Abstract
A decade has passed since the discovery of stomatal defense, and the field has expanded considerably with significant understanding of the basic mechanisms underlying the process.
Plants and microbes have long coevolved in a constant battle to overcome the mechanisms of defense and attack from both sides. Plants have developed means to prevent pathogen attack by hampering invasion of plant tissues and actively warding off pathogen colonization. On the other hand, pathogens have evolved strategies to mask their presence and/or evade host defenses. Plant-microbe interaction starts with molecular recognition of each other, leading to a cascade of signaling events with the final output of plant resistance or susceptibility to the pathogen. In this molecular war, epidermis of plants is the first barrier that pathogens need to overtake. Natural openings on the leaf surface, such as stomata, provide an entry site to pathogens. Plants have evolved a mechanism to close stomata upon sensing microbe-associated molecular patterns (MAMPs). This mechanism is known as stomatal defense. A decade has passed since the discovery of stomatal defense, and the field has expanded considerably with significant understanding of the basic mechanisms underlying the process. Here, we give a perspective of these findings and their implications in the understanding of plant-microbe interactions.
It has been long recognized that infection of plants by foliar pathogens involves pathogen penetration into inner tissues, a niche conducive for living, where they obtain water and nutrients from internal cells. Routes for pathogen penetration into the leaves include stomatal pores, hydathodes, and wounds (either accidental or direct breaching of the cuticle by the pathogen or its vector). The vast majority of contemporary experiments designed to understand mechanisms of pathogenesis in plants has relied on inoculation by artificial wounding or direct infiltration of inocula into the apoplast. Although these are valuable approaches to dissect plant diseases, they preclude thorough understanding of a key step for the establishment of disease: pathogen internalization into the host plant. In the last decade, we came to realize how dynamic and complex this entry process is. It requires active, inducible responses on both the host and the pathogen, as well as specific environmental conditions at the time of penetration. Perhaps these strict requirements contribute to the fact that widespread diseases are rare events in nature.
A large number of pathogens use the stomatal pore as a site for penetration into inner leaf tissues. In fact, some pathogens, such as the bacterium Xanthomonas campestris pv armoraciae (Hugouvieux et al., 1998), the oomycete Plasmopara viticola (Allègre et al., 2007), and species of the fungus Puccinia (Shafiei et al., 2007; Grimmer et al., 2012) are specialized to internalize into leaves only through stomata. Earlier observations provided some clues that stomatal closure might diminish bacterial disease severity in a biologically relevant context. For instance, reduced number of lesions developed on dark- or abscisic acid (ABA)-treated tomato (Solanum lycopersicum) plants after inoculation with X. campestris pv vesicatoria (Ramos and Volin, 1987). Furthermore, direct comparison of disease severity after surface-inoculation and apoplastic-infiltration in this pathosystem revealed that bacterium penetration through stomata may be a control point for disease progression (Ramos and Volin, 1987). Characterization of either bacterial or plant mutants also provided indications for possible stomatal control of bacterial infection. The coronatine (COR)-deficient mutant of Pseudomonas syringae pv tomato (Pst) causes less disease when inoculated on the leaf surface than when inoculated directly into the apoplast of Arabidopsis (Arabidopsis thaliana) or tomato (Mittal and Davis, 1995). Similarly, the flagellin-sensitive2 (fls2) mutant of Arabidopsis, which lacks the receptor for bacterial flagellin, is more susceptible than the wild-type plant only when surface-inoculated with Pst DC3000 (Zipfel et al., 2004). These previous studies set the foundation for a direct demonstration that guard cells surrounding the stomatal pore can sense microbes and close the pore, a process that is now known as stomatal defense or stomatal immunity (Melotto et al., 2006; Sawinski et al., 2013).
A number of recent reviews have discussed topics ranging from signaling networks that regulates stomatal defense to mechanisms of endogenous and exogenous signal integration in guard cells (Arnaud and Hwang, 2015; Murata et al., 2015; Cotelle and Leonhardt, 2016; Lee et al., 2016). In addition, the use of thermoimaging technology in crop breeding programs and precision agriculture to assess pathogen-induced stomatal movement has been highlighted (Ishimwe et al., 2014; Singh et al., 2016). Furthermore, stomatal closure in response to fungus- and plant-derived elicitors (e.g. chitin, chitosan, and oligogalacturonic acid) and stomatal opening in response to fungal metabolites (e.g. fusicoccin) has been extensively reviewed (Grimmer et al., 2012; Arnaud and Hwang, 2015; Murata et al., 2015). We refer readers to these excellent reviews. Here, we focus on significant advances toward a mechanistic understanding of stomatal defense and the impact of this discovery on the study of plant-bacterial interactions.
BACTERIUM-TRIGGERED STOMATAL CLOSURE
Stomata open and close daily, reflecting the internal circadian rhythm of plants. However, bacteria can trigger stomatal closure under bright daylight (Melotto et al., 2006; Gudesblat et al., 2009; Schellenberg et al., 2010; Roy et al., 2013), suggesting that stomatal guard cells can perceive bacteria and trigger a signaling cascade that overrides the natural circadian rhythm of stomatal movement. Bacterium-triggered stomatal closure is a fast response (<1 h) and the basic mechanism underlying this process includes the following.
Recognition of Bacteria
Plant perception of bacteria begins with the recognition MAMPs by cognate pattern recognition receptors (PRRs). The most widely studied example of such recognition is flagellin perception by the FLS2 receptor in Arabidopsis. Although flagellin recognition has a prominent role in stomatal defense during the Arabidopsis-P. syringae pv tomato DC3000 interaction (Zeng and He, 2010), the existence of other MAMP-PRR pairs that function in stomatal defense is likely. For instance, stomata of the fls2 mutant still close in response to lipopolysaccharide and Escherichia coli O157:H7 (Melotto et al., 2006). However, tools to characterize the importance of other MAMP-PRR are not completely developed as in many cases either the MAMP or the PRR is not known. The l-type lectin receptor kinases have been implicated in Arabidopsis stomatal response to Pst (Desclos-Theveniau et al., 2012, Singh et al., 2012); however, the cognate ligand(s) have not been described. Thus, the contribution of this bacterial recognition system to stomatal defense cannot be fully assessed. Similarly, purified lipopolysaccharide from various bacterial strains trigger stomatal closure (Melotto et al., 2006), but the role of its potential cognate receptor LIPOPOLIGOSACCHARIDE-SPECIFIC REDUCED ELICITATION (Ranf et al., 2015) has not been described yet. Another characterized MAMP-PRR pair is the elongation factor Tu (EF-Tu) and EF-Tu RECEPTOR (Zipfel et al., 2006). Purified elf26, an EF-Tu-derived peptide, closes the stomatal pore in the Arabidopsis ecotypes Col-0 and Ws4 (Desikan et al., 2008); however, the EF-Tu receptor mutant efr-1 still retains the wild-type stomatal closure phenotype in response to P. syringae (Zeng and He, 2010). It is probably due to the response triggered by other MAMPs, such as flagellin. It has been noted that elf peptides from different bacteria differ in their potency in inducing stomatal closure. Zeng and He (2010) observed that elf18, an EF-Tu-derived peptide from E. coli, is more potent than that of Pst in closing the stomatal pore of Col-0. Although additional experimentation in a biologically relevant context is still needed, emerging evidence suggests that, in principle, Arabidopsis guard cells may respond to different bacterial species at various degrees in part depending on the natural variations of bacterial MAMPs.
