DEL1 affects rice growth and leaf senescence mediated by PECTATE LYASE-LIKE genes.
Abstract
To better understand the molecular mechanisms behind plant growth and leaf senescence in monocot plants, we identified a mutant exhibiting dwarfism and an early-senescence leaf phenotype, termed dwarf and early-senescence leaf1 (del1). Histological analysis showed that the abnormal growth was caused by a reduction in cell number. Further investigation revealed that the decline in cell number in del1 was affected by the cell cycle. Physiological analysis, transmission electron microscopy, and TUNEL assays showed that leaf senescence was triggered by the accumulation of reactive oxygen species. The DEL1 gene was cloned using a map-based approach. It was shown to encode a pectate lyase (PEL) precursor that contains a PelC domain. DEL1 contains all the conserved residues of PEL and has strong similarity with plant PelC. DEL1 is expressed in all tissues but predominantly in elongating tissues. Functional analysis revealed that mutation of DEL1 decreased the total PEL enzymatic activity, increased the degree of methylesterified homogalacturonan, and altered the cell wall composition and structure. In addition, transcriptome assay revealed that a set of cell wall function- and senescence-related gene expression was altered in del1 plants. Our research indicates that DEL1 is involved in both the maintenance of normal cell division and the induction of leaf senescence. These findings reveal a new molecular mechanism for plant growth and leaf senescence mediated by PECTATE LYASE-LIKE genes.
The development of higher plants is accompanied by the complex processes of cell division and cell expansion and finally the emergence of many specialized organs, tissues, and cells. As part of cell development in plants, cell walls perform essential functions, such as providing tensile strength, shape, and protection, as well as helping the cell to maintain its internal pressure (Cosgrove, 2005). In growing plants, the cell wall is both resistant to the high turgor pressure that drives growth and also flexible enough to yield and expand selectively in response to that pressure (Cosgrove, 2005; Xiao et al., 2014). This process involves a series of molecular mechanisms, including disruption of the intermolecular adhesion, polymer lysis, religation and rearrangements, and/or cleavage of polymer glycosyl linkages by enzymes (McQueen-Mason and Cosgrove, 1995; Van Sandt et al., 2007; Anderson et al., 2010; Park and Cosgrove, 2012).
Pectins are major components of the plant primary cell wall and middle lamella and have several functions, including involvement in maintaining plant growth and development, promoting cell-to-cell adhesion, providing structural support in soft tissues, defense responses, and influencing wall porosity and thickness, etc (Ridley et al., 2001; Iwai et al., 2002; Ogawa et al., 2009; Wolf et al., 2009; Hongo et al., 2012). Pectins may be the most complex polysaccharide family in the living world, being composed of as many as 17 different monosaccharides and having more than 20 different linkages (Bonnin et al., 2014). According to the current understanding of pectins at the structure/function level, there are two conceptual models of structural modification during plant growth (Atmodjo et al., 2013). First, the large number of linkages and structural motifs allow the domains of pectins to interact indirectly and/or via covalent bonds (Dick-Pérez et al., 2011; Tan et al., 2013). Second, pectins can depend on reversible calcium-mediated cross linking between stretches of demethylated homogalacturonan (HG) to coordinate cell wall networks (Vincken et al., 2003; Xiao et al., 2014). HG is secreted in a highly methylesterified form and selectively demethylesterified by pectin methylesterases (PME; Hongo et al., 2012). The demethylesterified HG can either form a rigid gel by Ca2+-pectate cross-linked complexes, or become more susceptible to cleavage by two classes of pectin-degrading enzyme, namely pectin/pectate lyase (PL/PEL; EC 4.2.2.10; EC 4.2.2.2) and polygalacturonase (PG; EC 3.2.1.15) (Wolf et al., 2009; Hongo et al., 2012). Hence, the methylesterification status of HG can regulate cellular growth and cell shape, and affect plant growth and development (Wolf et al., 2009; Peaucelle et al., 2011).
PELs, a family of endo-acting depolymerizing enzymes, are responsible for the α-1,4-glycosidic linkages in demethylesterified HG by β-elimination and produces 4,5-unsaturated oligogalacturonides (OGs) at their nonreducing ends (Palusa et al., 2007). PELs have been extensively studied in plant pathogenic bacteria such as Erwinia chrysanthemi, which causes soft-rot diseases (Barras et al., 1994). In plants, multiple functions have been identified for PEL, and there is some suggestion that it is involved in pollen, anthers, pistils, and developing tracheary elements (Wing et al., 1990; Rogers et al., 1992; Wu et al., 1996; Kulikauskas and McCormick, 1997; Domingo et al., 1998; Milioni et al., 2001), fruit softening and ripening (Dominguez-Puigjaner et al., 1997; Medina-Escobar et al., 1997; Nunan et al., 2001; Pua et al., 2001), lateral root emergence (Laskowski et al., 2006), cotton fiber elongation (Wang et al., 2010), leaf senescence (Wu et al., 2013), and susceptibility to plant pathogens (Vogel et al., 2002). It has also been shown to be expressed in a wide range of tissues (Palusa et al., 2007; Sun and van Nocker, 2010). In addition, recent research has revealed that the overexpression of aspen PtxtPL1-27 can increase the solubility of wood matrix polysaccharides (Biswal et al., 2014). The modification of pectins may be important in the field of biotechnology for improving woody biomass (Biswal et al., 2014).
The large family of PECTATE LYASE-LIKE (PLL) genes all exhibit redundant or unique functions in plant evolution, and this diversity may enhance plasticity in adaptation to changing environments (Sun and van Nocker, 2010). In rice (Oryza sativa), genome prediction has suggested that there are 14 PLL genes, but only one of these (ospse1) has been analyzed genetically (Wu et al., 2013). In this study, we report on the isolation and characterization of a recessive mutant, dwarf and early-senescence leaf1 (del1), in rice. The mutation of DEL1 led to a phenotype involving abnormal plant growth and leaf senescence. DEL1 encodes a PEL precursor and is a member of a multigene family in rice. The loss of function of DEL1 decreased total PEL activity, increased the degree of methylesterified HG, and perturbed cell wall composition and structure, resulting in a reduced number of cells and triggering reactive oxygen species (ROS) activity. Expression profiling indicated the genes involved in cell wall function and senescence are altered in expression in the del1 plants. Our findings suggest that DEL1 is a critical gene for plant growth and leaf senescence in rice.
RESULTS
del1 Exhibits Significant Size Reduction in the Whole Plant
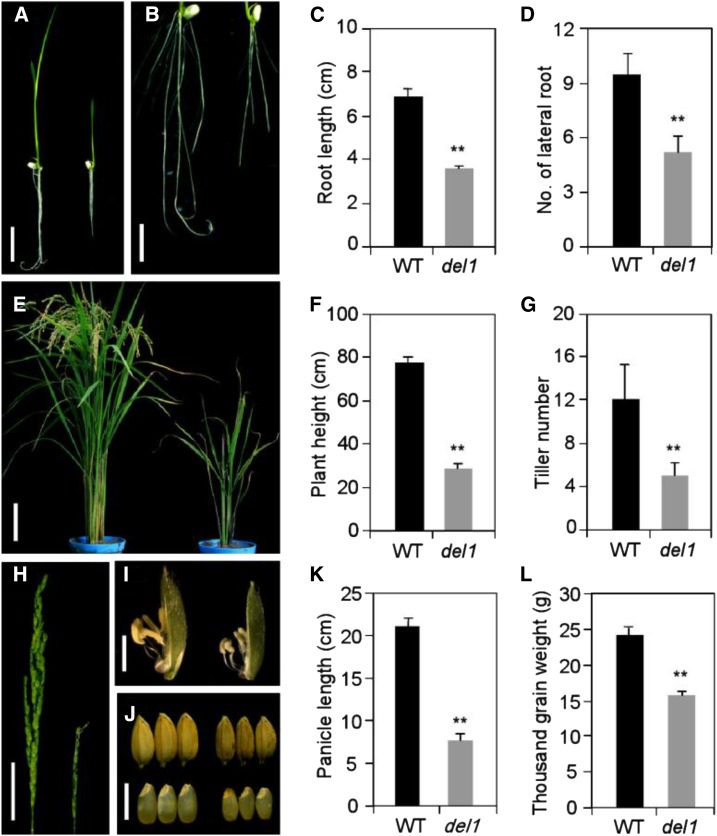
The del1 mutant was isolated from an ethyl methanesulfonate-mutagenized japonica cultivar, Nipponbare. del1 exhibited dwarfism at the seedling stage (5 d after germination), and its main root length was clearly reduced, which reached 47.8% of that in the wild type (Fig. 1, A and C). Meanwhile, the number of lateral roots in del1 decreased significantly to just 44.7% of that in the wild type (Fig. 1, B and D). The plant height of del1 was also shorter than that of the wild type throughout the growth period, being only about 36.8% of that of the wild type at the mature stage (Fig. 1, E and F; Supplemental Figs. S1 and S2), and the internode length, thickness, and diameter of del1 were significantly reduced (Supplemental Fig. S3). Compared with the wild type, the tiller number and panicle length were also clearly reduced in del1 plants (Fig. 1, E, G, H, and K). Moreover, grain development also changed significantly, with grain size and grain weight being diminished in del1 plants (Fig. 1, I, J, and L; Supplemental Table S1). Besides these phenotypic findings, the heading date of del1 was also notably retarded, along with decreases in leaf length and leaf width (Supplemental Table S1). These phenotypes clearly suggest that plant size was significantly lower in del1 plants.
Figure 1.
Comparison of phenotype between wild-type and del1 plants. A, Wild-type (Nipponbare, left) and del1 plants (right) 5 d after sowing. Scale bar = 2 cm. B, Root length of wild-type (left) and del1 plants (right) 5 d after sowing. Scale bar = 1 cm. C and D, Statistical analysis of root length and lateral root number between wild-type and del1 plants. Twenty plants were measured. Error bars indicate sd; **P < 0.01 (Student’s t test). E, Wild-type (left) and del1 plants (right) at maturity. Scale bar = 10 cm. F and G, Statistical analysis of plant height and tiller number between wild-type and del1 plants. Twenty plants were measured. Error bars indicate sd; **P < 0.01 (Student’s t test). H, Phenotype of panicle between wild-type (left) and del1 (right) plants. Scale bar = 5 cm. I, Floret with the lemma removed, wild-type (left) and del1 (right). Scale bar = 0.5 cm. J, Mature seed and brown rice of wild type (left) and del1 (right). Scale bar = 0.5 cm. K and L, Statistical analysis of panicle length and thousand grain weight between wild-type and del1 plants. Twenty panicles were measured. Error bars indicate sd; **P < 0.01 (Student’s t test).
