Abstract
Recent advances in the stomatal biology of CAM plants are reviewed, and key opportunities for future progress are identified.
Crassulacean acid metabolism (CAM) is a major physiological syndrome that has evolved independently in numerous land plant lineages. CAM plants are of great ecological significance, and there is increasing interest for their water-use efficiency and drought resistance. Integral to the improvement in water-use efficiency that CAM affords is a unique pattern of stomatal conductance, distinguished by primarily nocturnal opening and often extensive diurnal flexibility in response to environmental factors. Here, we assess how recent research has shed new light on the functional biology of CAM plant stomata and integration within the broader physiology and ecology of succulent organisms. Divergences in stomatal sensitivity to environmental and endogenous factors relative to C3 species have been a key aspect of the evolution of functional CAM. Stomatal traits of CAM plants are closely coordinated with other leaf functional traits, and structural specialization of CAM stomatal complexes may be of undiagnosed functional relevance. We also highlight how salient results from ongoing work on C3 plant stomatal biology could apply to CAM species. Key questions remaining relate to the interdependence between stomatal and mesophyll responses and are particularly relevant for bioengineering of CAM traits or bioenergy crops to exploit enhanced water-use efficiency and productivity on marginal land. With the increasing availability of powerful analytical tools and the emergence of new model systems for the study of the molecular basis of physiological traits in CAM plants, many exciting avenues for future research are open to intrepid investigators.
CAM is a celebrated example of a convergent physiological syndrome (i.e. a characteristic combination of traits), having evolved independently on numerous occasions across the land plants (Smith and Winter, 1996). Furthermore, thanks in part to their ability to withstand multiple, synergistic stressors (Lüttge, 2010), CAM plants have successfully invaded diverse environmental spaces ranging from deserts to cloud forests. In many tropical and subtropical vegetation types, CAM is a dominant ecophysiological syndrome, and CAM plants represent at least 6% of higher plant species richness (Dodd et al., 2002).
The physiological mechanisms and ecological significance of the gas exchange rhythms of plants performing CAM have been the subject of curiosity and investigation for not just decades, but centuries (De Saussure, 1804; Heyne, 1815; Osmond, 1978; Ting, 1987; Faak, 2000). The quintessential feature of CAM is nocturnal primary carbon assimilation by the enzyme phospho-enol-pyruvate carboxylase (PEPC), producing malic acid that is stored in mesophyll cell vacuoles and subsequently decarboxylated during the light period to provide CO2 for refixation by Rubisco (Winter and Smith, 1996). While a few lineages are capable of performing CAM in tissues lacking stomata, including some aquatic plants with leaves with no stomata (“astomatous”; Keeley, 1998) and epiphytic orchids with astomatous chlorophyllous roots (Goh et al., 1983), in most cases, CAM involves the delivery of CO2 to the mesophyll via stomata that are open in the dark (Winter and Smith, 1996). Nonnegligible nocturnal stomatal conductance is increasingly recognized as an important physiological phenomenon in many C3 plants (Zeppel et al., 2012; de Dios et al., 2013; Forster, 2014; Matimati et al., 2014; Zeppel et al., 2014; Cirelli et al., 2016; Resco de Dios et al., 2016), but stomata of CAM plants displaying primarily nocturnal CO2 assimilation clearly must differ from those of C3 plants in their responsiveness to environmental and endogenous stimuli.
The global CAM flora combines great ecological diversity with a wide variety of evolutionary backgrounds, and comparative studies of variation in the stomatal biology of different CAM lineages allow two overarching questions to be distilled. First, what characteristics unite the functional biology of CAM plant stomata? Were there multiple evolutionary routes to the same phenomenology, or do all CAM plants share the same molecular and metabolic basis for stomatal behavior? Second, how does variation in stomatal form and function among CAM species underpin physiological adaptation to the wide range of environmental niches these plants have come to occupy?
Researchers have adopted a multiplicity of approaches to shed light on these questions, spurred both by the enduring appeal of CAM as a “curiosity” (Osmond, 1978) and the rapidly growing interest in the application and engineering of CAM plants for bioenergy production (Borland et al., 2011, 2014, 2015; Owen and Griffiths, 2014; DePaoli et al., 2014; Yang et al., 2015). Simultaneously, the wider field of stomatal biology has experienced a renaissance in recent years, with numerous advances being made through both empirical and theoretical work. Although this research has generally been carried out in a C3 context, lessons can be carried through to the CAM world. Here, we provide a general synthesis of current understanding of CAM stomatal biology and identify key opportunities for future research.
PATTERNS OF STOMATAL CONDUCTANCE
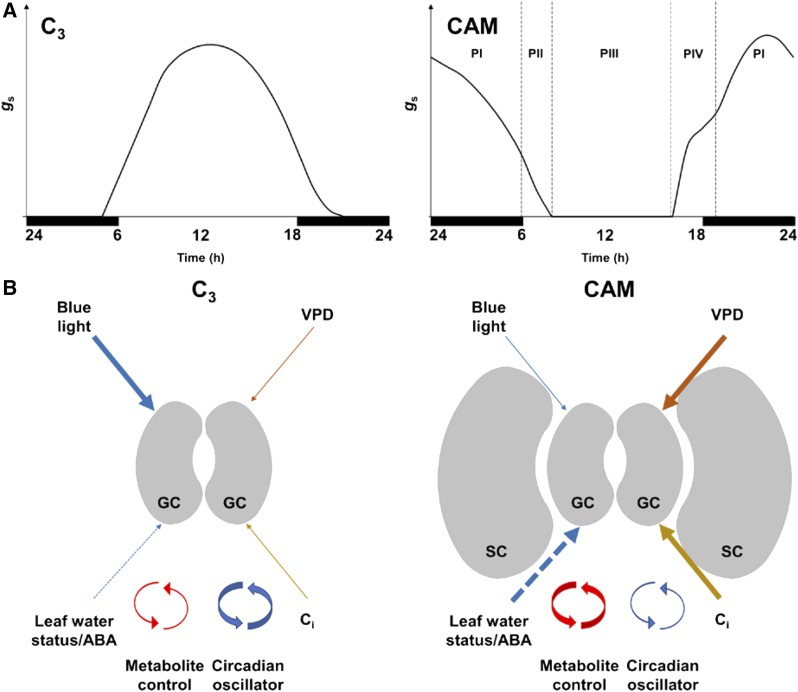
The four classical phases of CAM, driven by changes in carbon metabolism, coincide with changes in stomatal conductance across the diurnal cycle (Fig. 1; Osmond, 1978). Stomatal conductance is typically highest during the dark period (Phase I), in association with nocturnal CO2 assimilation by PEPC. During the dark period, mesophyll factors are often more important in limiting the rate of nocturnal assimilation than is stomatal conductance (Winter, 1985; Winter et al., 1985). Around dawn, there is often a spike in stomatal conductance and some direct fixation of CO2 by Rubisco (Phase II), which continues to fix CO2 released by decarboxylation of malic acid behind closed stomata during most of the light period (Phase III). During the late afternoon, if environmental conditions are favorable there may be a period of stomatal opening with direct Rubisco-mediated fixation of CO2 (Phase IV). However, this canonical pattern of gas exchange is subject to a large amount of interspecific, intraspecific, and intraindividual variation. One of the most remarkable features of CAM is its plasticity in response to environmental variability. The expression of the classical phases of CAM is modulated in response to recent and current environmental conditions (Dodd et al., 2002; Owen and Griffiths, 2013). Under low water availability and high evaporative demand, for instance, Phase IV stomatal opening may be completely abolished.
Figure 1.