Downstream Signaling
Since 2006, there have been extensive efforts by various groups to elucidate the signaling cascade that occurs downstream of bacterial recognition. This signaling cascade is largely mediated by (1) secondary messengers such as reactive oxygen species (ROS), nitric oxide (NO), and calcium; (2) regulators of innate immune response such as MPK3, MPK4, and MPK6; and (3) plant hormones. While ROS/NO production and [Ca2+]cyt oscillation have been documented in guard cells after MAMP recognition (Melotto et al., 2006; Desclos-Theveniau et al., 2012; Arnaud and Hwang, 2015), biosynthesis and accumulation of hormones in the guard cell have not been fully demonstrated due to technical impediments to directly quantify hormone concentration in this specialized cell type. Only recently, a fluorescence resonance energy transfer-based reporter system, ABAleons, has been developed in Arabidopsis that enables temporal and spatial mapping of ABA concentration changes in response to various cues (Waadt et al., 2014). The research community would really benefit if similar real-time, in planta reporter systems are available for other hormones that play a role in stomatal defense (see Outstanding Questions). Nonetheless, guard cells do respond to plant hormones. Pharmacological and genetic evidence supports that ABA and salicylic acid (SA) are positive regulators, while (+)-7-iso-jasmonoyl-l-Ile (JA-Ile) is a negative regulator of stomatal defense (see below).
ABA has long been recognized to induce stomatal closure under drought stress, thereby minimizing water loss through the leaves. However, the role of this hormone in Arabidopsis defense against P. syringae differs depending on the stage of infection. At the postinvasive stage of the disease, ABA enhances plant susceptibility via suppression of both callose deposition and SA-mediated plant resistance (de Torres-Zabala et al., 2007; Ton et al., 2009). However, at the preinvasion stage, ABA promotes resistance to bacterial infection as it favors stomatal defense (Inoue and Kinoshita, 2017; Eisenach and de Angeli, 2017; Vialet-Chabrand et al., 2017). For instance, purified MAMP and live Pst DC3000 do not induce stomatal closure in the ABA-deficient aba3-1 mutant (Melotto et al., 2006). Similarly, the notabilis mutant of tomato, which lacks a functional ABA biosynthesis enzyme, 9-cis-epoxycarotenoid dioxygenase, is also compromised in Pst DC3000-induced stomatal closure (Du et al., 2014). Furthermore, the core signaling components of the ABA pathway that lead to stomatal closure in Arabidopsis (Joshi-Saha et al., 2011) are involved in Pst-triggered stomatal closure. In particular, these components include (1) pyrabactin resistance1/pyrabactin resistance1-like/regulatory components of ABA receptors; (2) protein phosphatase 2CA; (c) OPEN STOMATA1 (OST1); (4) the ABA signaling-related secondary messengers ROS, NO, Ca2+, and G-protein α-subunit; and (5) the membrane channels SLOW ANION CHANNEL-ASSOCIATED1 (SLAC1) and K+ channels (Melotto et al., 2006; Zhang et al., 2008; Montillet et al., 2013; Lim et al., 2014; Guzel Deger et al., 2015; Sierla et al., 2016). Thus, current experimental evidence suggests a prominent role of ABA signaling in stomatal defense (Inoue and Kinoshita, 2017; Eisenach and de Angeli, 2017; Vialet-Chabrand et al., 2017). However, the specific step of ABA biosynthesis or signaling needed for stomatal defense is not clear. Montillet et al. (2013) suggested that flg22 and ABA converge at the SLAC1 level, while Guzel Deger et al. (2015) provided evidence that these pathways converge at the OST1 level that is upstream of SLAC1. An ABA-independent pathway in the early signaling leading to stomatal defense has also been proposed based on the fact that high concentration of flg22 (10 µm) closes the stomatal pore of ost-1 epidermal peels, and 100 nm flg22 does not activate OST1 on Arabidopsis cell suspension (Montillet et al., 2013; Montillet and Hirt, 2013). Now that the ABAleons reporter system is available (Waadt et al., 2014), the question as to whether ABA concentration changes in response to bacterial elicitors can be addressed at a single-cell resolution in planta (see Outstanding Questions).
The immune signal SA is also required for stomatal defense, as evidenced by the fact that ics1, eds5/sid1/scord3 (two SA synthesis mutants), and npr1 (an SA signaling mutant) are defective in stomatal defense (Melotto et al., 2006; Zeng and He, 2010; Zeng et al., 2011). Furthermore, the SA-responsive genes ICS1, EDS1, and PAD4 are induced in guard cells within 1 h after exposure to flg22 (Zheng et al., 2015). Direct measurement of SA concentration in guard cell is currently not possible due to current technical challenges. The two main factors that have prevented the success of this measurement are that (1) a large amount of isolated guard cells (> 150 mg) would be needed to quantify SA using the standard HPLC method (X.-y. Zheng and X. Dong, personal communication), and (2) SA synthesis seems to be rapidly induced in guard cells during stomatal defense (<1 h; Zheng et al., 2015). The current procedure to isolate guard cells cannot be completed in <2 h (Obulareddy et al., 2013). While it is clear that SA can induce stomatal closure, it is yet to be determined whether SA is produced in the guard cells per se or transported from other cell types during stomatal defense.
