Guard cells in hornwort develop wall thickenings, open, die, and collapse, similar to the oldest fossil plants, and in synchrony with sporogenesis, drying of internal fluids, and sporophyte maturation.
Abstract
As one of the earliest plant groups to evolve stomata, hornworts are key to understanding the origin and function of stomata. Hornwort stomata are large and scattered on sporangia that grow from their bases and release spores at their tips. We present data from development and immunocytochemistry that identify a role for hornwort stomata that is correlated with sporangial and spore maturation. We measured guard cells across the genera with stomata to assess developmental changes in size and to analyze any correlation with genome size. Stomata form at the base of the sporophyte in the green region, where they develop differential wall thickenings, form a pore, and die. Guard cells collapse inwardly, increase in surface area, and remain perched over a substomatal cavity and network of intercellular spaces that is initially fluid filled. Following pore formation, the sporophyte dries from the outside inwardly and continues to do so after guard cells die and collapse. Spore tetrads develop in spore mother cell walls within a mucilaginous matrix, both of which progressively dry before sporophyte dehiscence. A lack of correlation between guard cell size and DNA content, lack of arabinans in cell walls, and perpetually open pores are consistent with the inactivity of hornwort stomata. Stomata are expendable in hornworts, as they have been lost twice in derived taxa. Guard cells and epidermal cells of hornworts show striking similarities with the earliest plant fossils. Our findings identify an architecture and fate of stomata in hornworts that is ancient and common to plants without sporophytic leaves.
Stomata occur in all major groups of extant land plants except liverworts, but they are found on sporangia (capsules) only in hornworts and mosses. The scattered stomata on hornwort sporophytes resemble those in late Silurian and Devonian fossil plants in terms of size, distribution, and mature morphology and, therefore, are of critical interest in deciphering the origin and evolution of these important structures in land plants (Edwards et al., 1998; Renzaglia et al., 2000, 2007; Berry et al., 2010; Ligrone et al., 2012a).
In 2002, Lucas and Renzaglia experimented with hornwort stomata and concluded that, once open, they are locked in position. They suggested that stomata are involved in sporophyte drying, thereby facilitating dehiscence and spore dispersal. More recent studies have supported this concept in both hornworts (Pressel et al., 2014; Field et al., 2015) and mosses (Merced and Renzaglia, 2013, 2014; Chater et al., 2016). In angiosperms, stomatal movement involves a response to environmental cues through active changes in guard cell turgor pressure by hormonal signaling that produces rapid osmotic change. Reports of stomata closing in response to abscisic acid (ABA) and CO2 in Physcomitrella, Funaria, Selaginella, and ferns suggest that the physiological capacity for active movement and the presence of the ABA signaling pathway are present in early land plants (Chater et al., 2011, 2013; Ruszala et al., 2011; Cai et al., 2017). However, recent studies contradict that hypothesis and show that stomatal responses to leaf water status are controlled passively in ferns and lycophytes, with ABA signaling associated with drought stress and sex determination, not stomatal closure (Brodribb and McAdam, 2011; McAdam and Brodribb, 2013; McAdam et al., 2016). Support for passive closing of stomata is seen in mutants of Ceratopteris that are not sensitive to ABA but respond the same way to low leaf water status as nonmutants (McAdam et al., 2016). This gradualistic model proposes that stomata evolved the ability to close to ABA in seed plants by coopting the ABA mechanism that was already in place in early land plants (Sussmilch et al., 2017). Active movement of stomata appears to be absent in hornworts (Lucas and Renzaglia, 2002; Pressel et al., 2014; Villarreal and Renzaglia, 2015).
Unique among land plants, the hornwort sporophyte is an elongating sporangium that grows from a basal meristem and continuously produces new sporogenous tissue that is bathed in mucilage until sporophyte dehiscence (Villarreal and Renzaglia, 2006, 2015; Ligrone et al., 2012a; Pressel et al., 2014). This highly coordinated upward process results in progressive spore maturation and release, synchronized with dehiscence at the tip of the cylindrical sporophyte. The development of stomata also is basipetal, beginning at the sporophyte base and progressing upward. Thus, within a single hornwort sporophyte, progressive and continuous development may be followed from base to tip (Renzaglia, 1978). We hypothesized that stomata on a growing sporangium that is filled with mucilage would demonstrate structural, developmental, and compositional features that are distinct from those on vegetative organs.