Summary of differences in stomatal biology of C3 and CAM plants. A, Representative curves of day/night rhythms in stomatal conductance (gs) in C3 and CAM plants. The phases of the classical CAM gas exchange rhythm (Phases I–IV sensu; Osmond, 1978) are displayed. B, Schematic comparison of factors affecting regulation of stomatal aperture in C3 and CAM plants. Thicknesses of arrows indicate relative importance of factors in typical species of either photosynthetic pathway. The response of guard cells (GC) to blue light appears to be weaker or absent in many CAM species (e.g. Tallman et al., 1997), while strong sensitivity to leaf-air VPD occurs in many CAM plants (e.g. Lange and Medina, 1979). Leaf (or whole-plant) water status, often acting on stomatal aperture in an ABA-dependent manner, may have a stronger effect in CAM species (Jewer et al., 1981). Intercellular CO2 concentration (Ci) is a key driver of stomatal rhythms in CAM plants (e.g. Wyka et al., 2005), and this is intimately linked to the importance of metabolite control in CAM (Dever et al., 2015). While the circadian oscillator still plays a role in shaping CAM stomatal rhythms (von Caemmerer and Griffiths, 2009), it is perhaps of less direct importance than in C3 plants. Structural modifications are common in stomata of both C3 and CAM species, but specialized stomatal complex morphologies with large subsidiary cells appear to be overrepresented among CAM plants (see “Stomatal Structure-Function Relationships”).
Additionally, two frequently observed modes of CAM do not conform to the textbook four-phase gas exchange profile: “CAM cycling” and “CAM idling” (Sipes and Ting, 1985). CAM cycling involves the nocturnal operation of respiratory recycling and diurnal stomatal opening for direct Rubisco-mediated assimilation and most often occurs as a facultative trait in C3-CAM or “weak CAM” species (Silvera et al., 2010). Meanwhile, under CAM idling, stomata remain closed throughout the day and night, with a proportion of respiratory CO2 being refixed. CAM idling is often induced under extreme seasonal drought stress in “strong CAM” species, maximizing water retention (Silvera et al., 2010). This capacity for close environmental tracking on both diurnal and seasonal bases maximizes integrated water-use efficiency and is therefore an important contributor to the ecological success of CAM plants in stressful habitats (Fig. 1).
STOMATAL SENSITIVITY TO ENDOGENOUS STIMULI: CONTROL OF, AND BY, CAM
Stomata and mesophyll cell processes may be controlled by distinct circadian clocks in C3 species (Hubbard and Webb, 2016), and understanding the interplay between these cycles could provide insights for the coordination of CAM, as well as the interplay between responses to internal and external signals via metabolite feedback, internal CO2 availability (Ci), and environmental cues (Fig. 1).
The circadian rhythm of CAM plants involves the same system of clock genes as have been intensively studied in Arabidopsis (Arabidopsis thaliana; Boxall et al., 2005; Hubbard and Webb, 2016) and controls diurnal oscillations in physiological processes including photosynthetic enzyme activity (Nimmo, 2000; Hartwell, 2005). However, there is evidence for an important role for metabolite control of the temporal dynamics of the CAM cycle. For instance, manipulation of key decarboxylation and metabolite regeneration processes in Kalanchoë fedtschenkoi had a direct disruptive effect on the mesophyll circadian clock (Dever et al., 2015). Furthermore, reducing the capacity of CAM leaves to synthesize malic acid at night (by removal of external CO2 supply) showed that associated reductions in metabolite concentrations could override circadian control of PEPC kinase (Borland et al., 1999). However, the extent that the guard cell circadian cycle is synchronized with, or driven by, the mesophyll CAM cycle, remains to be determined.
Additional insights have been gained from combined measurements of gas exchange and carboxylation enzyme coregulation under continuous light. In Mesembryanthemum crystallinum, the timing of circadian rhythms of stomatal conductance, CO2 assimilation, and Rubisco/PEPC continued to be synchronized across light and dark cycles (Davies and Griffiths, 2012). However, stomatal conductance was lower when Rubisco carboxylation predominated at the end of the light period and higher when PEPC carboxylation predominated at the end of the dark periods, perhaps suggesting that guard cells are responding to the extent of CO2 drawdown and intercellular CO2 concentration (Ci; Davies and Griffiths, 2012).
Sensing CO2 concentrations has long been implicated in both C3 and CAM stomatal movements; intuitively, responding to Ci would seem to be the signal most likely to regulate the inverse stomatal cycle associated with CAM. At the beginning of Phase I of CAM, stomatal opening is thought to be driven by reduced Ci when PEPC activity increases at dusk (Wyka et al., 2005; Griffiths et al., 2007; von Caemmerer and Griffiths, 2009). In the morning, stomatal closure is then reinforced by the decarboxylation of stored malate during Phase III. This, coupled with respiration, can cause Ci to increase up to 100 times atmospheric concentration (Cockburn, 1979; Spalding et al., 1979). The reopening of stomata to initiate Phase IV is associated with the end of malic acid breakdown and, hence, internal CO2 limitation.
The responsiveness of CAM stomata to changing ambient CO2 transients was investigated in relation to degree of leaf succulence and commitment to the CAM cycle (von Caemmerer and Griffiths, 2009). The stomata of the more succulent Kalanchoë daigremontiana were more responsive to a CO2 transient reduction at night, whereas stomata in the less succulent Kalanchoë pinnata were more responsive during daytime Phase IV gas exchange. When CO2 uptake and malic accumulation were reduced overnight, and subsequent Ci regeneration lowered during Phase III, stomata still closed and showed little instantaneous response to CO2 transients, suggesting that circadian control of stomata remains a key factor controlling the CAM cycle in both species (von Caemmerer and Griffiths, 2009). However, there is still a lack of clarity in defining the interplay between circadian inputs from guard cells and mesophyll metabolism and how sensing of Ci and metabolites is transduced by stomata in CAM plants. The major advances in our understanding of the mechanism of CO2 sensing and regulation of stomatal conductance in C3 plants (Chater et al., 2015; Engineer et al., 2016) provide an excellent springboard for exploration of the role of equivalent genetic systems in CAM species. Abraham et al. (2016) have already demonstrated that there is a concerted shift in the temporal expression of components of CO2 signaling pathways in the constitutive CAM species Agave americana relative to C3 Arabidopsis. The generality of this observation among other CAM systems should now be explored and the regulatory mechanisms further elucidated.
STOMATAL RESPONSES TO EXTERNAL STIMULI
In addition to circadian control of stomatal and mesophyll processes, environmental tracking by CAM plant stomata is mediated by the integration of endogenous and exogenous signals by guard cells, as in C3 species (Assmann and Jegla, 2016).
The role of blue light in the stomatal movements of CAM plants has also not been fully resolved (Kinoshita, 2017). While there is some evidence for the involvement of blue light signaling in the regulation of stomatal conductance and malate decarboxylation in CAM bromeliads (Ceusters et al., 2014) and for the induction of CAM in Clusia minor (Grams and Thiel, 2002), other studies performed with facultative CAM plants have concluded that blue light regulates stomatal conductance of these plants only when they are in the C3 mode (Lee and Assmann, 1992; Tallman et al., 1997). Moreover, the results of transcriptomic analysis of the constitutive CAM plant A. americana were not consistent with a role for stomatal regulation by blue light (Abraham et al., 2016). This apparent divergence in stomatal regulation in different CAM lineages could hint at the existence of multiple mechanistic routes to CAM-like stomatal function.
Both leaf water potential and the humidity of the leaf microenvironment also affect stomatal conductance. Declining leaf water potential is a powerful driver of stomatal closure in C3 plants (Rodriguez-Dominguez et al., 2016). Although comparative data are quite limited, succulent CAM plant stomata tend to close at much higher (less negative) water potentials than those of co-occurring C3 plants (Osmond, 1978), consistent with evidence that succulent plants tend to avoid, or be isolated from, drought stress (Nobel, 1988; J. Males and H. Griffiths, unpublished data). Complete stomatal closure can therefore occur throughout both the light and dark periods (CAM idling).