Several derivatives of jasmonates (JAs) are naturally present in plants (Staswick and Tiryaki, 2004), some of which are biologically active for regulating JA-associated biological responses (Thines et al., 2007; Chini et al., 2007; Yan et al., 2007). In particular, methylated JA (MeJA) has been used extensively to elucidate JA-dependent responses. Although some studies have provided evidence that MeJA closes the stomatal pore (Suhita et al., 2004; Munemasa et al., 2007; Desclos-Theveniau et al., 2012; Hua et al., 2012; Yan et al., 2015), this could not be verified by other research groups (Speth et al., 2009; Montillet et al., 2013; Savchenko et al., 2014). This inconsistency might be explained by the fact that an endogenous ABA threshold is needed for MeJA-induced stomatal closure (Hossain et al., 2011). It is possible that plant growth conditions used in these studies resulted in different basal ABA levels, which could influence the different stomatal responses observed by these groups. ABA concentration in the plant is known to be highly dependent on the air relative humidity (Okamoto et al., 2009). It is also possible that the recently proposed link between ABA and JA responses by the modulation of MYC2 transcriptional activity via PLY6 ABA receptor (Aleman et al., 2016) and the JASMONATE ZIM-DOMAIN12 (JAZ12) degradation via three RING ligase KEEP ON GOING in an ABA-dependent manner (Pauwels et al., 2015) may contribute to this issue. Nonetheless, conclusions drawn solely from pharmacological evidence can be confounded by the fact that the chemical applied (e.g. MeJA) may be further metabolized in the plant and the functional output (i.e. stomatal closure) is a pleiotropic effect of multiple stimuli. At the moment, this alternative has not been explored for MeJA-induced stomatal closure. Contrary to what has been observed for MeJA, the role of JA-Ile as a negative regulator of stomatal defense is strongly supported in the literature. Evidence includes (1) COR, which is a molecular mimic of JA-Ile (Zhao et al., 2003; Staswick and Tiryaki, 2004), induces stomatal opening, and repress pathogen-associated molecular pattern-triggered stomatal closure (Mino et al., 1987; Melotto et al., 2006; Zhang et al., 2008; Montillet et al., 2013; Panchal et al., 2016b); (2) JA-Ile, but not jasmonic acid, induces stomatal opening with the same potency as COR in Ipomea tricolor (Okada et al., 2009); (3) the coronatine insensitive1 (coi1) mutant of Arabidopsis that lacks the functional receptor for both COR and JA-Ile (Sheard et al., 2010) has constitutively smaller stomatal aperture than the wild-type plant (Panchal et al., 2016a).
Functional Output
The outcome of stomatal defense is a reduction of pathogen penetration into the plant. Reduced pathogen entry into the plant diminishes the severity of foliar diseases or, in the case of human pathogens, reduced leafy vegetable contamination, as will be discussed below. In the context of a plant-microbe interaction, stomatal closure or opening appears to depend on the strength of the opposing signals from the plant and the microbe, which could vary depending on the specific plant-microbe combination. For instance, using the same experimental setup and environmental conditions, Panchal et al. (2016a) found that high relative humidity negatively affects stomatal defense against P. syringae, whereas Roy et al. (2013) demonstrated that two human pathogens, E. coli O157:H7 and Salmonella enterica serovar Typhimurium SL1344, could still induce significant stomatal closure under high relative humidity. Furthermore, increasing the concentration of flg22 (Felix et al., 1999) could override the effect of high humidity on the opening of the stomatal pore (Roy et al., 2013), illustrating that the relative strength of the opposing signals contribute to the final output. Thus, a detailed description of the experimental setup could facilitate the comparison and interpretation of results from various stomatal bioassays (Chitrakar and Melotto, 2010; Montano and Melotto, 2017).
MECHANISMS OF BACTERIUM COUNTERDEFENSE AT STOMATA
If stomatal defense is a natural form of disease resistance, one would expect that highly adapted pathogens may have evolved virulence mechanisms to counter stomatal defense. Indeed, several pathogens are able to overcome stomatal defense using secreted virulence factors (Fig. 1). Unlike many fungi and oomycetes that can directly penetrate the leaf epidermis and viruses that are injected into the leaves by their insect vectors, many bacteria rely only on wounds or natural openings to colonize leaves. It is therefore understandable that developing mechanisms to open the stomatal pore may be particularly important for these pathogens to enter the apoplast. Production of phytotoxins and the type III secretion system have emerged as important factors to overcome stomatal defense by bacterial pathogens. The chemical nature and mode of action of these molecules vary as discussed below.
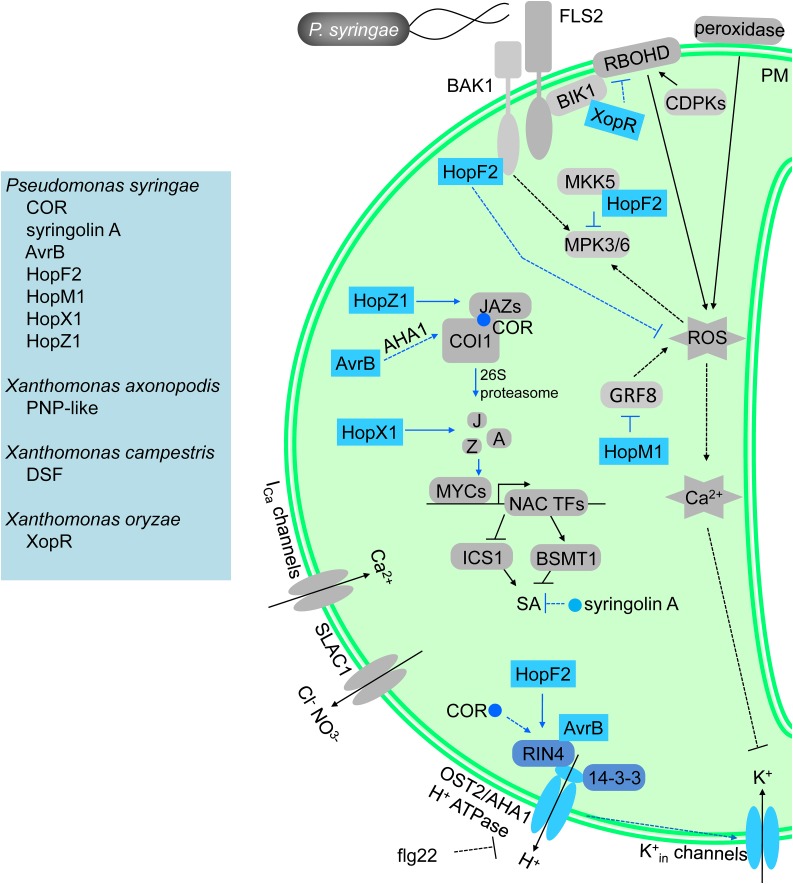
Figure 1.
