In Brief
An elderly woman presented with dyspnea and a large right-sided pleural effusion. After a large-volume thoracentesis, she developed acute hypoxemic respiratory failure and was transferred to the intensive care unit. Her case provides an opportunity to review the pathophysiology of reexpansion pulmonary edema and discuss how an understanding of pleural pressure monitoring may guide safe and maximal fluid removal during thoracentesis.
The Clinical Challenge
An 80-year-old woman presented to the emergency department with 3 weeks of progressive dyspnea and orthopnea. Her past medical history was notable for a 30 pack-year smoking history. She denied fevers, cough, and chest pain.
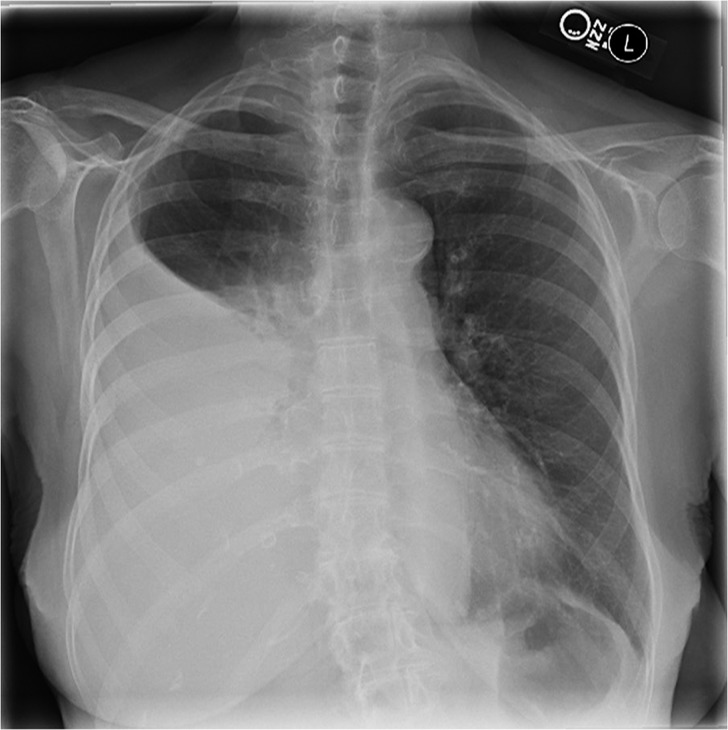
The patient was afebrile, with an oxygen saturation of 87% on ambient air. Physical examination was notable for decreased breath sounds in the right posterior lung field with associated dullness to percussion. A chest radiograph (Figure 1) demonstrated a large right pleural effusion that was confirmed to be free-flowing by bedside ultrasound, as no septations were seen and dynamic changes in the shape of the effusion were observed with respiration.
Figure 1.
Posterior-anterior chest radiograph demonstrating a large right-sided pleural effusion.
Thoracentesis was performed with ultrasound guidance with removal of 2 L of serosanguinous fluid over 15 minutes. Two hours after the procedure, the patient developed shortness of breath and worsening hypoxemia requiring 1.0 FiO2 via a non-rebreather mask to maintain an oxygen saturation of greater than 88%. She was transferred to the intensive care unit for further management.
Questions
1. What are the physiologic and pathophysiologic mechanisms of pleural fluid formation and development of a pleural effusion?
2. What are the characteristics and proposed mechanisms of reexpansion pulmonary edema?
3. Can pleural pressure monitoring during thoracentesis reduce the risk of reexpansion pulmonary edema?
Clinical Reasoning
In health, a small amount of fluid (roughly 0.3 ml/kg) continually bathes the pleural space (1). This fluid ensures mechanical coupling between the lung, chest wall, and diaphragm. A pleural effusion is caused by an imbalance between microvascular filtration and pleural fluid clearance, the latter driven predominately by lymphatic drainage through the parietal pleura.
