Abstract
Rationale: Patients without a known history of lung disease presenting with a spontaneous pneumothorax are generally diagnosed as having primary spontaneous pneumothorax. However, occult diffuse cystic lung diseases such as Birt-Hogg-Dubé syndrome (BHD), lymphangioleiomyomatosis (LAM), and pulmonary Langerhans cell histiocytosis (PLCH) can also first present with a spontaneous pneumothorax, and their early identification by high-resolution computed tomographic (HRCT) chest imaging has implications for subsequent management.
Objectives: The objective of our study was to evaluate the cost-effectiveness of HRCT chest imaging to facilitate early diagnosis of LAM, BHD, and PLCH.
Methods: We constructed a Markov state-transition model to assess the cost-effectiveness of screening HRCT to facilitate early diagnosis of diffuse cystic lung diseases in patients presenting with an apparent primary spontaneous pneumothorax. Baseline data for prevalence of BHD, LAM, and PLCH and rates of recurrent pneumothoraces in each of these diseases were derived from the literature. Costs were extracted from 2014 Medicare data. We compared a strategy of HRCT screening followed by pleurodesis in patients with LAM, BHD, or PLCH versus conventional management with no HRCT screening.
Measurements and Main Results: In our base case analysis, screening for the presence of BHD, LAM, or PLCH in patients presenting with a spontaneous pneumothorax was cost effective, with a marginal cost-effectiveness ratio of $1,427 per quality-adjusted life-year gained. Sensitivity analysis showed that screening HRCT remained cost effective for diffuse cystic lung diseases prevalence as low as 0.01%.
Conclusions: HRCT image screening for BHD, LAM, and PLCH in patients with apparent primary spontaneous pneumothorax is cost effective. Clinicians should consider performing a screening HRCT in patients presenting with apparent primary spontaneous pneumothorax.
Keywords: lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome, pulmonary Langerhans cell histiocytosis, pneumothorax
Diffuse cystic lung diseases are a group of pathophysiologically heterogeneous diseases characterized by the formation of multiple air-filled spaces (cysts) in the pulmonary parenchyma. The differential diagnosis for these diseases is broad and encompasses a wide variety of etiologies (1). The most common cystic lung diseases encountered in clinical practice include lymphangioleiomyomatosis (LAM), Birt-Hogg-Dubé syndrome (BHD), pulmonary Langerhans cell histiocytosis (PLCH), and cystic lymphoid interstitial pneumonia/follicular bronchiolitis (LIP/FB). Although the clinical features vary among these disorders, an increased risk of cyst rupture leading to the development of recurrent pneumothoraces is common to all.
Primary spontaneous pneumothorax refers to a pneumothorax that occurs in patients without underlying lung disease. The incidence of primary spontaneous pneumothorax varies from 1.2 to 9.8 per 100,000 women and 7.4 to 24 per 100,000 men per year (2, 3). It is estimated that more than 20,000 patients suffer from a spontaneous pneumothorax in the United States every year (4). Underlying pulmonary disorders are now being increasingly identified in patients presenting with an apparent primary spontaneous pneumothorax (5).
The distinction between primary spontaneous pneumothorax and spontaneous pneumothorax secondary to an underlying lung disease (secondary spontaneous pneumothorax) is important, as recommendations regarding interventions to prevent recurrence differ between these categories. In patients with primary spontaneous pneumothorax, interventions such as pleurodesis to prevent recurrences are recommended after a second episode of spontaneous pneumothorax, whereas pleurodesis is recommended after the first episode in patients with secondary spontaneous pneumothorax (4).
Recent reports suggest that diffuse cystic lung diseases are the underlying etiology in a significant proportion of patients presenting with an apparent primary spontaneous pneumothorax. Studies from China and Holland have shown that BHD is found in 5–10% of patients in those countries presenting with an apparent primary spontaneous pneumothorax (6, 7). The prevalence of LAM has been estimated to be greater than 5% in the restricted demographic of nonsmoking women aged between 25 and 54 years presenting with an apparent primary spontaneous pneumothorax (8). Early diagnosis of these disorders can lead to prudent, anticipatory management approaches, including early pleurodesis to prevent future pneumothoraces.
The advent of high-resolution computed tomography (HRCT) chest imaging has markedly increased the ability to diagnose diffuse cystic lung diseases. An expert radiologist can accurately diagnose PLCH, LAM, and BHD in 74, 91, and 93% of cases, respectively, on the basis of HRCT analysis alone (9). Routine CT chest imaging is not currently recommended in patients presenting with a primary spontaneous pneumothorax, however (4).
For this study, we hypothesized that screening for the presence of LAM, BHD, and PLCH by HRCT in patients presenting with apparent primary spontaneous pneumothorax will lead to a reduction in the number of future pneumothoraces and will prove cost effective. Some of the results of this study have been previously reported in the form of an abstract at the American Thoracic Society annual meeting in May 2016 (10).
Methods
Simulation Model
We constructed a Markov state-transition simulation model using a standard software program (Decision Maker; Boston, MA) to analyze decision trees and perform sensitivity analyses (Figures 1 and 2). Our base case was a 35-year-old nonsmoking woman presenting to the emergency room with a spontaneous pneumothorax.
