, 32% of those with severe curves had surgical correction. However, at age 12 years, 68% of those with severe curves reported surgical correction, remaining above 70% through age 19.
Table 1.
Prevalence of Scoliosis Curves from 1 through 19 Years
| Age at report (Years) | All Participants | No Scoliosis | Scoliosis <40° | Scoliosis ≥40° No surgery | Scoliosis ≥40° Surgery | Any Scoliosis N (%)* |
|---|---|---|---|---|---|---|
| 1 | 27 | 23 | 4 | - | - | 4 (14) |
| 2 | 159 | 138 | 21 | - | - | 21 (13) |
| 3 | 253 | 194 | 58 | 1 | - | 59 (23) |
| 4 | 285 | 206 | 75 | 4 | - | 79 (28) |
| 5 | 310 | 208 | 98 | 4 | - | 102 (33) |
| 6 | 304 | 184 | 112 | 8 | - | 120 (39) |
| 7 | 278 | 146 | 119 | 12 | 1 | 132 (47) |
| 8 | 255 | 120 | 114 | 18 | 3 | 135 (53) |
| 9 | 246 | 101 | 114 | 21 | 10 | 145 (59) |
| 10 | 248 | 74 | 121 | 32 | 21 | 174 (70) |
| 11 | 198 | 52 | 92 | 24 | 30 | 146 (73) |
| 12 | 165 | 35 | 67 | 20 | 43 | 130 (79) |
| 13 | 152 | 31 | 53 | 18 | 50 | 121 (80) |
| 14 | 126 | 27 | 46 | 11 | 42 | 99 (79) |
| 15 | 127 | 22 | 38 | 16 | 51 | 105 (83) |
| 16 | 97 | 16 | 35 | 8 | 38 | 81 (84) |
| 17 | 95 | 14 | 34 | 10 | 37 | 81 (85) |
| 18 | 90 | 12 | 34 | 11 | 33 | 78 (87) |
| 19 | 94 | 17 | 35 | 6 | 36 | 77 (82) |
The percentages reflect the presence of scoliosis for all participants seen longitudinally at the respective ages.
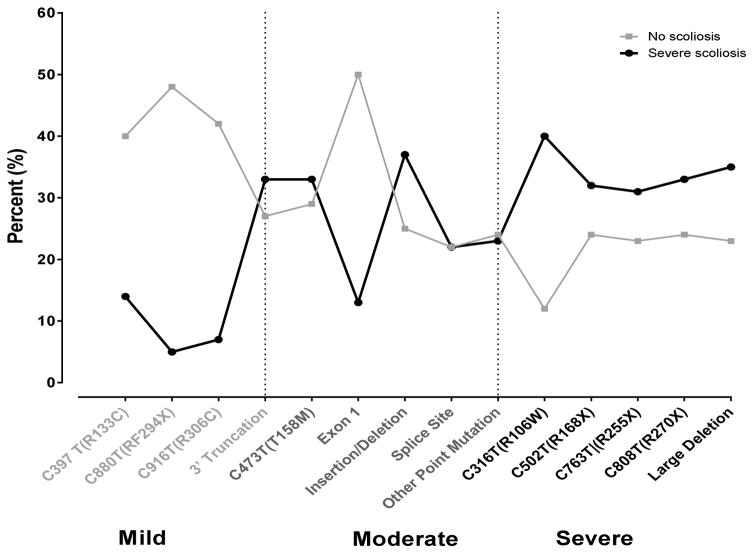
In terms of the actual age distribution for the 168 participants who had surgical correction, the majority (86%) occurred between age 9 and 15, the median age was 12 years (min=5, max=21). Only four individuals had surgery at age 19 or beyond. The proportion of severe scoliosis based on mutation is displayed in the Figure 1. Categorized mutations based on phenotypic similarity (mild, moderate and severe) showed an increasing trend of severe scoliosis from mild to severe, while the proportion without scoliosis trended to decrease.
Figure 1.
Scoliosis by MECP2 mutation
Scoliosis frequency is displayed by relevant mutations. The data are presented as those participants with “No scoliosis” (◆---◆), “Scoliosis <40°” (■---■), and “Scoliosis ≥40°” (▲---▲).
Table 2 shows the relationship between mutation type and curve severity. There was no mean age difference at baseline between three mutation groups (p=0.45). A logistic regression model was used to examine whether mutation type was associated with severe scoliosis in all subjects. After adjusting for age at enrollment, the proportion of severe scoliosis was 2.4 times and 2.9 times higher in moderate and severe mutation carriers than mild type carriers, respectively.