Symptomatic reexpansion pulmonary edema occurs in less than 1% of patients after large-volume thoracentesis (2). Reexpansion pulmonary edema occurs within 24 hours of the drainage procedure and is characterized by hypoxemia and alveolar infiltrates in the reexpanded lung and even rarely in the contralateral lung (3).
There is growing enthusiasm for using pleural manometry to measure changes in pleural pressure during a thoracentesis (4). Excessively negative intrapleural pressure often signals the inability of the lung to fully reexpand, either due to poor compliance of the lung or visceral pleural entrapment, and identifies a point at which further fluid removal may increase the risk of reexpansion edema, procedural discomfort, and the development of a pneumothorax ex vacuo.
The Clinical Solution
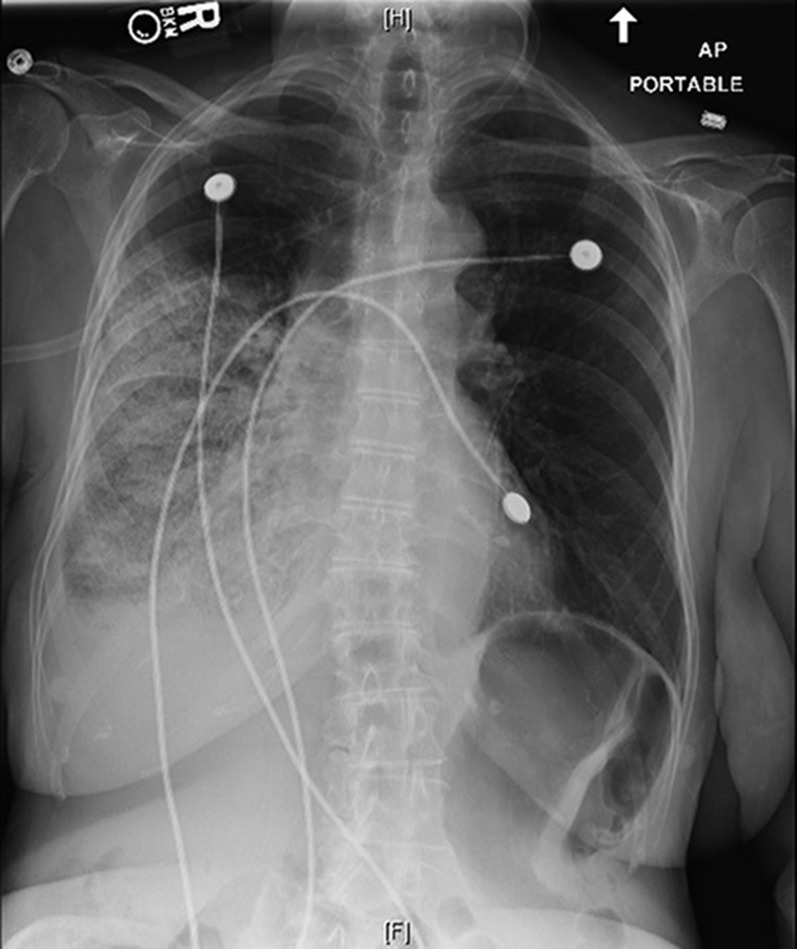
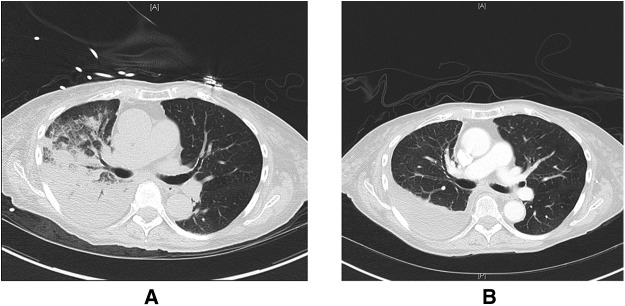
Imaging obtained on arrival to the intensive care unit showed improvement in the patient’s right pleural effusion with the interval development of airspace disease throughout the mid and lower right lung fields (Figure 2). Her presentation was consistent with reexpansion pulmonary edema precipitated by large-volume thoracentesis. Supplemental oxygen was administered, and the patient was given a single dose of an intravenous diuretic resulting in a negative 500-ml fluid balance over 24 hours. More aggressive diuresis was avoided, as cases of hypotension and cardiovascular collapse have been reported in the setting of reexpansion pulmonary edema (5). The patient experienced rapid clinical improvement, and follow-up chest CT obtained 5 days after her thoracentesis showed resolution of the right-sided ground-glass opacities and airspace disease and a persistent pleural effusion (Figure 3).
