Abstract
Streptococcus pneumoniae is an important global pathogen that causes a wide range of clinical disease in children and adults. Pneumococcal pneumonia is by far the common presentation of noninvasive and invasive pneumococcal disease and affects the young, the elderly, and the immunocompromised disproportionately. Patients with chronic pulmonary diseases are also at higher risk for pneumococcal infections. Substantial progress over the century has been made in the understanding of pneumococcal immunobiology and the prevention of invasive pneumococcal disease through vaccination. Currently, two pneumococcal vaccines are available for individuals at risk of pneumococcal disease: the 23-valent pneumococcal polysaccharide vaccine (PPV23) and the 13-valent pneumococcal protein-conjugate vaccine (PCV13). The goal of pneumococcal vaccination is to stimulate effective antipneumococcal antibody and mucosal immunity response and immunological memory. Vaccination of infants and young children with pneumococcal conjugate vaccine has led to significant decrease in nasal carriage rates and pneumococcal disease in all age groups. Recent pneumococcal vaccine indication and schedule recommendations on the basis of age and risk factors are outlined in this Focused Review. As new pneumococcal vaccine recommendations are being followed, continued efforts are needed to address the vaccine efficacy in the waning immunity of the ever-aging population, the implementation of vaccines using two different vaccines under very specific schedules and their real world clinical and cost effectiveness, and the development of next generation pneumococcal vaccines.
Keywords: Streptococcus pneumoniae, pneumonia, PCV-13 vaccine, pneumococcal vaccines
Streptococcus pneumoniae is an endemic global pathogen that causes a wide range of clinical disease in children and adults. Noninvasive pneumococcal disease encompasses otitis, sinusitis, and community-acquired pneumonia. As the name implies, pneumococcal pneumonia is a common presentation of noninvasive pneumococcal disease, resulting in 900,000 cases and 400,000 hospitalizations annually in the United States; mortality ranges from 5 to 7% (1).
Invasive pneumococcal disease implies invasion of pneumococcus into a normally sterile site, leading to complications such as bacteremia, empyema, meningitis, endocarditis, and osteomyelitis. The Centers for Disease Control and Prevention estimates an annual invasive pneumococcal disease incidence of 10.6/100,000 U.S. population, primarily occurring in adults. The majority of these cases start with streptococcal pneumonia as a primary focus. Pneumococcus is the most common cause of community-acquired pneumonia in adults, composing at least 25% of documented cases, with bacteremia present 20% of the time.
Epidemiology and Risk Factors
Although invasive pneumococcal disease is much less prevalent than noninvasive disease, it confers significant mortality risk (up to 10% for meningitis and 15% for bacteremia), and survivors can be left with significant sequelae (2). Furthermore, given the widespread use of empiric antibiotics and frequent lack of timely culture or urinary antigen data, the true burden of primary and invasive pneumococcal disease may be significantly underestimated (3).
Certain groups are at particularly high risk for invasive pneumococcal disease: young children, the elderly, and those with high-risk comorbid diseases or substance habits. The Centers for Disease Control and Prevention’s Active Bacterial Core Surveillance data from 2013 demonstrated increased rates of invasive pneumococcal disease in children younger than age 5 years (9.6/100,000 cases) and in adults 65 years or older (31/100,000 cases) (4).
The elderly in particular have been consistently shown to have a marked increased risk for invasive pneumococcal disease (5, 6). Functional or anatomic asplenia confers very high risk, particularly in younger patients with sickle cell anemia; mortality from invasive pneumococcal disease in asplenic patients is more than 50% (7, 8). Patients being treated for underlying solid or hematologic malignancies have high rates of invasive pneumococcal disease, although, interestingly, less than one-fifth of these infections occur during periods of neutropenia (9, 10). Antiretroviral therapy has significantly reduced the overall burden of invasive pneumococcal disease in individuals with HIV; however, the risk of invasive pneumococcal disease remains 35 times higher in HIV-infected individuals than in non–HIV-infected adults (11).
Independent of age, the presence of other comorbid chronic conditions such as cardiovascular disease, chronic obstructive pulmonary disease (COPD), asthma, renal insufficiency, and diabetes mellitus increases the risk for invasive pneumococcal disease (9, 12). Patients with common pulmonary conditions like COPD and asthma have a two- to sixfold risk for invasive pneumococcal disease compared with the general population (9, 12). In addition, active smoking confers increased risk, as do alcohol and intravenous drug use (9, 13–15).
Although pneumococcal pneumonia is a leading cause of community-acquired pneumonia irrespective of comorbidity, individuals with chronic lung diseases, particularly those with COPD, are at increased risk of pneumococcal community-acquired pneumonia and invasive pneumococcal disease, are prone to higher rates of complications and mortality, and suffer prolonged recovery after such illnesses (16). The reasons for this are many, including reduced innate immunity in diseased airways, systemic inflammation, ongoing smoking, acute exacerbations, and intermittent use of systemic corticosteroids.
In addition, there is ongoing concern about the increased risk for community-acquired pneumonia in the setting of chronic use of inhaled corticosteroids, an important component of maintenance therapy in COPD and asthma (17). Because death from pneumococcal pneumonia, even in absence of invasive disease, is more frequent in patients with underlying chronic pulmonary conditions, appropriate and timely vaccination against pneumococcus is an essential component of preventative care in this population.
Pneumococcal Vaccine Development: A Historical Perspective
S. pneumoniae was first discovered in the late 19th century by U.S. Army physician George Sternberg and French scientist Louis Pasteur, when it was recognized as a major pathogenic cause of bacterial pneumonia (18). In 1902, German scientist Friedrich Neufeld discovered that antiserum containing different types of S. pneumonia–specific antibodies caused a type-specific capsular swelling, or “Quellung reaction,” which allowed identification of multiple pneumococcal serotypes. Polysaccharides that were identified coursing along the exterior of the bacterium could be targeted for vaccine development. British physician Sir Almroth Wright conducted the first large clinical trial of a whole-cell pneumococcal vaccine. This trial was largely unsuccessful, bringing vaccine development to a relative halt for the next three decades (19).
In 1945, Alexander Fleming, a former aide of Wright, won a Nobel Prize for the discovery of the antibacterial properties of penicillin. That same year, after a more complete understanding of the pneumococcal capsular structure had been established, the first tetravalent polysaccharide pneumococcal vaccine was developed and tested in a large population of Army recruits (20). Although this study did show significant improvement in rates of infection with vaccination (Table 1), enthusiasm waned with the success of penicillin. As reports emerged of resistant pneumococcal infections in immunosuppressed, pediatric, and adult populations, with up to one-quarter of patients dying despite appropriate antibiotic treatment (21–23), there was renewed interest in effective prophylaxis.
Table 1.
