Abstract
Interstitial lung diseases, especially lymphoproliferative disorders such as follicular bronchiolitis and lymphoid interstitial pneumonia, are commonly seen in association with Sjögren syndrome. Although the predominant computed tomographic (CT) findings in patients with lymphoid interstitial pneumonia/follicular bronchiolitis include poorly defined centrilobular nodules and ground-glass attenuation, cystic changes can be seen in approximately two-thirds of these patients. The objective of this study was to define the clinical, radiological, and histopathological features of cyst-predominant lymphoid interstitial pneumonia/follicular bronchiolitis in patients with Sjögren syndrome. We present four patients who were referred to our institution with diffuse cystic changes on chest CT imaging. All four had a presumptive diagnosis of lymphangioleiomyomatosis but were subsequently found to have Sjögren syndrome. The diagnosis was established based on the clinical symptoms of xerostomia and xerophthalmia along with serologic detection of antinuclear antibodies, rheumatoid factor, anti-Sjögren's syndrome–related antigen A (SSA)/Ro antibodies, and anti-Sjögren's syndrome–related antigen B (SSB)/La antibodies. The cystic pattern associated with Sjögren syndrome had a characteristic appearance on chest CT images. Typical features included a wide variation in cyst size, internal structure within cysts, geographic simplification of parenchymal architecture producing a “dissolving lung appearance,” perivascular and often basilar-predominant distribution, and frequent association with ground-glass opacities and nodules. In a compatible clinical context, we submit that these findings can be sufficiently distinctive to obviate the need for lung biopsy, even in the absence of confirmatory serological studies or lip biopsy. Clinicians should consider occult Sjögren syndrome in the differential diagnosis of patients presenting with idiopathic diffuse cystic lung disease.
Keywords: Sjögren syndrome, diffuse cystic lung disease, lymphoid interstitial pneumonia, follicular bronchiolitis
Sjögren syndrome is a chronic inflammatory multisystem autoimmune exocrinopathy that can occur in isolation (primary Sjögren) or in combination with other rheumatologic conditions, such as rheumatoid arthritis, systemic lupus erythematosus, or systemic sclerosis (secondary Sjögren) (1). The estimated prevalence of interstitial lung disease in Sjögren syndrome is variably reported as 9 to 90% (2–4) and is greatly affected by the mode of ascertainment (e.g., rheumatology clinic versus pulmonary clinic) and detection (chest radiograph or computed tomography [CT] imaging).
Case series demonstrate that the patterns of lung involvement in Sjögren syndrome include nonspecific interstitial pneumonia, organizing pneumonia, usual interstitial pneumonia, amyloidosis, and lymphoproliferative pulmonary disorders (5). Lymphoid interstitial pneumonia (LIP) and follicular bronchiolitis (FB) are two of the more common lymphocyte-predominant pulmonary pathologies associated with Sjögren syndrome (1).
The predominant high-resolution CT findings in patients with LIP/FB include poorly defined centrilobular nodules and ground-glass attenuation. However, cystic changes are also seen in approximately two-thirds of patients with LIP/FB (6). Cyst-predominant LIP/FB as the presenting manifestation of Sjögren syndrome is not well described.
Methods
Here we define the clinical, laboratory, thoracic imaging, and histopathologic features of four adult patients who were referred to our University of Cincinnati Interstitial Lung Disease Center with diffuse cystic lung disease and a presumptive diagnosis of lymphangioleiomyomatosis (LAM). All four patients were subsequently found instead to have primary Sjögren syndrome. All data presented in this report were abstracted from the medical records. Some of the results of this analysis have been previously reported in the form of an abstract (7).
Results
The clinical characteristics of the cohort are displayed in Table 1. All patients were women, with a mean age of 41 years (range, 29–46 yr). Dyspnea on exertion was the most common clinical symptom present in three out of the four patients, followed by cough (two patients) and episodic pleuritic chest pain (two patients). None of the patients had a history of pneumothorax. All patients were either nonsmokers (two out of four) or former smokers (two out of four), with a maximum cigarette smoke exposure of 10 pack-years. On further questioning, all four patients had symptomatic keratoconjuctivitis sicca and xerostomia. In addition, all patients had serologic elevations of rheumatoid factor and antinuclear antibody and were positive for anti-Sjögren's syndrome–related antigen A (SSA)/Ro antibodies. Three patients were also positive for anti-Sjögren's syndrome–related antigen B (SSB)/La antibodies.