Cell Number Is Reduced in del1 Plants
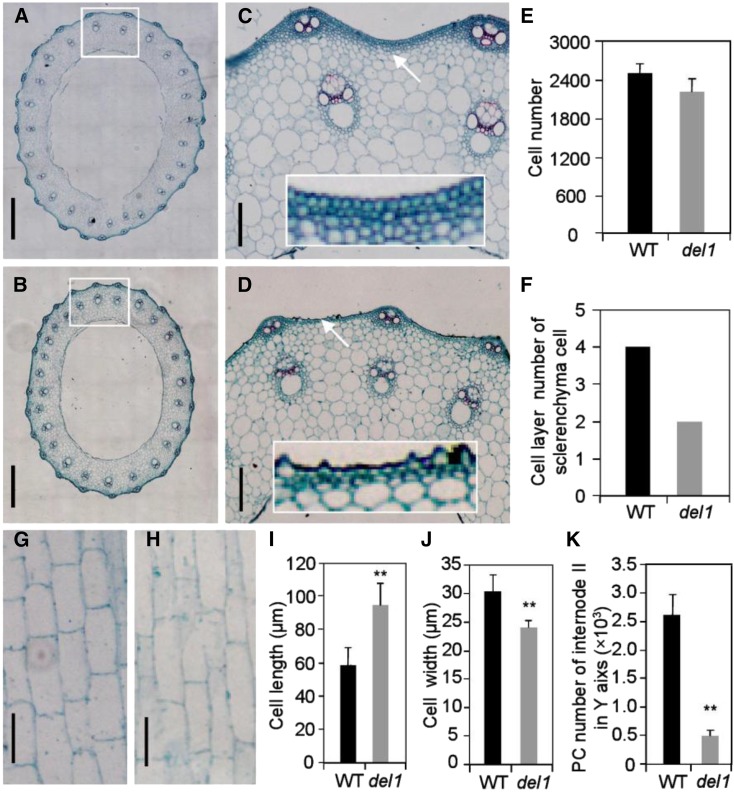
The size of a plant organ is determined by its cell size and number of cells, which are related to cell expansion and cell division, respectively (Krizek, 2009). To determine the causes of organ size reduction in del1 plants, we conducted microscopic observation on the second culms and compared the findings between the wild type and del1 using paraffinized sections. Cross sections of culms revealed that the size of sclerenchyma cells in del1 was less than that in the wild type (Fig. 2, A–D). Statistical analysis showed that the cell number of del1 was only 88.56% of that in the wild type (Fig. 2E). Moreover, the number of layers of sclerenchyma cells in del1 was reduced by two (Fig. 2, C, D, and F). Longitudinal sections of culms in del1 displayed a significant change in cell size (Fig. 2, G and H). The cell length in del1 was 59.6% longer, and the cell width was 20.8% narrower than that in the wild type (Fig. 2, I and J). Further investigation of the total number of parenchyma cells showed that del1 had only 19% of the number in the wild type (Fig. 2K).
Figure 2.
Histological characterization of culms in wild-type and del1 plants. A to D, Cross sections of internode II of wild type (A) and del1 (B). Scale bar = 500 μm. C, Magnification of A; D, magnification of B; white rectangle shows a magnification of the sclerenchyma cell layer. Scale bar = 50 μm. E and F, Statistical analysis of cell number and sclerenchyma cell layer number between wild-type and del1 plants, means ± sd of five independent replicates. G and H, Longitudinal sections of internode II of wild type (G) and del1 (H). Scale bar = 50 μm. I and J, Statistical analysis of the cell length and cell width between wild-type and del1 plants, mean ± sd of 30 cells. **P < 0.01 (Student’s t test). K, Number of parenchyma cells (PCs) for internode II of wild-type and del1 plants, mean ± sd of five independent replicates.
Cell Cycle Progression Is Delayed in del1 Plants
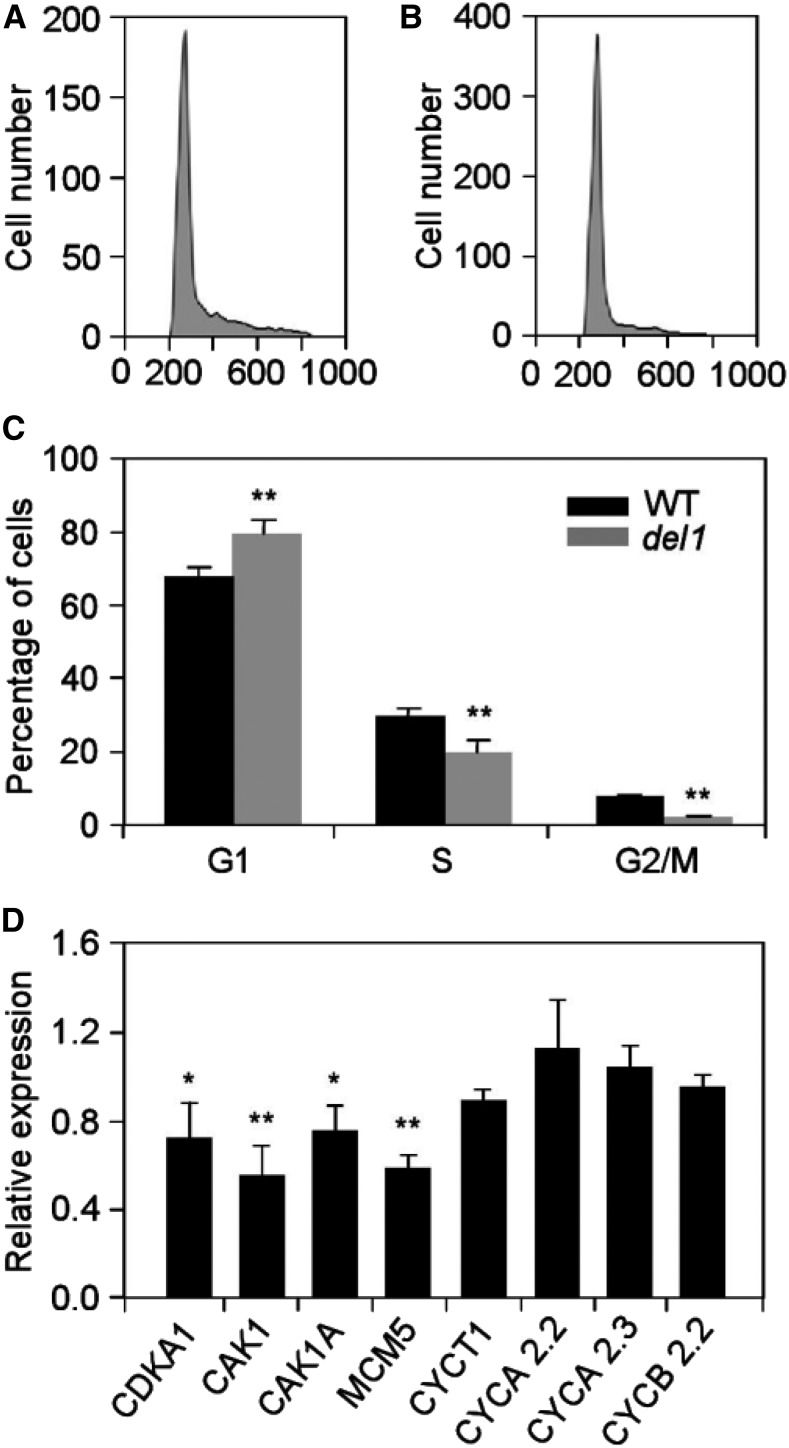
To determine whether the decline in cell number in del1 was affected by the cell cycle, we further investigated the cell cycle progression by flow cytometry. The results of the suspension cell lines of del1 revealed a significant increase in the number of cells in the G1 phase and decreases in the S and G2/M phases of the cell cycle, implying that the cell cycle was delayed at the G1 phase (Fig. 3, A–C). We also analyzed the expression of cell-cycle-related genes in wild-type and del1 plants using real-time reverse transcription (RT)-PCR. The expression levels of genes related to the G1 phase of the cell cycle, CDKA1, CAK1, CAK1A, MCM5, and CYCT1, were down-regulated by ∼10% to 45%, while little difference was observed for genes related to the G2 phase of the cell cycle in del1 mutants (Fig. 3D). Therefore, we conclude that the mutation of DEL1 delays cell cycle progression at the G1 phase.
Figure 3.
Cell cycle analysis of wild-type and del1 plants. A and B, Flow karyotype histogram of wild-type (A) and del1 (B) leaves. C, Quantification of the DNA profiles of wild-type and del1 plants. D, Relative expression levels of cell cycle-related genes in wild-type and del1 plants, mean ± sd of three independent replicates. *P < 0.05, **P < 0.01 (Student’s t test).
del1 Displays Early Leaf Senescence
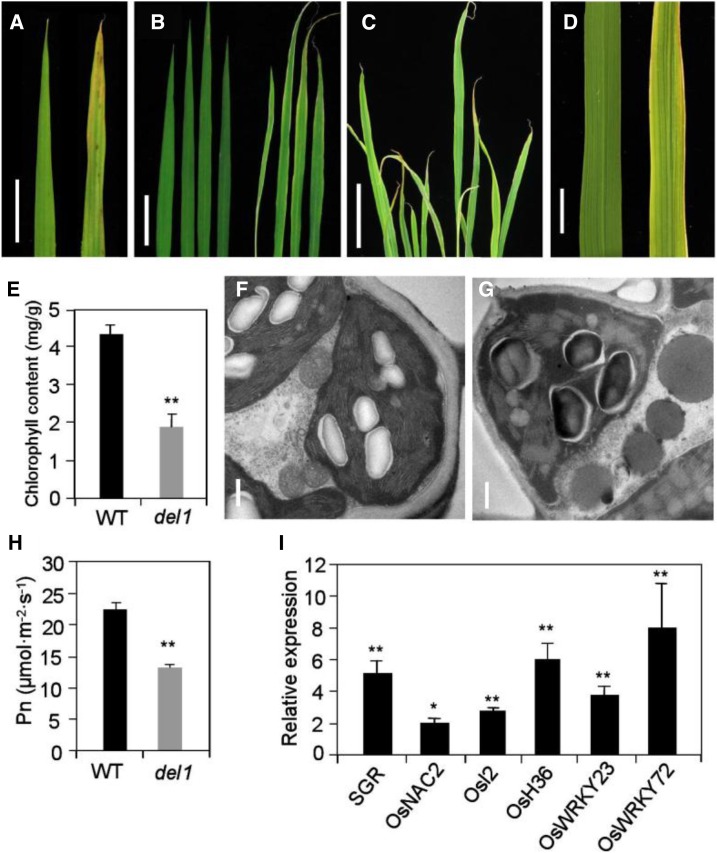
del1 also exhibited a phenotype of early senescence, which became increasingly apparent as the plants developed (Fig. 4, A–D). The mutant leaf apex and leaf margin exhibited a faint yellow color from 5 d after germination (Fig. 4A). With the development of plants, the young leaves were pale white and then turned green upon maturation, while the old leaves exhibited withering and cracking (Fig. 4, B–D). The chlorophyll content in del1 plants was significantly lower, being only 44.0% of that in the wild type (Fig. 4E). In addition, transmission electron microscopy (TEM) revealed the presence of well-developed mesophyll cells and membrane-intact chloroplasts in fully developed wild-type leaves, whereas the disordered arrangement of grana thylakoid and the degradation of chloroplasts was observed in del1 plants (Fig. 4, F and G). Moreover, the photosynthetic rate in del1 plants was only 58.5% of that in the wild type (Fig. 4H). All these results indicate that leaf senescence occurred in del1 plants.
Figure 4.
Leaf phenotype and identification of leaf senescence in DEL1. A to D, Leaf phenotype from young (A), tillering (B and C), and heading (D) stages. Scale bars = 1, 5, 5, and 1 cm in A, B, C, and D, respectively. E, Statistical analysis of chlorophyll content between wild-type and del1 plants, mean ± sd of five independent replicates. **P < 0.01 (Student’s t test). F and G, Transmission electron microscopy analysis of senescence leaves of wild-type (F) and del1 plants (G). Scale bar = 0.5 μm. H, Statistical analysis of photosynthesis rate between wild-type and del1 plants, mean ± sd of five independent replicates. **P < 0.01 (Student’s t test). I, Relative expression levels of senescence-related genes and transcription factors in wild-type and del1 plants, mean ± sd of three independent replicates. *P < 0.05, **P < 0.01 (Student’s t test).