Pneumococcal vaccine studies
| Study | Population | Methods | Primary Outcome | Results | Conclusions | Other |
|---|---|---|---|---|---|---|
| MacLeod and Hodges, 1945 (20) | 17,035 male army recruits; aged 18–32 yr | Tetravalent polysaccharide vaccine (serotypes 1, 2, 5, 7) vs. placebo | Vaccine-type PNA incidence | 84% RR reduction for vaccine-type PNA | Vaccine reduces incidence of vaccine-type pneumonia | Herd immunity conferred to the nonimmunized. |
| Riley et al., 1977 (24) | 11,958 healthy adults from Papua New Guinea | 14-valent polysaccharide vaccine (serotypes 1, 2, 3, 4, 5, 6, 7, 8, 12, 14, 18, 23, 25, 46) vs. placebo | PNA incidence | 16% RR reduction for all PNA (P > 0.05) | Vaccination reduced all-cause mortality and mortality due to pneumonia | |
| Overall mortality | 21% RR reduction for all-cause mortality (P < 0.05) | |||||
| PNA mortality | 43% RR reduction for PNA-related mortality (P < 0.05) | |||||
| Simberkoff et al., 1986 (25) | 2,295 high-risk adults; mean age, 61 yr; followed up for 2.93 yr | 14-valent polysaccharide vaccine vs. placebo | Overall mortality | 24% RR increase | Vaccination of “high-risk” subjects with PPSV14 is not protective against pneumococcal infections | High risk includes 1+ of the following: >55 yr old; chronic renal, hepatic, cardiac, or pulmonary disease; alcoholism; diabetes mellitus. |
| Proven S. pneumoniae infections | 100% RR increase | |||||
| Probable S. pneumoniae infections | 27% RR increase | |||||
| Total S. pneumoniae infections | 54% RR increase | |||||
| Shapiro et al., 1991 (60) | Case-control study; 2,108 subjects; mean age, 68 yr | Case = confirmed pneumococcal infection and indication for vaccination. Control subjects were matched for age and vaccine indication | (VE against vaccine-type IPD | VE (all patients) = 56% (P < 0.00001) | Vaccination reduces vaccine-type IPD infection | Vaccine was not protective against non–vaccine-type pneumococcal infections. VE waned as time from vaccination increased and with increasing age of subject. |
| VE (immunocompetent) = 61% (P < 0.00001) | ||||||
| VE (immune compromised) = 21% (P = 0.48) | ||||||
| Black et al., 2000 (46) | 37,868 healthy infants; randomized to vaccination (series of 4 immunizations); follow up 3.5 yr | PCV7 vs. meningococcus conjugated vaccine | Vaccine-type IPD | VE = 97.4% (P < 0.001) (fully vaccinated) | Vaccination reduces IPD in healthy infants | Non–vaccine-type infections were also prevented by vaccine (VE = 89.1%, P < 0.001). Trial enrollment was stopped early due to high efficacy at the interim analysis. |
| VE = 85.7% (P < 0.05) (partially vaccinated) | ||||||
| Black et al., 2002 (61) | 37,868 healthy infants; randomized to vaccination (series of 4 immunizations); follow up 3.5 yr | PCV7 vs. meningococcus conjugated vaccine | Clinical PNA | Vaccine effectiveness = 6.0% (P = 0.13) | Vaccination may prevent pneumonia in healthy infants | Vaccine effectiveness calculated by Cox regression with the time to first event as the primary outcome. Intention to treat data shown. |
| PNA and X-ray performed | Vaccine effectiveness = 8.9% (P = 0.03) | |||||
| PNA and positive X-ray | Vaccine effectiveness = 17.7% (P = 0.01) | |||||
| PNA and perihilar infiltrate | Vaccine effectiveness = 11.1% (P = 0.35) | |||||
| Jackson et al., 2003 (51) | Retrospective cohort study; assessed 47,365 outpatients from Washington State; all >65 yr old; followed up for 3 yr | Prior vaccination with PPSV23 vs. no prior vaccination | Hospitalization due to CAP | HR, 1.14 (95% CI, 1.02–1.28) | PPSV23 does not prevent pneumonia but does reduce rates of pneumococcal bacteremia | Influenza vaccination status (in immunocompetent subjects) was the only variable to be associated with a statistically significant reduction in the rate of death from any cause. |
| “Outpatient” CAP | HR, 1.04 (95% CI, 0.96–1.13) | |||||
| Pneumococcal bacteremia | HR, 0.56 (95% CI, 0.33–0.93) | |||||
| French et al., 2010 (68) | 496 subjects (88% HIV infected) with previous IPD; follow up 4.5 yr | PCV7 × 2 (given 4 wk apart) vs. placebo | Recurrent IPD | HR, 0.31 (95% CI, 0.11–0.84) | PCV7 is protective against recurrent IPD in HIV-infected subjects | HRs were adjusted for age, sex, viral load, CD4+ cell count. There was no change in mortality in non–vaccine-type IPD. |
| Jackson et al., 2013 (66) | 835 healthy adults with no prior pneumococcal vaccination; age, 60–64 yr | PCV13 vs. PPSV23 | Antipneumococcal OPA titers at 1 mo and 1 yr postvaccination | OPA titers were higher for 8 of 12 common serotypes, 1 mo postvaccination. Titers decreased at 1 yr but remained higher than baseline. | PCV13 shows a higher immune response at 1 mo compared with PPSV23 | OPA titers have not been directly shown to protect from pneumococcal infection. |
| Jackson et al., 2013 (67) | 936 adults; age > 70 yr; previously received PPSV23 >5 yr earlier | PCV13 × 2 (1 yr apart) versus PPSV23 followed by PCV13 1 year later | OPA titers at 1 mo after each vaccination | OPA titers were higher in the PCV13 group for 10 of 12 common serotypes at 1 mo post initial vaccination. OPA titers were higher in the PCV13 group for 11 of 12 common serotypes at 1 mo post second vaccination. | With prior PPSV23 vaccination, PCV13 is more immunogenic than PPSV23 | OPA titers have not been directly shown to protect from pneumococcal infection. |
| Bonten et al., 2015 (69) | 84,496 healthy adults; >65 yr old; no prior pneumococcal vaccination; follow up 4 yr | PCV13 vs. placebo | Prevention of vaccine-type CAP | VE = 37.7% (P = 0.006) | PCV13 is effective in preventing vaccine-type CAP in older, healthy patients | Secondary outcomes that were also statistically significant include: IPD, bacteremic and nonbacteremic CAP. Importantly, there was no difference in mortality and no difference in CAP from all causes. |
Definition of abbreviations: CAP = community-acquired pneumonia; CI = confidence interval; HR = hazard ratio; IPD = invasive pneumonococcal disease; OPA = opsonophagocytic activity; PNA = pneumonia; RR = relative risk; S. pneumoniae = Streptococcus pneumoniae; VE = vaccine efficacy.
In 1977, a 14-valent purified polysaccharide vaccine was approved for use in the United States, targeting the 14 serotypes responsible for more than 75% of infections at the time. This vaccine significantly reduced morbidity and mortality in healthy adults but notably did not reduce overall rates of pneumonia (24).
Over the next decade, it became increasingly apparent that many clinically important pneumococcal infections were occurring despite vaccination, particularly in vulnerable populations, raising numerous concerns about further antimicrobial resistance, shifting serotype dominance, and the efficacy of polysaccharide vaccine preparations. In particular, elderly adults with higher rates of comorbid disease seemed to derive little benefit from the 14-valent polysaccharide vaccine, with no differences seen in rates of pneumococcal pneumonia/bronchitis, all-cause pneumonia, or overall mortality during a follow-up period of almost 3 years (25). This was attributed to the fact that more than half of this susceptible patient population did not mount or sustain adequate levels of antibodies deemed to be protective against pneumococcal infection.