Malate has been proposed as a mesophyll to guard cell signal in the regulation of stomatal aperture in response to mesophyll turgor and light-dark transitions in C3 plants (Araújo et al., 2011; Lawson et al., 2014; Costa et al., 2015), while oxaloacetate has been shown to be an effective inhibitor of guard cell anion channel activity (Wang and Blatt, 2011). The involvement of organic anions in stomatal regulation has interesting implications for CAM plants, in which malate can accumulate to high concentrations during Phase I (Osmond, 1978). The importance of abscisic acid (ABA), which is synthesized and mobilized in roots and shoots in response to declining water potential, in regulating stomatal closure in CAM plants, compared to C3 plants, remains to be determined (Cutler, 2017; Jezek and Blatt, 2017). Jewer et al. (1981) suggested that stomata of CAM plants might be hypersensitive to ABA, which would be consistent with strategies for avoiding soil water deficits (tissue water potentials usually > −1 MPa), water storage, and rapid recharge in succulent tissues. Recent progress in our understanding of the role of ABA in the evolution of stomatal responses should be brought to bear on CAM plants (Negin and Moshelion, 2016). The debate over the origins of signaling pathways for both ABA and CO2 continues, and contrasting observations in ferns (which do contain CAM lineages; Ong et al., 1986; Winter et al., 1986) await resolution (McAdam and Brodribb, 2012; compare Chater et al., 2015; Franks and Britton-Harper, 2016).
An apparent feed-forward response of transpiration to rising leaf-air vapor pressure deficit (VPD), in which stomata seem to respond directly to humidity rather than indirectly via leaf water status, has been observed in some CAM lineages, with important consequences for assimilation rates and water-use efficiency under contrasting humidity regimes (Lange and Medina, 1979; Osmond et al., 1979; Martin and Siedow, 1981; von Willert et al., 1985; Lüttge et al., 1986; Herppich, 1997). Epiphytic CAM species might be expected to show particularly high levels of stomatal sensitivity to VPD, given the special adaptive value this would have in highly water-limited epiphytic environments (see discussion of integrated leaf traits below). Indeed, in C3 plants, stomatal sensitivities to VPD and leaf water potential are often strongly correlated with leaf or petiole hydraulic conductances and their sensitivity to tissue water potential (Brodribb and Jordan, 2008; Ocheltree et al., 2013, 2014; Klein, 2014; Tombesi et al., 2014; Bartlett et al., 2016). The mechanisms underlying stomatal sensitivity to VPD remain a controversial and active area of research, with the possibility of liquid- and/or vapor-phase signals being involved alongside ABA synthesis and signaling within guard cells (Peak and Mott, 2011; Bauer et al., 2013; Buckley and Mott, 2013; Mott and Peak, 2013; McAdam et al., 2016). Because of the potentially significant metabolic and signaling interactions between guard cells and the mesophyll, integrated investigation of stomatal sensitivity and the dynamic responses of the critical extravascular component of leaf hydraulic conductance in CAM (and C3) species is highly desirable (Sack et al., 2016; Trifiló et al., 2016). Analysis of the spatiotemporally dynamic expression patterns of aquaporins and of possible interactions between stomatal physiology and mesophyll osmotic properties could be especially fruitful (Pou et al., 2013; Martorell et al., 2015).
A final factor that has been demonstrated to influence stomatal conductance in CAM plants is temperature, with optimal CAM activity usually associated with narrow and relatively low (usually ∼15–25°C) nocturnal temperature windows (Yamori et al., 2014). Both thermoperiodic effects (Ting et al., 1967) and instantaneous leaf temperature effects (Nobel and Hartsock, 1979) have been reported. Given the known importance of nocturnal leaf temperature for the efficiency of malate synthesis and decarboxylation (e.g. Neales, 1973; Moradshahi et al., 1977; Nobel and Hartsock, 1984), water-use efficiency should be maximized through the regulation of stomatal conductance in line with temperature.
GUARD CELL METABOLISM
Guard cell metabolism in C3, C4, and CAM plants continues to be a fast-paced area of research with many critical questions awaiting resolution (Daloso et al., 2016; Santelia and Lunn, 2017). The similarities between guard cell metabolism in C3 plants and the metabolism of mesophyll cells of CAM plants are striking, which led Cockburn (1981) to suggest that a transfer of guard cell-like metabolism to mesophyll cells was a central event in evolutionary origins of CAM. More recent work has highlighted the importance of organic acids in C3 guard cell function (e.g. Wang and Blatt, 2011; Penfield et al., 2012; Daloso et al., 2015; Medeiros et al., 2016).
Controlled ion fluxes are fundamental to the operation of stomatal movements (Chen et al., 2012; Minguet-Parramona et al., 2016; Eisenach and de Angeli, 2017; Jezek and Blatt, 2017). In comparing the day-night transcriptomic profiles of C3 Arabidopsis and the constitutive CAM plant A. americana, Abraham et al. (2016) showed that there was a coordinated shift in the temporal expression patterns of key ion channels in A. americana. Notably, orthologous vacuolar chloride channel genes displayed reciprocal expression in the C3 and CAM species, which could help to drive appropriate charge balancing.
The presence of Rubisco in the guard cells of some CAM plants needs further investigation in the context of the emerging role of guard cell photosynthesis in the regulation of stomatal conductance in C3 plants (Madhavan and Smith, 1982; Azoulay-Shemer et al., 2015). Tallman (2004) suggested that guard cell photosynthesis could be supplied with large amounts of CO2 from the mesophyll during Phase III of CAM, establishing a strong sink for NADPH and thus inhibiting the degradation of guard cell endogenous ABA, which promotes stomata closure (Lind et al., 2015). In this way, guard cell photosynthesis in CAM plants could assist in the maintenance of negligible diurnal stomatal conductance during the light period.
Santelia and Lawson (2016), citing earlier work carried out by Pantoja and Smith (2002), recently highlighted the absence of the correlation between malate currents across the guard cell tonoplast and cytosolic calcium concentrations across CAM species that would be expected if they shared a uniform regulatory mechanism. This apparent diversity in stomatal physiology could have important consequences for our understanding of the evolution of complex syndromes like CAM. Further empirical studies of this topic are needed to advance our understanding of the imposition of daytime stomatal closure in CAM plants. Cell-specific perturbation of metabolic function offers an exciting opportunity in this respect (Lawson et al., 2014).
COORDINATION OF STOMATAL TRAITS WITH LEAF TRAIT NETWORKS
CAM species have rarely been included in analyses of leaf economic trait variation, partly because succulence is one trait which uncouples leaf mass-based relationships (Grubb et al., 2015; J. Males and H. Griffiths, unpublished data). However, in a survey of leaf economic, anatomical, and hydraulic traits in the Bromeliaceae, we found that CAM bromeliads tended to show lower stomatal density and conductance as well as lower leaf hydraulic conductance, photosynthetic capacity, and nutrient content, and higher leaf mass per unit area (J. Males and H. Griffiths, unpublished data). Variation in stomatal traits appears to be accommodated within a network of coordinated leaf traits in CAM species in the same way as has been observed in C3 plants (Reich et al., 1997, 1999; Wright et al., 2004, 2005; Donovan et al., 2011; Vasseur et al., 2012; Díaz et al., 2016). Recent modeling and empirical studies have highlighted the importance of the alignment of variation in stomatal, xylem, and veinal traits in angiosperms for optimal physiological function (Brodribb et al., 2013, 2016; Fiorin et al., 2016; Carins Murphy et al., 2016; Scoffoni et al., 2016). It would be particularly interesting to explore the degree of coordination between Phase I (nighttime) and Phase IV (daytime) stomatal and mesophyll conductances in CAM plants. Although few data are available, it is expected that mesophyll conductance is generally low in CAM plants due to their succulent anatomy with tight cell-packing (Maxwell et al., 1997; Nelson and Sage, 2008). Campany et al. (2016) recently showed that coupled responses of stomatal and mesophyll conductances to light improved carbon gain during sunfleck events in shade leaves of a Eucalyptus species. Similar effects are likely to be important in CAM epiphytes of the humid tropics with sunfleck-driven carbon economies.