A simplified diagram of microbial virulence factors that manipulate MAMP-induced stomatal closure. NADPH oxidase RBOHD mediates flg22-induced ROS production and stomatal defense through BIK1-/CDPKs-regulated phosphorylation (Feng et al., 2012; Dubiella et al., 2013; Gao et al., 2013; Kadota et al., 2014; Li et al., 2014). Type III peroxidase also contributes to flg22-induced ROS production (Daudi et al., 2012; O’Brien et al., 2012). MAMP-induced stomatal closure includes accumulation of ROS and NO, cytosolic calcium oscillations, activation of S-type anion channels, and inhibition of K+in channels (Klüsener et al., 2002; Melotto et al., 2006; Desikan et al., 2008; Zhang et al., 2008; Zeng and He, 2010; Macho et al., 2012; Montillet et al., 2013). COR induces COI1-JAZ interaction, mediates JAZ degradation, and activates the expression of NAC TFs, which inhibit the expression of ICS1 and induce expression of BSMT1, thereby leading to decreased SA level (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007, 2009; Katsir et al., 2008; Melotto et al., 2008; Sheard et al., 2010; Zheng et al., 2012; Gimenez-Ibanez et al., 2017). COR also may induce stomatal opening through RIN4, which interacts with H+-ATPase AHA1 and AHA2, resulting in induction of K+in channels (Zhang et al., 2008; Liu et al., 2009). Syringolin A inhibits SA signaling via its proteasome inhibitor activity (Misas-Villamil et al., 2013). Multiple bacterial effectors HopM1, HopF2, HopX1, AvrB, HopZ1, and XopR could disrupt stomatal defense. HopM1 disrupts the function of GRF8 (a 14-3-3 protein), resulting in reduction of MAMP-induced ROS production (Lozano- Durán et al., 2014). HopF2 directly targets BAK1, MKK5, and RIN4, resulting in inhibition of ROS production (Wang et al., 2010; Wilton et al., 2010; Hurley et al., 2014; Zhou et al., 2014). AvrB enhances the interaction of RIN4 and AHA1, which could promote the interaction between COI1 and JAZs, leading to stomatal opening (Zhou et al., 2015; Lee et al., 2015). HopX1 and HopZ1 induce stomatal opening after PTI-mediated stomatal closure, through a COI1-independent and COI1-dependent manner, respectively (Jiang et al., 2013; Gimenez-Ibanez et al., 2014; Ma et al., 2015). XopR interacts with BIK1 and suppresses stomatal closure possibly by inhibition of RBOHD (Wang et al., 2016).
Phytotoxins
COR is a phytotoxin produced by several pathovars of P. syringae including Pst DC3000 (Bender et al., 1999) and can effectively prevent MAMP-triggered stomatal closure (Melotto et al., 2006, Zhang et al., 2008, Montillet et al., 2013; Panchal et al., 2016b). A putative signaling cascade by which COR prevents bacterium-triggered stomatal closure has been elucidated (Fig. 1). As a molecular mimic of JA-Ile, COR promotes physical interaction between the F-box protein COI1 and the transcriptional repressors JAZ, leading to ubiquitination and proteasome-mediated degradation of JAZ proteins (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). Degradation of JAZ proteins derepresses bHLH transcription factors, such as MYC2, MYC3, and MYC4, leading to activation of downstream transcriptional responses (reviewed in Zhang et al., 2017). COR activation of JA signaling induces the expression of three homologous NAC transcription factor genes, ANAC019, ANAC055, and ANAC072, which are the direct targets of MYC2 (Zheng et al., 2012). Genetic characterization of nac null mutants shows that NACs mediate COR-induced stomatal reopening and bacterial multiplication in plant tissues by inhibiting the accumulation of SA. Specifically, these NACs exert an inhibitory effect by repressing the expression of genes involved in SA biosynthesis and activating the expression of genes involved in SA metabolism, resulting in overall depletion of SA in infected plants (Zheng et al., 2012; Gimenez-Ibanez et al., 2017). A very similar mechanism has also been described in tomato. JA and COR activate the expression of the NAC tomato homolog, JASMONIC ACID2-LIKE. This transcription factor binds to and activates the expression of SAMT1 and SAMT2, which encode enzymes that deactivate SA by methylation, thereby suppressing the accumulation of SA and promoting stomatal opening (Du et al., 2014).
Interestingly, another member of the NAC family of transcription factors, ANA032 acts as both a positive regulator of SA signaling and a negative regulator of JA signaling (Allu et al., 2016). ANAC032 directly binds to the promoter of MYC2 (positive regulator of JA signaling) and NIMIN1 (negative regulator of SA signaling) and concomitantly suppresses their transcription within 6 h of Pst DC3000 infection (Allu et al., 2016). Accordingly, overexpression of ANAC032 in Arabidopsis inhibits COR-dependent reopening of stomata (Allu et al., 2016).
COR has also been shown to trigger cellular responses that do not rely on the canonical COI1-JAZ signaling pathway to manipulate guard cell movement. For example, COR requires RPM1-INTERACTING4 (RIN4), a negative regulator of plant innate immunity, to open the stomatal pore as evidenced by the facts that neither Pst DC3000 nor COR was able to reopen stomata of rpm1/rps2/rin4 mutants (Liu et al., 2009; Zhou et al., 2015; Lee et al., 2015). The perception of flg22 via the FLS2 receptor leads to phosphorylation of the plasma membrane H+-ATPases and subsequent alkalization of the apoplast, which, along with the induction of ROS via NADPH oxidase RbohD, triggers stomatal closure (Liu et al., 2009; Li et al., 2014). RIN4 interacts with the plasma membrane H+-ATPases AHA1 and AHA2, leading to their inhibition and acidification of the apoplast through hyperpolarization of the plasma membrane and subsequent induction of inward K+ channels, promoting stomatal opening (Zhang et al., 2008; Liu et al., 2009). COR was shown to reverse the inhibitory effects of flg22 on K+ currents to promote stomatal opening (Zhang et al., 2008).
Another virulence factor that impacts stomatal defense is syringolin A, a peptide toxin produced by P. syringae pv syringae that has a proteasome inhibitory function (Groll et al., 2008). Syringolin A promotes stomatal opening (Schellenberg et al., 2010). Unlike Pst DC3000, P. syringae pv syringae does not induce an initial stomatal closure on either its bean host or Arabidopsis (Schellenberg et al., 2010; Panchal et al., 2016a). This finding suggests that this bacterium constitutively produces syringolin A and that syringolin A is a stronger signal than the MAMPs produced by P. syringae pv syringae (see discussion on the strength of the signal above) and/or that these plants do not recognize P. syringae pv syringae MAMPs efficiently. More recently, Misas-Villamil et al. (2013) demonstrated that syringolin A diffuses from the site of infection and suppresses SA signaling thereby decreasing immune responses in adjacent tissues. This might be the mechanism involved in syringolin A inhibition of MAMP-induced stomatal closure because syringolin A inhibits the proteasome-mediated turnover of NPR1, an important component of SA signaling to induce stomatal defense (Schellenberg et al., 2010).
The phytopathogenic bacterium X. campestris pv campestris is also capable of interfering with stomatal closure induced by MAMP or ABA signaling (Gudesblat et al., 2009). X. campestris pv campestris does so by inducing the production of diffusible signal factor that is involved in bacterium-to-bacterium signaling (Gudesblat et al., 2009). How diffusible signal factor mediates stomatal reopening upon bacterial invasion is still unknown. Another Xanthomonas species, X. axonopodis pv citri produces a compound, known as plant natriuretic peptide-like, that can open stomata during plant infection, which correlates with enhanced disease symptoms (Gottig et al., 2008). Plant natriuretic peptide-like controls stomatal aperture in a cGMP-dependent manner (Gottig et al., 2008).