Immunologic Basis and Host Response to Pneumococcal Vaccines
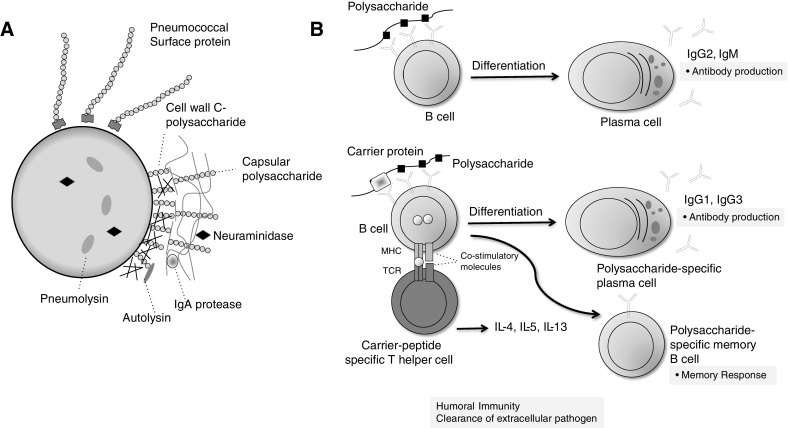
S. pneumoniae is a complex bacterium with 92 different polysaccharide capsular serotypes identified to date (26). The human airway uses numerous mechanisms to protect from colonization and invasive pneumococcal infection. Innate immune defenses, such as mucociliary escalator and an array of pattern recognition receptors that recognize bacterial proteins, help with the initial protection against the bacteria (27, 28). The antiphagocytic bacterial capsule is considered to be the most important determinant of pneumococcal virulence and is important for colonization of the nasopharynx (29–31) (Figure 1). Pneumococcal cell wall fragments and capsular polysaccharides are recognized by antibodies that bind and activate the complement system (32). All pneumococci serotypes can establish a carrier state and colonize in the human nasopharynx during the first few months of life. Children are the main reservoir, with colonization rates of up to 50%.
Figure 1.
(A) Pneumococcus bacteria and virulence factors including capsular polysaccharide. (B) Immune response to polysaccharide and protein-polysaccharide conjugate vaccines. MHC = major histocompatibility complex; TCR = T cell receptor. Adapted by permission from Reference 48.
The ability of pneumococci to move to another body site is limited to 20 of the 92 serotypes (33). Moreover, the development of symptoms of disease in the host involves the interactions between multiple bacterial virulence determinants and the host immune response. Typically, it is the very young and very old individuals who are most susceptible to systemic infection, as an effective immune response to S. pneumoniae relies on both innate and adaptive cellular components of the immune system.
The host immune response to pneumococcal lung disease is often characterized as intense and inflammatory in nature. In the unvaccinated population, and in the absence of preexisting serotype-specific antibodies, the infected host relies on innate immune defenses that stimulate the recruitment of polymorphonuclear cells. This response initially involves phagocytosis and intracellular killing by resident alveolar macrophages, followed by infiltration of neutrophil granulocytes into infected lungs.
Studies in innate immunity have shed light on the importance of complement, surfactant proteins, antimicrobial peptides, and several classes of pattern recognition receptors that recognize pathogen-associated molecular patterns and bacterial virulence factors on the pneumococci, which include the antiphagocytic polysaccharide capsule (27, 32, 34–39). Other important bacterial determinants include the pili, various adhesions, and toxins, including pneumolysin and autolysin (40). The innate immune response helps trigger subsequent acquired humoral and cellular responses.
Humoral response is important to help eradicate the bacteria during primary infection, although these responses are not as robust during primary infection. Anticapsular polysaccharide antibodies are believed to represent the single most important protective mechanism against invasive disease. In fact, antibodies to pneumococcal polysaccharides were the basis of serum therapy in which passively transferred, serotype-specific antipneumococcal serum was shown to reduce mortality from pneumococcal pneumonia by half (41).
With vaccination, B-cell responses are induced by vaccine antigen to produce antibodies that can bind to the bacteria. It is the acquired humoral immunity against the bacterial capsule that is central to vaccination strategies against pneumococcal pneumonia (34, 42). Immunologic protection is mediated through opsonophagocytic antibodies directed against bacterial capsular polysaccharides that define the pneumococcal serotypes and serve as virulence factors (43). Cell-mediated response (in the form of T-cell help and cytokine production) is needed to augment the humoral B-cell response to generate optimal robust and longer-lasting antipneumococcal protective antibody response. Once a humoral response has been generated, either in the setting of vaccination or with previous infection, the antipneumococcal antibodies contribute to a more immediate and effective neutralization of the bacteria.
The development of pneumococcal polysaccharide vaccines for adults and the efficacy of pneumococcal polysaccharide-protein conjugate vaccines in infants and children have confirmed that antibodies to polysaccharide antigens can provide excellent protection against invasive disease caused by pneumococci of the same serotype as well as that caused by cross-reacting serotypes (44–46).
Capsular polysaccharide antigens elicit antibodies that are isotype restricted to IgM and IgG2 and, to a lesser extent, IgG1. These IgM responses are typically short lived, and the IgG2 responses from polysaccharides are weak compared with what is induced by conjugate-based vaccines that are typically dominated by IgG1 subclasses that bind to complement better and are of higher affinity (47). Early pneumococcal vaccines were based on the use of capsular polysaccharides that induced type-specific antibodies that activated and fixed complement, promoting bacterial opsonization and phagocytosis. Antibodies to pneumococcus are also believed to represent the primary mechanism of naturally acquired resistance to colonization. Appropriately triggered B cells also release IgA, which is deposited on mucosal surfaces to protect against pneumococcal colonization.
Vaccines composed of purified capsular polysaccharides, which have been available for more than 50 years, are not immunogenic in young children and the elderly. Moreover, capsular polysaccharides do not yield an anamnestic response, despite multiple exposures, so that antibody titers do not boost beyond their original level after primary immunization. This is due to the inability of polysaccharides to recruit cognate CD4+ T-cell help through T-cell receptor recognition of peptide–major histocompatibility complex class II on the surface of antigen-presenting cells.
However, the regularly spaced repeating epitopes expressed by polysaccharides induce multivalent membrane immunoglobulin cross-linking on the B-cell surface. This immunological crosslinking on the B-cell surface by polysaccharide repeating epitopes mediates improved B-cell proliferative responses; however, on its own, and without proper T-cell help and cytokine signaling, this immunological crosslinking does not induce good memory response, which leads to decreased and suboptimal subsequent immune responses.