STOMATAL STRUCTURE-FUNCTION RELATIONSHIPS
CAM has arisen in a wide range of taxonomic and morpho-anatomical backgrounds, and this is reflected in the various stomatal complex morphologies found in different CAM lineages. When variation is considered among the angiosperms at the family level, using the APG IV classification (Angiosperm Phylogeny Group, 2016) and anatomical data from the DELTA database (Watson and Dallwitz, 1992), the proportional occurrence of different stomatal complex morphologies shows several potentially important differences between CAM and C3 lineages. None of the monocot families with CAM elements display anomocytic stomata (lacking subsidiary cells), whereas 26% of exclusively C3 monocot families do. Tetracytic stomata (four subsidiary cells) are nearly twice as common in CAM families as in C3 families. Among the dicots, anomocytic stomata are also less common in CAM families, and there are relatively more CAM families with paracytic stomata (two subsidiary cells). The overrepresentation of CAM in families with more specialized stomatal complexes in both monocots and dicots has not been investigated from a functional perspective. However, it is well established that the presence of subsidiary cells in C3 and C4 species can enhance the kinetics of stomatal movements (Franks and Farquhar, 2007), and systematic differences in stomatal kinetics and sensitivity may occur between CAM species with contrasting stomatal morphologies. Empirical and theoretical work in the C3 context also suggests that stomatal size could be an important determinant of the rapidity of stomatal movements (Drake et al., 2013; Lawson and Blatt, 2014; Raven, 2014), although this relationship may be modulated by guard cell morphology (McAusland et al., 2016). These trait linkages are potentially of great evolutionary and ecological importance and could easily be tested for in CAM plants. It is interesting to note that among the few fern lineages to have evolved CAM, modified polocytic and pericytic stomatal complexes occur, wherein the guard cell pair is surrounded either completely or partially by one or two subsidiary cells (e.g. Patel et al., 1975; Sen and Hennipman, 1981).
When compared with their nearest C3 relatives, CAM lineages show no consistent differences in guard cell ultrastructure (Faraday et al., 1982) but do tend to display a shift toward lower stomatal densities and lower maximal conductances in CAM plants (Ting et al., 1972; Kluge and Ting, 1978; Gibson, 1982; Zambrano et al., 2014; J. Males and H. Griffiths, unpublished data). These reductions have widely been interpreted as adaptive xeromorphic traits in their own right, but there is now accumulating evidence for a developmental constraint that generates a robust negative relationship between stomatal density and the sizes of guard cells and mesophyll cells (Brodribb et al., 2013). Since CAM is dependent on the presence of highly vacuolate succulent cells for malate storage, low stomatal densities could be a necessary trade-off. Further investigation of the coordination of stomatal traits, cell sizes, succulence, and perhaps genome sizes (Beaulieu et al., 2008) could prove illuminating.
STOMATA ON THE RUGGED CAM ADAPTIVE LANDSCAPE
CAM is now often discussed as a continuum of intergrading and flexible photosynthetic modes rather than a monolithic, discrete trait (Silvera et al., 2010; Winter et al., 2015). The existence of a wide range of CAM types and the occurrence of evolutionary reversions from CAM to C3 (Teeri, 1982a, 1982b; Silvera et al., 2009; Givnish et al., 2014) is a reflection of a rugged adaptive landscape with multiple peaks. While the description of the C3-C4 adaptive landscape as “Mount Fuji-like” (Heckmann et al., 2013) is a simplified abstraction, there are convincing accounts of the demonstrable increases in fitness associated with each step between full-C3 and full-C4 metabolism in independent C4 origins (Christin et al., 2011, 2013; Griffiths et al., 2013; Heckmann et al., 2013; Sage et al., 2013; Schlüter and Weber, 2016). In the absence of the wealth of phylogenetic and physiological information enjoyed by the C4 community, and despite the possibility that C4 and CAM represent alternative evolutionary pathways from similar starting points (Edwards and Ogburn, 2012), the picture for CAM is far murkier (Hancock and Edwards, 2014). Succulence has been identified as an anatomical prerequisite for CAM (Sage, 2002; Zambrano et al., 2014; Heyduk et al., 2016), but beyond this there is little clarity regarding the relative timing of the acquisition of component traits of the CAM syndrome or the extent to which different types of CAM could represent independent adaptive peaks. In particular, the involvement of stomatal innovation in convergent origins of CAM is unclear. How does the capacity for stomatal flexibility vary among the C3 sister taxa of CAM lineages? During evolutionary transitions from C3 to CAM, do any less obvious changes in stomatal biology occur prior to the appearance of the inverse stomatal rhythm? Is the answer to this question the same for lineages that have only evolved weak CAM (CAM cycling) as for those that have evolved strong CAM? Concerted efforts to improve phylogenetic resolution in critical lineages in which C3-to-CAM transitions have occurred, a more accurate diagnosis of “cryptic” low-level CAM, and targeted surveys of stomatal physiological traits and molecular biology in representative taxa would all be important preliminary steps toward unraveling these long-standing evolutionary puzzles.
CONCLUSIONS AND FUTURE PERSPECTIVES
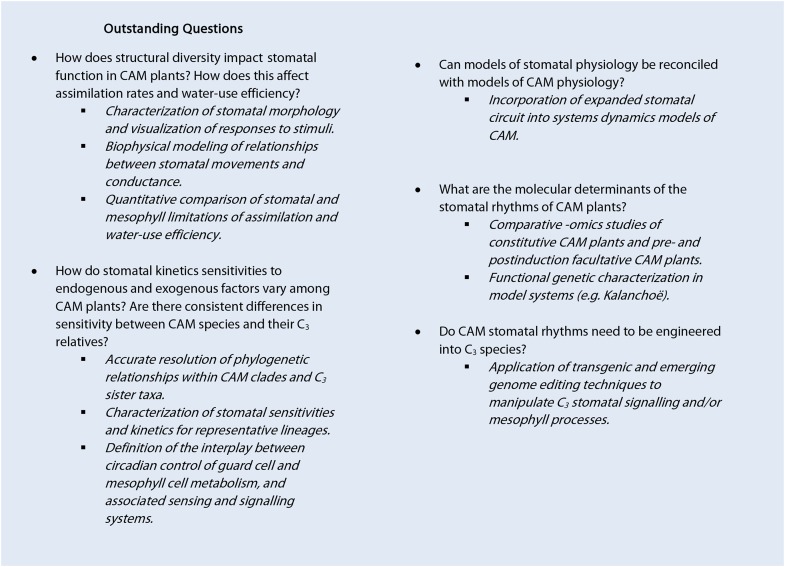
CAM is a major ecophysiological syndrome that has been repeatedly identified as providing high potential for sustainable production under climate change (Borland et al., 2011, 2014, 2015; Owen and Griffiths, 2014; Yang et al., 2015). Harnessing this potential is contingent upon a comprehensive understanding of the underlying physiology of CAM. Recent work has contributed to our knowledge of how stomatal specialization is involved in the unique metabolic flexibility and water-use efficiency afforded by CAM, while insights gained from work on the stomatal biology of non-CAM plants can also be reinterpreted from a CAM perspective. However, there is still much to be learned about the functioning of CAM stomata (see Outstanding Questions). One promising route for future research will be to make use of known C3-CAM intermediates and facultative CAM species as tools for exploring the molecular changes associated with the commencement of CAM stomatal rhythms (Winter and Holtum, 2014; Brilhaus et al., 2016). The identification of gradients in the relative contributions of C3 and CAM along the linear leaves of C3-CAM intermediate monocot species is another naturally occurring system ripe for further investigation (Popp et al., 2003; Freschi et al., 2010). Increasingly sensitive technologies will improve the ease of in situ and ex situ physiological characterization (e.g. Barkla and Rhodes, 2017), and robust transcriptomic methodologies will be crucial for elucidating the molecular genetic basis of divergences in stomatal function along the CAM continuum and under variable environments. Finally, the integration of recently developed physiological models of CAM (Owen and Griffiths, 2013; Bartlett et al., 2014; Hartzell et al., 2015) with more detailed models of stomatal conductance could be a powerful way of exploring the significance of variation in stomatal traits for carbon gain and water-use efficiency.