Table 2.
Mutation Type and Scoliosis Severity*
| Categorized mutation type | Age at enrollment: Mean ±SD | Scoliosis ≥40° or Surgery: N (%) | Scoliosis <40° or No scoliosis: N (%) | Odds ratio (95% CI), p-value |
|---|---|---|---|---|
| Mild | 10.4±8.8 | 39 (16) | 199 (31) | 1 |
| Moderate | 9.7±8.3 | 87 (36) | 208 (32) | 2.40 (1.54,3.73), p=0.050 |
| Severe | 9.5±9.5 | 117 (48) | 235 (37) | 2.93 (1.91,4.51), p=0.0001 |
There were 28 subjects without a MECP2 mutation; 8 in Scoliosis ≥40° or Surgery and 20 in Scoliosis <40° or No scoliosis
In order to examine factors associated with the onset and progression of scoliosis, we excluded those 138 subjects who had already developed severe scoliosis at baseline visit since those potential risk factors are mostly age dependent, as well as scoliosis. This cohort included 113 subjects who developed severe scoliosis during the follow-up and 662 had not. At baseline, while 76% of those 113 subjects had some level of scoliosis, 71% of those 662 subjects did not have any scoliosis. Hence, for further analysis, we adjusted for MECP2 mutation, scoliosis status and age at baseline. Table 3 shows the baseline model.
Table 3.
Baseline Cox regression model
| Factor | Severe scoliosis | Hazard ratio (95% CI), p-value | |
|---|---|---|---|
| N(%) or Mean ±SD | |||
| Scoliosis at baseline | None | 27 (5) | 1 |
| 1 to <20° Cobb angle | 47 (26) | 6.68 (3.97, 11,23), p<0.0001 | |
| 20 to <40° Cobb angle | 39 (41) | 20.3 (11.3, 36.5), p<0.0001 | |
| Mutation | Mild | 21 (10) | 1 |
| Moderate | 34 (14) | 1.31 (0.76, 2.27), p=0.34 | |
| Severe | 54 (19) | 1.84 (1.10, 3.08), p=0.021 | |
| Age at baseline | 8±4 | 0.73 (0.66, 0.81), p<0.0001 | |
We examined neuromuscular, clinical characteristics, menarche and puberty at baseline. The effect of each factor was examined by including that in the baseline model (Table 4). Being able to sit and walk without aid were associated with decreasing the risk of severe scoliosis, compared to those requiring aid for sitting or walking (54% and 68% reduction, respectively). Breath-holding was also associated with decreasing 37% of risk of severe scoliosis, compared to those not breath-holding. Conversely, those without purposeful hand use was associated with increasing (80%) risk, compared to those without any problem in using hands. Higher CSS score showed an association with increasing the risk. Also, 31% of the girls had puberty at baseline, and of those, 20% developed severe scoliosis, showing an association with almost three times higher risk of developing severe scoliosis compared to those without puberty. Hence, we further divided those without puberty at baseline into having puberty and not yet during the follow up.
Table 4.
Baseline characteristics and the onset of severe scoliosis: Univariate Cox regression analysis using the baseline model
| Factor | Severe Scoliosis | Hazard ratio (95% CI), p-value | |
|---|---|---|---|
| N(%) or Mean ±SD | |||
| Muscle hypertonia | Hypertonic | 36 (17) | 1.15 (0.48,2.71), p=0.76 |
| Hypotonic | 52 (17) | 1.45 (0.63,3.34), p=0.39 | |
| Mixed or Variable | 17 (15) | 1.76 (0.68, 4.56), p=24 | |
| Normal | 8 (6) | 1 | |
| Contractures | Yes | 16 (21) | 0.99 (0.54,1.83), p=0.98 |
| No | 97 (14) | 1 | |
| Sitting Ability | Able to sit without aid | 77 (11%) | 0.46 (0.29, 0.71), p=0.0005 |
| With support and aid | 34 (35%) | 1 | |
| Walks without aid | Yes | 26 (6) | 0.32 (0.20, 0.52), p<0.0001 |
| No | 87 (24) | 1 | |
| Holds breath | Yes | 77 (15) | 0.63 (0.41, 0.97), p=0.034 |