Table 1.
Clinical features of the patients in our analysis
| Patient No. | Sex | Age (yr) | Clinical Symptoms at Presentation | Smoking History | Sicca Symptoms | Lip Biopsy | Laboratory Values (Normal Values) |
|---|---|---|---|---|---|---|---|
| 1 | F | 46 | Dyspnea on exertion | Nonsmoker | Yes | Not performed | RF, 180 (0–13.9 IU/ml) |
| ANA, 1:320 (<1:40) | |||||||
| SSA positive | |||||||
| SSB negative | |||||||
| 2 | F | 44 | Chronic cough | 5 pack-year former smoker | Yes | Nondiagnostic | RF, 22.1 (0–13.9 IU/ml) |
| ANA, 1:640 (<1:40) | |||||||
| SSA, >8 (0–0.9) | |||||||
| SSB, >8 (0–0.9) | |||||||
| 3 | F | 29 | Dyspnea on exertion and pleuritic chest pain | Nonsmoker | Yes | Not performed | RF, 81 (<18 IU/ml) |
| ANA, strong positive | |||||||
| SSA, strong positive | |||||||
| SSB, strong positive | |||||||
| 4 | F | 44 | Dyspnea on exertion, chronic cough, pleuritic chest pain | 10 pack-year former smoker | Yes | Not performed | RF, 202 (0–13.9 IU/ml) |
| ANA, 1:320 (<1:40) | |||||||
| SSA, >8 (0–0.9) | |||||||
| SSB, >8 (0–0.9) |
Definition of abbreviations: ANA = antinuclear antibody; RF = rheumatoid factor; SSA = Sjögren's syndrome–related antigen A; SSB = Sjögren's syndrome–related antigen B.
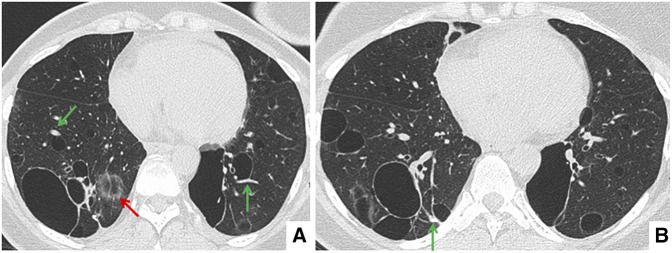
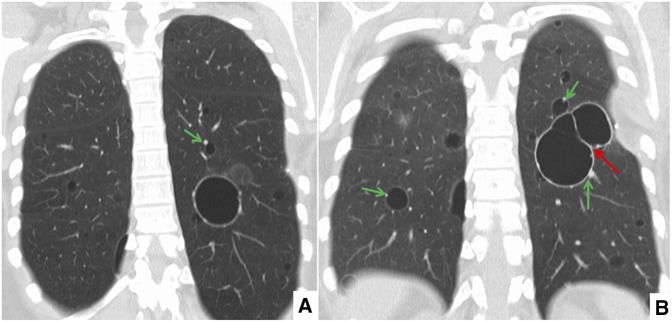
Representative high-resolution CT axial (Figure 1) and coronal (Figure 2) images are shown with the chest CT findings summarized in Table 2. CT images from all four patients showed multiple cysts of varying sizes in either a diffuse (two out of four patients) or lower-lobe–predominant (two out of four patients) distribution. In all four cases, cysts were predominantly perivascular and often bordered by an eccentric vessel (Figures 1 and 2, green arrows). Some cysts contained internal septations, vessels, or other intracystic densities (Figure 2B, red arrow). In other areas, geographically well-defined regions of the lung appeared to be fading into the background, as if dissolving (Figure 1A, red arrow).
Figure 1.
(A, B) Representative axial high-resolution chest computed tomography images showing multiple cysts of varying size and distribution. The majority of the cysts are associated with eccentric vessels (green arrows). In some areas, geographically well-defined regions of the lung appeared to be fading into the background, as if dissolving (A, red arrow).
Figure 2.
(A, B) Representative coronal high-resolution chest computed tomography images showing multiple cysts of varying size and distribution. The majority of the cysts are associated with eccentric vessels (green arrows) and some of the cysts contained internal septations (B, red arrow).
Table 2.