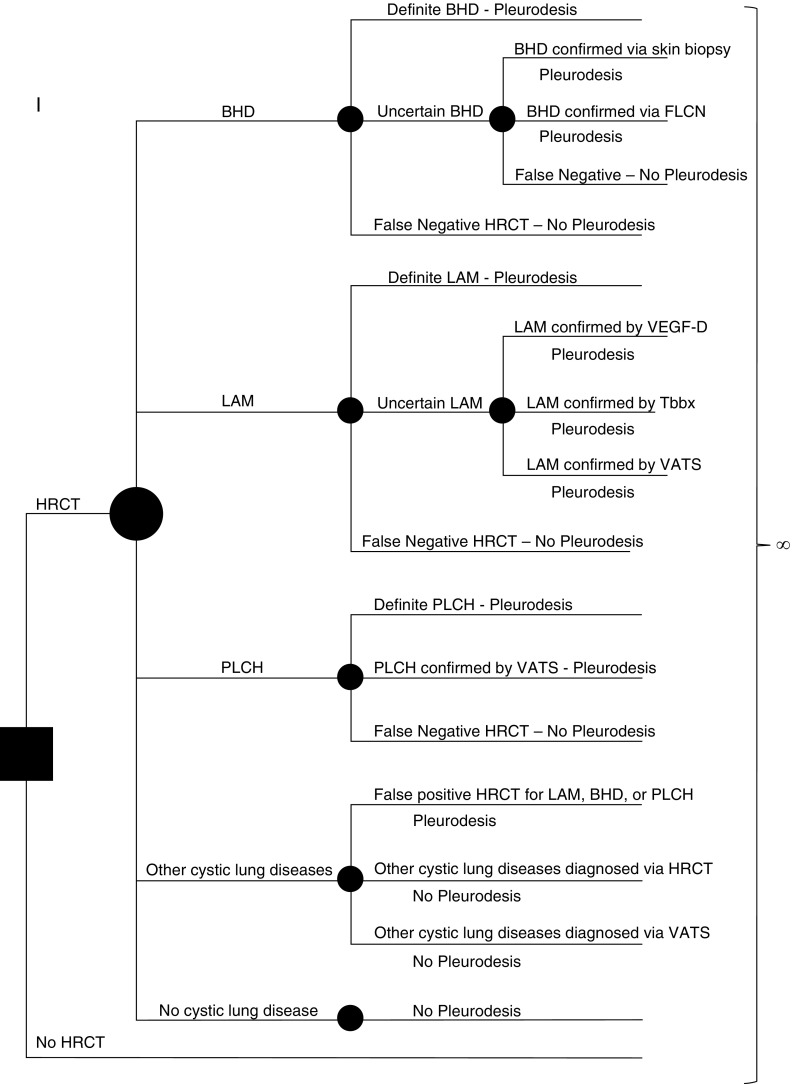
Figure 1.
Simplified schematic of the model used in our analysis. The solid square represents the decision node where the initial decision is made by the practicing clinician—to obtain a high-resolution computed tomography (HRCT) scan or not. Each solid circle represents a chance node, where the likelihood of a given outcome is defined by a probability entered into the model. Patients presenting with a pneumothorax can have one of the following five scenarios: underlying Birt-Hogg-Dubé syndrome (BHD), underlying lymphangioleiomyomatosis (LAM), underlying pulmonary Langerhans cell histiocytosis (PLCH), other cystic lung diseases, or no cystic lung disease. The probability of the presence of each of these five scenarios is entered into the model. Further testing for diagnostic confirmation is based on the sensitivity and specificity of HRCT to accurately diagnose LAM, BHD, or PLCH. The performance characteristics of each diagnostic test are also considered when assigning a correct diagnosis, and the rates of false-positive and false-negative diagnoses are accounted for at each step. Each arm of the tree ultimately ends in the Markov node (∞ symbol). In each state, costs and quality adjustment factors are applied and tabulated. FLCN = folliculin; Tbbx = transbronchial biopsy; VATS = video-assisted thoracoscopic surgery; VEGF-D = vascular endothelial growth factor-D.
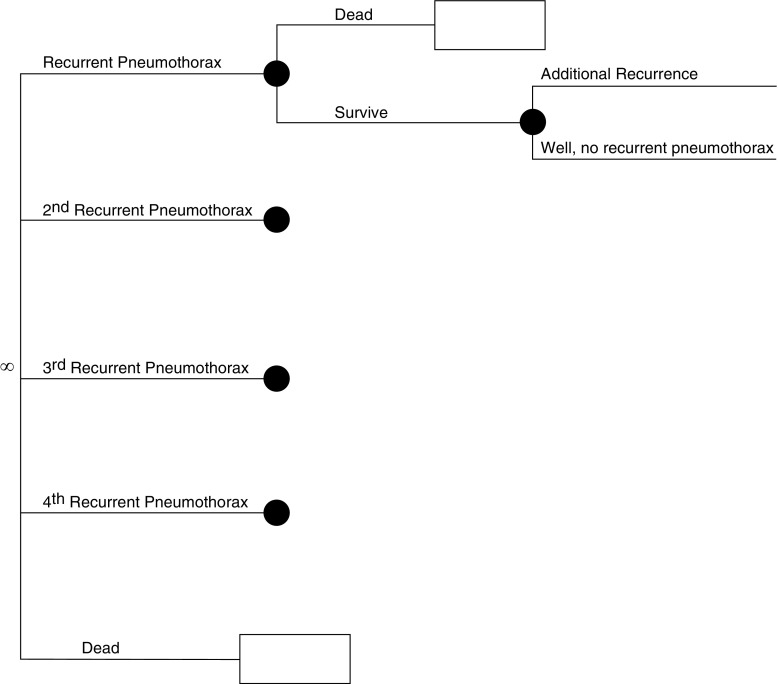
Figure 2.