Type III Secreted Effectors
In addition to phytotoxins, pathogenic bacteria also produced type-III-secretion-system effectors (T3SEs) that can overcome stomatal defense by either inhibiting MAMP-triggered stomatal closure or actively inducing stomatal opening. The T3SE HopM1 of P. syringae disrupts the function of a 14-3-3 protein, GRF8, leading to reduction in MAMP-triggered ROS burst and stomatal defense (Lozano-Durán et al., 2014). However, it is not clear in this case whether stomatal opening is a consequence of HopM1-mediated ROS suppression. Likewise, the P. syringae effector HopF2 inhibits flg22-induced ROS and stomatal defense (Hurley et al., 2014). HopF2 is an ADP-ribosyltransferase; however, the ADP-ribosyltransferase activity of HopF2 is not required for the ability of HopF2 to disable stomatal defense, suggesting the existence of another biochemical activity of HopF2 (Hurley et al., 2014). The XopR effector from Xanthomonas oryzae pv oryzae strain PXO99A also inhibits flg22-induced stomatal closure; however, the mechanism remains elusive (Wang et al., 2016).
Induction of stomatal opening through plasma membrane polarization was attributed to the P. syringae effector AvrB. AvrB-induced stomatal opening requires the canonical JA signaling pathway (Zhou et al., 2015). AvrB interacts with RIN4, which leads to the activation of the plasma membrane H+-ATPase AHA1 (Lee et al., 2015). This may potentially generate an unknown signal that promotes the interaction between COI1 and JAZ proteins to degrade JAZ and enhance JA signaling. In addition, AvrB induces MYC-mediated expression of ANAC019, ANAC055, and ANAC072 genes in Arabidopsis and the tomato NAC homolog JASMONIC ACID2-LIKE gene to repress SA responses (Du et al., 2014; Zhou et al., 2015; Gimenez-Ibanez et al., 2017). Therewith, like COR, AvrB appears to regulate stomatal opening through manipulating the SA-JA antagonism. Likewise, the HopZ1 effector from P. syringae pv syringae (which does not produce COR) also induces JAZ protein degradation. HopZ1 possesses acetyltransferase activity, directly interacts with JAZ proteins, and promotes JAZ acetylation and degradation to activate JA signaling in soybean and Arabidopsis (Jiang et al., 2013). Moreover, HopZ1 can induce stomatal opening after flg22-mediate stomatal closure in Arabidopsis (Ma et al., 2015). Finally, the HopX1 Cys protease effector from P. syringae pv tabaci (which also does not produce COR) can interact with and promote degradation of JAZ proteins, in a COI1-independent manner, resulting in stomatal opening (Gimenez-Ibanez et al., 2014). These findings indicate that induction of JA signaling response is a common target for effectors to overcome stomatal defense and provide insights on a variety of mechanisms that lead to JAZ degradation and JA response in plants.
Unknown Bacterial Factors
The human pathogen Salmonella enterica serovar Typhimurium SL1344 can migrate to open stomata of lettuce to access internal leaf cells and colonize the apoplast (Kroupitski et al., 2009). A recent study shows that SL1344, like Pst DC3000, causes a transient stomatal closure (Roy et al., 2013). The mechanisms underlying stomatal closure and reopening mediated by SL1344 are not understood, but it appears that not only plant pathogens, but also some human pathogens may have evolved mechanisms to modulate plant stomatal movements as part of their colonization strategy of the phyllosphere (Roy et al., 2013; Melotto et al., 2014). As such, the study of stomatal defense may have implications beyond plant diseases.
ENVIRONMENTAL CONDITIONS THAT INFLUENCE STOMATAL DEFENSE
As several abiotic environmental conditions also promote stomatal closure (e.g. darkness, low humidity, low temperature, high CO2), one would expect that these conditions may prevent pathogen penetration into leaves, unless the pathogen has evolved the ability to override the effect of these environmental stimuli. For instance, at night, most land plants close their stomata, which could potentially decrease pathogen infection. Indeed, the COR-deficient mutant Pst DC3118 colonizes leaf apoplast less efficiently in the dark as compared to plants inoculated under light (Panchal et al., 2016b). This finding suggests that, similar to MAMPs, darkness could effectively diminish P. syringae penetration into leaves. The mechanism underlying this process has begun to be elucidated. The transcription factors CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) are two key regulators of the circadian clock in Arabidopsis. Disruption of the normal clock activity by either knocking out both genes (double mutant cca1-1 lhy-20) or by overexpressing either of them results in plants that are no longer able to properly close stomata in response to dark or P. syringae (Zhang et al., 2013). Zhang et al. (2013) also observed that these genetically engineered plants are more susceptible to P. syringae at day 3 after both spray- and pressure-inoculation. The COR-producing Pst DC3000, however, opens dark-closed stomata and efficiently invades the leaf at night (Panchal et al., 2016b).
Alternatively, there are environmental conditions (e.g. light, high humidity) that promote stomatal opening, and pathogens might take advantage and penetrate leaves in these situations. Indeed, high humidity compromises Pst DC3118-triggered stomatal closure (Panchal et al., 2016a). High humidity also decreases guard cell sensitivity to flg22, ABA, and SA (Roy et al., 2013; Panchal et al., 2016a) by simultaneously inducing JA signaling and repressing SA signaling in guard cells (Panchal et al., 2016a). Interestingly, SA accumulates to higher levels at night and JA accumulates to higher levels during the day in Arabidopsis (Goodspeed et al., 2012; Grundy et al., 2015; Zheng et al., 2015; Inoue and Kinoshita, 2017; Eisenach and de Angeli, 2017; Vialet-Chabrand et al., 2017). It is tempting to speculate that the endogenous fluctuation of these plant hormones within a 24-h period could contribute to dark-induced stomatal closure and light-induced stomatal opening.
Curiously, high humidity does not compromise E. coli O157:H7-induced stomatal closure (Roy et al., 2013). It is possible that P. syringae have evolved variants of MAMPs (e.g. elf18) that are less potent in triggering stomatal closure compared to those from E. coli O157:H7 (Zeng and He, 2010). If this is the case, high humidity is sufficient to overcome P. syringae-triggered stomatal closure, but not E. coli O157:H7-triggered stomatal closure.
It is important to note that pathogen penetration into leaves through stomata depends not only on the pore being open, but also on the pathogen behavior on the leaf surface. For instance, directional movement of bacterial pathogens on the leaf surface can be assisted by chemotaxis toward signaling molecules (reviewed in Vorholt, 2012; Melotto and Kunkel, 2013). Relative humidity may have great influence on the accumulation and diffusion of these signals and, consequently, on directional bacterial movement on the leaf surface.