Leaf senescence is usually accompanied by a change of expression of many genes, including those for transcription factors (Wang et al., 2015). To confirm senescence in the del1 plants, the expression levels of senescence-associated genes (SAGs) and related transcription factors (Osl2, OsH36, SGR, OsNAC2, OsWRKY23, and OsWRKY72) were determined by real-time RT-PCR. The expression levels of Osl2, OsH36, SGR, OsNAC2, OsWRKY23, and OsWRKY72 mRNAs were 2.0 to 8.0 times higher than in wild-type leaves (Fig. 4I). The up-regulated expression patterns of SAGs and transcription factors further support the notion that early leaf senescence occurred in del1 plants.
ROS Accumulation and Programmed Cell Death Are Enhanced in del1 Plants
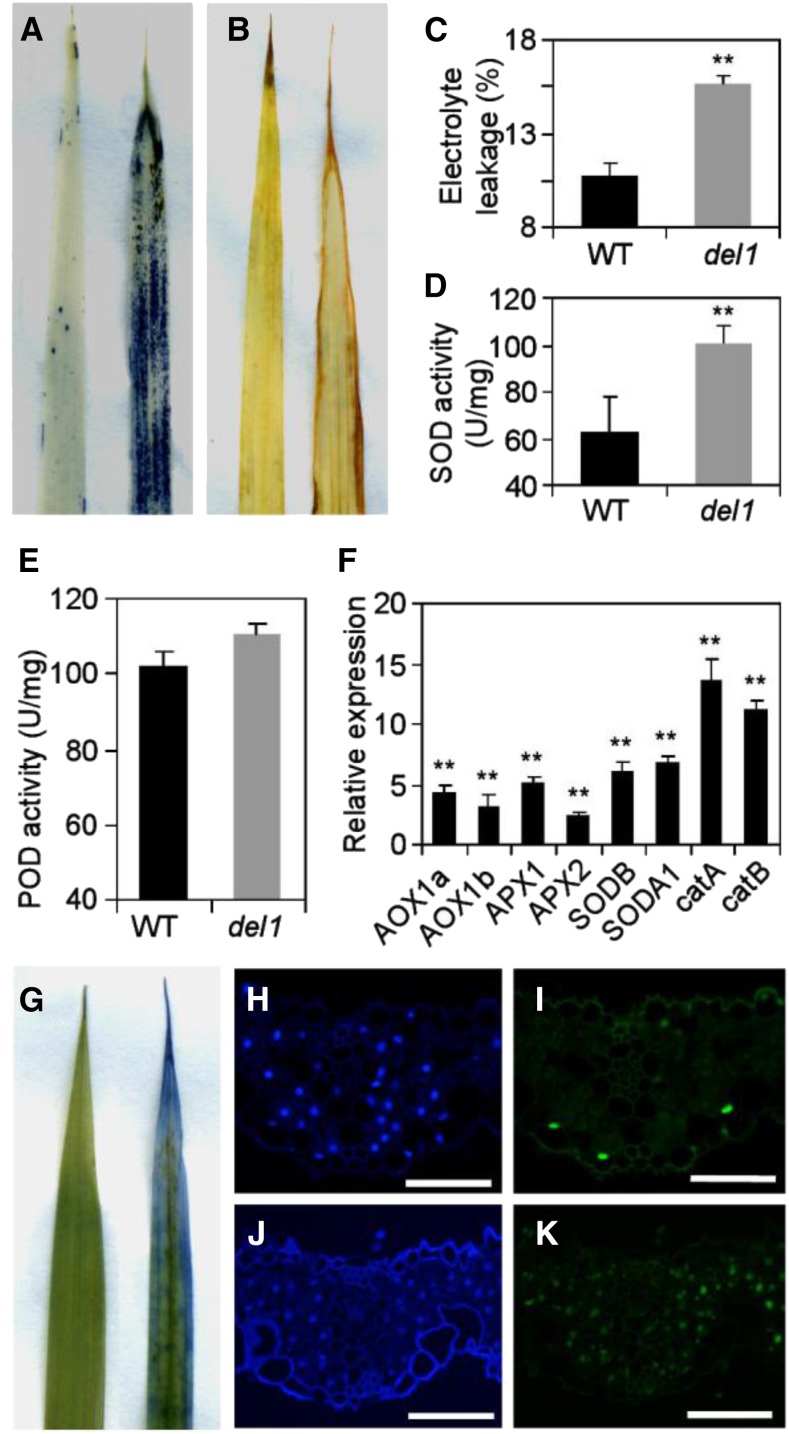
The accumulation of ROS can lead to leaf senescence (Khanna-Chopra, 2012). Therefore, we performed nitro blue tetrazolium (NBT) staining and 3,3′-diaminobenzidine (DAB) staining tests to detect O2− and H2O2 accumulation, respectively. Extensive NBT staining was observed in del1 plants, whereas the staining was minimal in wild-type leaves (Fig. 5A). A brown color was seen for the DAB staining, correlating with the area of leaf senescence, but there was no sign of this in wild-type leaves (Fig. 5B). These results revealed that ROS accumulated in del1 plants. During senescence, plant cell membrane damage can lead to a change in electrolyte leakage (Blum and Ebercon, 1981). In del1 plants, the electrolyte leakage was 45.8% higher than that in the wild type (Fig. 5C), suggesting that del1 lost more membrane integrity during development than the wild type.
Figure 5.
ROS accumulation and enhancement of PCD in wild-type and del1 leaves. A and B, NBT and DAB staining of leaves between the wild-type (left) and del1 plants (right). C to E, Statistical analysis of electrolyte leakage (C), SOD activity (D), and POD activity (E) in leaves between wild-type and del1 plants, mean ± sd of five independent replicates. **P < 0.01 (Student’s t test). F, Relative expression levels of ROS detoxification-related genes in wild-type and del1 plants, mean ± sd of three independent replicates. **P < 0.01 (Student’s t test). G, Trypan blue staining of leaves in wild-type (left) and del1 plants (right). H to K, TUNEL assay of leaves. DAPI staining of wild-type (H) and del1 plants (J). Positive results of wild-type (I) and del1 plants (K). Scale bar = 50 μm.
Plant senescence can lead to the synthesis of antioxidative enzymes to remove ROS (Miller et al., 2010). Therefore, we quantitatively determined the activity of these enzymes. The results indicated that the activities of superoxide dismutase (SOD) and peroxidase (POD) increased in del1 plants, at 100.3 and 110.4 U/mg fresh weight in del1 leaves, which were much higher than the levels of 62.2 and 102.3 U/mg fresh weight in the wild-type leaves, respectively (Fig. 5, D and E). In the process of leaf senescence, ROS-scavenging systems may play an important role in ROS detoxification (Tan et al., 2014). ROS-scavenging-related genes (alternative oxidases [AOXs], ascorbate peroxidase [APX], SOD, and catalase [CAT]) were up-regulated during leaf senescence (Tan et al., 2014). As expected, the expression levels of ROS-scavenging-related genes (AOX1a, AOX1b, APX1, APX2, SODB, SODA1, catA, and catB) were 2.5 to 13.6 times higher than in the wild type (Fig. 5F).
Leaf senescence is the final stage of leaf development, and it is considered to be a type of programmed cell death (PCD; Nooden and Leopold, 1978). Trypan blue staining revealed that the leaf apex and leaf margin of del1 were colored blue, but the counterpart wild type remained green (Fig. 5G), suggesting that some of the cells in the leaf apex and leaf margin of the del1 plant were dead. One basic feature of PCD is the condensation of nuclear chromatin, which is caused by endonucleolytic degradation of nuclear DNA (Simeonova et al., 2000). We accordingly used the TUNEL assay to determine whether the del1 plants induced PCD. A few of the nuclei in the wild type were TUNEL positive; in contrast, most nuclei in the del1 leaf sections were TUNEL positive (Fig. 5, H–K), indicating that DNA degradation was widespread in the del1 leaves.
Map-Based Cloning of DEL1
To determine the molecular basis for the del1 phenotypes, a map-based cloning approach was employed to isolate the corresponding gene. The mapping population was generated by crossing DEL1 with Taichung Native 1, a wild-type indica variety with DNA polymorphism with japonica. All the F1 plants exhibited a normal phenotype matching that of the wild type. In the F2 segregating populations, normal and mutant phenotypes showed a typical segregation ratio of 3:1 (Supplemental Table S2). This finding suggests that del1 is controlled by a single recessive nuclear gene.
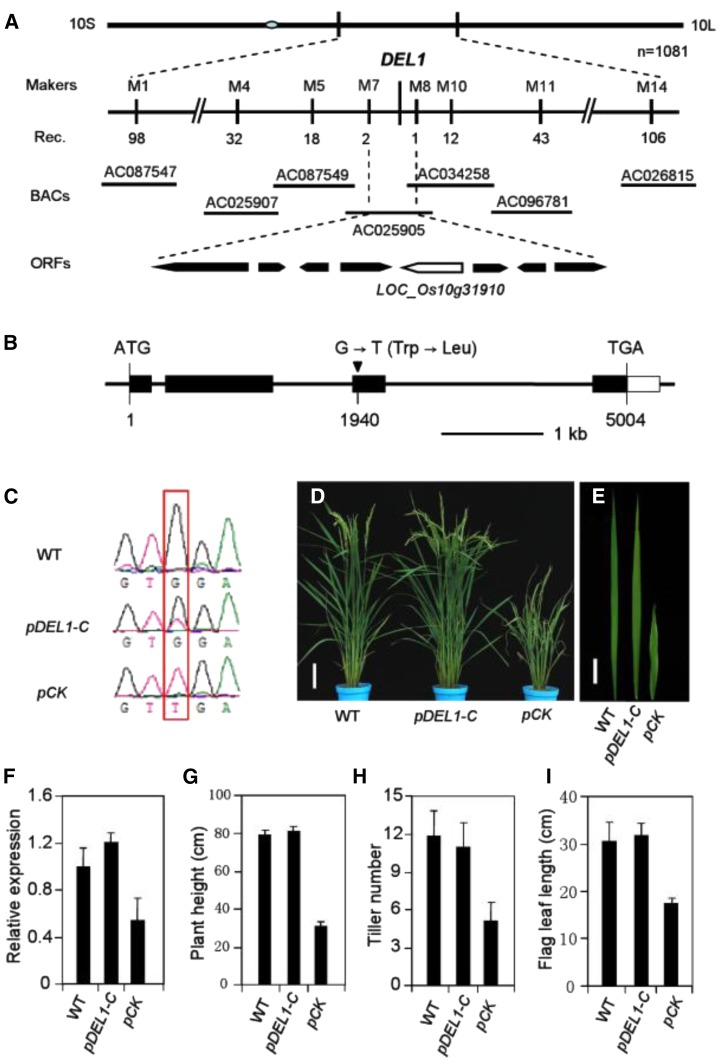
Thirty F2 plants with the del1 phenotype were used for primary mapping, and DEL1 was found to be located on the long arm of chromosome 10 between markers M1 and M14. Furthermore, by using 1081 homozygous mutant plants, DEL1 was further narrowed down to an ∼45-kb region with molecular markers as shown in Supplemental Table S5. This 45-kb region is in the BAC AC025905 and contains eight putative open reading frames (ORFs), as annotated by the MSU Rice Genome Annotation Project (Fig. 6A). Upon sequencing analysis of these eight ORFs, one base pair mutation was found at position 1940 of ORF LOC_Os10g31910. This change from G to T causes a substitution at the 365th amino acid residue from Trp to Leu (Fig. 6B). By examining this base in six other rice varieties, we found that they all exhibited the wild-type genotype (Supplemental Fig. S4).