Supplementary Material
Glossary
- CAM
Crassulacean acid metabolism
- PEPC
phospho-enol-pyruvate carboxylase
- ABA
abscisic acid
- VPD
vapor pressure deficit
Footnotes
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Abraham PE, Yin H, Borland AM, Weighill D, Lim SD, De Paoli HC, Engle N, Jones PC, Agh R, Weston DJ, et al. (2016) Transcript, protein and metabolite temporal dynamics in the CAM plant Agave. Nat Plants 2: 16178. [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group (2016) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot J Linn Soc 181: 1–20 [Google Scholar]
- Araújo WL, Fernie AR, Nunes-Nesi A (2011) Control of stomatal aperture: a renaissance of the old guard. Plant Signal Behav 6: 1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Jegla T (2016) Guard cell sensory systems: recent insights on stomatal responses to light, abscisic acid, and CO2. Curr Opin Plant Biol 33: 157–167 [DOI] [PubMed] [Google Scholar]
- Azoulay-Shemer T, Palomares A, Bagheri A, Israelsson-Nordstrom M, Engineer CB, Bargmann BOR, Stephan AB, Schroeder JI (2015) Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J 83: 567–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla BJ, Rhodes T (2017) Use of infrared thermography for monitoring crassulacean acid metabolism. Funct Plant Biol http://dx.doi.org/10.1071/FP16210 [DOI] [PubMed] [Google Scholar]
- Bartlett MK, Klein T, Jansen S, Choat B, Sack L (2016) The correlations and sequence of plant stomatal, hydraulic, and wilting responses to drought. Proc Natl Acad Sci USA 113: 13098–13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett MS, Vico G, Porporato A (2014) Coupled carbon and water fluxes in CAM photosynthesis: modeling quantification of water use efficiency and productivity. Plant Soil 383: 111–138 [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179: 975–986 [DOI] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Jenkins GI, Wilkins MB, Nimmo HG (1999) Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in crassulacean acid metabolism. Plant Physiol 121: 889–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Hartwell J, Weston DJ, Schlauch KA, Tschaplinski TJ, Tuskan GA, Yang X, Cushman JC (2014) Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci 19: 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Barrera Zambrano VA, Ceusters J, Shorrock K (2011) The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytol 191: 619–633 [DOI] [PubMed] [Google Scholar]
- Borland AM, Wullschleger SD, Weston DJ, Hartwell J, Tuskan GA, Yang X, Cushman JC (2015) Climate-resilient agroforestry: physiological responses to climate change and engineering of crassulacean acid metabolism (CAM) as a mitigation strategy. Plant Cell Environ 38: 1833–1849 [DOI] [PubMed] [Google Scholar]
- Boxall SF, Foster JM, Bohnert HJ, Cushman JC, Nimmo HG, Hartwell J (2005) Conservation and divergence of circadian clock operation in a stress-inducible Crassulacean acid metabolism species reveals clock compensation against stress. Plant Physiol 137: 969–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilhaus D, Bräutigam A, Mettler-Altmann T, Winter K, Weber AP (2016) Reversible burst of transcriptional changes during induction of Crassulacean Acid Metabolism in Talinum triangulare. Plant Physiol 170: 102–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ (2008) Internal coordination between hydraulics and stomatal control in leaves. Plant Cell Environ 31: 1557–1564 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ, Carpenter RJ (2013) Unified changes in cell size permit coordinated leaf evolution. New Phytol 199: 559–570 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Carins Murphy MR (2016) Xylem and stomata, coordinated through time and space. Plant Cell Environ http://dx.doi.org/10.1111/pce.12817 [DOI] [PubMed] [Google Scholar]
- Buckley TN, Mott KA (2013) Modelling stomatal conductance in response to environmental factors. Plant Cell Environ 36: 1691–1699 [DOI] [PubMed] [Google Scholar]
- Campany CE, Tjoelker MG, von Caemmerer S, Duursma RA (2016) Coupled response of stomatal and mesophyll conductance to light enhances photosynthesis of shade leaves under sunflecks. Plant Cell Environ 39: 2762–2773 [DOI] [PubMed] [Google Scholar]
- Carins Murphy MR, Jordan GJ, Brodribb TJ (2016) Cell expansion not cell differentiation predominantly co-ordinates veins and stomata within and among herbs and woody angiosperms grown under sun and shade. Ann Bot (Lond) 118: 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Borland AM, Taybi T, Frans M, Godts C, De Proft MP (2014) Light quality modulates metabolic synchronization over the diel phases of crassulacean acid metabolism. J Exp Bot 65: 3705–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang YK, McLachlan DH, Casson S, Isner JC, Wilson I, et al. (2015) Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr Biol 25: 2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-H, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci USA 110: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin P-A, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF (2011) Complex evolutionary transitions and the significance of c(3)-c(4) intermediate forms of photosynthesis in Molluginaceae. Evolution 65: 643–660 [DOI] [PubMed] [Google Scholar]
- Cirelli D, Equiza MA, Lieffers VJ, Tyree MT (2016) Populus species from diverse habitats maintain high night-time conductance under drought. Tree Physiol 36: 229–242 [DOI] [PubMed] [Google Scholar]
- Cockburn W. (1981) The evolutionary relationship between stomatal mechanism, crassulacean acid metabolism and C4 photosynthesis. Plant Cell Environ 4: 417–418 [Google Scholar]
- Cockburn W. (1979) Relationships between stomatal behaviour and internal carbon dioxide concentration in Crassulacean acid metabolism plants. Plant Physiol 63: 1029–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JM, Monnet F, Jannaud D, Leonhardt N, Ksas B, Reiter IM, Pantin F, Genty B (2015) Open all night long: the dark side of stomatal control. Plant Physiol 167: 289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daloso DM, Antunes WC, Pinheiro DP, Waquim JP, Araújo WL, Loureiro ME, Fernie AR, Williams TCR (2015) Tobacco guard cells fix CO2 by both Rubisco and PEPcase while sucrose acts as a substrate during light-induced stomatal opening. Plant Cell Environ 38: 2353–2371 [DOI] [PubMed] [Google Scholar]
- Daloso DM, Dos Anjos L, Fernie AR (2016) Roles of sucrose in guard cell regulation. New Phytol 211: 809–818 [DOI] [PubMed] [Google Scholar]
- Davies BN, Griffiths H (2012) Competing carboxylases: circadian and metabolic regulation of Rubisco in C3 and CAM Mesembryanthemum crystallinum L. Plant Cell Environ 35: 1211–1220 [DOI] [PubMed] [Google Scholar]
- Eisenach C, de Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios VR, Turnbull MH, Barbour MM, Ontedhu J, Ghannoum O, Tissue DT (2013) Soil phosphorous and endogenous rhythms exert a larger impact than CO2 or temperature on nocturnal stomatal conductance in Eucalyptus tereticornis. Tree Physiol 33: 1206–1215 [DOI] [PubMed] [Google Scholar]
- DePaoli HC, Borland AM, Tuskan GA, Cushman JC, Yang X (2014) Synthetic biology as it relates to CAM photosynthesis: challenges and opportunities. J Exp Bot 65: 3381–3393 [DOI] [PubMed] [Google Scholar]