STOMATAL DEVELOPMENT AND IMMUNITY
It has long been observed that viral infection of plant tissues alters normal stomatal development, leading to fewer stomata in infected leaves. One of the earliest reports on this phenomenon shows that sugar beet infected with Beet yellow virus has lower stomatal density on both upper and lower leaf surfaces as compared to the mock control (Hall and Loomis, 1972). Virus-induced reduction of stomatal density seems to be a systemic response (Murray et al., 2016). Additionally, Murray et al. (2016) reported that systemic reduction of stomatal index and density only occurred in two compatible interactions, Nicotiana tabacum-Tobacco mosaic virus (TMV) and Arabidopsis-Turnip vein-clearing virus, but not in two incompatible interactions, namely, Nicotiana glutinosa-TMV and Chenopodium quinoa-TMV. Reduction of stomatal index and density correlated with reduction in plant leaf transpiration and water loss in TMV-inoculated N. tabacum (Murray et al., 2016). A possible link between viral infection and stomatal development may be a microtubule-associated protein, MPB2C, found in both N. tabacum and Arabidopsis (Kragler et al., 2003; Ruggenthaler et al., 2009). MPB2C was identified as an interactor of the TMV-movement protein (Kragler et al., 2003) and overexpression of this protein in Arabidopsis results in increased number of stomata organized in clusters and resistance to Oilseed rape mosaic virus, a TMV close relative (Ruggenthaler et al., 2009).
Interestingly, overexpression of Pst DC3000 effectors, AvrPto or AvrPtoB, also impairs stomatal patterning in Arabidopsis (Meng et al., 2015), where stomata occur in clusters similar to what has been observed by Ruggenthaler et al. (2009). AvrPto and AvrPtoB target several plant kinases including members of the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) protein family, including SERK1, SERK2, and BAK1 (Brassinosteroid insensitive1-Associated Kinase, also known as SERK3; Meng et al., 2015). These three SERK proteins have redundant functions in regulating stomatal development responses (Cheung and Wu, 2015; Meng et al., 2015), whereas BAK1 is also a common signaling component of plant immunity (Chinchilla et al., 2007). The Arabidopsis bak1-5 mutant shows higher stomatal index and density than the wild-type Col-0 and is also susceptible to the Plectosphaerella cucumerina BMM fungus (Jordá et al., 2016). The molecular mechanisms by which pathogens manipulate stomatal development and how this process is linked to stomatal defense are beginning to be elucidated (see Outstanding Questions). At this moment, it is clear that bacterial pathogens can manipulate stomatal movement to their own benefit. However, whether the interference of stomatal density and patterns by viruses via movement protein or bacteria via T3SEs are strategies that promote disease by these diverse pathogens still need further investigation.
CONCLUSION
There has been impressive progress made toward understanding the mechanisms of stomatal defense in the past decade, thanks to contributions from many laboratories. It is clear now that MAMP perception and signal transduction is an important and innate function of stomatal guard cells. One could imagine that as the first lineage of plant stomata appeared, they would have to “deal with” both biotic and abiotic stresses. Therefore, it is conceivable that both biotic and abiotic signals have had substantial contributions in shaping the evolution of plant stomata, possibly including their shape, density, and the remarkable complexity of the guard cell signaling network. It may be said that without the discovery of the defense function of stomata and a deep understanding of the signal transduction pathway involved in stomatal defense, we might have never completely appreciated the many sophisticated behaviors of stomata. Much needs to be learned about stomatal defense and its cross talk with other functions of stomata in the coming decade!
Glossary
- MAMP
microbe-associated molecular pattern
- COR
coronatine
- PRR
pattern recognition receptor
- MeJA
methylated jasmonate
- T3SE
type-III-secretion-system effector
Footnotes
This research topic in the M.M. lab and S.Y.H. lab is supported by grants from the U.S. National Institute of Allergy and Infectious Disease (5R01AI068718, M.M. and S.Y.H.), the U.S. Department of Agriculture–National Institute of Food and Agriculture (2015-67017-23360, M.M. and S.Y.H.), the Center for Produce Safety (CPF43206, M.M.), University of California Davis-FAPESP SPRINT (award 40747474, M.M.), the National Science Foundation (IOS-1557437, S.Y.H.), and the Gordon and Betty Moore Foundation (GBMF3037, S.Y.H.).
Articles can be viewed without a subscription.
References
- Aleman F, Yazaki J, Lee M, Takahashi Y, Kim AY, Li Z, Kinoshita T, Ecker JR, Schroeder JI (2016) An ABA-increased interaction of the PYL6 ABA receptor with MYC2 transcription factor: A putative link of ABA and JA signaling. Sci Rep 6: 28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allègre M, Daire X, Héloir M-C, Trouvelot S, Mercier L, Adrian M, Pugin A (2007) Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytol 173: 832–840 [DOI] [PubMed] [Google Scholar]
- Allu AD, Brotman Y, Xue GP, Balazadeh S (2016) Transcription factor ANAC032 modulates JA/SA signalling in response to Pseudomonas syringae infection. EMBO Rep 17: 1578–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud D, Hwang I (2015) A sophisticated network of signaling pathways regulates stomatal defenses to bacterial pathogens. Mol Plant 8: 566–581 [DOI] [PubMed] [Google Scholar]
- Bender CL, Alarcón-Chaidez F, Gross DC (1999) Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63: 266–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2015) Stomatal patterning: SERKs put the mouths in their right place. Curr Biol 25: R838–R840 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Chitrakar R, Melotto M (2010) Assessing stomatal response to live bacterial cells using whole leaf imaging. J Vis Exp pii: 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelle V, Leonhardt N (2016) 14-3-3 Proteins in guard cell signaling. Front Plant Sci 6: 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bögre L, Grant M (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos-Theveniau M, Arnaud D, Huang TY, Lin GJ, Chen WY, Lin YC, Zimmerli L (2012) The Arabidopsis lectin receptor kinase LecRK-V.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathog 8: e1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, Grefen C, Cheung MK, Meixner AJ, Hooley R, et al. (2008) The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One 3: e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Zhai Q, Deng L, Li S, Li H, Yan L, Huang Z, Wang B, Jiang H, Huang T, et al. (2014) Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. Plant Cell 26: 3167–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte CP, Schulze WX, Romeis T (2013) Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, de Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Feng F, Yang F, Rong W, Wu X, Zhang J, Chen S, He C, Zhou JM (2012) A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485: 114–118 [DOI] [PubMed] [Google Scholar]
- Gao X, Chen X, Lin W, Chen S, Lu D, Niu Y, Li L, Cheng C, McCormack M, Sheen J, et al. (2013) Bifurcation of Arabidopsis NLR immune signaling via Ca2+-dependent protein kinases. PLoS Pathog 9: e1003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Boter M, Fernández-Barbero G, Chini A, Rathjen JP, Solano R (2014) The bacterial effector HopX1 targets JAZ transcriptional repressors to activate jasmonate signaling and promote infection in Arabidopsis. PLoS Biol 12: e1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Boter M, Ortigosa A, García-Casado G, Chini A, Lewsey MG, Ecker JR, Ntoukakis V, Solano R (2017) JAZ2 controls stomata dynamics during bacterial invasion. New Phytol 213: 1378–1392 [DOI] [PubMed] [Google Scholar]
- Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF (2012) Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci USA 109: 4674–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottig N, Garavaglia BS, Daurelio LD, Valentine A, Gehring C, Orellano EG, Ottado J (2008) Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proc Natl Acad Sci USA 105: 18631–18636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer MK, John Foulkes M, Paveley ND (2012) Foliar pathogenesis and plant water relations: A review. J Exp Bot 63: 4321–4331 [DOI] [PubMed] [Google Scholar]
- Groll M, Schellenberg B, Bachmann AS, Archer CR, Huber R, Powell TK, Lindow S, Kaiser M, Dudler R (2008) A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature 452: 755–758 [DOI] [PubMed] [Google Scholar]
- Grundy J, Stoker C, Carré IA (2015) Circadian regulation of abiotic stress tolerance in plants. Front Plant Sci 6: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA (2009) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol 149: 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MR (2015) Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol 208: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AE, Loomis RS (1972) An explanation for the difference in photosynthetic capabilities of healthy and Beet yellows virus-infected sugar beets (Beta vulgaris L.). Plant Physiol 50: 576–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2011) Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol 156: 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, Liao H, Duan Y, Zhu Z, Guo Y, Chen Z, Gong Z (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V, Barber CE, Daniels MJ (1998) Entry of Xanthomonas campestris pv. campestris into hydathodes of Arabidopsis thaliana leaves: A system for studying early infection events in bacterial pathogenesis. Mol Plant Microbe Interact 11: 537–543 [DOI] [PubMed] [Google Scholar]
- Hurley B, Lee D, Mott A, Wilton M, Liu J, Liu YC, Angers S, Coaker G, Guttman DS, Desveaux D (2014) The Pseudomonas syringae type III effector HopF2 suppresses Arabidopsis stomatal immunity. PLoS One 9: e114921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T (2017) Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol 174: 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimwe R, Abutaleb K, Ahmed F (2014) Applications of thermal imaging in agriculture—a review. Adv Remote Sens 3: 128–140 [Google Scholar]
- Jiang S, Yao J, Ma KW, Zhou H, Song J, He SY, Ma W (2013) Bacterial effector activates jasmonate signaling by directly targeting JAZ transcriptional repressors. PLoS Pathog 9: e1003715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordá L, Sopeña-Torres S, Escudero V, Nuñez-Corcuera B, Delgado-Cerezo M, Torii KU, Molina A (2016) ERECTA and BAK1 receptor like kinases interact to regulate immune responses in Arabidopsis. Front Plant Sci 7: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Saha A, Valon C, Leung J (2011) A brand new START: Abscisic acid perception and transduction in the guard cell. Sci Signal 4: re4. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI (2002) Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol 130: 2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragler F, Curin M, Trutnyeva K, Gansch A, Waigmann E (2003) MPB2C, a microtubule-associated plant protein binds to and interferes with cell-to-cell transport of tobacco mosaic virus movement protein. Plant Physiol 132: 1870–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S (2009) Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol 75: 6076–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Bourdais G, Yu G, Robatzek S, Coaker G (2015) Phosphorylation of the plant immune regulator RPM1-INTERACTING PROTEIN4 enhances plant plasma membrane H+-ATPase activity and inhibits flagellin-triggered immune responses in Arabidopsis. Plant Cell 27: 2042–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim YJ, Kim M-H, Kwak JM (2016) MAPK cascades in guard cell signal transduction. Front Plant Sci 7: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Lim CW, Luan S, Lee SC (2014) A prominent role for RCAR3-mediated ABA signaling in response to Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis. Plant Cell Physiol 55: 1691–1703 [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Durán R, Bourdais G, He SY, Robatzek S (2014) The bacterial effector HopM1 suppresses PAMP-triggered oxidative burst and stomatal immunity. New Phytol 202: 259–269 [DOI] [PubMed] [Google Scholar]
- Ma K-W, Jiang S, Hawara E, Lee D, Pan S, Coaker G, Song J, Ma W (2015) Two serine residues in Pseudomonas syringae effector HopZ1a are required for acetyltransferase activity and association with the host co-factor. New Phytol 208: 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho AP, Boutrot F, Rathjen JP, Zipfel C (2012) Aspartate oxidase plays an important role in Arabidopsis stomatal immunity. Plant Physiol 159: 1845–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Kunkel BN (2013) Virulence strategies of plant pathogenic bacteria. In Rosenberg E, Stackebrand E, DeLong EF, Thompson F, Lory S, eds, The Prokaryotes: Prokaryotic Physiology and Biochemistry, Ed 4. Springer-Verlag, Berlin, pp 61–82 [Google Scholar]
- Melotto M, Mecey C, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, et al. (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Panchal S, Roy D (2014) Plant innate immunity against human bacterial pathogens. Front Microbiol 5: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L (2015) Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Curr Biol 25: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mino Y, Matsushita Y, Sakai R (1987) Effect of coronatine on stomatal opening in leaves of broad bean and Italian ryegrass. Ann Phytopathol Soc Jpn 53: 53–55 [Google Scholar]
- Mittal S, Davis KR (1995) Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol Plant Microbe Interact 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Misas-Villamil JC, Kolodziejek I, Crabill E, Kaschani F, Niessen S, Shindo T, Kaiser M, Alfano JR, van der Hoorn RA (2013) Pseudomonas syringae pv. syringae uses proteasome inhibitor syringolin A to colonize from wound infection sites. PLoS Pathog 9: e1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano J, Melotto M (2017) Stomatal bioassay to characterize bacterial-stimulated PTI at the pre-invasion phase of infection. In Shan L, He P, eds, Methods in Molecular Biology: Plant Pattern Recognition Receptors, Vol 1578 Humana Press, New York, pp 233–241 [DOI] [PubMed] [Google Scholar]
- Montillet JL, Hirt H (2013) New checkpoints in stomatal defense. Trends Plant Sci 18: 295–297 [DOI] [PubMed] [Google Scholar]
- Montillet J-L, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M, Garcia AV, Douki T, Bigeard J, Laurière C, et al. (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11: e1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y (2007) The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol 143: 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S (2015) Diverse stomatal signaling and the signal integration mechanism. Annu Rev Plant Biol 66: 369–392 [DOI] [PubMed] [Google Scholar]