Figure 6.
Map-based cloning and identification of DEL1. A, Fine mapping of DEL1. The del1 locus was mapped to a 45-kb region on chromosome 10. B, Schematic diagram of DEL1. Black rectangles represent exons. Black inverted triangle represents mutant site. C, Sequencing analysis of the DEL1 transcripts in T0 transgenic lines. D and E, Phenotype of the complementation transgenic line: Wild type (left), complementation transgenic line (middle), and empty vector control (right). Scale bars = 10 cm and 4 cm, respectively. F, Expression levels of DEL1 detected by qRT-PCR in wild-type and transgenic plants, mean ± sd of three independent replicates. G to I, Statistical analysis of plant height (G), tiller number (H), and flag leaf length (I) in wild-type and transgenic plants, mean ± sd of 10 independent replicates.
To confirm that LOC_Os10g31910 is responsible for the del1 phenotype, a 7843-bp wild-type DEL1 DNA fragment containing the promoter region and the entire ORF was introduced into del1 plants by Agrobacterium tumefaciens-mediated transformation. Analysis of independent transgenic plants by PCR using primers flanking the mutation site, together with further sequencing, revealed a double peak (G and T) at the mutation site, confirming the presence of normal DEL1 gene sequence (Fig. 6C). The transgenic plants rescued the phenotypes of del1, whereas in all of those transformed by pCAMBIA1300, there was a failure to rescue the mutant phenotype, confirming that disruption of the DEL1 gene was responsible for the del1 mutant phenotype (Fig. 6, D–I). In addition, RNAi transgenic plants exhibited early senescence leaves and a significantly reduced size (Supplemental Fig. S5, A–E). The expression of DEL1 was significantly decreased in RNAi plants (Supplemental Fig. S5F). Therefore, we conclude that LOC_Os10g31910 is the DEL1 gene.
DEL1 Encodes a Pectate Lyase Precursor
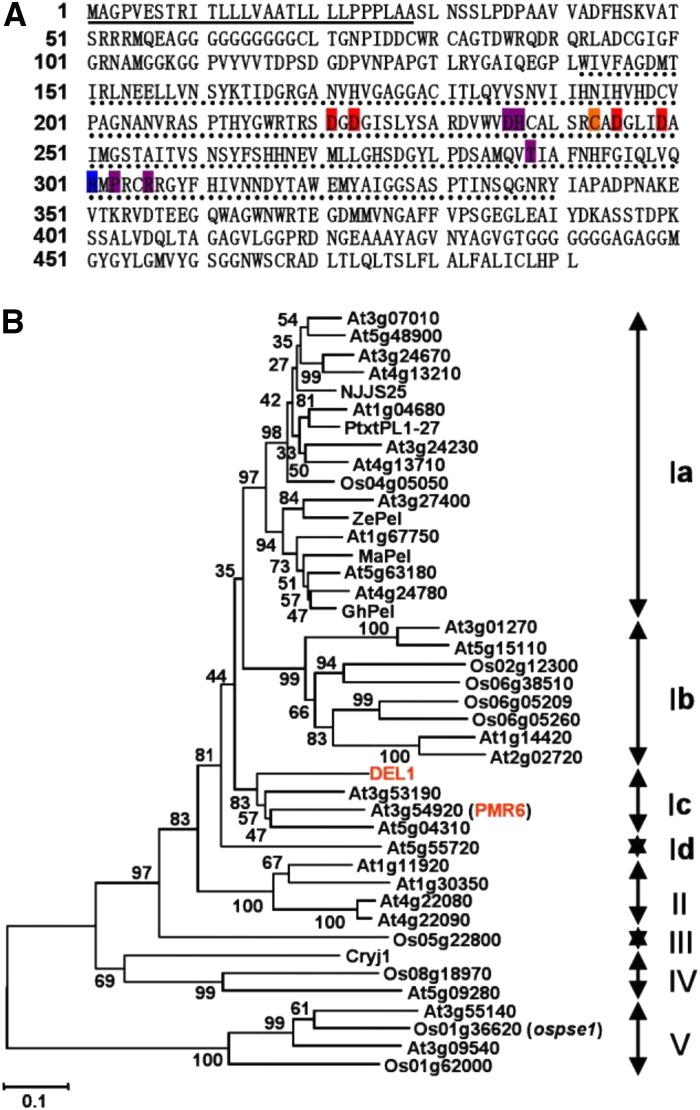
Sequence analysis indicated that DEL1 cDNA is 1476 bp in length and encodes a protein of 491 amino acid residues (Fig. 7A). A search using SignalP and Pfam revealed that the DEL1 protein contains a 28-amino acid signal peptide and one predicted PelC domain (Fig. 7A). Multiple sequence alignment analyses revealed that DEL1 contains all the conserved residues of PELs known to be involved in Ca2+ binding, substrate binding, and catalysis and has stronger similarity with plant PelC than Erwinia chrysanthemi (Supplemental Fig. S6).
Figure 7.
Prediction of the primary sequence and phylogenetic analysis of DEL1. A, The deduced amino acid sequence of DEL1. Numbers on the left refer to the positions of amino acid residues. The signal peptide is indicated with an underline; the PelC domain is shown by a dotted line; the conserved residues involved in Ca2+ binding (red background), disulfide bonds (orange background), catalysis (blue background), and substrate binding (purple background). B, Phylogenetic tree of PEL in Arabidopsis, rice, and other plants. The numbers at each node represent the bootstrap support (percentage), and scale bar is an indicator of genetic distance based on branch length.
An unrooted phylogenetic tree was built among PEL members of rice, Arabidopsis (Arabidopsis thaliana), and seven known plant PELs using the neighbor-joining method, which revealed that PELs were divided into five major clades, with clade I being further divided into four subclades (Fig. 7B). DEL1 belongs to Ic and is in the same clade as PMR6 (a powdery mildew susceptibility) of Arabidopsis (Vogel et al., 2002).
DEL1 Was Highly Expressed in Elongating Tissues
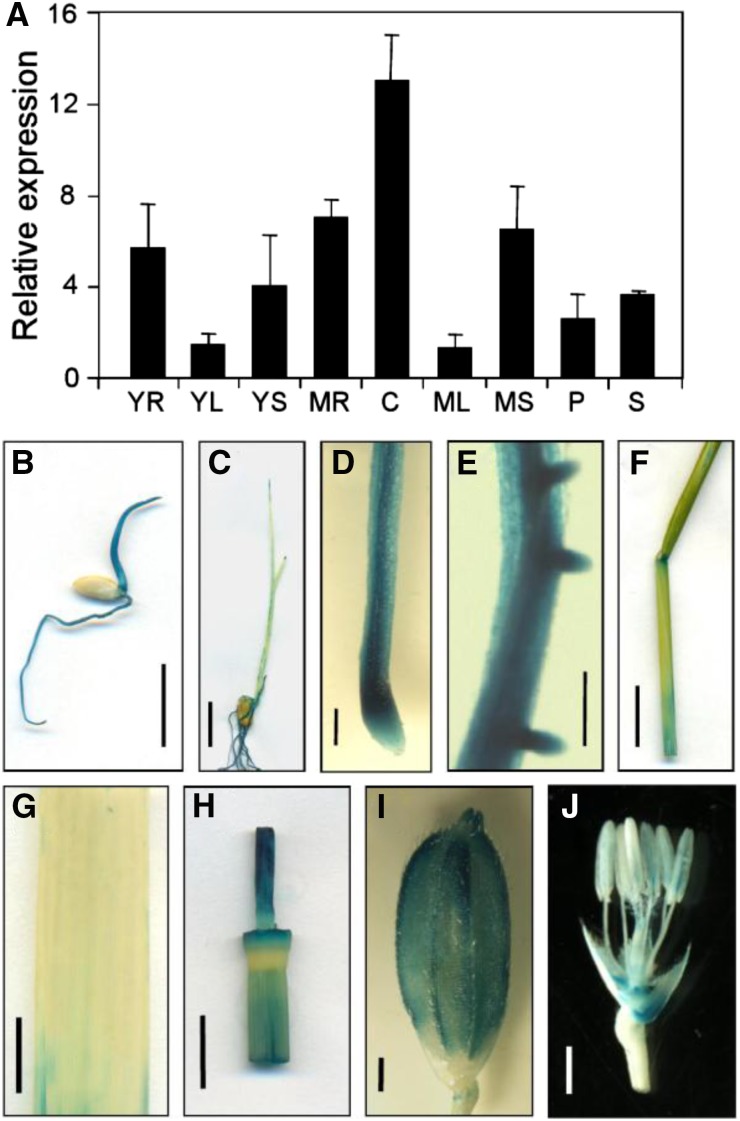
To analyze the spatial and developmental expression of the DEL1 genes, we isolated RNA from different tissues, including young roots, young sheaths, young leaves, mature roots, mature sheaths, mature leaves, culms, panicles, and spikelets, and determined the transcript levels of the DEL1 genes by real-time RT-PCR. The results revealed that DEL1 was ubiquitously expressed in all the tissues examined at the young and mature stages, and high levels of DEL1 expression were observed in elongating tissues, such as culms and roots (Fig. 8A).
Figure 8.
Expression analysis of DEL1. A, Transcription level of DEL1 in various organs, mean ± sd of three independent replicates. YR, Young root; YL, young leaf; YS, young sheath; MR, mature root; C, culm; ML, mature leaf; MS, mature sheath; P, panicle; S, spikelet. B to J, GUS analysis of DEL1 expression: B and C, Four and seven days after germination of the young plant, scale bar = 1 cm; D, root, scale bar = 500 μm; E, lateral root, scale bar = 250 μm; F, mature sheath, scale bar = 1 cm; G, mature leaf, scale bar = 1 cm; H, culm, scale bar = 1 cm; I, spikelet, scale bar = 1 cm; J, lemma and palea were removed in I, scale bar = 500 μm.
In addition, to analyze the spatial expression pattern of DEL1, we expressed the GUS gene under the control of the native promoter of the DEL1 gene. Six independent DEL1:GUS transgenic lines were analyzed, and all of them exhibited similar results. The GUS activity was expressed in all of the tissues and was consistent with the qRT-PCR data (Fig. 8, B–J).
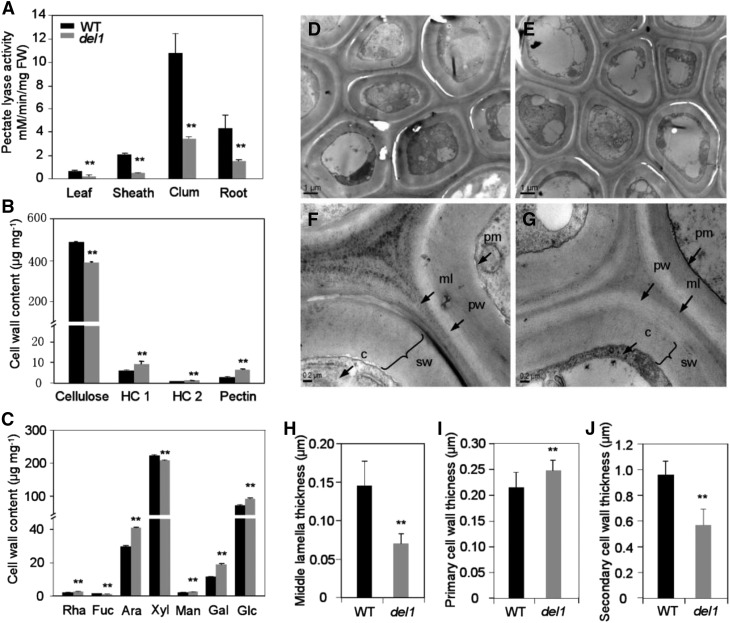
Mutation of DEL1 Decreased Total PEL Activity and Altered Cell Wall Composition and Structure
Since PelC is the only domain identified in DEL1, it may be critical for its function. Therefore, we determined the total PEL activity. As expected, PEL enzyme activity was significantly decreased in del1 plants. Especially in culms, the PEL activity was only 32.0% of that in the wild type (Fig. 9A). This result is consistent with the real-time RT-PCR (Fig. 8A) and the ProDEL1:GUS expression results (Fig. 8, B–J).