- De Saussure T. (1804) Recherches Chimiques sur la Végétation. Chez la Ve. Nyon, Paris [Google Scholar]
- Dever LV, Boxall SF, Kneřová J, Hartwell J (2015) Transgenic perturbation of the decarboxylation phase of Crassulacean acid metabolism alters physiology and metabolism but has only a small effect on growth. Plant Physiol 167: 44–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, et al. (2016) The global spectrum of plant form and function. Nature 529: 167–171 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Maherali H, Caruso CM, Huber H, de Kroon H (2011) The evolution of the worldwide leaf economics spectrum. Trends Ecol Evol 26: 88–95 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53: 569–580 [DOI] [PubMed] [Google Scholar]
- Drake PL, Froend RH, Franks PJ (2013) Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot 64: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. Int J Plant Sci 173: 724–733 [Google Scholar]
- Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel WJ, Iba K, Schroeder JI (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21: 16–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faak M. editor (2000) Alexander von Humboldt. Reise durch Venezuela. Auswahl aus den amerikanischen Resietagebüchern. Beiträge zur Alexander von Humboldt-Forschung 12. Akademie-Verlag, Berlin [Google Scholar]
- Faraday CD, Thomson WW, Platt-Aloia KA(1982) Comparative ultrastructure of guard cells of C3, C4 and CAM plants. In IP Ting, M Gibbs, eds, Crassulacean Acid Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 18–26 [Google Scholar]
- Fiorin L, Brodribb TJ, Anfodillo T (2016) Transport efficiency through uniformity: organization of veins and stomata in angiosperm leaves. New Phytol 209: 216–227 [DOI] [PubMed] [Google Scholar]
- Forster MA. (2014) How significant is nocturnal sap flow? Tree Physiol 34: 757–765 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Britton-Harper ZJ (2016) No evidence of general CO2 insensitivity in ferns: one stomatal control mechanism for all land plants? New Phytol 211: 819–827 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L, Takahashi CA, Cambui CA, Semprebom TR, Cruz AB, Mioto PT, de Melo Versieux L, Calvente A, Latansio-Aidar SR, Aidar MPM, Mercier H (2010) Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water shortage. J Plant Physiol 167: 526–533 [DOI] [PubMed] [Google Scholar]
- Gibson AC. (1982) The anatomy of succulence. In IP Ting M, Gibbs, eds, Crassulacean Acid Metabolism. American Society of Plant Physiologists, Rockville, MD, pp: 1–17 [Google Scholar]
- Givnish TJ, Barfuss MHJ, Van Ee B, Riina R, Schulte K, Horres R, Gonsiska PA, Jabaily RS, Crayn DM, Smith JA, et al. (2014) Adaptive radiation, correlated and contingent evolution, and net species diversification in Bromeliaceae. Mol Phylogenet Evol 71: 55–78 [DOI] [PubMed] [Google Scholar]
- Goh CJ, Arditti J, Avadhani PN (1983) Carbon fixation in orchid aerial roots. New Phytol 95: 367–374 [Google Scholar]
- Grams TEE, Thiel S (2002) High light-induced switch from C(3)-photosynthesis to Crassulacean acid metabolism is mediated by UV-A/blue light. J Exp Bot 53: 1475–1483 [PubMed] [Google Scholar]
- Griffiths H, Cousins AB, Badger MR, von Caemmerer S (2007) Discrimination in the dark. Resolving the interplay between metabolic and physical constraints to phosphoenolpyruvate carboxylase activity during the crassulacean acid metabolism cycle. Plant Physiol 143: 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H, Weller G, Toy LFM, Dennis RJ (2013) You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ 36: 249–261 [DOI] [PubMed] [Google Scholar]
- Grubb PJ, Marañón T, Pugnaire FI, Sack L (2015) Relationships between specific leaf area and leaf composition in succulent and non-succulent species of contrasting semi-desert communities in south-eastern Spain. J Arid Environ 118: 69–83 [Google Scholar]
- Hancock L, Edwards EJ (2014) Phylogeny and the inference of evolutionary trajectories. J Exp Bot 65: 3491–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J. (2005) The co-ordination of central plant metabolism by the circadian clock. Biochem Soc Trans 33: 945–948 [DOI] [PubMed] [Google Scholar]
- Hartzell S, Bartlett MS, Virgin L, Porporato A (2015) Nonlinear dynamics of the CAM circadian rhythm in response to environmental forcing. J Theor Biol 368: 83–94 [DOI] [PubMed] [Google Scholar]
- Heckmann D, Schulze S, Denton A, Gowik U, Westhoff P, Weber APM, Lercher MJ (2013) Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Herppich WB. (1997) Stomatal responses to changes in air humidity are not necessarily linked to nocturnal CO2 uptake in the CAM plant Plectranthus marrubioides Benth. (Lamiaceae). Plant Cell Environ 20: 393–399 [Google Scholar]
- Heyduk K, McKain MR, Lalani F, Leebens-Mack J (2016) Evolution of a CAM anatomy predates the origins of Crassulacean acid metabolism in the Agavoideae (Asparagaceae). Mol Phylogenet Evol 105: 102–113 [DOI] [PubMed] [Google Scholar]
- Heyne B. (1815) On the deoxidation of the leaves of Cotyledon calycina. Trans Linn Soc Lond 11: 213–215 [Google Scholar]
- Hubbard KE, Webb AAR (2016) Circadian rhythms in stomata: physiological and molecular aspects. In S Mancuso S, Shabala, eds, Rhythms in Plants: Dynamics Responses in a Dynamic Environment. Springer, Cham, Switzerland, pp 231–255 [Google Scholar]
- Inoue S-i, Kinoshita T (2017) Blue light regulation of stomatal opening and the plasma membrane H1-ATPase. Plant Physiol 174: 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewer PC, Incoll LD, Howarth GL (1981) Stomatal responses in isolated epidermis of the crassulacean acid metabolism plant Kalanchoe daigremontiana Hamet et Perr. Planta 153: 238–245 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley JE. (1998) CAM photosynthesis in submerged aquatic plants. Bot Rev 64: 121–175 [Google Scholar]
- Klein T. (2014) The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol 28: 1313–1320 [Google Scholar]
- Kluge M, Ting IP (1978) Crassulacean Acid Metabolism. Analysis of an Ecological Adaptation. Springer, Berlin [Google Scholar]
- Lange OL, Medina E (1979) Stomata of the CAM plant Tillandsia recurvata respond directly to humidity. Oecologia 40: 357–363 [DOI] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Lee DM, Assmann SM (1992) Stomatal responses to light in the facultative Crassulacean acid metabolism species Portulacaria afra. Physiol Plant 85: 35–42 [Google Scholar]
- Lind C, Dreyer I, López-Sanjurjo EJ, von Meyer K, Ishizaki K, Kohchi T, Lang D, Zhao Y, Kreuzer I, Al-Rasheid KA, et al. (2015) Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr Biol 25: 928–935 [DOI] [PubMed] [Google Scholar]
- Lüttge U. (2010) Ability of crassulacean acid metabolism plants to overcome interacting stresses in tropical environments. AoB Plants 2010: plq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U, Stimmel K-H, Smith JAC, Griffiths H (1986) Comparative ecophysiology of CAM and C3 bromeliads. II. Field measurements of gas exchange of CAM bromeliads in the humid tropics. Plant Cell Environ 9: 377–383 [Google Scholar]