The lack of anamnestic response by capsular polysaccharide can be overcome by the use of conjugate vaccines that elicit more of an IgG1 (and IgG3) response to polysaccharide and produce a stronger immune response because polysaccharides coexpressed with carrier proteins interact better with major histocompatibility complex and mediate cognate CD4+ T-cell help for polysaccharide-specific B-cell activation. In the context of appropriate costimulatory signals, the triggering of carrier-peptide–specific T cells results in T-cell help, with the production of both plasma cells and memory B cells. This allows for enhanced immunogenicity with a more rapid and robust production of antibodies and the establishment of T-cell–dependent immunological memory (48). This process stimulates good antibody response, mucosal immunity, and immunologic memory and systemic anamnestic IgG response in all children and adults.
Polysaccharide-based vaccines have been shown to result in decreased memory B-cell frequency, whereas conjugate protein-based vaccines increase serotype-specific memory B-cell responses, highlighting that these vaccines induce important T-cell–dependent memory responses (49).
Given the importance of cellular T- and B-cell function in eliciting an effective and protective anticapsular antibody response, patients with impaired immune systems, such as those with HIV infection, splenic dysfunction, cancer, solid-organ transplantation, or iatrogenic immunosuppressive medications, are highly at risk for invasive pneumococcal infections. The polysaccharide vaccine has been widely evaluated in these populations, but due to its low immunogenicity, its efficacy has been suboptimal or even absent (50–52). Age-related immunosenescence mechanisms in elderly people have been described to explain the increased susceptibility of the aging population to pneumococcus (37). In these populations, the conjugate vaccine should provide improved immunogenicity; however, there is a paucity of evidence surrounding the clinical efficacy of conjugate pneumococcal vaccines in adults with varying immunocompromising conditions (50).
Herd Effect
Individuals who are otherwise personally vulnerable to pneumococcal infection derive protection from the presence of herd immunity in the immunocompetent population. The maintenance of indirect (or herd) immunity depends on the sustained ability of the immune response to prevent acquisition of the bacteria by individuals in the population who are transmitters (53). There is strong evidence that pneumococcal nasopharyngeal carriage is an immediate and essential precursor for pneumococcal disease and the source of transmission between individuals (54). The pneumococcal nasopharyngeal carriage rate is age dependent, starting early in the first year of life and peaking at 55% around 3 years of age, with a steady decline into adulthood.
The protective herd effect of the vaccine is hypothesized to be a direct consequence of the reduction in nasopharyngeal carriage of vaccine-type strains in immunized children, with subsequent interruption of transmission to their nonimmunized contacts (55). The presumed mechanism of herd immunity has been attributed to mucosal IgA antibodies or serum antibodies that leak into the mucosa to alter and decrease the bacterial population in the nasopharynx (56).
As a result, the elderly have indirectly benefited considerably from the introduction of conjugate vaccines in pediatric population. Deaths and hospitalizations related to pneumonia have all decreased substantially with reduction in invasive pneumococcal disease and nonbacteremic pneumococcal pneumonia among adults 65 years of age or older, comparing prevaccination and postvaccination eras (57). Even though herd immunity limits the transmission of the bacteria, populations at risk, including the elderly and the immunosuppressed, do not intrinsically mount excellent antipneumococcal responses. There is still a need to achieve a more effective direct vaccination in the adult population.
Pneumococcal Vaccination in the Modern Age
PPSV23
In 1983, the 23-valent polysaccharide vaccine (PPSV23) was approved for use in the United States (Figure 2) for adults and children older than 2 years of age. This new formulation was developed after worldwide surveillance showed a high frequency of pneumococcal bacteremia and meningitis by serotypes not covered in the previous 14-valent vaccine (58). Use of PPSV23 has repeatedly been shown to provide a significant reduction in the rates of invasive pneumococcal disease, however, sadly, without impact on overall mortality or in overall rates of pneumonia (59). Historically, rates of pneumococcal meningitis have been too low for determination of vaccination efficacy and do not factor largely into published data, even in metaanalysis; thus, the majority of the measured benefit is seen in cases of bacteremic pneumococcal pneumonia.
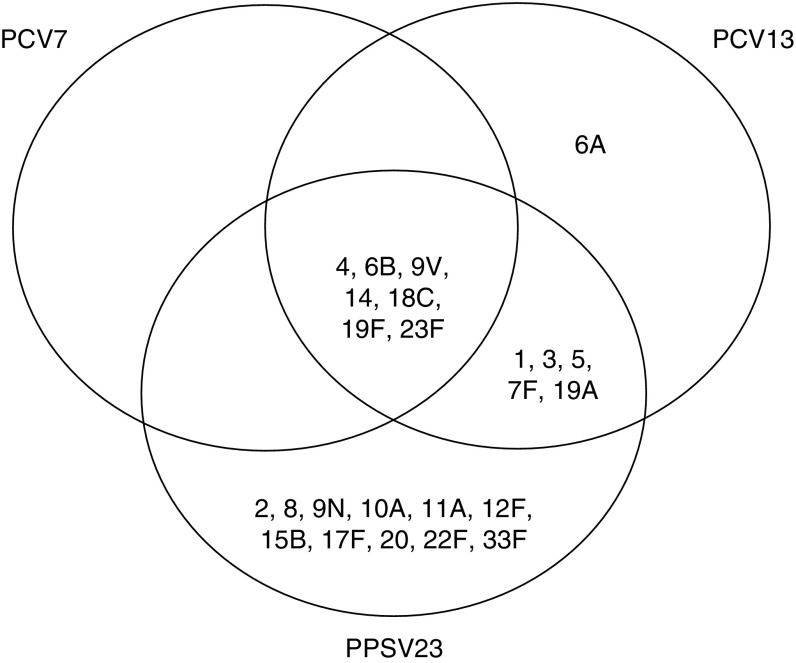
Figure 2.
Pneumococcal serotypes in vaccines.
As previously discussed, immunocompromised individuals are significantly less likely to benefit from vaccination when compared with immunocompetent individuals, borne out by studies of clinical infection rates (60). Moreover, even in patients without frank immunocompromise, vaccine efficacy degrades with age. For example, the vaccine efficacy of an immune-competent 80-year-old man is 67% within 3 years of being vaccinated; more than 5 years after vaccination, the same man has a vaccine efficacy of only 32%.
PCV7
Conjugated vaccinations had been developed previously for prevention of Haemophilus and Meningococcus infections with good efficacy, which led to the development and approval of a seven-valent conjugated vaccine in 2000 for pediatric use. In large prospective trials, the conjugated vaccine was shown not only to reduce pneumococcal bacteremia (46) but also to decrease the risk of otitis media and pneumonia (61). A population-based survey between 1998 and 2005 was able to demonstrate a 30% decrease in the incidence of pneumococcal meningitis. The biggest differences were seen in the very young (<2 yr old, decrease by 64%) and the elderly (>65 yr old, decrease by 54%) (62).
The effect of herd immunity was seen in years to follow, with rates of pneumococcal disease decreasing in older patients who had never received conjugated vaccine, often by significant magnitude (45). In a 10-year study after routine PCV7 vaccination of infants, reduction in the rates of invasive pneumococcal disease and pneumococcal pneumonia were shown in all age groups (57), with drastic reductions in nonbacteremic pneumococcal pneumonia in infants (47%) and adults 65 years and older (54%). Prevention of at least 750,000 hospitalizations over a 6-year period was attributed to the PCV7 vaccine, primarily from herd protection in adult populations. A study in South Africa similarly showed a decrease in invasive pneumococcal disease rates in children and adults after pediatric vaccination with PCV7 (63).