Simplified schematic of the model used in our analysis. This figure depicts the continuation of our model. In the Markov node (∞ symbol), patients progress through various health states dictated by probabilities entered into the model. These probabilities are different, depending on how the individual entered the Markov node (e.g., a patient with cystic lung disease [lymphangioleiomyomatosis (LAM), Birt-Hogg-Dubé syndrome (BHD), or pulmonary Langerhans cell histiocytosis (PLCH)] who has undergone pleurodesis has a lower probability of recurrent pneumothorax than a patient who has not). In each cycle (defined as 1 mo), patients are subjected to risk of recurrent pneumothorax or age/sex-related death, as well as disease-specific additional mortality as applicable to patients with LAM and PLCH. In each state, costs and quality adjustment factors are applied and tabulated. These cycles repeat until all patients are dead. Outcomes can then be compared for each strategy tested (high-resolution computed tomography [HRCT] vs. no HRCT).
After successful management of the pneumothorax (by observation or pleural drainage), we evaluated two distinct strategies for follow-up care. The first used the current standard recommendations for management of spontaneous pneumothorax and no further diagnostic tests (4). The alternate strategy consisted of a screening HRCT to evaluate for the presence of BHD, LAM, and PLCH.
Cystic LIP/FB is another commonly encountered diffuse cystic lung disease in clinical practice, typically seen in association with Sjögren syndrome (11). Although patients with cystic LIP/FB can also suffer from multiple pneumothoraces (12), the exact risk of recurrent events is not well established in these patients. For this reason, we did not model cystic LIP/FB as a separate diagnosis in our analysis and assumed that these patients will behave similar to patients with primary spontaneous pneumothorax.
On the basis of our interpretation of HRCT images, we characterized diagnostic status as: (1) definite, based on characteristic HRCT findings; (2) uncertain, warranting further diagnostic evaluation; or (3) alternative, suggestive of a diagnosis other than LAM, PLCH, or BHD. Patients with a definite diagnosis of LAM, BHD, or PLCH proceeded to receive pleurodesis.
Patients with uncertain diagnosis underwent confirmatory testing in a stepwise manner from least to most invasive methods. For patients with suspected BHD, the confirmatory tests were skin biopsy to look for fibrofolliculomas and/or folliculin (FLCN) mutation analysis. For suspected LAM, the confirmatory tests used were a stepwise testing of serum vascular endothelial growth factor-D (VEGF-D), transbronchial lung biopsy, and video-assisted thoracoscopic surgery (VATS)-guided surgical lung biopsy. For suspected PLCH, the diagnostic confirmation was achieved by VATS-guided surgical lung biopsy.
If diagnosed with LAM, BHD, or PLCH, the patients underwent pleurodesis. Patients with negative confirmatory tests had no other intervention performed and were managed as primary spontaneous pneumothorax. Performance characteristics (sensitivity, specificity) of each test were taken into consideration to calculate probabilities of achieving an accurate diagnosis. Costs for tests and procedures, as well as procedural complications and short-term reductions in quality of life (QOL) associated with the procedures, were included in the analysis.
The cycle length used in our analysis was 1 month, during which the patients were exposed to recurrent pneumothoraces as well as age- and sex-related death. A discount rate of 3% per year was applied by convention (13). All analyses of cost-effectiveness were done from a societal perspective.
Simplifying Assumptions
We made the following simplifying assumptions in our model:
-
1.
Confirmatory testing and pleurodesis, if indicated, would occur within 1 month of the initial presentation.
-
2.
All patients with a diagnosis other than BHD, LAM, or PLCH will follow a clinical course similar to patients with primary spontaneous pneumothorax.
-
3.
All patients, after being diagnosed with BHD, LAM, or PLCH, will undergo pleurodesis to prevent recurrent pneumothorax.
-
4.
We assumed that no more than four recurrences of pneumothorax could occur in any given patient.
-
5.
A short-term reduction in QOL was applied during the month in which a pneumothorax occurred.
Summary of Data Used in Our Model
BHD
On the basis of prior results, we estimated a BHD prevalence of 5% among patients presenting with an apparent primary spontaneous pneumothorax (6, 7). The HRCT pattern in BHD consists of multiple, round to lentiform-shaped cysts that present in a basilar and subpleural distribution with normal intervening lung parenchyma (14, 15). In an analysis of 89 patients with diffuse cystic lung diseases, expert radiologists were able to accurately diagnose BHD in 93% of cases on the basis of imaging features alone (9).
Because not every facility has access to an expert thoracic radiologist, we assumed a conservative HRCT accuracy rate of 80% for our base case analysis. Of the 20% of cases in which HRCT is nondiagnostic, the diagnosis can be established in many patients by either biopsy of a skin lesion showing fibrofolliculomas or genetic testing revealing a pathogenic FLCN mutation (14, 16). However, skin lesions are not present in all patients with BHD, and genetic testing is not 100% accurate (17), and thus there can be a small number of false negatives even after these confirmatory tests.
We assumed for our analysis that the false-negative rate of these confirmatory analyses (skin biopsy and FLCN testing) was 10%. Rarely, a patient can have BHD without radiologically apparent cysts (18). Therefore, we applied a small percentage (5%) false-negative rate on the ability to diagnose BHD on the basis of HRCT (see Table E1 in the online supplement).
Although no longitudinal pulmonary function data exist for BHD, these patients typically have well-preserved respiratory function and follow a benign clinical course (14). Thus, no increase in annual mortality attributed to BHD was used in our analysis.