Figure 9.
The levels of PEL activity and cell wall composition and structure in wild-type and del1 plants. A, Analysis of PEL activity in wild-type and del1 plants, mean ± sd of five independent replicates. **P < 0.01 (Student’s t test). B, Comparison of cell wall composition between wild-type and del1 plants, mean ± sd of five independent replicates. **P < 0.01 (Student’s t test). HC 1, Hemicellulose 1; HC 2l hemicellulose 2. C, Neutral monosaccharide composition between wild-type and del1 plants, mean ± se of five independent replicates, **P < 0.01 (Student’s t test). D and E, Transmission electron microscopy micrographs of the bundle sheath fiber cells of wild-type (D) and del1 (E) plants, scale bar = 1 μm. F, Magnification in D, and G. magnification in E, scale bar = 0.2 μm. ml, Middle lamella; pw, primary cell wall; sw, secondary cell wall; pm, plasma membrane; c, cytoplasm. H to J, Statistical analysis of the middle lamella (H), primary cell wall (I), and secondary cell wall (J) thicknesses of bundle sheath fiber cells between the wild-type and del1 plants, mean ± sd of 30 cells, **P < 0.01 (Student’s t test).
The morphological phenotypes suggest that the cell wall structure in the mutant plants may also be altered. We therefore analyzed the structure of wild-type and del1 plants by TEM. The results revealed that the wall thicknesses of bundle sheath fiber cells in del1 culms were altered (Fig. 9, D–G). Specifically, the thicknesses of the middle lamella and secondary cell wall were reduced by 51.6% and 40.6%, respectively, and that of the primary cell wall was 15.6% higher than those of the wild type (Fig. 9, H–J). Similarly in roots, the thicknesses (sclerenchyma cell) of the middle lamella and secondary cell wall were reduced by 18.5% and 41.4%, respectively, and that of the primary cell wall was 12.7% higher than those of the wild type (Supplemental Fig. S7).
To determine whether changes in the cell wall structure affected the wall composition, we analyzed the cellulose, hemicellulose, and pectin contents and compared them between wild-type and del1 culms. The cellulose content of del1 was reduced by ∼20%, while the contents of hemicellulose 1 (HC 1), hemicellulose 2 (HC 2), and pectin were increased by 58.3, 27.3, and 117.2%, respectively (Fig. 9B). In addition, the levels of seven neutral monosaccharides were also determined and compared between the wild-type and del1 plants. All the neutral monosaccharides in del1 exhibited significant increases, except for Xyl (Fig. 9C). These results suggest that the DEL1 mutation causes complex compositional and structural alterations in cell walls.
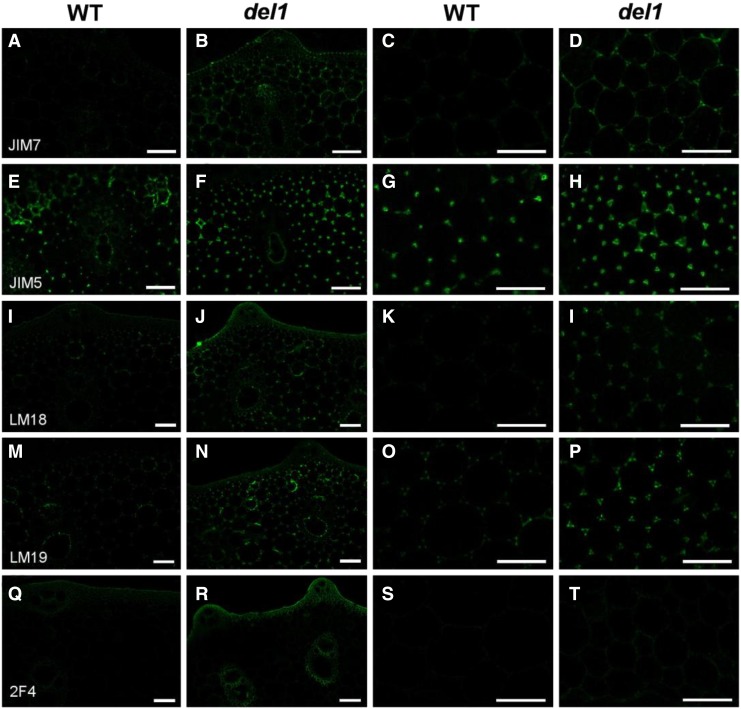
Mutation of DEL1 Increased the Degree of HG Methylesterification
Given that PEL can catalyze the α-1,4 glycosidic linkages of demethylated HG by β-elimination, we used immunohistochemical techniques to discern in situ aspects of cell wall microstructures and locate polymers precisely. The second culms from wild-type and del1 plants were probed using JIM5, LM18, and LM19 antibodies, which are used to recognize partially demethylesterified and unesterified HG; JIM7 antibody, which labels moderately high extent of methylesterified HG; and 2F4 antibody, which binds to HG with degrees of methylesterification up to 40% (Verhertbruggen et al., 2009; Held et al., 2011). As shown in Figure 10, immunolabeling of culm sections with JIM7, LM18, LM19, and 2F4 indicated an apparent increase in the recognition of HG epitopes, while JIM5 exhibited a slight increase, suggesting that the degree of methylesterified HG was higher in del1 plants.
Figure 10.
Immunohistochemical localization of HG in culm sections of wild-type and del1 plants. A to T, Immunolocalization of HG of wild-type (A and C) and del1 plants (B and D) with JIM7, wild-type (E and G) and del1 plants (F and H) with JIM5, wild-type (I and K) and del1 plants (J and L) with LM18, wild-type (M and O) and del1 plants (N and P) with LM19, and wild-type (Q and S) and del1 plants (R and T) with 2F4. Scale bar = 50 μm.
Activation of Cell Wall- and Senescence-Related Genes Revealed by Transcriptome Analysis
To further unravel the function of DEL1 in rice, 10-d-old wild-type and del1 plants were used for transcriptome analysis. A total of 491 differentially expressed genes (DEGs) were found with a P value < 0.05 (Supplemental Table S3). Among these genes, 332 were up-regulated and 159 were down-regulated in del1 plants (Supplemental Table S3). Clusters of orthologous groups of proteins functional catalog showed that in DEGs were assigned to 39.2% and 50.9% of the up- and down-regulated genes, respectively (Supplemental Table S3). Most of the genes up-regulated in del1 were involved in secondary metabolite biosynthesis, transport, and catabolism, carbohydrate transport and metabolism, and lipid transport and metabolism (Supplemental Table S3). The majority of the down-regulated genes were associated with translation, ribosomal structure and biogenesis, secondary metabolites biosynthesis, transport, and catabolism, and cell wall/membrane/envelope biogenesis (Supplemental Table S3). Of note, a set of the DEGs associated with cell wall and senescence were significantly altered in del1 plants, such as the PME gene (OsPME1), β-expansin gene (OsEXPB2), peroxidase precursor gene (OsPOX1), protochlorophyllide oxidoreductase B gene (OsPORA), etc. (Kim et al., 2012; Sakuraba et al., 2013; Zou et al., 2015; Fang et al., 2016; Supplemental Table S4). These results suggested that the mutation of DEL1 might affect the expression of cell wall- and senescence-related genes in an indirect manner.
DISCUSSION
PEL is an endogenous pectin-degrading enzyme that is capable of cleaving α-1,4-glycosidic linkages in demethylated pectin by β-elimination (Palusa et al., 2007). It is a ubiquitous enzyme in higher plants and is encoded by at least 26, 22, and 14 genes in Arabidopsis, poplar (Populus trichocarpa), and rice, respectively (Palusa et al., 2007). Although several PLL genes are considered to play potentially diverse physiological roles in plants, such as being expressed in anthers and pollen, and being involved in fruit softening and ripening, pathogen defense, and tissue/plant growth and development, their molecular mechanism in monocots remains largely unknown. Here, we identify DEL1 as a PEL precursor in model monocot rice using a forward genetic approach; it contains a PelC domain and represents a multigene family in rice. DEL1 is crucial for plant growth and leaf senescence. The loss of function of DEL1 reduced the total PEL enzymatic activity, increased the degree of methylesterified HG, and perturbed the cell wall structure and composition, resulting in retardation of the cell cycle and enhancement of ROS activity. These results reveal a dual molecular function of PLL genes in rice.
DEL1 Impacts Plant Growth by Regulating Cell Cycle Progression
Cell division/expansion is a fundamental, dynamic cellular process driving plant growth and development, and it enables the plant and various organs to develop to suitable sizes (Duan et al., 2012). The orderly development process involves many genes and pathways that affect plant organ size by altering cell number, cell size, or both (Krizek, 2009). Although recent studies have uncovered some key regulators and genes that affect plant organ size, the intrinsic mechanisms responsible for organ size variation are yet to be fully understood (Krizek, 2009; Duan et al., 2012). In this study, we identified a PLL gene, DEL1, that appeared to play an important role in the control of organ size in rice. In del1 plants, various organs were dramatically reduced in size, including in terms of root length, leaf size, plant height, grain size, and panicle and internode length (Fig. 1; Supplemental Table 1). Inside the organs, paraffinized sections of internodes revealed that cell number was lower in the del1 plants (Fig. 2), suggesting that cell division is inhibited in the mutant.
Cell division requires a range of complicated processes that must be strictly executed in a spatially and temporally controlled manner (Dewitte and Murray, 2003). The inhibition of cell division could alter cell cycle progression and affect plant development (Dewitte and Murray, 2003). Our study demonstrates that the cell cycle in the G1 phase was significantly retarded in del1 plants (Fig. 3, A–C). Recent evidence has indicated that a variety of cell cycle regulators play roles as targets coupling cell proliferation with development, and their proper functioning is crucial for patterning (Ramirez-Parra et al., 2005). Consistent with the flow cytometric results, the expression levels of genes regulating the G1 phase of the cell cycle were down-regulated in del1, and no significant difference was observed in the G2 phase (Fig. 3D). These results strongly suggest that DEL1 controls plant growth by regulating cell cycle progression.
DEL1 Contributes to Early Leaf Senescence through ROS Accumulation
Leaf senescence is a complex process that involves many highly organized molecular and cellular changes, such as the disintegration of chloroplasts; down-regulation of photosynthesis; degradation of nucleic acids, proteins, and lipids; and recycling of nutrients (Lim et al., 2007). This process is controlled by both internal and external factors. To date, although many SAGs and/or transcription factors have been identified, the complex mechanisms responsible for leaf senescence in rice remain poorly understood. Recently, Wu et al. (2013) identified the novel leaf senescence gene OsPSE1, which encodes a PEL. Mutation of OsPSE1 displays a premature senescence phenotype. In our study, we identified a PLL gene, DEL1, which also appears to play a general role in leaf senescence. TEM observation, physiological analysis, and TUNEL assays showed that leaf senescence was induced in del1 plants, resulting in the withering and cracking of leaves (Fig. 4). Moreover, the up-regulated expression levels of SAGs and transcription factors also demonstrate leaf senescence in del1 plants (Fig. 4I).