PCV13
Figure 2 shows the serotypes that were included in the development of PCV13 (seven serotypes from PCV7, five that were in PPSV23, and one previously uncovered serotype). In February 2010, the U.S. Food and Drug Administration approved PCV13 for use in children 6 weeks to 71 months old, for the prevention of invasive pneumococcal disease and otitis media. Data from the post-PCV7 era showed that although rates of vaccine-type invasive pneumococcal disease, pneumonia, and otitis media were decreasing, rates of disease from nonvaccine serotypes were becoming more common. A large retrospective cohort study identified more than 30,000 cases of invasive pneumococcal disease over a 9-year time period (64). There was a reduction in overall and PCV7-type invasive pneumococcal disease of 45 and 94%, respectively.
A study of a pediatric population showed that only 15% of identified invasive pneumococcal disease infections were caused by PCV7 serotypes. The study also showed, however, a stable overall rate of invasive pneumococcal disease in this population when compared with pre-PCV7 data, suggesting no change in overall disease burden (65).
In June 2012, the U.S. Food and Drug Administration approved PCV13 for all adults older than 50 years, for the prevention of pneumonia and invasive pneumococcal disease. Much of this recommendation was supported by evidence from immunogenicity trials in which more than 800 vaccine-naive subjects aged 60 to 64 years old were randomized to receive one dose of either PCV13 or PPSV23 (66). The primary outcome was 1-month postvaccination opsonophagocytic activity (OPA) titers that are believed, but not proven, to be associated with in vivo protection from pneumococcal infection. At 1 month, subjects given PCV13 had increased OPA titers compared with the subjects given PPSV23, specifically for the shared serotypes between the two vaccines. Titers were shown to decrease at 1 year postvaccination, although they still remained higher than prevaccination levels.
A similar study was performed looking at the effect of PCV13 (vs. PPSV23) on subjects older than 70 years who were previously vaccinated with PPSV23 more than 5 years earlier (67). OPA titers were higher in the PCV13 “boosted” group for 10 of 12 serotypes common to the two vaccines, implying that a patient’s immunity against pneumococcal infection could be enhanced with a combined strategy of polysaccharide vaccination followed by conjugate vaccine, particularly in the face of waning immunity.
In June 2012, PCV13 was recommended by the Advisory Committee on Immunization Practices (ACIP) for adults older than 18 years with immune-compromising conditions. This recommendation was based largely on a 2010 randomized controlled trial that studied the secondary prevention of invasive pneumococcal disease in nearly 500 subjects, most of whom had HIV (68). In this population, two doses of PCV7 resulted in a significant decrease in the rate of recurrent invasive pneumococcal disease over 5 years.
As of August 2014, the ACIP has recommended the routine use of PCV13, in series with PPSV23, for all patients 65 years and older. The Community Acquired Pneumonia Immunization Trial in Adults (CAPiTA) study, conducted between 2008 and 2010, randomized 85,000 Dutch subjects 65 years and older to receive one dose of PCV13 or placebo (69). The subjects had never been vaccinated and were otherwise immune competent. It is important to note that in the CAPiTA cohort, 12% of subjects were current smokers, and 10% reported a known diagnosis of chronic lung disease. They used a serotype-specific urinary antigen assay to differentiate vaccine-type and non–vaccine-type infections. After a mean follow up of 4 years, the vaccinated group developed 49 episodes of vaccine-type confirmed community-acquired pneumonia compared with 90 episodes in the placebo group, representing 45% vaccine efficacy. There were also significant decreases in the rates of invasive pneumococcal disease and confirmed noninvasive community-acquired pneumonia (P = 0.007). For invasive pneumococcal disease, vaccine efficacy was 75%.
Current Recommendations for Pneumococcal Vaccination
For the clinician, there are multiple branch points to consider when approaching pneumococcal vaccination of an individual patient. Here, we will first consider age groups, followed by vaccination status, then clinical risk factors (Table 2).
Table 2.
Pneumococcal disease risk assessment
| Immunocompetent individuals with high-risk comorbidities | Chronic heart disease (with exception of isolated hypertension) |
| Chronic lung disease | |
| Diabetes mellitus | |
| Chronic liver disease or cirrhosis | |
| Smoking | |
| Alcoholism | |
| Cerebrospinal fluid leak | |
| Cochlear implants | |
| Functional or anatomical asplenia | Sickle cell disease and other hemoglobinopathies |
| Congenital or acquired asplenia/splenic dysfunction | |
| Immunosuppressed individuals | HIV |
| Chronic renal failure and nephrotic syndrome | |
| Immunosuppressant diseases and/or associated immunosuppressant drugs or radiation therapy (malignant neoplasms, leukemias, lymphomas, solid organ transplants) | |
| Congenital immunodeficiencies (B- or T-cell deficiencies, complement deficiencies, phagocytic disorders) |
Pediatric Population
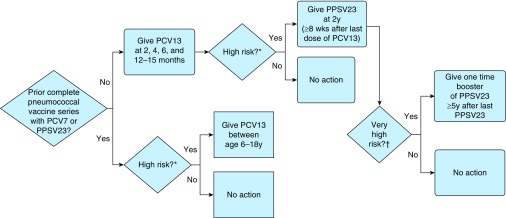
The ACIP currently recommends routine vaccination for children aged 2 to 59 months with PCV13 as a four-dose series (at age 2, 4, 6, and 12–15 mo) (Figure 3). There are resources available to assist with phasing non–vaccine-naive patients from primarily PCV7-based regimens over to the current PCV13-based regimens (70).
Figure 3.
Advisory Committee on Immunization Practices recommendations for pediatric pneumococcal vaccination. *See Table 2 for relevant high-risk comorbidities and immunocompromised conditions. †Functional or anatomic asplenia and immunocompromised conditions. Adapted from CDC guidelines for appropriate overlap schedules for individuals who received partial series of PCV7 and/or PPSV23 (70).
Special Pediatric Scenarios
-
1.
For children aged 6 to 18 years who previously completed an appropriate vaccination regimen with either PCV7 or PPSV23, and who are at higher risk of pneumococcal infection, a one-time dose of PCV13 can be administered.
-
2.
For children aged 2 to 18 years who previously completed an appropriate vaccination regimen with PCV13, and who are at higher risk of pneumococcal infection, a dose of PPSV23 can be given at age 2 years, at least 8 weeks after last dose of PCV13.
-
3.
For those children with specific high-risk conditions (functional or anatomic asplenia, sickle cell disease, HIV, other immunocompromised conditions; see Table 2), an additional PPSV23 one-time booster can be given 5 years later than the initial PPSV23 vaccine.