Figure 2.
Portable anterior-posterior chest radiograph demonstrating new airspace pulmonary infiltrates in the right mid and lower lung fields consistent with reexpansion pulmonary edema after thoracentesis.
Figure 3.
Representative images from computed tomography scans of the chest before and after therapy for reexpansion pulmonary edema. (A) Ground-glass opacities and airspace disease in the right lung after thoracentesis consistent with reexpansion pulmonary edema. (B) Resolution of these abnormalities and a residual right-sided pleural effusion after diuresis and supportive care.
Analysis of her pleural fluid demonstrated an exudative effusion with negative cytology and cultures. Diagnostic testing was negative for tuberculosis. She was discharged home without the need for supplemental oxygen, and close follow up was planned given the high index of suspicion for malignancy.
The Science behind the Solution
Pleural Space Anatomy
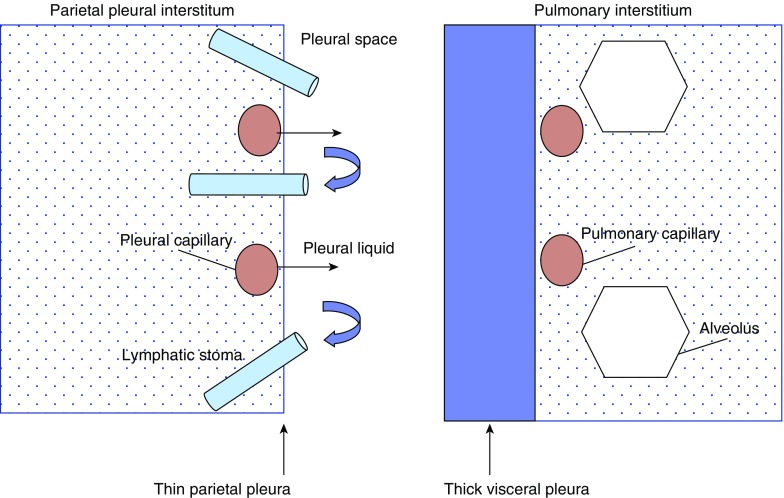
An understanding of pleural anatomy is important when considering the function of the pleural space in both health and disease. A simplified schematic of pleural space anatomy is provided in Figure 4.
Figure 4.
Simplified schematic of pleural space anatomy. Note the anatomic features of the parietal pleura, which suggest its central role in pleural fluid flow, including capillaries close to the pleural space, a thin pleural lining, and the presence of lymphatic stomata. Normal pleural fluid originates from parietal pleural microvascular filtrate and is drained from the pleural space by the hydraulic pumping action of parietal pleural lymphatics. Pleural fluid can also develop from movement across the visceral pleura in settings of elevated left atrial pressure and interstitial pulmonary edema.
The pleural space in humans is approximately 10 to 20 μm wide and is bordered by the parietal and visceral pleural linings. Several anatomic differences between these two linings suggest that the parietal pleura plays a more central role in normal pleural fluid flow. In humans, both the parietal and visceral pleura receive blood flow from the systemic circulation. However, microvascular fluid filtration pressure is lower in the visceral pleura because the bronchial circulation, which supplies the visceral pleura, drains into low-pressure pulmonary veins. The visceral pleura is also thicker, creating a longer distance between visceral pleural microvessels and the pleural surface. Finally, only the parietal pleura contains lymphatic stomata between mesothelial cells that communicate directly with the lymphatics and provide a drainage system for pleural fluid (6).