ROS are byproducts of various metabolic processes such as photosynthesis and respiration in chloroplasts, mitochondria, and peroxisomes (Apel and Hirt, 2004). They can cause oxidative damage to thylakoid membranes and other cellular components and assume several important roles in leaf senescence (Wang et al., 2015). In the present study, staining by NBT and DAB indicated the accumulation of O2− and H2O2 in del1 plants (Fig. 5, A and B), and there was also an elevated level of electrolyte leakage, which is a marker of cell membrane damage (Fig. 5C). Meanwhile, the activities of SOD and POD were increased in del1 plants (Fig. 5, D and E). These results indicate that leaf senescence in del1 plants is triggered by the accumulation of ROS. Under normal physiological conditions, cells control ROS levels by balancing the generation of ROS with their elimination by the ROS scavenging system, but the overproduction of ROS can trigger retrograde signaling from chloroplasts to the nucleus, resulting in alteration of the expression of nuclear-encoded genes (Tan et al., 2014). Consistent with this, the expression of ROS-scavenging genes was significantly up-regulated (Fig. 5F). In addition, transcriptome analysis also revealed that a set of the DEGs associated with senescence was significantly altered in del1 plants (Supplemental Table S4).
Taking these findings together, it is clear that ROS accumulation-mediated chloroplast membrane breakage and chloroplast degradation play an important role in leaf senescence in the del1 plants.
Possible Molecular Mechanism of DEL1 Responsible for the Mutant Phenotypes
PLL genes have been shown to exhibit a broad range of functions in plants, which depend on the degradation of demethylesterified HG and alteration of cell wall composition and structure (Yadav et al., 2009; Biswal et al., 2014). In the process of fruit ripening, the cell wall needs to loosen the xyloglucan-cellulose network and pectin solubilization, this processes increasing the access of degradative enzymes to their substrates (Brummell, 2006). For pathogen defense, the accumulation of pectin increases hydrogen bonding in the extracellular matrix, resulting in decreased nutrient availability to the pathogen (Vogel et al., 2002). The decrease of de-esterified pectin in cotton fiber can promote its elongation (Wang et al., 2010). Our study also demonstrates that the mutant phenotype of del1 depends on the alteration of cell wall composition and structure. Despite our failure to purify the fusion DEL1 protein via prokaryotic expression in Escherichia coli or transient expression in Nicotiana benthamiana (data not shown), the mutant phenotype indicated that DEL1 protein plays an important role in rice growth and leaf senescence.
Many cell wall-related mutants display abnormal growth and morphogenesis (Pien et al., 2001). It has been speculated that cell wall biogenesis and modification are tightly associated with cell growth via key genes at the interface of morphogenesis, the cell cycle, and cell wall biogenesis (Somerville et al., 2004; Zhang et al., 2010). In rice, the Brittle Culm 12 gene, encoding a kinesin-4 protein, has been implicated in cell cycle progression, cellulose microfibril deposition, and wall composition (Zhang et al., 2010). In our study, the del1 mutant exhibited cell wall abnormalities and the retardation of cell cycle progression, which indicates that DEL1 is at the interface between the cell cycle and cell wall biogenesis.
The dynamic interactions of plant cells depending on the status of pectin in the cell wall form an important regulatory mechanism of growth and development (Wolf et al., 2012). The degree of HG methylesterification has a large effect on the physical properties of the cell wall (Held et al., 2011). A high degree of HG methylesterification impedes the formation of calcium-mediated cross linking complexes, which is thought to promote cellular expansion by increasing wall flexibility (Wolf et al., 2009). Our findings revealed that the degree of methylesterified HG was increased, as determined by immunohistochemical assay, which indicates that the cell wall loosened and expanded in the del1 plants (Fig. 10). Consistent with this, the cell length was significantly increased and the expression levels of expansion were all dramatically up-regulated in the del1 plants (Fig. 2, G and H; Supplemental Fig. S8). These results indicate that the function of DEL1 affects not only cell number, but also cell expansion.
In addition, our findings also revealed an early leaf senescence phenotype in comparison to that in wild-type plants. This initially appears paradoxical, but it could potentially be explained by the alterations of cell wall structure. Pectin OGs are oligomers of α-1,4-linked galacturonosyl residues and are released by PEL- and PG-mediated breakdown of HG (Côté et al., 1998). Oligogalacturonides can produce ROS signal molecules and trigger many abiotic stress responses in plants (Ferrari et al., 2013). In our study, mutation in DEL1 significantly increased ROS content. We speculate that DEL1 may participate in the OG-mediated ROS pathway that affects leaf senescence.
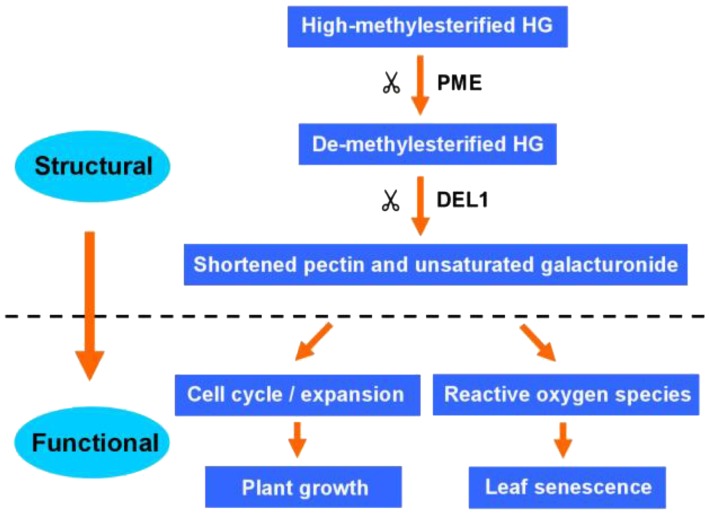
We hypothesize a conceptual model that by tuning HG methylesterification, cell wall component and structure might act as downstream components of the regulatory networks that control cell cycle and expansion, as well as ROS, enabling normal growth and leaf senescence in rice (Fig. 11).
Figure 11.
A schematic model of DEL1 function in rice. Homogalacturonan is secreted in a highly methylesterified form and selectively demethylesterified by PME. The demethylesterified HG might be cleaved by DEL1 and other PELs or PGs. The alternative of the cell wall regulated the cell cycle/expansion and ROS, enabling normal rice growth and leaf senescence process.
MATERIALS AND METHODS
Plant Materials
The rice (Oryza sativa) del1 mutant was isolated from ethyl methanesulfonate-treated japonica cultivar Nipponbare, as described previously (Guo et al., 2006). An F2 mapping population was generated from a cross between del1 and an indica cultivar, Taichung Native 1 (TN1). All plants were cultivated in paddies in Hangzhou (HZ, 119°54′ E, 30°04′ N) and Hainan (HN, 110°00′ E, 18°31′ N), during rice-growing seasons.
Paraffin Sectioning and TEM Analysis
For paraffin sectioning, the samples were fixed in 50% ethanol, 0.9 m glacial acetic acid, and 3.7% formaldehyde overnight at 4°C, then dehydrated with a graded series of ethanol, infiltrated with xylene, and embedded in paraffin (Sigma-Aldrich). The specimens were sectioned (8 μm thickness) with a Leica RM2245, transferred onto glass slides with poly-l-lysine-coated, deparaffinized with xylene series, and dehydrated through an ethanol series (Ren et al., 2016). The sample section was conducted using a Nikon Eclipse 90i microscope.
For TEM, the samples were fixed in 2.5% glutaraldehyde in PBS for at least 4 h and washed in PBS three times. Then, they were postfixed with 1% (w/v) OsO4 for 2 h after extensive washing in PBS, dehydrated with a graded ethanol series, and infiltrated with Suprr Kit (Sigma-Aldrich). The specimens were then sectioned (70 nm ultrathin) with a Leica EM UC7 ultratome, and the sections were stained by uranyl acetate and alkaline lead citrate for 10 min before being observed by TEM with a Hitachi model H-7650.
Flow Cytometric Analysis
Cell cycle analysis was performed in accordance with the method described by Galbraith et al. (1983). In brief, suspension cells were cut using a razor blade and placed in ice-cold Galbraith’s buffer (45 mm MgCl2, 30 mm sodium citrate, 20 mm MOPS, and 0.1% Triton X-100, pH 7.0). The homogenates were then passed through a 20-µm mesh to remove cellular debris. After staining with 4′,6-diamino-phenylindole (DAPI; 2 µg mL−1), the nuclei were analyzed using FACSAria II flow cytometer (BD Biosciences). A total of 30,000 events were recorded, and three independent flow cytometric experiments were carried out and analyzed using the ModFit LT (Verity Software House) and FlowJo V10 software (Tree Star).
Chlorophyll Content and Net Photosynthesis Rate
A total 0.2 g of samples from wild-type and del1 leaves were extracted with 80% acetone. The chlorophyll content was determined according to the method previously described (Yang et al., 2016). Net photosynthesis rate was measured using a Walz GFS-3000 (Eichenring). Parameter settings were accordance with the manufacturer’s instructions.
Histochemical Staining and ROS-Scavenging Enzyme Assays
DAB and NBT staining was used to detect the accumulation of ROS, as previously described (Blum and Ebercon, 1981). Trypan blue staining was performed as described previously (Koch and Slusarenko, 1990). Electrolyte leakage was determined in accordance with a previous study (Zhou and Guo, 2009). Assays of the activities of SOD and POD were conducted in accordance with previously described methods (Wang et al., 2013).
TUNEL Assays
The TUNEL assays were performed as described previously (Huang et al., 2007). The leaves were fixed in 4% paraformaldehyde in 0.1 m PBS containing 0.1% Tween 20 and Triton X-100 at 4°C overnight and embedded in paraplasts. The sections of leaves (8 μm thickness) were treated using a Fluorescein In Situ Cell Death Detection Kit (Roche). The green fluorescence of fluorescein and blue fluorescence of DAPI was analyzed using a Carl Zeiss LSM 710 laser-scanning confocal microscope (Gottingen).
Gene Cloning and Complementation
For genetic analysis, 1081 F2 mutant plants were used for fine mapping of the DEL1 locus. The molecular markers of polymorphism are listed in Supplemental Table S5. The markers were designed using Primer 5 software (Primer-E). Gene prediction and sequence analysis were performed using the publicly available rice databases, including Gramene (http://www.gramene.org) and the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/index.shtml). For the complementation construct, a 7,843-bp genomic DNA fragment containing the entire DEL1 coding region, a 1,998-bp upstream sequence, and an 841-bp downstream region was amplified using KOD FX (Toyobo) from Nipponbare genomic DNA and inserted into the binary vector pCAMBIA 1300. The recombinant binary vector was introduced into Agrobacterium tumefaciens strain EHA105 by electroporation and transformed into the del1 mutant callus as described previously (Hiei et al., 1994). For the RNAi construct, a 336-bp fragment was amplified by PCR from Nipponbare cDNA and inserted into the vector pTCK303. The recombinant binary vector was transformed into the Nipponbare callus. The primer sequences used in this study are listed in Supplemental Table S5.
Phylogenetic Analysis and Protein Domain Identification
Annotated proteins of DEL1 domains were identified in the Pfam database (http://pfam.xfam.org/). The signal peptide was predicted using SignalP version 4.1 (Petersen et al., 2011). Homologous protein sequences of DEL1 were identified using the NCBI BLAST server (http://www.ncbi.nlm.nih.gov/). Amino acid sequence alignments were conducted using ClustalX 2.1 (http://www.clustal.org/) and DNAMAN version 5.0 (Lynnon Biosoft). A phylogenetic tree was constructed using MEGA4 software (http://www.megasoftware.net/).