| No | 36 (13) | 1 | |
| Periodic breathing | Yes | 87 (16) | 0.69 (0.44, 1.10), p=0.12 |
| No | 26 (12) | 1 | |
| Hand clumsiness | Uses utensils/cup, may be | ||
| 17 (12) | 1.26 (0.61, 2.59), p=0.53 | ||
| adaptive | |||
| Finger feeds only | 16 (11) | 1.91 (0.94, 3.89), p=0.08 | |
| No purposeful hand use | 64 (22) | 1.80 (1.02, 3.19), p=0.044 | |
| Plays with toys or activates switches purposefully | 16 (9) | 1 | |
| Feeding difficulties | Occasional choking/gagging | 25 (13) | 1.27 (0.72, 2.22), p=0.41 |
| >30 minutes to feed | 32 (15) | 1.13 (0.66, 1.91), p=0.66 | |
| Oral and gastrostomy feeding | 15 (24) | 1.43 (0.73, 2.78), p=0.30 | |
| Gastrostomy only | 12 (29) | 0.97 (0.47, 2.03), p=0.94 | |
| None | 29 (11) | 1 | |
| Any sleep problems | Yes | 57 (13) | 1.15 (0.78, 1.69), p=0.49 |
| No | 56 (17) | 1 | |
| Constipation | Yes | 97 (16) | 1.25 (0.71, 2.20), p=0.45 |
| No | 16 (9) | 1 | |
| menarche | Yes | 10 (7) | 0.50 (0.16, 1.50), p=0.21 |
| No | 103 (16) | 1 | |
| Puberty | Yes | 47 (20) | 2.98 (1.61, 5.52), p=0.0005 |
| No | 66 (12) | 1 | |
| Total CSS | 25±6 | 1.06 (1.03, 1.10), p=0.0004 | |
| Total MBA | 52±11 | 1.01 (0.99, 1.03), p=0.17 | |
A backward selection from the model including all significant factors in Table 4 included three characteristics, in addition to the baseline model (Table 5): being able to walk without aid and breath-holding (decreasing risk) and puberty (increasing risk). This implies that breathing and puberty can be good predictors of the onset and progression of severe scoliosis, in addition to MECP2 mutation, the status of scoliosis, age, and the ability to walk at baseline.
Table 5.
Multiple Cox regression model for the onset of severe scoliosis
| Factor | Hazard ratio (95% CI), p-value | |
|---|---|---|
| Scoliosis at baseline | None | 1 |
| 1 to <20° Cobb angle | 4.94 (2.87, 8,52), p<0.0001 | |
| 20 to <40° Cobb angle | 14.5 (7.87, 26.8), p<0.0001 | |
| Mutation | Mild | 1 |
| Moderate | 1.39 (0.79, 2.44), p=0.26 | |
| Severe | 1.53 (0.90, 2.61), p=0.12 | |
| Age at baseline | 0.66 (0.58, 0.76), p<0.0001 | |
| Walks without aid | Yes | 0.32 (0.20, 0.52), p<0.0001 |
| No | 1 | |
| Holds breath | Yes | 0.57 (0.37, 0.86), p=0.008 |
| No | 1 | |
| Puberty | During follow-up | 3.56 (1.44, 8.84), p=0.006 |
| At baseline | 8.28 (2.90, 23.7), p<0.0001 | |
| No | 1 | |
Discussion
Scoliosis is a major concern in individuals with RTT. The prior report from the US Natural History study18 was based on cross-sectional data revealing a significant association with greater clinical severity and loss or absence of independent ambulation. In the present study, longitudinal assessment provided the opportunity to expand these analyses across time as well as increasing the number of individuals under study from 554 to 913. Considering the new onset of scoliosis and progression during the follow up period, we found that being unable to sit and walk without support, hand clumsiness, lack of breath-holding, and onset of puberty were associated with increasing the risk after adjusting for the severity of the MECP2 mutation, the status of scoliosis, and age at baseline. In multivariate analysis, lack of breath-holding and puberty remained as independent risk factors, as well as the inability to walk independently. Absence of breath-holding as an indication of increased risk for severe scoliosis is a completely unanticipated finding. This will deserve increased attention in future studies to ascertain if this is verifiable.