The pulmonary interstitium, which is bordered by the visceral pleura, is a physiologically distinct compartment from the pleural space and contains the alveolar–capillary interface. Although they are distinct compartments, conditions that drop pleural pressure may also drop pulmonary interstitial pressure.
Pleural Liquid Flow
It is useful to remember that throughout the body the flow of fluid between the microcirculation and the surrounding interstitium is governed by Starling forces. As described in the equation below, fluid flow is determined by the balance between hydrostatic pressure and protein osmotic pressure.
where K is the filtration coefficient of the capillary wall, Pmv is the hydrostatic pressure in the microvascular compartment, Ppmv is the hydrostatic pressure in the perimicrovascular compartment, σ is the reflection coefficient for total protein (which defines how easily a protein can move across a pore of a certain size), πmv is protein osmotic pressure in the microvascular compartment, and πpmv is protein osmotic pressure in the perimicrovascular compartment.
Analysis of pleural fluid protein concentrations suggests that pleural fluid is primarily produced from microvascular filtration of systemic blood flow into the parietal pleura interstitium (6). This filtration is driven by a hydrostatic pressure gradient between the systemic capillaries and the microvascular interstitium (Pmv > Ppmv). Microvascular filtrate is then “pulled” through the mesothelial lining of the parietal pleura into the pleural space by a small pressure gradient (approximately −10 cm H2O) between the parietal pleura interstitium and the subatmospheric pressure of the pleural space. This negative pressure results from a combination of pleural surface pressure (a balance between the opposite recoil pressures of the lung and chest wall) and pleural liquid pressure (which is generated largely by the strong pumping action of the parietal lymphatics). The parietal pleural lymphatics have been characterized as vacuum pumps that open into the pleural space and generate subatmospheric pressure through a combination of rhythmic contractions of smooth muscle lining the lymphatic walls and oscillations in intrathoracic pressure with respiration (1, 7).
For years, Starling forces were believed to drive reabsorption of pleural fluid through the visceral pleura. We now understand from studies tracking the flow of radiolabeled proteins in sheep that pleural fluid flows down an intrapleural pressure gradient and enters the parietal pleural lymphatics through stomata in the parietal pleural lining (8). This flow is driven by the hydraulic pressure created by the lymphatic pumps.
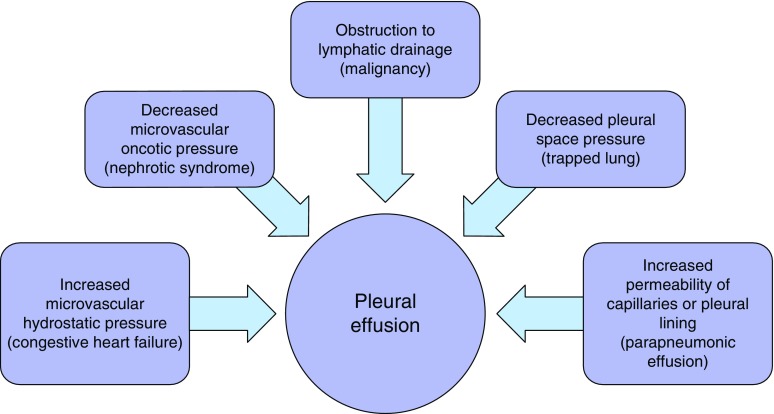
The development of a pleural effusion represents an imbalance between pleural fluid filtration and lymphatic clearance. Physiologic causes of pleural fluid accumulation can be viewed in terms of the variables outlined in the Starling equation, as depicted in Figure 5.
Figure 5.
Physiologic mechanisms of pleural fluid accumulation. A representative example of each mechanism is included in parentheses.
Reexpansion Pulmonary Edema
Symptomatic reexpansion pulmonary edema is an uncommon complication of a thoracentesis, occurring in less than 1% of procedures in recent large case series (2, 9). The exact pathophysiology of reexpansion pulmonary edema is unclear, and many of the studies exploring potential mechanisms are now several decades old. Nevertheless, limited data suggest that both disruption of the alveolar–capillary barrier and excessive hydrostatic forces contribute to the development of reexpansion pulmonary edema.