Adult Population
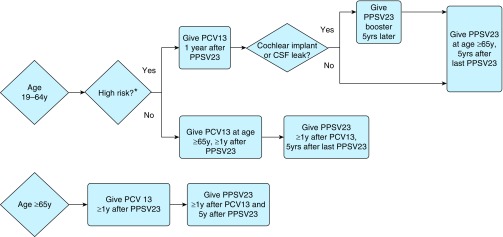
Many individuals will have received pneumococcal vaccination at some point prior as an adult (age 19 years or older), requiring a more complex algorithm to be discussed below, but in the case of truly vaccine-naive individuals, the vaccine schedule is again predicated on age and comorbid conditions (Figure 4) (71). As with the pediatric population, there are comorbid conditions that increase risk for pneumococcal infection in adults and impact vaccination recommendations between ages 19 and 65 years (Table 2). Those with otherwise intact immunity but with high-risk comorbid conditions should receive a dose of PPSV23 at the earliest relevant opportunity. Notably, this includes active smokers, irrespective of presence of lung disease. Their subsequent vaccine schedule would otherwise follow that for the adult population.
Figure 4.
Advisory Committee on Immunization Practices recommendations for adult pneumococcal vaccination: vaccine-naive individuals. *Includes patients with functional/anatomic asplenia, immunocompromising conditions, cerebrospinal fluid (CSF) leak, or cochlear implants (see Table 2). †Includes patients with normal immunity but certain comorbid conditions.
Those with immunocompromising conditions, such as acquired or functional asplenia, congenital or acquired immunosuppression, chronic renal failure, nephrotic syndrome, or underlying malignancy, should also first receive PCV13, followed by PPSV23 8 weeks later, but should also receive a second PPSV23 5 years after the initial PPSV23 dose. Once again, if PPSV23 has been given first, PCV13 may still be given 1 year after the initial PSV23, with the second PPSV23 still occurring 5 years after the initial vaccination. All individuals should receive an additional dose of PPSV23 at age 65 years, provided it has been 5 years since the last dose. Immunocompetent individuals with either cochlear implants or cerebrospinal fluid leak follow a similar schedule.
Otherwise, vaccine-naive individuals should receive one dose of the PCV13 vaccine at age 65 years (or older, if none prior) followed by one dose of the PPSV23 vaccine 6 to 12 months later. The minimum interval between the two vaccines should be 8 weeks, and the PPSV23 vaccine can be given more than 12 months beyond the 6- to 12-month interval if this window is missed. However, many individuals will already have received one, if not more, doses of PPSV23 in adulthood. In this case, timing and sequence of PCV13 and any subsequent PPSV23 must be more deliberate (Figure 5).
Figure 5.
Advisory Committee on Immunization Practices recommendations for adult pneumococcal vaccination: individuals with prior PPSV23 vaccination. *Includes patients with functional/anatomic asplenia, immunocompromising conditions, cerebrospinal fluid (CSF) leak, or cochlear implants (see Table 2).
It is important to note that current ACIP guidelines recommend very specific intervals between both types of vaccines. Because conjugate vaccines stimulate memory B cells, it was believed that administration of conjugate vaccine should prime the immune system for an improved secondary response to a polysaccharide vaccine. However, a randomized trial that administered polysaccharide vaccine in the first 6 months before the PCV7 conjugate vaccine resulted in attenuated antibody concentration compared with PCV7 alone (72).
Although several smaller earlier studies did not show that priming with pneumococcal conjugate vaccine followed by polysaccharide vaccine enhanced immunogenicity, more recent larger studies showed improved response (72–74). These larger studies using PCV13 conjugate vaccine followed a year later by the polysaccharide-based PPSV23 resulted in higher antibody activities after a month of PPSV23 administration, but at 6 months the antibody levels in booster recipients fall to baseline (66, 75).
Although polysaccharide vaccines do not necessarily trigger anamnestic responses, revaccination with polysaccharide vaccines at the proper interval can result in sustained antipneumococcal antibody titers, especially in middle-aged and older adults (76, 77). There are studies to suggest that there is an inverse relationship between circulating antibodies before revaccination and the subsequent increase in antibody titers (78). However, there is also a suggestion that revaccination too soon after initial vaccination could be detrimental. Because of these findings, ACIP recommend PCV13 first, followed by a minimum of 8 weeks before the PPSV23. For those who already have received PPSV23, current recommendations advocate waiting 1 year before receiving PCV13. More investigations are needed to determine the clinical implications of frequent vaccinations.
Discussion and Future Directions
Over the past 100 years, substantial progress has been made in the prevention of invasive pneumococcal disease. Current recommendations reflect a series of changes made over a relatively short span of time, on the basis of growing data and experience with both polysaccharide and conjugate vaccines in multiple different populations. With so many nearly simultaneous changes in vaccine strategy, it remains a challenge to appropriately attribute the source of greatest effect. It is clear that prior PCV7 vaccination strategies in children over the past 2 decades yielded substantial indirect impacts on the prevalence of adult vaccine-type pneumococcal disease, and therefore the switchover to preferential use of PCV13 in the pediatric population may be expected to yield more of the same, although that remains to be proven.
At this point, we are still inferring future benefits from data presently available. The original impetus for use of PCV13 in the elderly population was the robust immunogenicity data in this population. This fact, in conjunction with clinical trial results in immunocompromised populations, was the foundation for the present substantial shift in adult immunization practice. Although this strategy appears to be well supported by the compelling results observed in CAPiTA, with 45% efficacy in prevention of community-acquired pneumonia and 75% efficacy in prevention of invasive pneumococcal disease, there are some caveats to consider.
First, this study does not represent a true test of the current ACIP guidelines or comparison to prior recommended practice with PPSV23, as subjects were vaccine naive, and subjects received either PCV13 or placebo. Moreover, results may in part reflect herd-immunity impact of pediatric vaccination practices in the Netherlands, first with PCV7, then with PCV10. Finally, the population studied was predominantly white, and most patients were 65 to 75 years of age. As the average life expectancy continues to increase, we will need to confer vaccine efficacy to an ever-aging population for whom vaccine efficacy is known to wane over time (51, 60). A study in a more heterogeneous elderly population with more long-term follow up of vaccine titers would be instructive, as concern remains about waning immunity in the elderly as they move decades (or more) beyond last vaccination.
Although expanded serologic coverage makes intuitive sense, particularly for an aging and immunologically vulnerable population, it is unclear whether any further changes to vaccination protocol could outstrip the benefits of comprehensive measures to improve immunization adherence in both the pediatric and adult populations. With the use of two different vaccines administered under very specific schedules, implementation may prove to be a difficult task in the real world. It will be important to monitor immunization adherence as this combination strategy moves into general practice and to determine if these vaccines are indeed being administered as intended to most patients. More study is needed to see how effectively (and cost-effectively) these new pneumococcal vaccination strategies can be applied in populations that differ in age, sex, socioeconomic status, race, comorbidities, access to care, and immunologic status.
Finally, is eradication of invasive pneumococcal disease an attainable goal? For this to take place, there are several questions that need to be addressed. Should the aim of pneumococcal disease prevention be eradication of nasopharyngeal colonization, or should the goal be preventing bacterial invasion, leaving colonization unaffected to avoid selective colonization with more virulent organisms? These facts underline the key role for pneumococcal colonization in pathogenesis and prevention of pneumococcal infections, which justifies extensive consideration in decision making about mass vaccination and future vaccine strategies. Certainly, without complete eradication of pneumococcal carriage, the immunological pressure will ultimately select for noncovered bacterial serotypes, but will these serotypes cause invasive pneumococcal disease (79)?