Chest computed tomography imaging findings of the patients in our analysis
| Patient No. | Cyst Size | Cyst Shape | Cysts Associated with Eccentric Vessels | Cysts with Septations or Internal Structures | Distribution | Other Findings |
|---|---|---|---|---|---|---|
| 1 | 5 mm–5 cm | Round–oval cysts | Yes | Yes | Lower lobe predominant | Bilateral small nodules |
| 2 | 5 mm–5 cm | Round–oval cysts, some irregular shaped | Yes | Yes | Diffuse | Ground-glass attenuation and nodules |
| 3 | <1 cm | Round cysts | Yes | Yes | Diffuse | Mild ground glass attenuation |
| 4 | 5 mm–3 cm | Round–oval cysts | Yes | Yes | Lower lobe predominant | None |
Surgical lung biopsy was performed on three patients. Representative histopathological findings are shown in Figure 3, with a summary of the biopsy findings given in Table 3. Follicular bronchiolitis was identified in two patients (Figures 3A–3D), and lymphoid interstitial pneumonia was present in the third patient. Histopathologic features of the cysts (Figures 3A–3D) correlated well with the radiographic findings. The variably sized parenchymal cysts were often centered on bronchovascular bundles, with many of the cysts bordered by eccentric vessels (Figures 3A and 3C). In the pathologic evaluation of one patient, the cystic lung disease and FB was accompanied by a subpleural nodular focus of amyloidosis (Figure 3D).
Figure 3.
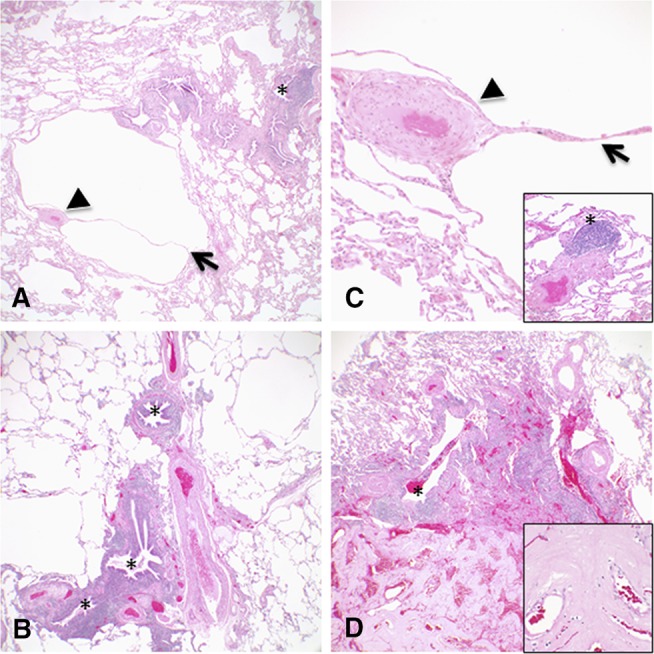
(A–D) Representative histologic images corresponding to the radiographic features showing multiple varying sized parenchymal cysts, some associated with eccentric vessels (arrowheads) and internal septations (arrows). Follicular bronchiolitis was present in the lung biopsies from all three patients characterized by lymphoid hyperplasia with reactive germinal centers cuffing bronchioles (*). One lung biopsy also had focal subpleural nodular amyloidosis diagnosed by accumulations of amorphous eosinophilic material that stained deep pink to red with Congo red stain and showed apple green birefringence with polarized light (D, bottom of image and inset). Original magnifications: 4× (A, B, and D); 20× (C, including inset); 40× (D, inset).
Table 3.
Histopathological findings in the lung biopsies from patients in our analysis
| Patient No. | Biopsy Type | Major Histopathological Pattern | Localized Cystic Change | Additional Features and Findings |
|---|---|---|---|---|
| 1 | Right lower lobe wedge biopsy | Follicular bronchiolitis | Present with some parenchymal cystic spaces associated with eccentric vessels and septations | Negative for features of lymphangioleiomyomatosis and pulmonary Langerhans cell histiocytosis by routine stains and immunohistochemical stains for HMB-45 and CD1a |
| 2 | Right middle and right lower lobe wedge biopsies | Follicular bronchiolitis | Present | Subpleural nodular focus of amyloidosis in right lower lobe; vasculopathy characterized by concentric mural thickening of small arteries without luminal occlusion; rare poorly formed small nonnecrotizing granulomas; chronic pleuritis; lymphocytic infiltrate composed of a mixture of B and T cells without restricted kappa or lambda immunoglobulin expression as assessed by immunohistochemical stains, supporting a reactive inflammatory process |
| 4 | Right middle and right lower lobe wedge biopsies | Lymphoid interstitial pneumonia | Present | Lymphoid interstitial pneumonia characterized by peribronchial and peribronchiolar lymphocytic infiltrate with scattered lymphoid follicles that focally also involve the alveolar septae |
Surgical lung biopsies were obtained from three of the four patients.