Experimental models of reexpansion pulmonary edema have demonstrated that sudden ventilation and perfusion of a chronically collapsed lung can precipitate increased permeability edema through a variety of mechanisms. Lung reexpansion promotes a robust influx of inflammatory cells to the newly aerated lung, and toxic mediators released from these cells may damage the alveolar–capillary barrier (10). Oxidative injury from free radical accumulation likely also contributes to disruption of the capillary endothelial lining. When chronically hypoxic alveolar cells in areas of atelectatic lung are exposed to a sudden increase in oxygen tension during lung reexpansion, there is an increase in the production of oxygen free radicals driven in part by recruitment of activated neutrophils (11). Increased local production of the chemokine IL-8 may worsen neutrophil accumulation and free radical production, as elevated levels of this protein have been found in both animal and human cases of reexpansion pulmonary edema (12, 13). Finally, rapid lung reexpansion (as can be seen during a large-volume thoracentesis) can result in a sudden increase in local perfusion due to a fall in pulmonary vascular resistance from both lung reexpansion and the reversal of hypoxic vasoconstriction. Dramatic increases in capillary transmural pressure can mechanically injure the basement membrane of the alveolar–capillary lining and precipitate increased permeability edema (so-called capillary stress failure) (14, 15).
Small clinical studies of pulmonary edema fluid in patients with reexpansion pulmonary edema have found protein profiles characteristic of hydrostatic edema suggesting that mechanisms that favor the formation of hydrostatic edema likely also play an important role in reexpansion pulmonary edema pathogenesis (15). Chronic atelectasis can induce local surfactant depletion and dysfunction, increasing alveolar surface tension and leading to areas of poorly compliant lung. Increased retractive forces in these poorly compliant alveoli may lower surrounding perimicrovascular hydrostatic pressure (Ppmv), predisposing to the formation of hydrostatic edema (16, 17). Furthermore, during lung reexpansion excessively negative intrapleural pressures (as can be seen during a large-volume thoracentesis in the setting of lung entrapment) may drop interstitial hydrostatic pressure and trigger the formation of hydrostatic edema by increasing the gradient for filtration across the microvascular barrier (15).
Pleural Pressure Monitoring
Several techniques are currently available to measure changes in pleural pressure with fluid removal during a thoracentesis (18). These range from a water manometer constructed from intravenous tubing (the most commonly used method at our institution), an electronic transducer attached to an intensive care unit monitor, and commercially produced handheld digital manometers. The pressure changes observed with these devices can help clinicians identify conditions when the lung is unable to fully reexpand and potentially guide the safe removal of a large fluid volume during thoracentesis.
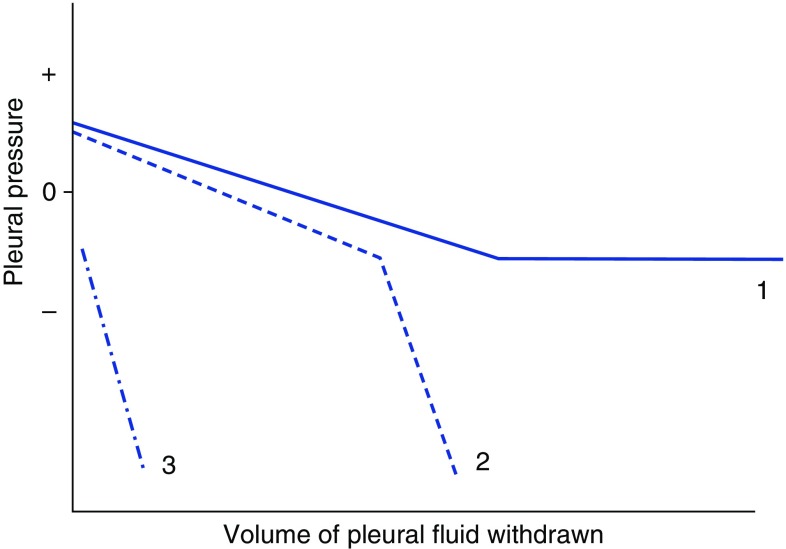