LAM
The prevalence of LAM has been estimated to vary between 5 and 30% of all spontaneous pneumothoraces in nonsmoking women aged 25–54 years (8). Because we are testing both men and women in our analysis, we started with a baseline LAM prevalence of 2.5% in our analysis. The characteristic HRCT pattern in LAM consists of multiple, round, thin-walled, uniform cysts distributed evenly in all lung zones (19). Expert radiologists can accurately diagnose LAM in 72–91% of cases on the basis of HRCT features alone (9, 20, 21).
We conservatively estimated in our analysis that 80% of the patients with LAM can be diagnosed based on HRCT features (Table E2). According to the European Respiratory Society guidelines, the diagnosis of LAM can be established with certainty on the basis of a compatible chest CT, along with the presence of chylous effusions, angiomyolipomas or lymphangiomyomas, or a personal/family history of tuberous sclerosis (22). Serum VEGF-D is a useful diagnostic biomarker, and if elevated to greater than 800 pg/ml, specificity for the diagnosis of LAM approaches 100% (23, 24). Tissue confirmation may be needed if the diagnosis of LAM is not fully established based on above steps.
Small series suggest that LAM can be diagnosed in approximately 60% of cases by transbronchial biopsy (25). In the remaining cases, surgical lung biopsy is required to achieve diagnostic certainty. In our analysis, we used a stepwise strategy, where patients who were unsure of a diagnosis after noninvasive assessments underwent a transbronchial biopsy followed by a VATS-guided surgical lung biopsy (if the transbronchial biopsy was negative).
Of the patients needing a biopsy, we estimated that 50% of patients will achieve a confirmatory diagnosis via transbronchial biopsy, and the remaining 50% will require surgical lung biopsy. The natural history of LAM is characterized by a progressive decline in lung function, with a reported median survival time of 29 years (26). These data were used to determine annual mortality rates for patients with LAM.
PLCH
The prevalence of PLCH among patients presenting with a spontaneous pneumothorax is not known. The incidence of PLCH is reported to be one to two cases per million, which is almost certainly an underestimate (27). The average survival among patients with PLCH is estimated to be 12.5 years (28). On the basis of these estimates, the prevalence of PLCH should be 12.5–25 cases per million (prevalence = incidence rate × average disease duration).
On the basis of the United States census in 2014 (29), this equates to a total of 3,986–7,972 patients with PLCH in the United States. Sixteen percent of patients with PLCH have a pneumothorax in their lifetime (30). With an average survival of 12.5 years (28), this equates to an annual pneumothorax rate of 1.28% (16/12.5) among patients with PLCH. Thus approximately 51 to 102 episodes of spontaneous pneumothoraces can be attributed to PLCH every year in the United States. With an overall annual spontaneous pneumothorax incidence of 20,000 in the United States (4), we estimate that PLCH accounts for 0.25–0.5% of all spontaneous pneumothoraces every year. For our analysis, we estimated the prevalence of PLCH to be 0.5% among patients presenting with an apparent primary spontaneous pneumothorax.
The characteristic HRCT features of PLCH include upper and middle lung zone predominant irregular, bizarre-shaped cysts with varying wall thickness (1). An expert radiologist can identify PLCH in 74% of the cases on the basis of HRCT features alone, and accuracy approaches 100% for the confident/typical cases (9). In our base case, we estimated conservatively that PLCH could be diagnosed based on HRCT features in 70% of the cases. For the remaining cases, we estimated that VATS-guided lung biopsy would confirm the diagnosis in 25% of cases, and 5% would be classified as false negatives (Table E3). The natural history of PLCH is characterized by a progressive decline in lung function, with an estimated median survival of 12.5 years after diagnosis (28). Data from this study were used to determine annual mortality rates for patients with PLCH.
Pneumothorax and risk of recurrence
In patients with primary spontaneous pneumothorax, the rate of recurrent pneumothorax is estimated to be approximately 30% (31). Pleurodesis after an initial event reduces the rate of recurrence to 4% in primary spontaneous pneumothorax (32). The rate of a second recurrent pneumothorax with and without pleurodesis in patients with primary spontaneous pneumothorax was assumed to be 0 and 15%, respectively (Table E4) (8).
The risk of recurrence after a sentinel event is estimated to be 75, 73, and 58% for BHD, LAM, and PLCH, respectively (30, 33, 34). No disease-specific data exist for rates of subsequent recurrent pneumothoraces in patients with BHD. The clinical phenotype of BHD regarding recurrent pneumothoraces is quite similar to LAM (14). Rates for recurrent pneumothoraces beyond a second pneumothorax have been defined for patients with LAM (Table E2) (34), and we used the same rates for our analysis for BHD (Table E1). In patients with PLCH, the risk of recurrence is reduced to nearly zero after surgical pleurodesis (Table E3) (30).
QOL
Death from a primary pneumothorax is a rare event; however, it can be associated with significant morbidity (2, 4). The impact of a pneumothorax and pleurodesis on QOL has been previously described, and we used the same values for our analysis (Table E4) (35). We assumed that the loss in QOL in each of the conditions mentioned above is temporary and lasts for one cycle length before returning back to baseline.
Both LAM and PLCH are progressive diseases associated with a gradual decline in lung function over time. Thus, an estimated reduction in QOL by a factor of 0.0017 per month was applied over time for both of these diseases (8). Because BHD is generally not a progressive disease (14), no incremental reduction in QOL was applied for BHD.