Gene Expression Analysis
Total RNA from roots, culms, sheaths, leaves and panicles at young and mature stages was extracted using the RNeasy plant mini kit (Qiagen). RNA reverse transcription was performed using ReverTra Ace real-time PCR-RT kit with gDNA remover (Toyobo). After synthesis, the cDNA reaction was diluted five times in TE buffer, and 1 μL was used for real-time RT-PCR using the SYBR Green PCR Master Mix kit (Applied Biosystems) and gene-specific primers (Supplemental Table S5) in ABI7900 (Applied Biosystems). The tissue-level expression pattern of DEL1 was analyzed using the GUS reporter gene. The promoter of DEL1 (2,038 bp upstream of ATG) was amplified from the genome DNA of Nipponbare and inserted into the binary vector pCAMBIA1305 in-frame with the GUS reporter gene. The binary vector was introduced into the Nipponbare callus to generate transgenic plants. The positive transgenic plants were used to analyze the GUS reporter gene. Different tissues of T1 plants were incubated at 37°C in 0.1 m X-Gluc in buffer [0.1 m Na2HPO4-NaH2P4, pH 7.0, 10% Triton X-100, 0.5 m EDTA-Na, and 50 mm K3Fe(CN)6] for ∼12 h to examine GUS activity. After being cleaning in 70% ethanol, the tissues were photographed under a light microscope.
Enzyme Activity Assays
PEL enzyme activity assays were routinely performed using a modified version of the method of Collmer et al. (1988). A total of 0.5 g of sample was suspended in 1.5 mL of extraction buffer (20 mm sodium phosphate, pH 7.0, 20 mm Cys/HCl, 1% polyvinylpyrrolidone, MW 360,000, and 1 mm phenylmethylsulfonyl fluoride) and centrifuged at 10,000g for 30 min. The supernatant was collected as the crude extract for enzyme assays.
The enzyme activity system consisted of 0.3% polygalacturonic acid in 0.07 m Tris-HCl buffer (pH 8.0), 0.1 m CaCl2, crude extract protein, and sterile water. The enzyme reaction was incubated at 37°C for 30 min, and then stopped by the addition of 9% ZnSO4⋅7H2O and 0.5 m NaOH. The control tubes received the enzyme after the addition of ZnSO4⋅7H2O and NaOH. One unit of PEL activity was defined as the amount of enzyme that produces 1 mm of 4,5-unsaturated product in 1 min under the assay conditions. The activity of PEL is expressed in units per mg of protein. Soluble protein concentrations were determined in accordance with Bradford method (Bradford, 1976), using bovine serum albumin as a standard.
Immunofluorescence Microscopy
The culms of wild-type and del1 plant at the same development stage were fixed and embedded in glycol methacrylate. Two-micrometer-thick sections were cut with a Leica RM2265. Immunolabeling was performed in accordance with the methodology of Willats et al. (2001). The primary antibodies JIM5, JIM7, LM18, LM19, and 2F4 were used at a 1:10 dilution in PBS containing 5% (w/v) fat-free milk powder (5% M/PBS) and the secondary antibody (goat anti-rat IgG coupled with fluorescein isothiocyanate) were used at dilution of 100-fold in 5% M/PBS. Sections incubated without primary antibody were used as controls to assess the autofluorescence of the samples. The labeled sections were observed using Carl Zeiss LSM 710 confocal microscope.
Compositional Analysis of Neutral Glycosyl Residues and Cellulose Content
Alcohol-insoluble residues of culms were prepared as described by Zhang et al. (2012). In brief, destarched alcohol-insoluble residues samples were hydrolyzed in 2 m trifluoroacetic acid at 121°C for 90 min. The samples were centrifuged to collect the supernatants, air-dried, and then dissolved with a 1 m ammonium hydroxide buffer containing NaBH4. The alditol acetate derivatives were analyzed using an Agilent 7890 GC system equipped with a 5975C MSD (gas chromatography-mass spectrometry; Song et al., 2013). To measure the crystalline cellulose content, the pellets remaining after trifluoroacetic acid treatment were hydrolyzed with Updegraff reagent (Updegraff, 1969). The samples were subsequently centrifuged and the supernatant removed. The pellets were then treated with 72% sulfuric acid. The cellulose content was quantified via an anthrone assay, and three replicates were included.
Cell Wall Extraction and Measurement of Polysaccharide Content
Cell wall materials and fractionation components were analyzed in accordance with the methods of Zhong and Lauchli (1993). The samples were ground in liquid nitrogen to a fine power and suspended in 75% ethanol for 25 min in an ice-cold water bath. The samples were then centrifuged at 12,000 rpm for 15 min and removed the supernatant. Next, the pellet was suspended and washed with precooled acetone, followed by methanol:chloroform and then with methanol. The remaining pellet as a crude cell wall fraction was freeze-dried and stored at 4°C for future use.
The cell wall extracts were fractionated into three parts: pectin, HC 1, and HC 2. First, the pectin fraction was extracted twice using 0.5% ammonium oxalate buffer containing 0.1% NaBH4 (pH 4) in a boiling water bath for 1 h. Next, the pellets were subjected to triple extractions with 4% KOH containing 0.1% NaBH4 at room temperature for 8 h each, followed by a similar extraction with 24% KOH containing 0.1% NaBH4. The HC 1 and HC 2 fractions were referred to as hemicellulose material (Yang et al., 2008). The uronic acid content in the pectin fraction was assayed in accordance with the method of Blumenkrantz and Asboe-Hansen (1973) using GalUA (GalA) as a standard.
Transcriptome Sequencing and Data Analysis
The RNA samples from 10-d-old wild-type and del1 plants were used for transcriptome sequencing, and each sample was pooled for total RNA isolation with three biological replicates. Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies). Transcriptome sequencing was performed in Biomarker Biotechnology Corporation using the Illumina system HiSeq2500 (Illumina) according to the standard procedure. Transcriptome assembly was performed according to the protocol described previously (Yu et al., 2013). Differentially expressed genes were defined by using IDEG6 (Romualdi et al., 2003), at a significance level of P < 0.05. Functional annotation analysis of DEGs in wild-type and del1 plants was performed by DAVID Web tools (Huang et al., 2009).
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: DEL1 (LOC_Os10g31910), PMR6 (At3g54920), NJJS25 (AF339024), PtxtPL1-27 (EU379971), ZePel (Y09541), MaPel (AAF19195), GhPel (ADB90478), CryjI (BAA05542), SGR (LOC_Os09g36200), OsNAC2 (LOC_Os01g66120), Osl2 (LOC_Os04g52450), OsH36 (LOC_Os05g39770), OsWRKY23 (LOC_Os01g53260), OsWRKY72 (LOC_Os11g29870), CDKA1 (LOC_Os03g02680), CAK1 (LOC_Os06g07480), CAK1A (LOC_Os06g22820), CMC5 (LOC_Os02g55410), CYCT1 (LOC_Os02g24190), CYCA2.2 (LOC_Os12g31810), CYCA2.3 (LOC_Os01g13260), CYCB2.2 (LOC_Os06g51110), AOX1a (LOC_Os04g51150), AOX1b (LOC_Os04g51160), APX1 (LOC_Os03g17690), APX2 (LOC_Os07g49400), SODB (LOC_Os06g05110), SODA1 (LOC_Os05g25850), catA (LOC_Os02g02400), catB (LOC_Os06g51150), OsEXPA1 (LOC_Os04g15840), OsEXPA2 (LOC_Os01g60770), OsEXPA5 (LOC_Os02g51040), OsEXPA10 (LOC_Os04g49410), OsEXPA32 (LOC_Os08g44790), OsEXPB3 (LOC_Os10g40720), OsEXPB5 (LOC_Os04g46650), OsEXPB9 (LOC_Os10g40090), and OsEXPB11 (LOC_Os02g44108).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Wild-type (Nipponbare, left) and del1 plants (right) at the tillering stage.
Supplemental Figure S2. Statistical analysis of plant height from 30 to 110 d between wild-type and del1 plants.
Supplemental Figure S3. Internode assessment of culms in the wild-type and del1 plants.
Supplemental Figure S4. Mutation position of wild type, del1, and six other varieties.
Supplemental Figure S5. The phenotype of wild-type and RNAi transgenic plants.
Supplemental Figure S6. Protein sequence alignment of DEL1 and homologs.
Supplemental Figure S7. Cell wall structure in wild-type and del1 roots
Supplemental Figure S8. Expression analysis of cell expansion-related genes in culms.
Supplemental Table S1. The basic agronomic traits in wild-type and del1 plants.
Supplemental Table S2. Segregation analysis of F2 phenotype from crosses of del1 and five crop strains (NPB, TN1, ZF802, NJ06, and 93-11).
Supplemental Table S3. Clusters of orthologous groups of proteins functional catalog of differentially expressed genes in del1 plants
Supplemental Table S4. Cell wall function and senescence related genes identified by RNA-seq analysis as being differentially in del1 plants.
Supplemental Table S5. Primers used for PCR in this study.
Supplementary Material
Acknowledgments
We are grateful to Dr. Yihua Zhou (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for help with the monosaccharide and cellulose analyses, Dr. Yong Hu (Capital Normal University) for help with the flow cytometric analysis, and Dr. Paul Knox (Centre for Plant Science, University of Leeds, UK) for kindly providing the protocol for the immunofluorescence assay.
Glossary
- HG
homogalacturonan
- OG
oligogalacturonide
- TEM
transmission electron microscopy
- SAG
senescence-associated gene
- DEG
differentially expressed gene
Footnotes
Articles can be viewed without a subscription.
This research was supported by the National Natural Science Foundation of China (Grant No. 91435105, 3161143006, 91535205), National Key Basic Research Program (grant no. 2013CBA014), and the “Science and technology innovation project” of the Chinese Academy of Agriculture Sciences.
These authors contributed equally to the article.