- Madhavan S, Smith BN (1982) Localization of ribulose bisphosphate carboxylase in the guard cells by an indirect, immunofluorescence technique. Plant Physiol 69: 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CE, Siedow JN (1981) Crassulacean acid metabolism in the epiphyte Tillandsia usneoides L. (Spanish moss). Plant Physiol 68: 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell S, Medrano H, Tomàs M, Escalona JM, Flexas J, Diaz-Espejo A (2015) Plasticity of vulnerability to leaf hydraulic dysfunction during acclimation to drought in grapevines: an osmotic-mediated process. Physiol Plant 153: 381–391 [DOI] [PubMed] [Google Scholar]
- Matimati I, Verboom GA, Cramer MD (2014) Do hydraulic redistribution and nocturnal transpiration facilitate nutrient acquisition in Aspalathus linearis? Oecologia 175: 1129–1142 [DOI] [PubMed] [Google Scholar]
- Maxwell K, von Caemmerer S, Evans JR (1997) Is a low internal conductance to CO2 diffusion a consequence of succulence in plants with Crassulacean acid metabolism? Aust J Plant Physiol 24: 777–786 [Google Scholar]
- McAdam SAM, Brodribb TJ (2012) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ (2016) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 39: 485–491 [DOI] [PubMed] [Google Scholar]
- McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol 211: 1209–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros DB, Martins SCV, Cavalcanti JHF, Daloso DM, Martinoia E, Nunes-Nesi A, DaMatta FM, Fernie AR, Araújo WL (2016) Enhanced photosynthesis and growth in atquac1 knockout mutants are due to altered organic acid accumulation and an increase in both stomatal and mesophyll conductance. Plant Physiol 170: 86–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradshahi A, Vines HM, Black CC (1977) Carbon dioxide exchange and acidity levels in detached pineapple, Ananas comosus (L.) Merr., leaves during the day at various temperatures, oxygen and carbon dioxide concentrations. Plant Physiol 59: 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Peak D (2013) Testing a vapour-phase model of stomatal responses to humidity. Plant Cell Environ 36: 936–944 [DOI] [PubMed] [Google Scholar]
- Neales TF. (1973) The effect of night temperature on CO2 assimilation, transpiration, and water use efficiency in Agave americana L. Aust J Biol Sci 26: 705–714 [Google Scholar]
- Negin B, Moshelion M (2016) The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci 251: 82–89 [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage RF (2008) Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. J Exp Bot 59: 1841–1850 [DOI] [PubMed] [Google Scholar]
- Nimmo HG. (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5: 75–80 [DOI] [PubMed] [Google Scholar]
- Nobel PS. (1988) Environmental Biology of Cacti and Agaves. Cambridge University Press, Cambridge, UK [Google Scholar]
- Nobel PS, Hartsock TL (1979) Environmental influences on open stomates of a Crassulacean acid metabolism plant, Agave deserti. Plant Physiol 63: 63–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel PS, Hartsock TL (1984) Physiological responses of Opuntia ficus-indica to growth temperature. Physiol Plant 60: 98–105 [Google Scholar]
- Ocheltree TW, Nippert JB, Kirkham MB, Prasad PVV (2013) Partitioning hydraulic resistance in Sorghum bicolor leaves reveals unique correlations with stomatal conductance during drought. Funct Plant Biol 41: 25–36 [DOI] [PubMed] [Google Scholar]
- Ocheltree TW, Nippert JB, Prasad PVV (2014) Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant Cell Environ 37: 132–139 [DOI] [PubMed] [Google Scholar]
- Ong BL, Kluge M, Friemert V (1986) Crassulacean acid metabolism in the epiphytic ferns Drymoglossum piloselloides and Pyrrosia longifolia: studies on responses to environmental signals. Plant Cell Environ 9: 547–557 [Google Scholar]
- Osmond CB. (1978) Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol 29: 379–414 [Google Scholar]
- Osmond CB, Ludlow MM, Davis R, Cowan IR, Powles SB, Winter K (1979) Stomatal responses to humidity in Opuntia inermis in relation to control of CO2 and H2O exchange patterns. Oecologia 41: 65–76 [DOI] [PubMed] [Google Scholar]
- Owen NA, Griffiths H (2013) A system dynamics model integrating physiology and biochemical regulation predicts extent of crassulacean acid metabolism (CAM) phases. New Phytol 200: 1116–1131 [DOI] [PubMed] [Google Scholar]
- Owen NA, Griffiths H (2014) Marginal land bioethanol yield potential of four crassulacean acid metabolism candidates (Agave fourcroydes, Agave salmiana, Agave tequilana and Opuntia ficus-indica) in Australia. Glob Change Biol Bioenergy 6: 687–703 [Google Scholar]
- Pantoja O, Smith JAC (2002) Sensitivity of the plant vacuolar malate channel to pH, Ca2+ and anion-channel blockers. J Membr Biol 186: 31–42 [DOI] [PubMed] [Google Scholar]
- Patel JD, Raju EC, Fotedar RL, Kothari IL, Shah JJ (1975) Structure and histochemistry of stomata and epidermal cells in five species of Polypodiaceae. Ann Bot (Lond) 38: 611–619 [Google Scholar]
- Peak D, Mott KA (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ 34: 162–178 [DOI] [PubMed] [Google Scholar]
- Penfield S, Clements S, Bailey KJ, Gilday AD, Leegood RC, Gray JE, Graham IA (2012) Expression and manipulation of phosphoenolpyruvate carboxykinase 1 identifies a role for malate metabolism in stomatal closure. Plant J 69: 679–688 [DOI] [PubMed] [Google Scholar]
- Popp M, Janett H-P, Lüttge U, Medina E (2003) Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytol 157: 649–656 [DOI] [PubMed] [Google Scholar]
- Pou A, Medrano H, Flexas J, Tyerman SD (2013) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ 36: 828–843 [DOI] [PubMed] [Google Scholar]
- Raven JA. (2014) Speedy small stomata? J Exp Bot 65: 1415–1424 [DOI] [PubMed] [Google Scholar]
- Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, Bowman WD (1999) Generality of leaf trait relationships: a test across six biomes. Ecology 80: 1955–1969 [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94: 13730–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resco de Dios V, Loik ME, Smith R, Aspinwall MJ, Tissue DT (2016) Genetic variation in circadian regulation of nocturnal stomatal conductance enhances carbon assimilation and growth. Plant Cell Environ 39: 3–11 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Dominguez CM, Buckley TN, Egea G, de Cires A, Hernandez-Santana V, Martorell S, Diaz-Espejo A (2016) Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ 39: 2014–2026 [DOI] [PubMed] [Google Scholar]
- Sack L, Buckley TN, Scoffoni C (2016) Why are leaves hydraulically vulnerable? J Exp Bot 67: 4917–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. (2002) Are Crassulacean acid metabolism and C4 photosynthesis incompatible? Funct Plant Biol 29: 775–785 [DOI] [PubMed] [Google Scholar]
- Sage TL, Busch FA, Johnson DC, Friesen PC, Stinson CR, Stata M, Sultmanis S, Rahman BA, Rawsthorne S, Sage RF (2013) Initial events during the evolution of C4 photosynthesis in C3 species of Flaveria. Plant Physiol 163: 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Lawson T (2016) Rethinking guard cell metabolism. Plant Physiol 172: 1371–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Lunn J (2017) Transitory starch metabolism in guard cells: unique features for a unique function. Plant Physiol 174: 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter U, Weber APM (2016) The road to C4 photosynthesis: evolution of a complex trait via intermediary traits. Plant Cell Physiol 57: 881–889 [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Chatelet D, Pasquet-Kok J, Rawls M, Donoghue M, Edwards E, Sack L (2016) Hydraulic basis for the evolution of photosynthetic productivity. Nat Plants 2: 16072. [DOI] [PubMed] [Google Scholar]