Costs
All costs are expressed in 2014 U.S. dollars. Costs include both institutional and professional services. Average Medicare reimbursement for the corresponding Current Procedural Terminology or Diagnosis-related Group codes was used as a proxy for cost (Table E4). Indirect costs, such as lost income due to absence from work during hospital stays, were not included in the analysis.
Results
The results of the base case analysis are shown in Table 1. The strategy using HRCT screening for diffuse cystic lung diseases (BHD, LAM, and PLCH) was more costly, but also more effective. The marginal cost-effectiveness ratio of HRCT screening was $1,427 per quality-adjusted life-year (QALY) gained. Traditionally, a threshold of $50,000/QALY has been the benchmark for establishing the cost-effectiveness of a given strategy (36). Interventions less costly than $50,000/QALY are considered extremely cost effective.
Table 1.
Results of base case analysis showing that the strategy of high-resolution computed tomography screening at the time of initial pneumothorax is cost effective, with a marginal cost-effectiveness ratio of $1,426.72 per quality-adjusted life year gained
| Strategy | Cost ($) | Effectiveness (QALYs) | Marginal Cost ($) | Marginal Effectiveness (QALY) | mCER ($/QALY) |
|---|---|---|---|---|---|
| No HRCT | 49,866 | 40.77 | |||
| HRCT | 55,158 | 44.48 | 5,292.67 | 3.71 | 1,426.72 |
Definition of abbreviations: HRCT = high-resolution computed tomography; mCER = marginal cost-effectiveness ratio; QALY = quality-adjusted life-year.
Our analysis was most sensitive to uncertainty regarding the prevalence of diffuse cystic lung diseases in patients presenting with a spontaneous pneumothorax. In our base case analysis, diffuse cystic lung diseases accounted for 8% of cases of apparent primary spontaneous pneumothorax (5% attributed to BHD, 2.5% to LAM, and 0.5% to PLCH). To perform sensitivity analyses on the overall prevalence of diffuse cystic lung diseases, we linked the prevalence of LAM, PLCH, and BHD to maintain a constant proportion between each of these disorders, while at the same time allowing us to increase or decrease the overall prevalence of diffuse cystic lung diseases.
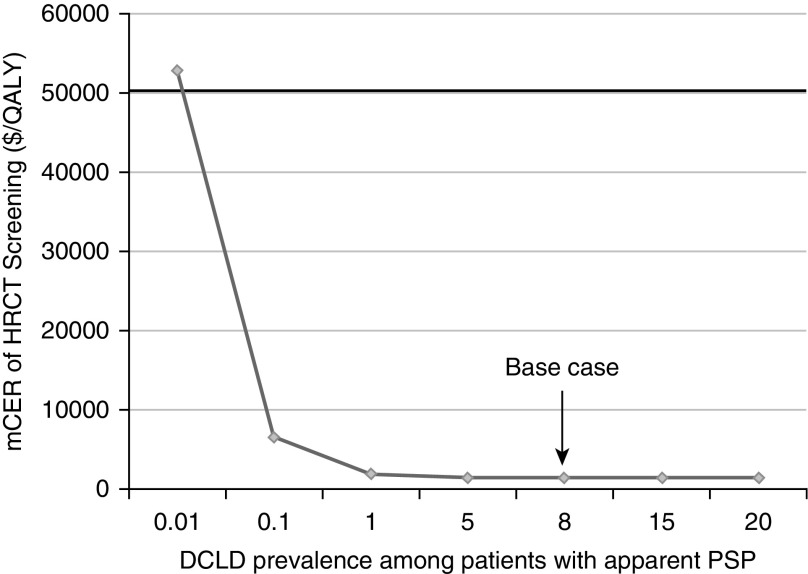
We performed sensitivity analyses over a wide range of prevalence rates for diffuse cystic lung diseases (0.01–20%). Not surprisingly, the strategy of HRCT screening became less costly and more effective as the prevalence of diffuse cystic lung diseases among patients presenting with an apparent primary spontaneous pneumothorax increased. HRCT screening had a marginal cost-effectiveness ratio less than $50,000/QALY unless the prevalence of diffuse cystic lung diseases was less than 0.01% (Figure 3). When prevalence was less than 0.01%, the HRCT screening strategy remained more effective but was associated with increased cost.
Figure 3.
Marginal cost-effectiveness ratio (mCER) of high-resolution computed tomography (HRCT) chest screening in relationship to the prevalence of diffuse cystic lung diseases (DCLDs) in patients presenting with an apparent primary spontaneous pneumothorax (PSP). This analysis demonstrates that as the prevalence of DCLDs among patients with an apparent PSP increases, it becomes more cost effective to screen for the presence of DCLDs. By convention, treatment strategies with an mCER of less than $50,000 per quality-adjusted life-year (QALY) gained are considered cost effective. On the basis of that threshold, the strategy for HRCT screening remains cost effective as long as DCLD prevalence among patients presenting with an apparent PSP is greater than 0.01%. Arrow denotes the base case in our analysis.