There is also a role for controlling drug-resistant bacteria with judicious use of antibiotics. The rapid increase in antibiotic resistance, high cost, and limited serotype coverage of the currently available pneumococcal vaccines will likely require the need for novel vaccine candidates that are more affordable and elicit protection against a broader range of pneumococcal strains. Moreover, efforts are needed for next-generation vaccine development that has longer-lasting immunity effects with broader serotype coverage that work well in the aging population.
Efforts in this area include optimization of cultivation conditions for bacteria capsule production for better yield and lower cost, the use of common pneumococcal proteins for broader coverage, and the use of immune adjuvants to improve mucosal and systemic immunity. Monitoring changes in the distribution of serotypes causing pneumococcal disease among children and adults should inform formulations of future higher-valent conjugate vaccines. Approaches that rely on targeting common proteins independent of serotypes, inactivated and live attenuated whole-cell vaccines, DNA vaccines and genetic engineering of glycoproteins to develop glycoconjugates hold the promise to confer broader serotype-independent protection against pneumococcal disease. (80).
Close scrutiny of the changing epidemiology of invasive pneumococcal disease and pneumonia will be required to evaluate the effectiveness and the continued utility of the current vaccination strategy and the future directions for pneumococcal disease prevention among older adults.
Acknowledgments
Acknowledgment
The authors thank Susan Ardito and Dr. Geoffrey Connors for their editorial assistance and review of the manuscript.
Footnotes
Supported by a Patterson Trust Clinical Research Award (C.S.D.C.), Yale Center of Clinical Investigation Clinical Translational Scholar Award (C.S.D.C.), National Heart, Lung, and Blood Institute grants HL126094 and HL103770 (C.S.D.C.), and a Clinical and Translational Science Award grant UL1 TR000142 (C.S.D.C.) from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The funding bodies had no part in the writing of the manuscript or the submission.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Atkinson W, Wolfe C, Hamborsky J, editors. Epidemiology and prevention of vaccine-preventable diseases. 12th ed. Washington DC: Public Health Foundation; 2012. [Google Scholar]
- 2.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 3.Austrian R. The pneumococcus at the millennium: not down, not out. J Infect Dis. 1999;179:S338–S341. doi: 10.1086/513841. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs) report Emerging Infections Program Network Streptococcus pneumoniae; 2013 [accessed 2013 Dec 6]. Available from: http://www.cdc.gov/abcs/reports-findings/survreports/spneu12.pdf.
- 5.van der Hoek W, Wielders CC, Schimmer B, Wegdam-Blans MC, Meekelenkamp J, Zaaijer HL, Schneeberger PM. Detection of phase I IgG antibodies to Coxiella burnetii with EIA as a screening test for blood donations. Eur J Clin Microbiol Infect Dis. 2012;31:3207–3209. doi: 10.1007/s10096-012-1686-7. [DOI] [PubMed] [Google Scholar]
- 6.Hjuler T, Poulsen G, Wohlfahrt J, Kaltoft M, Biggar RJ, Melbye M. Genetic susceptibility to severe infection in families with invasive pneumococcal disease. Am J Epidemiol. 2008;167:814–819. doi: 10.1093/aje/kwm376. [DOI] [PubMed] [Google Scholar]
- 7.Bisharat N, Omari H, Lavi I, Raz R. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182–186. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 8.Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, Schaffner W, Craig AS, Griffin MR. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 9.Kyaw MH, Rose CE, Jr, Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi: 10.1086/431521. [DOI] [PubMed] [Google Scholar]
- 10.Kumashi P, Girgawy E, Tarrand JJ, Rolston KV, Raad II, Safdar A. Streptococcus pneumoniae bacteremia in patients with cancer: disease characteristics and outcomes in the era of escalating drug resistance (1998-2002) Medicine (Baltimore) 2005;84:303–312. doi: 10.1097/01.md.0000180045.26909.29. [DOI] [PubMed] [Google Scholar]
- 11.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995-2000. J Infect Dis. 2005;191:2038–2045. doi: 10.1086/430356. [DOI] [PubMed] [Google Scholar]
- 12.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, Schaffner W, Craig AS, Griffin MR. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 13.Cruickshank HC, Jefferies JM, Clarke SC. Lifestyle risk factors for invasive pneumococcal disease: a systematic review. BMJ Open. 2014;4:e005224. doi: 10.1136/bmjopen-2014-005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med. 1999;107:12S–27S. doi: 10.1016/s0002-9343(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 15.Harboe ZB, Larsen MV, Ladelund S, Kronborg G, Konradsen HB, Gerstoft J, Larsen CS, Pedersen C, Pedersen G, Obel N, et al. Incidence and risk factors for invasive pneumococcal disease in HIV-infected and non-HIV-infected individuals before and after the introduction of combination antiretroviral therapy: persistent high risk among HIV-infected injecting drug users. Clin Infect Dis. 2014;59:1168–1176. doi: 10.1093/cid/ciu558. [DOI] [PubMed] [Google Scholar]
- 16.Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70:984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch W. The Micrococcus lanceolatus, with especial reference to the etiology of acute lobar pneumonia. Johns Hopkins Hospital Bulletin. 1892;iii:125–139. [Google Scholar]
- 19.Wright A, Morgan W, Colebrook L, Dodgson R. Observation on prophylactic inoculation against pneumococcus infections, and on the results that have been achieved by it. Lancet. 1914;10:87–95. [Google Scholar]
- 20.MacLeod CM, Hodges RG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. [PubMed] [Google Scholar]
- 21.Hansman D, Bullen M. A resistant pneumococcus. Lancet. 1967;2:264–265. doi: 10.1016/s0140-6736(75)91547-0. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs MR, Koornhof HJ, Robins-Browne RM, Stevenson CM, Vermaak ZA, Freiman I, Miller GB, Witcomb MA, Isaäcson M, Ward JI, et al. Emergence of multiply resistant pneumococci. N Engl J Med. 1978;299:735–740. doi: 10.1056/NEJM197810052991402. [DOI] [PubMed] [Google Scholar]
- 23.Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759–776. doi: 10.7326/0003-4819-60-5-759. [DOI] [PubMed] [Google Scholar]
- 24.Riley ID, Tarr PI, Andrews M, Pfeiffer M, Howard R, Challands P, Jennison G. Immunisation with a polyvalent pneumococcal vaccine: reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet. 1977;1:1338–1341. doi: 10.1016/s0140-6736(77)92552-1. [DOI] [PubMed] [Google Scholar]
- 25.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, Richmond AS, Smith RP, Schiffman G, Shepard DS, et al. Efficacy of pneumococcal vaccine in high-risk patients: results of a Veterans Administration Cooperative Study. N Engl J Med. 1986;315:1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 26.Dockrell DH, Whyte MK, Mitchell TJ. Pneumococcal pneumonia: mechanisms of infection and resolution. Chest. 2012;142:482–491. doi: 10.1378/chest.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calbo E, Garau J. Factors affecting the development of systemic inflammatory response syndrome in pneumococcal infections. Curr Opin Infect Dis. 2011;24:241–247. doi: 10.1097/QCO.0b013e3283463e45. [DOI] [PubMed] [Google Scholar]