Three distinct pleural elastance profiles with fluid removal can be identified with pleural manometry (Figure 6) (19). In the setting of a normal pleural lining and lung parenchyma, excess pleural fluid raises the normally subatmospheric pressure of the pleural space to a positive value. As fluid is removed, pleural pressure falls to its normal resting pressure of −5 to −10 cm H2O and then plateaus. In conditions of pleural disease, such as malignancy or inflammation, excessive fluid removal causes a steep drop in pleural pressure as full lung expansion is inhibited by an unmoving visceral pleura (termed lung entrapment). A similar elastance profile may be seen when a chronically atelectatic area of lung loses its normal compliance (due to the loss of functional surfactant as described above) and requires significant negative pressure to reexpand. Finally, when the lung is chronically unable to expand due to a thickened visceral pleura, negative pressure in the pleural space may actually exceed the negative pressure generated by the parietal pleural lymphatics and lead to the formation of an effusion ex vacuo (termed trapped lung). Identifying a negative pleural pressure even before fluid removal suggests the diagnosis of trapped lung.
Figure 6.
Three distinct pleural elastance curves can be encountered when monitoring pleural pressure changes during a thoracentesis. Curve 1 depicts a well-performed thoracentesis in the setting of normal underlying lung and visceral pleura, where an initially positive pleural pressure gradually returns to the resting subatmospheric pressure of the pleural space with fluid removal. Curve 2 depicts lung entrapment, where pleural pressure rapidly falls when a diseased visceral pleura prevents full lung reexpansion. A similar elastance profile may be encountered when chronic atelectasis creates an area of poorly compliant lung that requires significant negative pressure for reexpansion. Curve 3 represents a trapped lung, where an initially negative pleural pressure is encountered, indicating a chronically unexpanded lung and a pleural effusion ex vacuo.
Along with aiding in the identification of unexpandable lung, the use of pleural manometry may identify a point at which further fluid removal may be harmful. As pressure in the pleural space falls below the normal subatmospheric resting pressure of −5 to −10 cm H2O, the increasing transpleural gradient may entrain air from the outside along the needle track into the pleural space (creating a pneumothorax ex vacuo), cause procedural discomfort, and potentially lead to reexpansion pulmonary edema. Based on limited data, many clinicians use a pleural pressure of −20 cm H2O as a signal to halt further fluid removal.
Answers
1. What are the physiologic and pathophysiologic mechanisms of pleural fluid formation and development of a pleural effusion?
Physiologic pleural fluid originates from parietal pleural microvascular filtrate. A pleural effusion results from an imbalance between pleural fluid filtration and clearance by parietal pleural lymphatics.
2. What are the characteristics and proposed mechanisms of reexpansion pulmonary edema?
Reexpansion pulmonary edema presents as hypoxemia and alveolar infiltrates within 24 hours of pleural fluid drainage. Evidence suggests that both injury to the alveolar–capillary barrier and increased hydrostatic pressure in the reexpanding lung contribute to reexpansion pulmonary edema pathogensis.