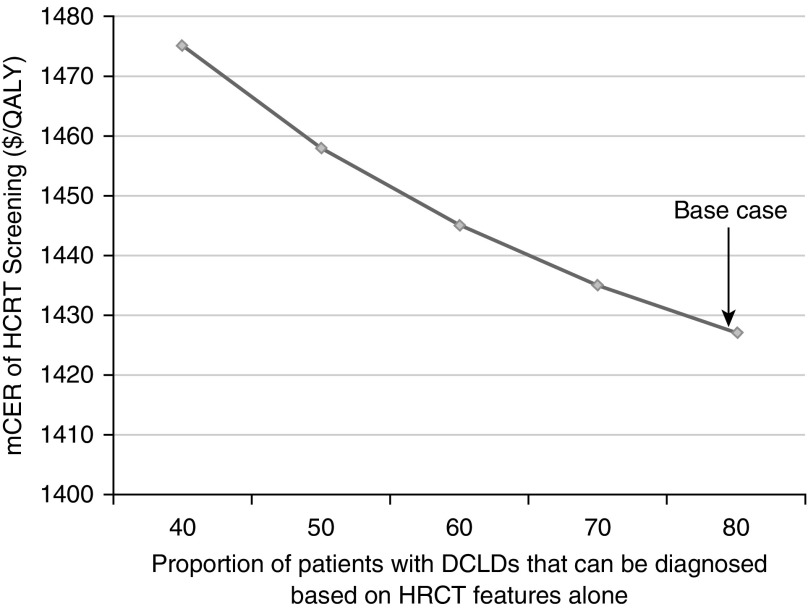
Our analysis was also sensitive to the ability to accurately diagnose BHD, LAM, and PLCH on the basis of HRCT characteristics alone. Similar to the manner in which we linked the prevalence of diffuse cystic lung diseases, we linked HRCT sensitivities for the diagnosis of LAM, PLCH, and BHD, thus allowing us to conduct sensitivity analysis on HRCT sensitivities for all three diffuse cystic lung diseases together. We performed sensitivity analysis on HRCT sensitivities ranging from 40 to 80%. As the ability to diagnose diffuse cystic lung diseases increased based on HRCT features alone, screening with HRCT became increasingly cost effective. However, the strategy of HRCT screening remained cost effective at all values tested for HRCT sensitivity (Figure 4). All other variables and costs used in the model were tested in our sensitivity analyses (Tables E1–E4) and did not significantly impact the results of our model.
Figure 4.
Marginal cost-effectiveness ratio (mCER) of high-resolution computed tomography (HRCT) chest screening in relationship to the ability to accurately diagnose the exact diffuse cystic lung disease (DCLD) on the basis of imaging features alone. This analysis demonstrates that as the sensitivity of HRCT to diagnose DCLD increases, the strategy of HRCT screening becomes more cost effective. However, at all sensitivity values of HRCT tested in our analysis, the strategy of HRCT screening remained cost effective, with an mCER substantially below the conventional cost-effectiveness threshold of $50,000 per quality-adjusted life-year (QALY) gained. Arrow denotes the base case in our analysis.
Discussion
The results of our model show that screening for the presence of diffuse cystic lung diseases in patients presenting with apparent primary spontaneous pneumothorax is cost effective. The strategy of HRCT screening is cost effective at disease prevalence rates as low as 0.01%.
Primary spontaneous pneumothorax is primarily seen in young patients, with the peak incidence ranging from 15 to 34 years old (2). The age range of first pneumothorax in patients with a diffuse cystic lung disease overlaps extensively with that of primary spontaneous pneumothorax. The median age for development of pneumothorax is 38, 35, and 29 years for BHD, LAM, and PLCH, respectively (30, 33, 34), yet it is important to note that pneumothoraces also occur in patients more than 50 years old with diffuse cystic lung diseases (34). Secondary spontaneous pneumothoraces are seen mainly in elderly patients, with a peak incidence after the age of 55 years (2).
Although consideration of a common underlying lung disorder, such as chronic obstructive lung disease or interstitial lung disease, in elderly patients presenting with a spontaneous pneumothorax often triggers chest CT imaging, in younger patients the diagnosis of primary spontaneous pneumothorax is often made without due consideration of a radiographically invisible diffuse cystic lung disease.
We did not perform our analysis on the basis of age distribution; rather, we studied the cost-effectiveness of HRCT screening based on disease prevalence. Our results indicate that the strategy of HRCT screening for diffuse cystic lung diseases remains cost effective at disease prevalence greater than 0.01%, which would almost certainly be the case for diffuse cystic lung diseases in all age groups. Thus, on the basis of prior work demonstrating the high prevalence of diffuse cystic lung diseases in young patients presenting with an apparent primary spontaneous pneumothorax (6–8), the overall prevalence of common and uncommon underlying lung disorders in older patients with a first pneumothorax, and our results demonstrating the cost-effectiveness of screening chest CT in the entire population, we submit that adult patients of all ages with an apparent primary spontaneous pneumothorax should undergo a screening chest CT examination to evaluate for the presence of underlying BHD, LAM, and PLCH.
In our model, all of the benefits from early diagnosis of diffuse cystic lung diseases were derived from early pleurodesis and prevention of recurrent pneumothoraces. In reality, early diagnosis of LAM, BHD, and PLCH provides other potential benefits that we did not account for in this analysis and which would likely have further biased the recommendations toward screening. For example, patients with BHD are at increased risk of renal cancers, and current recommendations call for screening for renal neoplasms after the age of 20 years (17, 37). By detecting BHD earlier, patients can be placed on regular surveillance programs, allowing for timely recognition of renal cancer. Identifying the index case of BHD in a family can also allow for screening of asymptomatic family members for the disease.
A recent randomized, placebo-controlled, double-blind trial has shown that treatment with sirolimus can stabilize lung function decline in patients with LAM (38). By establishing the diagnosis early, patients can be offered treatment with sirolimus while respiratory health is relatively well preserved, with the goal of avoiding further loss of lung function. Similarly, early detection of PLCH can facilitate smoking cessation counseling. More recently, mutations in the v-Raf murine sarcoma viral oncogene homolog B pathway have been discovered in a subset of patients with PLCH (39). Early detection of PLCH can enable ascertainment of underlying genetic mutations, which may someday provide access to targeted treatment options aimed at specific disease-causing mutations.