- Sen U, Hennipman E (1981) Structure and ontogeny of stomata in Polypodiaceae. Blumea 27: 175–201 [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC (2010) Evolution along the crassulacean acid metabolism continuum. Funct Plant Biol 37: 995–1010 [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K (2009) Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiol 149: 1838–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipes DL, Ting IP (1985) Crassulacean acid metabolism and crassulacean acid metabolism modifications in Peperomia camptotricha. Plant Physiol 77: 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JAC, Winter K (1996) Taxonomic distribution of Crassulacean acid metabolism. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer, Berlin, pp 1–13 [Google Scholar]
- Spalding MH, Stumpf DK, Ku MSB, Burris RH, Edwards GE (1979) Crassulacean acid metabolism and diurnal variations of internal CO2 and O2-concentrations in Sedum praealtum DC. Aust J Plant Physiol 6: 557–567 [Google Scholar]
- Tallman G. (2004) Are diurnal patterns of stomatal movement the result of alternating metabolism of endogenous guard cell ABA and accumulation of ABA delivered to the apoplast around guard cells by transpiration? J Exp Bot 55: 1963–1976 [DOI] [PubMed] [Google Scholar]
- Tallman G, Zhu J, Mawson BT, Amodeo G, Nouhi Z, Levy K, Zeiger E (1997) Induction of CAM in Mesembryanthemum crystallinum abolishes the stomatal response to blue light and light-dependent zeaxanthin formation in guard cell chloroplasts. Plant Cell Physiol 38: 236–242 [Google Scholar]
- Teeri JA. (1982a) Photosynthetic variation in the Crassulaceae. In I Ting M, Gibbs, eds, Crassulacean Acid Metabolism. American Society of Plant Physiologists, Rockville, MD, pp 244–259 [Google Scholar]
- Teeri JA. (1982b) Carbon isotopes and the evolution of C4 photosynthesis and crassulacean acid metabolism. In MH Nitecki, ed, Biochemical Aspects of Evolutionary Biology. University of Chicago Press, Chicago, pp 93–130 [Google Scholar]
- Ting IP. (1987) Stomata in plants with crassulacean acid metabolism. In E Zeiger, GD Farquhar, IR Cowan, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 353–366 [Google Scholar]
- Ting IP, Johnson HB, Szarek SR (1972) Net CO2 fixation in crassulacean acid metabolism plants. In CC Black, ed, Net Carbon Dioxide Assimilation in Higher Plants: Proceedings of the Joint Symposium of the Southern Section of the American Society of Plant Physiologists and Cotton. American Society of Plant Physiologists and Cotton, Raleigh, NC, pp 26–53 [Google Scholar]
- Ting IP, Thompson ML, Dugger WM (1967) Leaf resistance to water vapor transfer in succulent plants: Effect of thermoperiod. Am J Bot 54: 245–251 [Google Scholar]
- Tombesi S, Nardini A, Farinelli D, Palliotti A (2014) Relationships between stomatal behavior, xylem vulnerability to cavitation and leaf water relations in two cultivars of Vitis vinifera. Physiol Plant 152: 453–464 [DOI] [PubMed] [Google Scholar]
- Trifiló P, Raimondo F, Savi T, Lo Gullo MA, Nardini A (2016) The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. J Exp Bot 67: 5029–5039 [DOI] [PubMed] [Google Scholar]
- Vasseur F, Violle C, Enquist BJ, Granier C, Vile D (2012) A common genetic basis to the origin of the leaf economics spectrum and metabolic scaling allometry. Ecol Lett 15: 1149–1157 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Griffiths H (2009) Stomatal responses to CO2 during a diel Crassulacean acid metabolism cycle in Kalanchoe daigremontiana and Kalanchoe pinnata. Plant Cell Environ 32: 567–576 [DOI] [PubMed] [Google Scholar]
- von Willert DJ, Brinckmann E, Scheitler B, Eller BM (1985) Availability of water controls Crassulacean acid metabolism in succulents of the Richtersveld (Namib desert, South Africa). Planta 164: 44–55 [DOI] [PubMed] [Google Scholar]
- Wang Y, Blatt MR (2011) Anion channel sensitivity to cytosolic organic acids implicates a central role for oxaloacetate in integrating ion flux with metabolism in stomatal guard cells. Biochem J 439: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L, Dallwitz MJ (1992) The Families of Flowering Plants: Descriptions, Illustrations, Identification, and Information Retrieval. delta-intkey.com
- Winter K. (1985) Crassulacean acid metabolism. In J Barber, NR Baker, eds, Photosynthetic Mechanisms and the Environment. Elsevier, Amsterdam, The Netherlands, pp 329–387 [Google Scholar]
- Winter K, Holtum JAM (2014) Facultative crassulacean acid metabolism (CAM) plants: powerful tools for unravelling the functional elements of CAM photosynthesis. J Exp Bot 65: 3425–3441 [DOI] [PubMed] [Google Scholar]
- Winter K, Holtum JAM, Smith JAC (2015) Crassulacean acid metabolism: a continuous or discrete trait? New Phytol 208: 73–78 [DOI] [PubMed] [Google Scholar]
- Winter K, Medina E, Garcia V, Luisa Mayoral M, Muniz R (1985) Crassulacean acid metabolism in roots of a leafless orchid, Campylocentrum tyrridion Caray & Dunsterv. J Plant Physiol 118: 73–78 [DOI] [PubMed] [Google Scholar]
- Winter K, Osmond CB, Hubick KT (1986) Crassulacean acid metabolism in the shade. Studies on an epiphytic fern, Pyrrosia longifolia, and other rainforest species from Australia. Oecologia 68: 224–230 [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC (1996) An introduction to crassulacean acid metabolism. Biochemical principles and ecological diversity. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution. Springer, Berlin, Germany, pp 1–13 [Google Scholar]
- Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, et al. (2005) Assessing the generality of global leaf trait relationships. New Phytol 166: 485–496 [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827 [DOI] [PubMed] [Google Scholar]
- Wyka TP, Duarte HM, Lüttge UE (2005) Redundancy of stomatal control for the circadian photosynthetic rhythm in Kalanchoë daigremontiana Hamet et Perrier. Plant Biol (Stuttg) 7: 176–181 [DOI] [PubMed] [Google Scholar]
- Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119: 101–117 [DOI] [PubMed] [Google Scholar]
- Yang X, Cushman JC, Borland AM, Edwards EJ, Wullschleger SD, Tuskan GA, Owen NA, Griffiths H, Smith JAC, De Paoli HC, et al. (2015) A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytol 207: 491–504 [DOI] [PubMed] [Google Scholar]
- Zambrano VAB, Lawson T, Olmos E, Fernández-García N, Borland AM (2014) Leaf anatomical traits which accommodate the engagement of facultative crassulacean acid metabolism in tropical trees of the genus Clusia. J Exp Bot 65: 3513–3523 [DOI] [PubMed] [Google Scholar]
- Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT (2012) Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol 193: 929–938 [DOI] [PubMed] [Google Scholar]
- Zeppel MJB, Lewis JD, Phillips NG, Tissue DT (2014) Consequences of nocturnal water loss: a synthesis of regulating factors and implications for capacitance, embolism and use in models. Tree Physiol 34: 1047–1055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.