3. Can pleural pressure monitoring during thoracentesis reduce the risk of reexpansion pulmonary edema?
Pleural manometry can help identify unexpandable lung and may identify a point at which further fluid removal may increase the risk of reexpansion pulmonary edema. Many clinicians halt fluid removal if pleural pressure reaches −20 cm H2O, although data supporting this practice is limited.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–225. doi: 10.1183/09031936.97.10010219. [DOI] [PubMed] [Google Scholar]
- 2.Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656–1661. doi: 10.1016/j.athoracsur.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Kim JJ, Kim YH, Choi SY, Jeong SC, Moon SW. Contralateral reexpansion pulmonary edema with ipsilateral collapsed lung after pleural effusion drainage: a case report. J Cardiothorac Surg. 2015;10:68. doi: 10.1186/s13019-015-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feller-Kopman D. Point: should pleural manometry be performed routinely during thoracentesis? Yes. Chest. 2012;141:844–845. doi: 10.1378/chest.11-3231. [DOI] [PubMed] [Google Scholar]
- 5.Komatsu T, Shibata S, Seo R, Tomii K, Ishihara K, Hayashi T, Takahashi Y. Unilateral re-expansion pulmonary edema following treatment of pneumothorax with exceptionally massive sputum production, followed by circulatory collapse. Can Respir J. 2010;17:53–55. doi: 10.1155/2010/259195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiener-Kronish JP, Broaddus VC. Interrelationship of pleural and pulmonary interstitial liquid. Annu Rev Physiol. 1993;55:209–226. doi: 10.1146/annurev.ph.55.030193.001233. [DOI] [PubMed] [Google Scholar]
- 7.Negrini D, Ballard ST, Benoit JN. Contribution of lymphatic myogenic activity and respiratory movements to pleural lymph flow. J Appl Physiol (1985) 1994;76:2267–2274. doi: 10.1152/jappl.1994.76.6.2267. [DOI] [PubMed] [Google Scholar]
- 8.Broaddus VC, Wiener-Kronish JP, Berthiaume Y, Staub NC. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol (1985) 1988;64:384–390. doi: 10.1152/jappl.1988.64.1.384. [DOI] [PubMed] [Google Scholar]
- 9.Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70:127–132. doi: 10.1136/thoraxjnl-2014-206114. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Tanita T, Koike K, Fujimura S. Evidence of acute inflammatory response in reexpansion pulmonary edema. Chest. 1992;101:275–276. doi: 10.1378/chest.101.1.275. [DOI] [PubMed] [Google Scholar]
- 11.Jackson RM, Veal CF, Alexander CB, Brannen AL, Fulmer JD. Re-expansion pulmonary edema: a potential role for free radicals in its pathogenesis. Am Rev Respir Dis. 1988;137:1165–1171. doi: 10.1164/ajrccm/137.5.1165. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Fujishima S, Sawafuji M, Ishizaka A, Oguma T, Soejima K, Matsubara H, Tasaka S, Kikuchi K, Kobayashi K, et al. Importance of interleukin-8 in the development of reexpansion lung injury in rabbits. Am J Respir Crit Care Med. 2000;161:1030–1036. doi: 10.1164/ajrccm.161.3.9906039. [DOI] [PubMed] [Google Scholar]
- 13.Garantziotis S, Bhalla KS, Long GD, Vredenburgh JJ, Folz RJ. Fatal re-expansion pulmonary edema associated with increased lung IL-8 levels following high-dose chemotherapy and autologous stem cell transplant. Respiration. 2002;69:351–354. doi: 10.1159/000063260. [DOI] [PubMed] [Google Scholar]
- 14.West JB, Mathieu-Costello O. Stress-induced injury of pulmonary capillaries. Proc Assoc Am Physicians. 1998;110:506–512. [PubMed] [Google Scholar]
- 15.Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30:1921–1926. doi: 10.1007/s00134-004-2379-1. [DOI] [PubMed] [Google Scholar]
- 16.Finley TN, Tooley WH, Swenson EW, Gardner RE, Clements JA. Pulmonary surface tension in experimental atelectasis. Am Rev Respir Dis. 1964;89:372–378. doi: 10.1164/arrd.1964.89.3.372. [DOI] [PubMed] [Google Scholar]
- 17.Albert RK, Lakshminarayan S, Hildebrandt J, Kirk W, Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs’ lungs. J Clin Invest. 1979;63:1015–1018. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126:1764–1769. doi: 10.1378/chest.126.6.1764. [DOI] [PubMed] [Google Scholar]
- 19.Light RW, Jenkinson SG, Minh VD, George RB. Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis. 1980;121:799–804. doi: 10.1164/arrd.1980.121.5.799. [DOI] [PubMed] [Google Scholar]