In all four patients, the diagnosis of Sjögren syndrome was established based on the clinical symptoms of xerostomia and keratoconjuctivitis sicca, along with the demonstration of autoimmunity as evidenced by serologic detection of antinuclear antibodies, rheumatoid factor, and anti-SSA/Ro antibodies. Anti-SSB/La antibodies were also present in three of the four patients.
Discussion
In this report we describe four individuals who presented with cystic lung disease that was considered to be consistent with LAM by the referring service. However, on further evaluation in our clinic, each was confirmed to have primary Sjögren syndrome based on characteristic clinical features, serologies, and lung pathologic and radiographic findings.
Diffuse cystic lung disease is an uncommon clinical and radiographic presentation with a broad differential diagnosis (8). Although diseases such as LAM and pulmonary Langerhans cell histiocytosis remain the prototypical diffuse cystic lung diseases most commonly encountered in referral centers, cyst-predominant LIP/FB as the forme fruste of Sjögren syndrome should also be considered.
The diagnosis of Sjögren syndrome may be difficult to establish with certainty. Multiple diagnostic criteria and guidelines for its classification have been reported in the literature, with varying performance characteristics (9). The most common confirmatory findings are demonstration of autoimmunity via detection of serum anti-SSA/Ro and/or anti-SSB/La antibodies, or the presence of focal lymphocytic sialoadenitis demonstrated on lip biopsy. However, both of these confirmatory features suffer from a lack of sensitivity and specificity (10–12).
Few studies have evaluated the nature of cystic abnormalities on chest imaging in patients with Sjögren syndrome (1–3, 13). The cystic pattern associated with Sjögren syndrome has a characteristic appearance on high-resolution CT imaging. Typical features include a wide variation in cyst size, internal structure within cysts, geographic simplification of parenchymal architecture producing a “dissolving lung appearance,” perivascular and often basilar-predominant distribution, and frequent association with ground-glass opacities and nodules. We submit that in a compatible clinical context, these findings can be sufficiently distinctive to obviate the need for lung biopsy, in some cases even in the absence of confirmatory serological studies or lip biopsy.
It is noteworthy that all four patients in our analysis were women. This may represent a referral bias, as the basis for referral was suspicion of LAM, which is predominantly seen in women (8). However, Sjögren syndrome is well known to be a female-predominant disorder, with reported female-to-male ratios ranging from 9:1 to 20:1 for all comers (14, 15), and 5:1 to 9:1 in patients with Sjögren syndrome with pulmonary involvement (2, 5, 6). Indeed, in one study evaluating 80 patients with Sjögren syndrome and lung involvement, there were no men in the cohort (3). Mechanisms postulated to explain the remarkable sex bias in Sjögren syndrome have included alterations in immune system (16) and/or abnormal acinar cell apoptosis due to imbalanced androgen/estrogen ratios (17, 18), but there is no consensus in the literature on this important question.
In summary, cystic lung disease associated with Sjögren syndrome can have a characteristic appearance on thoracic imaging and can represent a forme fruste presentation of the disease. Clinicians should consider occult Sjögren syndrome in the differential diagnosis of patients presenting with idiopathic diffuse cystic lung disease and consider cyst radiographic features of internal cyst structure and association with eccentric vessels and geographic simplification of parenchymal architecture as useful clues to inform the diagnosis. Future identification of more sensitive and specific biomarkers than SSA, SSB antibodies, and labial salivary gland biopsy may improve the recognition of Sjögren syndrome in patients presenting with diffuse cystic lung disease.