- Murray RR, Emblow MS, Hetherington AM, Foster GD (2016) Plant virus infections control stomatal development. Sci Rep 6: 34507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP (2012) A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol 158: 2013–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulareddy N, Panchal S, Melotto M (2013) Guard cell purification and RNA isolation suitable for high-throughput transcriptional analysis of cell-type responses to biotic stresses. Mol Plant Microbe Interact 26: 844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Ito S, Marsubara A, Iwakura I, Egoshi S, Ueda M (2009) Total syntheses of coronatines by exo-selective Diels-Alder reaction and their biological activities on stomatal opening. Org Biomol Chem 7: 3065–3073 [Google Scholar]
- Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E (2009) High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol 149: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S, Chitrakar R, Thompson BK, Obulareddy N, Roy D, Hambright WS, Melotto M (2016a) Regulation of stomatal defense by air relative humidity. Plant Physiol 172: 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S, Roy D, Chitrakar R, Price L, Breitbach ZS, Armstrong DW, Melotto M (2016b) Coronatine facilitates Pseudomonas syringae infection of Arabidopsis leaves at night. Front Plant Sci 7: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L, Ritter A, Goossens J, Durand AN, Liu H, Gu Y, Geerinck J, Boter M, Vanden Bossche R, De Clercq R, et al. (2015) The RING E3 ligase KEEP ON GOING modulates JASMONATE ZIM-DOMAIN12 stability. Plant Physiol 169: 1405–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos LJ, Volin RB (1987) Role of stomatal opening and frequency on infection of Lycopersicum spp. by Xhantomonas campestris pv. vesicatoria. Phytopathology 77: 1311–1317 [Google Scholar]
- Ranf S, Gisch N, Schäffer M, Illig T, Westphal L, Knirel YA, Sánchez-Carballo PM, Zähringer U, Hückelhoven R, Lee J, et al. (2015) A lectin S-domain receptor kinase mediates lipopolysaccharide sensing in Arabidopsis thaliana. Nat Immunol 16: 426–433 [DOI] [PubMed] [Google Scholar]
- Roy D, Panchal S, Rosa BA, Melotto M (2013) Escherichia coli O157:H7 induces stronger plant immunity than Salmonella enterica Typhimurium SL1344. Phytopathology 103: 326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenthaler P, Fichtenbauer D, Krasensky J, Jonak C, Waigmann E (2009) Microtubule-associated protein AtMPB2C plays a role in organization of cortical microtubules, stomata patterning, and tobamovirus infectivity. Plant Physiol 149: 1354–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko T, Kolla VA, Wang CQ, Nasafi Z, Hicks DR, Phadungchob B, Chehab WE, Brandizzi F, Froehlich J, Dehesh K (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164: 1151–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawinski K, Mersmann S, Robatzek S, Böhmer M (2013) Guarding the green: Pathways to stomatal immunity. Mol Plant Microbe Interact 26: 626–632 [DOI] [PubMed] [Google Scholar]
- Schellenberg B, Ramel C, Dudler R (2010) Pseudomonas syringae virulence factor syringolin A counteracts stomatal immunity by proteasome inhibition. Mol Plant Microbe Interact 23: 1287–1293 [DOI] [PubMed] [Google Scholar]
- Shafiei R, Hang C, Kang J-G, Loake GJ (2007) Identification of loci controlling non-host disease resistance in Arabidopsis against the leaf rust pathogen Puccinia triticina. Mol Plant Pathol 8: 773–784 [DOI] [PubMed] [Google Scholar]
- Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu F-F, Sharon M, Browse J, et al. (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J (2016) Reactive oxygen species in the regulation of stomatal movements. Plant Physiol 171: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Ganapathysubramanian B, Singh AK, Sarkar S (2016) Machine learning for high-throughput stress phenotyping in plants. Trends Plant Sci 21: 110–124 [DOI] [PubMed] [Google Scholar]
- Singh P, Kuo Y-C, Mishra S, Tsai C-H, Chien C-C, Chen C-W, Desclos-Theveniau M, Chu P-W, Schulze B, Chinchilla D, et al. (2012) The lectin receptor kinase-VI.2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 24: 1256–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth EB, Melotto M, Zhang W, Assmann SM, He SY (2009) Crosstalk in pathogen and hormonal regulation of guard cell signaling. In Yoshioka K, Shinozaki K, eds, Signal Crosstalk in Plant Stress Response. Wiley-Blackwell, Hoboken, NJ, pp 96–112 [Google Scholar]
- Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14: 310–317 [DOI] [PubMed] [Google Scholar]
- Vorholt JA. (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10: 828–840 [DOI] [PubMed] [Google Scholar]
- Vialet-Chabrand S, Hills A, Wang Y, Griffiths H, Lew VL, Lawson T, Blatt MR, Rogers S (2017) Global sensitivity analysis of OnGuard models identifies key hubs for transport interaction in stomatal dynamics. Plant Physiol 174: 680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Hitomi K, Nishimura N, Hitomi C, Adams SR, Getzoff ED, Schroeder JI (2014) FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3: e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li J, Hou S, Wang X, Li Y, Ren D, Chen S, Tang X, Zhou JM (2010) A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell 22: 2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sun J, Fan F, Tan Z, Zou Y, Lu D (2016) A Xanthomonas oryzae pv. oryzae effector, XopR, associates with receptor-like cytoplasmic kinases and suppresses PAMP-triggered stomatal closure. Sci China Life Sci 59: 897–905 [DOI] [PubMed] [Google Scholar]
- Wilton M, Subramaniam R, Elmore J, Felsensteiner C, Coaker G, Desveaux D (2010) The type III effector HopF2Pto targets Arabidopsis RIN4 protein to promote Pseudomonas syringae virulence. Proc Natl Acad Sci USA 107: 2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, McLamore ES, Dong S, Gao H, Taguchi M, Wang N, Zhang T, Su X, Shen Y (2015) The role of plasma membrane H(+) -ATPase in jasmonate-induced ion fluxes and stomatal closure in Arabidopsis thaliana. Plant J 83: 638–649 [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chételat A, Reymond P, Pagni M, Dubugnon L, Farmer EE (2007) A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Brutus A, Kremer JM, Withers JC, Gao X, Jones AD, He SY (2011) A genetic screen reveals Arabidopsis stomatal and/or apoplastic defenses against Pseudomonas syringae pv. tomato DC3000. PLoS Pathog 7: e1002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xie Q, Anderson RG, Ng G, Seitz NC, Peterson T, McClung CR, McDowell JM, Kong D, Kwak JM, et al. (2013) Crosstalk between the circadian clock and innate immunity in Arabidopsis. PLoS Pathog 9: e1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang F, Melotto M, Yao J, He SY (2017) Jasmonate signaling and manipulation by pathogens and insects. J Exp Bot 68: 1371–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA (2003) Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J 36: 485–499 [DOI] [PubMed] [Google Scholar]
- Zheng X-Y, Spivey NW, Zeng W, Liu P-P, Fu ZQ, Klessig DF, He SY, Dong X (2012) Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11: 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X-Y, Zhou M, Yoo H, Pruneda-Paz JL, Spivey NW, Kay SA, Dong X (2015) Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc Natl Acad Sci USA 112: 9166–9173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wu S, Chen X, Liu C, Sheen J, Shan L, He P (2014) The Pseudomonas syringae effector HopF2 suppresses Arabidopsis immunity by targeting BAK1. Plant J 77: 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wu Y, Yang Y, Du M, Zhang X, Guo Y, Li C, Zhou JM (2015) An Arabidopsis plasma membrane proton ATPase modulates JA signaling and is exploited by the Pseudomonas syringae effector protein AvrB for stomatal invasion. Plant Cell 27: 2032–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]