Limitations
We did not consider the harmful effects of radiation exposure resulting from screening CT imaging in our analysis. The effective radiation dose resulting from a chest CT scan varies between 4 and 14 mSv, with an average dose of 7 mSv per chest CT (40, 41). This radiation dose is roughly the equivalent of exposure to 2 years of natural background radiation (42). Although the additional cancer risk resulting from exposure to 7 mSv of radiation varies depending on individual demographics (age, sex, etc.), and in general is considered small, a 35-year-old woman (our base case) will have an additional 0.08% risk of developing a cancer over her lifetime attributed to the screening CT examination (43). This risk can be reduced significantly with the use of low-dose CT scanning protocols, with an average radiation exposure of 1.5 mSv per CT examination (41). However, the efficacy of a low-dose chest CT scan in the diagnosis of diffuse cystic lung diseases needs to be better established before recommending low-dose chest CT imaging as the screening modality of choice.
As with all analyses of this type, our model relies on previously published data and extrapolations from other related disease states. We believe that this shortcoming is addressed by choosing a wide range of parameter estimates in the sensitivity analysis. Another limitation is our assumption that all patients with a diagnosis of LAM, BHD, or PLCH will agree to undergo pleurodesis. In reality, patients may choose conservative management for their first pneumothorax and wait for a recurrent event before considering pleurodesis (44). Similarly, we assumed that all patients with an uncertain diagnosis will agree to have confirmatory testing for diffuse cystic lung diseases, such as genetic testing for BHD. Genetic testing can have potential insurance and employment implications that may lead some patients to decline. Last, because we used Medicare data as a proxy for costs, our conclusions may not be valid for international centers.
Conclusions
By using decision-modeling techniques, we have shown that screening for the presence of diffuse cystic lung diseases by HRCT in patients presenting with an apparent primary spontaneous pneumothorax is cost effective. Clinicians treating patients with pneumothoraces should consider performing a screening HRCT in patients who present with apparent primary spontaneous pneumothorax.
Additional material
Supplementary data supplied by authors.
Footnotes
Author Contributions: N.G., D.L., F.X.M., D.P.S., and M.H.E. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part I. Am J Respir Crit Care Med. 2015;191:1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55:666–671. doi: 10.1136/thorax.55.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melton LJ, III, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379–1382. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 4.Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, Luketich JD, Panacek EA, Sahn SA, Group APC AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 5.Bintcliffe OJ, Hallifax RJ, Edey A, Feller-Kopman D, Lee YC, Marquette CH, Tschopp JM, West D, Rahman NM, Maskell NA. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med. 2015;3:578–588. doi: 10.1016/S2213-2600(15)00220-9. [DOI] [PubMed] [Google Scholar]
- 6.Ren HZ, Zhu CC, Yang C, Chen SL, Xie J, Hou YY, Xu ZF, Wang DJ, Mu DK, Ma DH, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178–183. doi: 10.1111/j.1399-0004.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.Johannesma PC, Reinhard R, Kon Y, Sriram JD, Smit HJ, van Moorselaar RJ, Menko FH, Postmus PE Amsterdam BHD working group. Prevalence of Birt-Hogg-Dubé syndrome in patients with apparently primary spontaneous pneumothorax. Eur Respir J. 2015;45:1191–1194. doi: 10.1183/09031936.00196914. [DOI] [PubMed] [Google Scholar]
- 8.Hagaman JT, Schauer DP, McCormack FX, Kinder BW. Screening for lymphangioleiomyomatosis by high-resolution computed tomography in young, nonsmoking women presenting with spontaneous pneumothorax is cost-effective. Am J Respir Crit Care Med. 2010;181:1376–1382. doi: 10.1164/rccm.200910-1553OC. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Meraj R, Tanase D, James LE, Seyama K, Lynch DA, Akira M, Meyer CA, Ruoss SJ, Burger CD, et al. Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases. Eur Respir J. 2015;46:1196–1199. doi: 10.1183/13993003.00570-2015. [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. HRCT screening for diffuse cystic lung diseases in patients presenting with spontaneous pneumothorax is cost-effective [abstract] Am J Respir Crit Care Med. 2016;104:A6261. [Google Scholar]