Footnotes
Author Contributions: N.G. led the writing group; N.G., K.A.W.-B., A.F., and F.X.M. wrote the manuscript; K.A.W.-B. provided the pathological images and descriptions.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part II. Am J Respir Crit Care Med. 2015;192:17–29. doi: 10.1164/rccm.201411-2096CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohrmann C, Uhl M, Warnatz K, Ghanem N, Kotter E, Schaefer O, Langer M. High-resolution CT imaging of the lung for patients with primary Sjogren’s syndrome. Eur J Radiol. 2004;52:137–143. doi: 10.1016/j.ejrad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Naniwa T, Hara M, Arakawa T, Maeda T. Pulmonary manifestations in Sjogren’s syndrome: correlation analysis between chest computed tomographic findings and clinical subsets with poor prognosis in 80 patients. J Rheumatol. 2010;37:365–373. doi: 10.3899/jrheum.090507. [DOI] [PubMed] [Google Scholar]
- 4.Strimlan CV, Rosenow EC, III, Divertie MB, Harrison EG., Jr Pulmonary manifestations of Sjögren’s syndrome. Chest. 1976;70:354–361. doi: 10.1378/chest.70.3.354. [DOI] [PubMed] [Google Scholar]
- 5.Parambil JG, Myers JL, Lindell RM, Matteson EL, Ryu JH. Interstitial lung disease in primary Sjögren syndrome. Chest. 2006;130:1489–1495. doi: 10.1378/chest.130.5.1489. [DOI] [PubMed] [Google Scholar]
- 6.Johkoh T, Müller NL, Pickford HA, Hartman TE, Ichikado K, Akira M, Honda O, Nakamura H. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999;212:567–572. doi: 10.1148/radiology.212.2.r99au05567. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N, Fischer A, McCormack FX. Diffuse multicystic lung disease as a presenting manifestation of Sjogren’s syndrome [abstract] Am J Respir Crit Care Med. 2015;191:A1585. [Google Scholar]
- 8.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease: part I. Am J Respir Crit Care Med. 2015;191:1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjögren’s syndrome. J Autoimmun. 2014;48-49:42–45. doi: 10.1016/j.jaut.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Meilof JF, Smeenk RJ. Autoantibodies and their target antigens in Sjögren’s syndrome. Neth J Med. 1992;40:140–147. [PubMed] [Google Scholar]
- 11.Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47:520–524. doi: 10.1002/art.10668. [DOI] [PubMed] [Google Scholar]
- 12.Langerman AJ, Blair EA, Sweiss NJ, Taxy JB. Utility of lip biopsy in the diagnosis and treatment of Sjogren’s syndrome. Laryngoscope. 2007;117:1004–1008. doi: 10.1097/MLG.0b013e31804654f7. [DOI] [PubMed] [Google Scholar]
- 13.Matsuyama N, Ashizawa K, Okimoto T, Kadota J, Amano H, Hayashi K. Pulmonary lesions associated with Sjögren’s syndrome: radiographic and CT findings. Br J Radiol. 2003;76:880–884. doi: 10.1259/bjr/18937619. [DOI] [PubMed] [Google Scholar]
- 14.Alamanos Y, Tsifetaki N, Voulgari PV, Venetsanopoulou AI, Siozos C, Drosos AA. Epidemiology of primary Sjögren’s syndrome in north-west Greece, 1982-2003. Rheumatology (Oxford) 2006;45:187–191. doi: 10.1093/rheumatology/kei107. [DOI] [PubMed] [Google Scholar]
- 15.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–369. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Lu MC, Hsieh MC, Koo M, Lai NS. Risk of Sjögren’s syndrome in Taiwanese female adults with irregular menstrual cycles: a population-based case-control study. Rheumatol Int. 2016;36:155–160. doi: 10.1007/s00296-015-3324-z. [DOI] [PubMed] [Google Scholar]
- 17.Porola P, Laine M, Virkki L, Poduval P, Konttinen YT. The influence of sex steroids on Sjögren’s syndrome. Ann N Y Acad Sci. 2007;1108:426–432. doi: 10.1196/annals.1422.045. [DOI] [PubMed] [Google Scholar]
- 18.Konttinen YT, Fuellen G, Bing Y, Porola P, Stegaev V, Trokovic N, Falk SS, Liu Y, Szodoray P, Takakubo Y. Sex steroids in Sjögren’s syndrome. J Autoimmun. 2012;39:49–56. doi: 10.1016/j.jaut.2012.01.004. [DOI] [PubMed] [Google Scholar]