- 28.Calbo E, Garau J. Of mice and men: innate immunity in pneumococcal pneumonia. Int J Antimicrob Agents. 2010;35:107–113. doi: 10.1016/j.ijantimicag.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–3761. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. Plos Pathog. 2009;5:e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson S, Musher DM, Chapman A, Goree A, Lawrence EC. Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J Infect Dis. 1985;152:4–13. doi: 10.1093/infdis/152.1.4. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell AM, Mitchell TJ. Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect. 2010;16:411–418. doi: 10.1111/j.1469-0691.2010.03183.x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 34.Casal J, Tarragó D. Immunity to Streptococcus pneumoniae: factors affecting production and efficacy. Curr Opin Infect Dis. 2003;16:219–224. doi: 10.1097/00001432-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Vernatter J, Pirofski LA. Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis. 2013;26:277–283. doi: 10.1097/QCO.0b013e3283608419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olliver M, Hiew J, Mellroth P, Henriques-Normark B, Bergman P. Human monocytes promote Th1 and Th17 responses to Streptococcus pneumoniae. Infect Immun. 2011;79:4210–4217. doi: 10.1128/IAI.05286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krone CL, van de Groep K, Trzciński K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. Lancet Respir Med. 2014;2:141–153. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 38.Paterson GK, Orihuela CJ. Pneumococci: immunology of the innate host response. Respirology. 2010;15:1057–1063. doi: 10.1111/j.1440-1843.2010.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koppe U, Suttorp N, Opitz B. Recognition of Streptococcus pneumoniae by the innate immune system. Cell Microbiol. 2012;14:460–466. doi: 10.1111/j.1462-5822.2011.01746.x. [DOI] [PubMed] [Google Scholar]
- 40.Steel HC, Cockeran R, Anderson R, Feldman C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediators Inflamm. 2013;2013:490346. doi: 10.1155/2013/490346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lord F, Hefron R. Pneumonia and serum therapy. New York: Commonwealth Fund; 1938. [Google Scholar]
- 42.Snapper CM. Mechanisms underlying in vivo polysaccharide-specific immunoglobulin responses to intact extracellular bacteria. Ann N Y Acad Sci. 2012;1253:92–101. doi: 10.1111/j.1749-6632.2011.06329.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim JO, Romero-Steiner S, Sørensen UB, Blom J, Carvalho M, Barnard S, Carlone G, Weiser JN. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornu C, Yzèbe D, Léophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine. 2001;19:4780–4790. doi: 10.1016/s0264-410x(01)00217-1. [DOI] [PubMed] [Google Scholar]
- 45.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 46.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Northern California Kaiser Permanente Vaccine Study Center Group. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 47.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 48.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 49.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–1416. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordonnier C, Averbuch D, Maury S, Engelhard D. Pneumococcal immunization in immunocompromised hosts: where do we stand? Expert Rev Vaccines. 2014;13:59–74. doi: 10.1586/14760584.2014.859990. [DOI] [PubMed] [Google Scholar]
- 51.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 52.Jackson ML, Neuzil KM, Thompson WW, Shay DK, Yu O, Hanson CA, Jackson LA. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39:1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rashid H, Khandaker G, Booy R. Vaccination and herd immunity: what more do we know? Curr Opin Infect Dis. 2012;25:243–249. doi: 10.1097/QCO.0b013e328352f727. [DOI] [PubMed] [Google Scholar]
- 54.Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. Epidemiologic evidence for serotype-specific acquired immunity to pneumococcal carriage. J Infect Dis. 2008;197:1511–1518. doi: 10.1086/587941. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien KL, Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine. 2003;21:1815–1825. doi: 10.1016/s0264-410x(02)00807-1. [DOI] [PubMed] [Google Scholar]
- 56.Fujihashi K, Sato S, Kiyono H. Mucosal adjuvants for vaccines to control upper respiratory infections in the elderly. Exp Gerontol. 2014;54:21–26. doi: 10.1016/j.exger.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2:e00309–e00310. doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbins JB, Austrian R, Lee CJ, Rastogi SC, Schiffman G, Henrichsen J, Mäkelä PH, Broome CV, Facklam RR, Tiesjema RH, et al. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 59.Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, Detsky AS, Kapoor WN. Efficacy of pneumococcal vaccination in adults: a meta-analysis of randomized controlled trials. Arch Intern Med. 1994;154:2666–2677. doi: 10.1001/archinte.1994.00420230051007. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 61.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–815. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Hsu HE, Shutt KA, Moore MR, Beall BW, Bennett NM, Craig AS, Farley MM, Jorgensen JH, Lexau CA, Petit S, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244–256. doi: 10.1056/NEJMoa0800836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, Madhi SA, Zell ER, Verani JR, O’Brien KL, et al. GERMS-SA Investigators. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 64.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 65.Hsu KK, Shea KM, Stevenson AE, Pelton SI Massachusetts Department of Public Health. Changing serotypes causing childhood invasive pneumococcal disease: Massachusetts, 2001-2007. Pediatr Infect Dis J. 2010;29:289–293. doi: 10.1097/INF.0b013e3181c15471. [DOI] [PubMed] [Google Scholar]
- 66.Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31:3577–3584. doi: 10.1016/j.vaccine.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 67.Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31:3585–3593. doi: 10.1016/j.vaccine.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 68.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362:812–822. doi: 10.1056/NEJMoa0903029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 70.Nuorti JP, Whitney CG Centers for Disease Control and Prevention (CDC) Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 71.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T Centers for Disease Control and Prevention (CDC) Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 72.Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, et al. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis. 2011;52:736–742. doi: 10.1093/cid/cir003. [DOI] [PubMed] [Google Scholar]
- 73.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis. 2009;49:1318–1325. doi: 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 74.Miernyk KM, Butler JC, Bulkow LR, Singleton RJ, Hennessy TW, Dentinger CM, Peters HV, Knutsen B, Hickel J, Parkinson AJ. Immunogenicity and reactogenicity of pneumococcal polysaccharide and conjugate vaccines in Alaska native adults 55-70 years of age. Clin Infect Dis. 2009;49:241–248. doi: 10.1086/599824. [DOI] [PubMed] [Google Scholar]
- 75.Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–3602. doi: 10.1016/j.vaccine.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 76.Manoff SB, Liss C, Caulfield MJ, Marchese RD, Silber J, Boslego J, Romero-Steiner S, Rajam G, Glass NE, Whitney CG, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J Infect Dis. 2010;201:525–533. doi: 10.1086/651131. [DOI] [PubMed] [Google Scholar]
- 77.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 78.Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine. 2012;30:4435–4444. doi: 10.1016/j.vaccine.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 79.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PW. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 80.Terra VS, Mills DC, Yates LE, Abouelhadid S, Cuccui J, Wren BW. Recent developments in bacterial protein glycan coupling technology and glycoconjugate vaccine design. J Med Microbiol. 2012;61:919–926. doi: 10.1099/jmm.0.039438-0. [DOI] [PubMed] [Google Scholar]