The onset of scoliosis in RTT was seen as early as age 1 year increasing dramatically over the first decade. By age 13 years, about 80% of this population had measurable scoliosis, yet only 70% of those had undergone surgical correction. The importance of phenotype-genotype correlations is critical for the occurrence of scoliosis and the need for surgical correction. Participants with mutations associated with greater clinical severity had much greater likelihood of surgery and those with lower clinical severity had a reduced likelihood of surgery. In addition, the occurrence of any scoliosis appears to be greater in individuals with greater clinical severity; those with lower clinical severity, particularly R133C, R294X, and R306C, had a much reduced occurrence of scoliosis (<15%). As demonstrated previously by Neul et al.25 and Bebbington et al.28 and more recently by Cuddapah et al.26, the degree of clinical severity shows a striking correlation with the common mutation types in keeping with that noted for the likelihood of requiring surgical intervention for severe scoliosis. The first cross-sectional report on scoliosis surgery indicated that 13% had spinal instrumentation. This longitudinal analysis revealed an increase to 18.5% indicating the significant importance of careful follow-up over time. This finding deserves close attention to determine whether it is related to longitudinal assessments, as in the present study, or whether this indicates a greater sensitivity on the part of parents and physicians to intervene with corrective surgery.
With all these factors in mind, the authors urge steadfast attention to scoliosis changes over time in this population. Although bracing has not been shown to be useful in modifying curvature progression in this population due to absence of any comparative clinical assessment, bracing has been shown to retard the progression of scoliosis in individuals with static encephalopathy29,30. As such, when curves reach 25°, we recommend implementation of bracing in hopes of retarding or minimizing further progression. However, once the curve reaches 40° or more, we strongly promote surgical intervention. As before, parents have been uniformly pleased with the overall results and most individuals who were ambulatory prior to surgery retained this capability. In addition, we recommend close adherence to the guidelines for scoliosis management which provide both evidence- and consensus-based approaches to scoliosis in those with RTT31.
In conclusion, the role of puberty, and not simply increasing age, and the specific mutation seem to be important factors in the occurrence and progression of scoliosis. These become important considerations for long-term management and will require increased vigilance both by family and those providing care.
Acknowledgments
This study was supported by NIH U54 grants RR019478 and HD061222, Office of Rare Disease Research, and IDDRC grant HD38985, funds from the International Rett Syndrome Foundation and the Civitan International Research Center. The Rett Syndrome Natural History Study (U54 HD061222) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the Eunice Kennedy Shriver Child Health and Human Development Institute (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest. The authors acknowledge the gracious participation and provision of information by the families of the reported participants.
Footnotes
The authors report no conflicts
Author Contributions
JK: Conceptualization, database review and analysis, statistical methodology, writing the final document.
JBL: Conceptualization, data acquisition, database review, revision and review of final document.
AKP: Conceptualization, data acquisition, database review, revision and review of final document. All other authors: Data acquisition, review of manuscript
Disclosures
The authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr. 1966;116(37):723–726. [PubMed] [Google Scholar]
- 2.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14(4):471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 3.Tarquinio DC, Motil KJ, Hou W, et al. Growth failure and outcome in Rett syndrome: specific growth references. Neurology. 2012;79(16):1653–1661. doi: 10.1212/WNL.0b013e31826e9a70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagberg B, Witt-Engerstrom I. Rett syndrome: a suggested staging system for describing impairment profile with increasing age towards adolescence. Am J Med Genet Suppl. 1986;1:47–59. doi: 10.1002/ajmg.1320250506. [DOI] [PubMed] [Google Scholar]
- 5.Naidu S, Murphy M, Moser HW, Rett A. Rett syndrome--natural history in 70 cases. Am J Med Genet Suppl. 1986;1:61–72. doi: 10.1002/ajmg.1320250507. [DOI] [PubMed] [Google Scholar]
- 6.Hennessy MJ, Haas RH. The orthopedic management of Rett syndrome. J Child Neurol. 1988;3(Suppl):S43–47. doi: 10.1177/0883073888003001s09. [DOI] [PubMed] [Google Scholar]