References
- Anderson CT, Carroll A, Akhmetova L, Somerville C (2010) Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol 152: 787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 [DOI] [PubMed] [Google Scholar]
- Barras F, Gijsegem FV, Chatterjee AK (1994) Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu Rev Phytopathol 32: 201–234 [Google Scholar]
- Biswal AK, Soeno K, Gandla ML, Immerzeel P, Pattathil S, Lucenius J, Serimaa R, Hahn MG, Moritz T, Jönsson LJ, et al. (2014) Aspen pectate lyase PtxtPL1-27 mobilizes matrix polysaccharides from woody tissues and improves saccharification yield. Biotechnol Biofuels 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21: 43–47 [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Bonnin E, Garnier C, Ralet MC (2014) Pectin-modifying enzymes and pectin-derived materials: Applications and impacts. Appl Microbiol Biotechnol 98: 519–532 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brummell DA. (2006) Cell wall disassembly in ripening fruit. Funct Plant Biol 33: 103–119 [DOI] [PubMed] [Google Scholar]
- Collmer A, Ried JL, Mount MS (1988) Assay methods for pectic enzymes. In Wood WA, Kellogg ST, eds, Methods in Enzymology. Academic Press, San Diego, CA, pp 329–335 [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Côté F, Ham KS, Hahn MG, Bergmann CW (1998) Oligosaccharide elicitors in host-pathogen interactions. Generation, perception, and signal transduction. Subcell Biochem 29: 385–432 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JAH (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Dick-Pérez M, Zhang Y, Hayes J, Salazar A, Zabotina OA, Hong M (2011) Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50: 989–1000 [DOI] [PubMed] [Google Scholar]
- Domingo C, Roberts K, Stacey NJ, Connerton I, Ruíz-Teran F, McCann MC (1998) A pectate lyase from Zinnia elegans is auxin inducible. Plant J 13: 17–28 [DOI] [PubMed] [Google Scholar]
- Dominguez-Puigjaner E, LLop I, Vendrell M, Prat S (1997) A cDNA clone highly expressed in ripe banana fruit shows homology to pectate lyases. Plant Physiol 114: 1071–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, Li S, Chen Z, Zheng L, Diao Z, Zhou Y, Lan T, Guan H, Pan R, Xue Y, et al. (2012) Dwarf and deformed flower 1, encoding an F-box protein, is critical for vegetative and floral development in rice (Oryza sativa L.). Plant J 72: 829–842 [DOI] [PubMed] [Google Scholar]
- Fang C, Zhang H, Wan J, Wu Y, Li K, Jin C, Chen W, Wang S, Wang W, Zhang H, et al. (2016) Control of leaf senescence by an MeOH-Jasmonates cascade that is epigenetically regulated by OsSRT1 in rice. Mol Plant 9: 1366–1378 [DOI] [PubMed] [Google Scholar]
- Ferrari S, Savatin DV, Sicilia F, Gramegna G, Cervone F, Lorenzo GD (2013) Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front Plant Sci 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220: 1049–1051 [DOI] [PubMed] [Google Scholar]
- Guo LB, Chu CC, Qian Q (2006) Rice mutants and functional genomics. Chin Bull Bot 23: 1–13 [Google Scholar]
- Held MA, Be E, Zemelis S, Withers S, Wilkerson C, Brandizzi F (2011) CGR3: A Golgi-localized protein influencing homogalacturonan methylesterification. Mol Plant 4: 832–844 [DOI] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6: 271–282 [DOI] [PubMed] [Google Scholar]
- Hongo S, Sato K, Yokoyama R, Nishitani K (2012) Demethylesterification of the primary wall by PECTIN METHYLESTERASE35 provides mechanical support to the Arabidopsis stem. Plant Cell 24: 2624–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Huang L, Sun Q, Qin F, Li C, Zhao Y, Zhou DX (2007) Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol 144: 1508–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99: 16319–16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Chopra R. (2012) Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 249: 469–481 [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi HS, Cho YC, Kim SR (2012) Cold-responsive regulation of a flower-preferential class III peroxidase gene, OsPOX1, in rice (Oryza sativa L.). J Plant Biol 55: 123–131 [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA. (2009) Making bigger plants: Key regulators of final organ size. Curr Opin Plant Biol 12: 17–22 [DOI] [PubMed] [Google Scholar]
- Kulikauskas R, McCormick S (1997) Identification of the tobacco and Arabidopsis homologues of the pollen-expressed LAT59 gene of tomato. Plant Mol Biol 34: 809–814 [DOI] [PubMed] [Google Scholar]
- Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R (2006) Expression profiling of auxin-treated Arabidopsis roots: Toward a molecular analysis of lateral root emergence. Plant Cell Physiol 47: 788–792 [DOI] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58: 115–136 [DOI] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ (1995) Expansin mode of action on cell walls. Analysis of wall hydrolysis, stress relaxation, and binding. Plant Physiol 107: 87–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Escobar N, Cárdenas J, Moyano E, Caballero JL, Muñoz-Blanco J (1997) Cloning, molecular characterization and expression pattern of a strawberry ripening-specific cDNA with sequence homology to pectate lyase from higher plants. Plant Mol Biol 34: 867–877 [DOI] [PubMed] [Google Scholar]
- Milioni D, Sado PE, Stacey NJ, Domingo C, Roberts K, McCann MC (2001) Differential expression of cell-wall-related genes during the formation of tracheary elements in the Zinnia mesophyll cell system. Plant Mol Biol 47: 221–238 [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33: 453–467 [DOI] [PubMed] [Google Scholar]
- Nooden LD, Leopold AC (1978) Phytohormones and the endogenous regulation of senescence and abscission. In Letham DS, Goodwin PB, Higgins TJV, eds, Phytohormones and Related Compounds: A Comprehensive Treatise. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands, 329–369. [Google Scholar]
- Nunan KJ, Davies C, Robinson SP, Fincher GB (2001) Expression patterns of cell wall-modifying enzymes during grape berry development. Planta 214: 257–264 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S, Swain SM (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21: 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palusa SG, Golovkin M, Shin SB, Richardson DN, Reddy ASN (2007) Organ-specific, developmental, hormonal and stress regulation of expression of putative pectate lyase genes in Arabidopsis. New Phytol 174: 537–550 [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ (2012) A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol 158: 1933–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook SA, Le Guillou L, Bron E, Kuhlemeier C, Höfte H (2011) Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr Biol 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat Methods 8: 785–786 [DOI] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98: 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua EC, Ong CK, Liu P, Liu JZ (2001) Isolation and expression of two pectate lyase genes during fruit ripening of banana (Musa acuminata). Physiol Plant 113: 92–99 [Google Scholar]
- Ramirez-Parra E, Desvoyes B, Gutierrez C (2005) Balance between cell division and differentiation during plant development. Int J Dev Biol 49: 467–477 [DOI] [PubMed] [Google Scholar]
- Ren D, Rao Y, Leng Y, Li Z, Xu Q, Wu L, Qiu Z, Xue D, Zeng D, Hu J, et al. (2016) Regulatory role of OsMADS34 in the determination of glumes fate, grain yield and quality in rice. Front Plant Sci 7: 1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Rogers HJ, Harvey A, Lonsdale DM (1992) Isolation and characterization of a tobacco gene with homology to pectate lyase which is specifically expressed during microsporogenesis. Plant Mol Biol 20: 493–502 [DOI] [PubMed] [Google Scholar]
- Romualdi C, Bortoluzzi S, D’Alessi F, Danieli GA (2003) IDEG6: A web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol Genomics 12: 159–162 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Rahman ML, Cho SH, Kim YS, Koh HJ, Yoo SC, Paek NC (2013) The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J 74: 122–133 [DOI] [PubMed] [Google Scholar]
- Simeonova E, Sikora A, Charzyńska M, Mostowska A (2000) Aspects of programmed cell death during leaf senescence of mono- and dicotyledonous plants. Protoplasma 214: 93–101 [Google Scholar]
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. (2004) Toward a systems approach to understanding plant cell walls. Science 306: 2206–2211 [DOI] [PubMed] [Google Scholar]
- Song XQ, Liu LF, Jiang YJ, Zhang BC, Gao YP, Liu XL, Lin QS, Ling HQ, Zhou YH (2013) Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Mol Plant 6: 768–780 [DOI] [PubMed] [Google Scholar]
- Sun L, van Nocker S (2010) Analysis of promoter activity of members of the PECTATE LYASE-LIKE (PLL) gene family in cell separation in Arabidopsis. BMC Plant Biol 10: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Eberhard S, Pattathil S, Warder C, Glushka J, Yuan C, Hao Z, Zhu X, Avci U, Miller JS, et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Tan Z, Wu F, Sheng P, Heng Y, Wang X, Ren Y, Wang J, Guo X, Zhang X, et al. (2014) A novel chloroplast-localized pentatricopeptide repeat protein involved in splicing affects chloroplast development and abiotic stress response in rice. Mol Plant 7: 1329–1349 [DOI] [PubMed] [Google Scholar]
- Updegraff DM. (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32: 420–424 [DOI] [PubMed] [Google Scholar]
- Van Sandt VS, Suslov D, Verbelen JP, Vissenberg K (2007) Xyloglucan endotransglucosylase activity loosens a plant cell wall. Ann Bot (Lond) 100: 1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP (2009) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr Res 344: 1858–1862 [DOI] [PubMed] [Google Scholar]
- Vincken JP, Schols HA, Oomen RJFJ, McCann MC, Ulvskov P, Voragen AGJ, Visser RGF (2003) If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol 132: 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14: 2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang G, Li Y, Ding M, Gong HY, Li YS (2013) Differential antioxidant responses to cold stress in cell suspension cultures of two subspecies of rice. Plant Cell Tissue Organ Cult 113: 353–361 [Google Scholar]
- Wang H, Guo Y, Lv F, Zhu H, Wu S, Jiang Y, Li F, Zhou B, Guo W, Zhang T (2010) The essential role of GhPEL gene, encoding a pectate lyase, in cell wall loosening by depolymerization of the de-esterified pectin during fiber elongation in cotton. Plant Mol Biol 72: 397–406 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Y, Hong X, Hu D, Liu C, Yang J, Li Y, Huang Y, Feng Y, Gong H, et al. (2015) Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice. J Exp Bot 66: 973–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Knox JP (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Wing RA, Yamaguchi J, Larabell SK, Ursin VM, McCormick S (1990) Molecular and genetic characterization of two pollen-expressed genes that have sequence similarity to pectate lyases of the plant pathogen Erwinia. Plant Mol Biol 14: 17–28 [DOI] [PubMed] [Google Scholar]
- Wolf S, Hématy K, Höfte H (2012) Growth control and cell wall signaling in plants. Annu Rev Plant Biol 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2: 851–860 [DOI] [PubMed] [Google Scholar]
- Wu Y, Qiu X, Du S, Erickson L (1996) PO149, a new member of pollen pectate lyase-like gene family from alfalfa. Plant Mol Biol 32: 1037–1042 [DOI] [PubMed] [Google Scholar]
- Wu HB, Wang B, Chen Y, Liu YG, Chen L (2013) Characterization and fine mapping of the rice premature senescence mutant ospse1. Theor Appl Genet 126: 1897–1907 [DOI] [PubMed] [Google Scholar]
- Xiao C, Somerville C, Anderson CT (2014) POLYGALACTURONASE INVOLVED IN EXPANSION1 functions in cell elongation and flower development in Arabidopsis. Plant Cell 26: 1018–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S, Yadav PK, Yadav D, Yadav KDS (2009) Pectin lyase: A review. Process Biochem 44: 1–10 [Google Scholar]
- Yang JL, Li YY, Zhang YJ, Zhang SS, Wu YR, Wu P, Zheng SJ (2008) Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol 146: 602–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xu J, Huang L, Leng Y, Dai L, Rao Y, Chen L, Wang Y, Tu Z, Hu J, et al. (2016) PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J Exp Bot 67: 1297–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Chen X, Wang Z, Wang S, Wang Y, Zhu Q, Li S, Xiang C (2013) Arabidopsis enhanced drought tolerance1/HOMEODOMAIN GLABROUS11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol 162: 1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Song XQ, Yu BS, Zhang BC, Sun CQ, Knox JP, Zhou YH (2012) Identification of quantitative trait loci affecting hemicellulose characteristics based on cell wall composition in a wild and cultivated rice species. Mol Plant 5: 162–175 [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang B, Qian Q, Yu Y, Li R, Zhang J, Liu X, Zeng D, Li J, Zhou Y (2010) Brittle Culm 12, a dual-targeting kinesin-4 protein, controls cell-cycle progression and wall properties in rice. Plant J 63: 312–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lauchli A (1993) Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J Exp Bot 44: 773–778 [Google Scholar]
- Zhou B, Guo Z (2009) Calcium is involved in the abscisic acid-induced ascorbate peroxidase, superoxide dismutase and chilling resistance in Stylosanthes guianensis. Biol Plant 53: 63–68 [Google Scholar]
- Zou HY, Wenwen YH, Zang GC, Kang ZH, Zhang ZY, Huang JL, Wang GX (2015) OsEXPB2, a β-expansin gene, is involved in rice root system architecture. Mol Breed 35: 41 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.