- 11.Gupta N, Wikenheiser-Brokamp KA, Fischer A, McCormack FX. Diffuse cystic lung disease as the presenting manifestation of Sjögren syndrome. Ann Am Thorac Soc. 2016;13:371–375. doi: 10.1513/AnnalsATS.201511-759BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ismael S, Wermert D, Dang-Tran KD, Venot M, Fagon JY, Diehl JL. Severe excessive dynamic airway collapse in a patient with primary Sjögren’s syndrome. Respir Care. 2014;59:e156–e159. doi: 10.4187/respcare.02929. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg JM. Clinical economics: a guide to the economic analysis of clinical practices. JAMA. 1989;262:2879–2886. doi: 10.1001/jama.262.20.2879. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam Cancer. 2013;12:387–396. doi: 10.1007/s10689-013-9660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tobino K, Hirai T, Johkoh T, Kurihara M, Fujimoto K, Tomiyama N, Mishima M, Takahashi K, Seyama K. Differentiation between Birt-Hogg-Dubé syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol. 2012;81:1340–1346. doi: 10.1016/j.ejrad.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjöld M, Hansen TV, Solly J, Maher ER European BHD Consortium. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt LS, Linehan WM. Molecular genetics and clinical features of Birt-Hogg-Dubé syndrome. Nat Rev Urol. 2015;12:558–569. doi: 10.1038/nrurol.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onuki T, Goto Y, Kuramochi M, Inagaki M, Bhunchet E, Suzuki K, Tanaka R, Furuya M. Radiologically indeterminate pulmonary cysts in Birt-Hogg-Dubé syndrome. Ann Thorac Surg. 2014;97:682–685. doi: 10.1016/j.athoracsur.2013.05.120. [DOI] [PubMed] [Google Scholar]
- 19.Tobino K, Johkoh T, Fujimoto K, Sakai F, Arakawa H, Kurihara M, Kumasaka T, Koike K, Takahashi K, Seyama K. Computed tomographic features of lymphangioleiomyomatosis: evaluation in 138 patients. Eur J Radiol. 2015;84:534–541. doi: 10.1016/j.ejrad.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Bonelli FS, Hartman TE, Swensen SJ, Sherrick A. Accuracy of high-resolution CT in diagnosing lung diseases. AJR Am J Roentgenol. 1998;170:1507–1512. doi: 10.2214/ajr.170.6.9609163. [DOI] [PubMed] [Google Scholar]
- 21.Koyama M, Johkoh T, Honda O, Tsubamoto M, Kozuka T, Tomiyama N, Hamada S, Nakamura H, Akira M, Ichikado K, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol. 2003;180:827–835. doi: 10.2214/ajr.180.3.1800827. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, et al. Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 23.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med. 2008;358:199–200. doi: 10.1056/NEJMc0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meraj R, Wikenheiser-Brokamp KA, Young LR, Byrnes S, McCormack FX. Utility of transbronchial biopsy in the diagnosis of lymphangioleiomyomatosis. Front Med. 2012;6:395–405. doi: 10.1007/s11684-012-0231-5. [DOI] [PubMed] [Google Scholar]
- 26.Oprescu N, McCormack FX, Byrnes S, Kinder BW. Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: a population-based registry. Lung. 2013;191:35–42. doi: 10.1007/s00408-012-9419-3. [DOI] [PubMed] [Google Scholar]
- 27.Histiocytosis Association. LCH in adults. [accessed 2016 Apr 9]. Available from: http://www.histio.org.
- 28.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N Engl J Med. 2002;346:484–490. doi: 10.1056/NEJMoa012087. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Census Bureau. Population estimates. 2014 [accessed 2016 Apr 15]. Available from: http://www.census.gov.
- 30.Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest. 2004;125:1028–1032. doi: 10.1378/chest.125.3.1028. [DOI] [PubMed] [Google Scholar]
- 31.O’Rourke JP, Yee ES. Civilian spontaneous pneumothorax: treatment options and long-term results. Chest. 1989;96:1302–1306. doi: 10.1378/chest.96.6.1302. [DOI] [PubMed] [Google Scholar]
- 32.Barker A, Maratos EC, Edmonds L, Lim E. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet. 2007;370:329–335. doi: 10.1016/S0140-6736(07)61163-5. [DOI] [PubMed] [Google Scholar]
- 33.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, Maurer J, McCormack FX, Sahn SA. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto T, Fukui T, Koyama H, Noguchi Y, Shimbo T. Optimal strategy for the first episode of primary spontaneous pneumothorax in young men: a decision analysis. J Gen Intern Med. 2002;17:193–202. doi: 10.1046/j.1525-1497.2002.10636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Detsky AS. Using cost-effectiveness analysis to improve the efficiency of allocating funds to clinical trials. Stat Med. 1990;9:173–184. doi: 10.1002/sim.4780090124. [DOI] [PubMed] [Google Scholar]
- 37.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part II. Am J Respir Crit Care Med. 2015;192:17–29. doi: 10.1164/rccm.201411-2096CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden AC, Hu X, Kip S, Parrilla Castellar ER, Rumilla KM, Vrana JA, Vassallo R, Ryu JH, Yi ES. BRAF V600E expression in Langerhans cell histiocytosis: clinical and immunohistochemical study on 25 pulmonary and 54 extrapulmonary cases. Am J Surg Pathol. 2014;38:548–551. doi: 10.1097/PAS.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 40.Wade JP, Weyman JC, Goldstone KE. CT standard protocols are of limited value in assessing actual patient dose. Br J Radiol. 1997;70:1146–1151. doi: 10.1259/bjr.70.839.9536906. [DOI] [PubMed] [Google Scholar]
- 41.Radiological Society of North America. Radiation dose in X-ray and CT exams. 2016 [accessed 2016 Apr 26]. Available from: http://www.radiologyinfo.org/en/info.cfm?pg=safety-xray.
- 42.Parry RA, Glaze SA, Archer BR. The AAPM/RSNA physics tutorial for residents: typical patient radiation doses in diagnostic radiology. Radiographics. 1999;19:1289–1302. doi: 10.1148/radiographics.19.5.g99se211289. [DOI] [PubMed] [Google Scholar]
- 43.The National Academies Press. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2 [accessed 2016 Apr 26]. Available from: https://www.nap.edu/catalog/11340/health-risks-from-exposure-to-low-levels-of-ionizing-radiation.
- 44.Young LR, Almoosa KF, Pollock-Barziv S, Coutinho M, McCormack FX, Sahn SA. Patient perspectives on management of pneumothorax in lymphangioleiomyomatosis. Chest. 2006;129:1267–1273. doi: 10.1378/chest.129.5.1267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.