- 7.Keret D, Bassett GS, Bunnell WP, Marks HG. Scoliosis in Rett syndrome. J Pediatr Orthop. 1988;8(2):138–142. [PubMed] [Google Scholar]
- 8.Loder RT, Lee CL, Richards BS. Orthopedic aspects of Rett syndrome: a multicenter review. J Pediatr Orthop. 1989;9(5):557–562. doi: 10.1097/01241398-198909010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Bassett GS, Tolo VT. The incidence and natural history of scoliosis in Rett syndrome. Dev Med Child Neurol. 1990;32(11):963–966. doi: 10.1111/j.1469-8749.1990.tb08118.x. [DOI] [PubMed] [Google Scholar]
- 10.Harrison DJ, Webb PJ. Scoliosis in the Rett syndrome: natural history and treatment. Brain Dev. 1990;12(1):154–156. doi: 10.1016/s0387-7604(12)80200-2. [DOI] [PubMed] [Google Scholar]
- 11.Holm VA, King HA. Scoliosis in the Rett syndrome. Brain Dev. 1990;12(1):151–153. doi: 10.1016/s0387-7604(12)80199-9. [DOI] [PubMed] [Google Scholar]
- 12.Guidera KJ, Borrelli J, Jr, Raney E, Thompson-Rangel T, Ogden JA. Orthopaedic manifestations of Rett syndrome. J Pediatr Orthop. 1991;11(2):204–208. doi: 10.1097/01241398-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Huang TJ, Lubicky JP, Hammerberg KW. Scoliosis in Rett syndrome. Orthop Rev. 1994;23(12):931–937. [PubMed] [Google Scholar]
- 14.Lidstrom J, Stokland E, Hagberg B. Scoliosis in Rett syndrome. Clinical and biological aspects. Spine (Phila Pa 1976) 1994;19(14):1632–1635. doi: 10.1097/00007632-199407001-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kerr AM, Webb P, Prescott RJ, Milne Y. Results of surgery for scoliosis in Rett syndrome. J Child Neurol. 2003;18(10):703–708. doi: 10.1177/08830738030180101201. [DOI] [PubMed] [Google Scholar]
- 16.Larsson EL, Aaro S, Ahlinder P, Normelli H, Tropp H, Oberg B. Long-term follow-up of functioning after spinal surgery in patients with Rett syndrome. Eur Spine J. 2009;18(4):506–511. doi: 10.1007/s00586-008-0876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ager S, Downs J, Fyfe S, Leonard H. Parental experiences of scoliosis management in Rett syndrome. Disabil Rehabil. 2009;31(23):1917–1924. doi: 10.1080/09638280902846392. [DOI] [PubMed] [Google Scholar]
- 18.Percy AK, Lee HS, Neul JL, et al. Profiling scoliosis in Rett syndrome. Pediatr Res. 2010;67(4):435–439. doi: 10.1203/PDR.0b013e3181d0187f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riise R, Brox JI, Sorensen R, Skjeldal OH. Spinal deformity and disability in patients with Rett syndrome. Dev Med Child Neurol. 2011;53(7):653–657. doi: 10.1111/j.1469-8749.2011.03935.x. [DOI] [PubMed] [Google Scholar]
- 20.Killian JT, Lane JB, Cutter GR, et al. Pubertal development in Rett syndrome deviates from typical females. Pediatr Neurol. 2014;51(6):769–775. doi: 10.1016/j.pediatrneurol.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaze DG, Percy AK, Skinner S, et al. Epilepsy and the natural history of Rett syndrome. Neurology. 2010;74(11):909–912. doi: 10.1212/WNL.0b013e3181d6b852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. Eur J Paediatr Neurol. 2002;6(5):293–297. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- 23.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68(6):944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neul JL, Lane JB, Lee HS, et al. Developmental delay in Rett syndrome: data from the natural history study. J Neurodev Disord. 2014;6(1):20. doi: 10.1186/1866-1955-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neul JL, Fang P, Barrish J, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70(16):1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuddapah VA, Pillai RB, Shekar KV, et al. Methyl-CpG-binding protein 2 (MECP2) mutation type is associated with disease severity in Rett syndrome. J Med Genet. 2014;51(3):152–158. doi: 10.1136/jmedgenet-2013-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IBM SPSS Statistics for Windows [computer program]. Version 21.0. Armonk, NY: IBM Corp; Released 2012. [Google Scholar]
- 28.Bebbington A, Anderson A, Ravine D, et al. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008;70(11):868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. [DOI] [PubMed] [Google Scholar]
- 29.Terjesen T, Lange JE, Steen H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev Med Child Neurol. 2000;42(7):448–454. doi: 10.1017/s0012162200000840. [DOI] [PubMed] [Google Scholar]
- 30.Koop SE. Scoliosis in cerebral palsy. Dev Med Child Neurol. 2009;51(Suppl 4):92–98. doi: 10.1111/j.1469-8749.2009.03461.x. [DOI] [PubMed] [Google Scholar]
- 31.Downs J, Bergman A, Carter P, et al. Guidelines for management of scoliosis in Rett syndrome patients based on expert consensus and clinical evidence. Spine (Phila Pa 1976) 2009;34(17):E607–617. doi: 10.1097/BRS.0b013e3181a95ca4. [DOI] [PubMed] [Google Scholar]