Here, we examined stomatal development and fate vis-a-vis spore differentiation and sporophyte maturation. We examined the composition of guard cell walls for the occurrence of arabinan-containing polysaccharides that allow for flexibility and resilience in actively moving stomata (Jones et al., 2003, 2005; Merced and Renzaglia, 2014). Finally, through measurements of 16 hornwort species from all seven genera with stomata, we assessed the existence of an evolutionary correlation between guard cell size and genome size in hornworts as occurs in angiosperms (Beaulieu et al., 2008; Hodgson et al., 2010).
This study identifies a developmental fate of guard cells in hornworts that involves pore development, early death, collapse, and increase in surface area and outer aperture width, all of which are associated with progressive drying of internal mucilage, spore differentiation, and sporophyte dehiscence. These findings come together with a paucity of arabinans in the cell walls and no correlation between guard cell and genome sizes to challenge the possibility of diurnally active stomata in hornworts. Stomata on hornworts are larger in width and depth than the surrounding epidermal cells, which is an unusual character in plants. The large collapsed hornwort stomata show similarities with the first fossil plants from rocks over 400 million years old. These earliest plants produced terminal sporangia and lacked leaves, as do hornworts, suggesting that the collapsed condition originated in the colonizing stages of plant evolution in the Upper Silurian and was conserved over hundreds of millions of years.
RESULTS
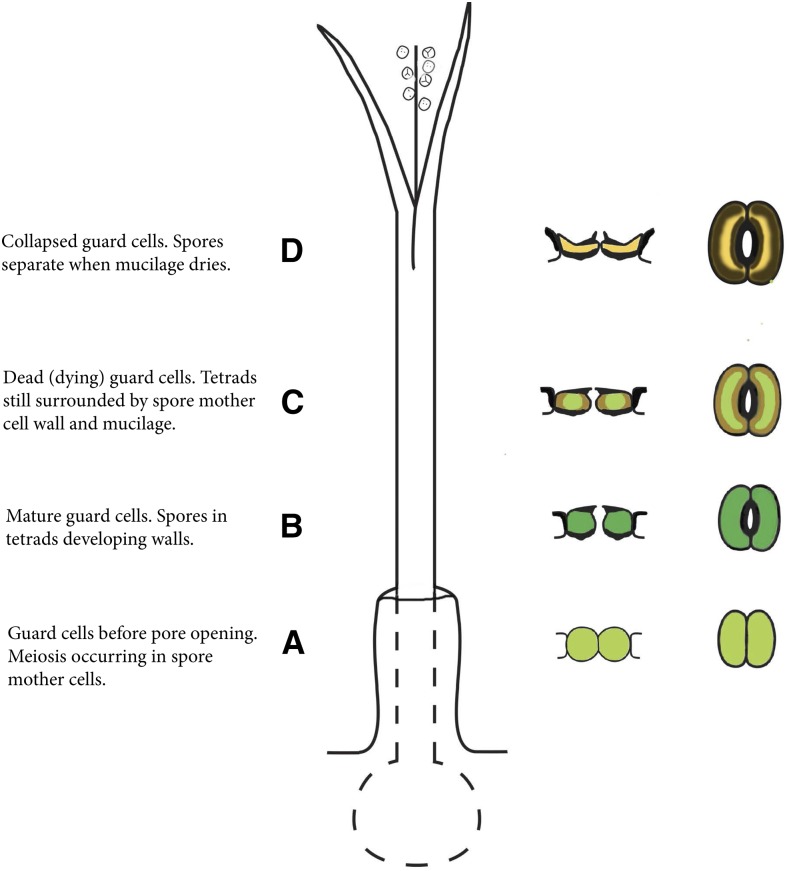
The general structure and development of an idealized hornwort sporophyte is presented in Figure 1. Stomatal condition, position, and color are indicated on the right of the diagram in developmental order from the base up.
Figure 1.
Diagrammatic representation of a hornwort sporophyte with progressive development and color of stomata indicated from the base upward. Once open, stomata never close, but the outer aperture increases slightly in width after guard cell collapse. The stomata color does not necessarily coincide with the overall color of the sporophyte because stomata die and collapse while the sporophyte is actively photosynthesizing. A, Developing stoma. B, Mature, living, and open stoma. C, Dead (dying) guard cells at the onset of collapse of the outer walls. D, Dead, collapsed, and slightly larger stoma. Above D, the sporophyte dries, leading to dehiscence into two valves along two parallel suture lines, mucilage dries around the spore tetrads, the spore mother cell wall adheres to the spore surfaces, and the spores separate for dispersal.
Guard Cell Wall Development
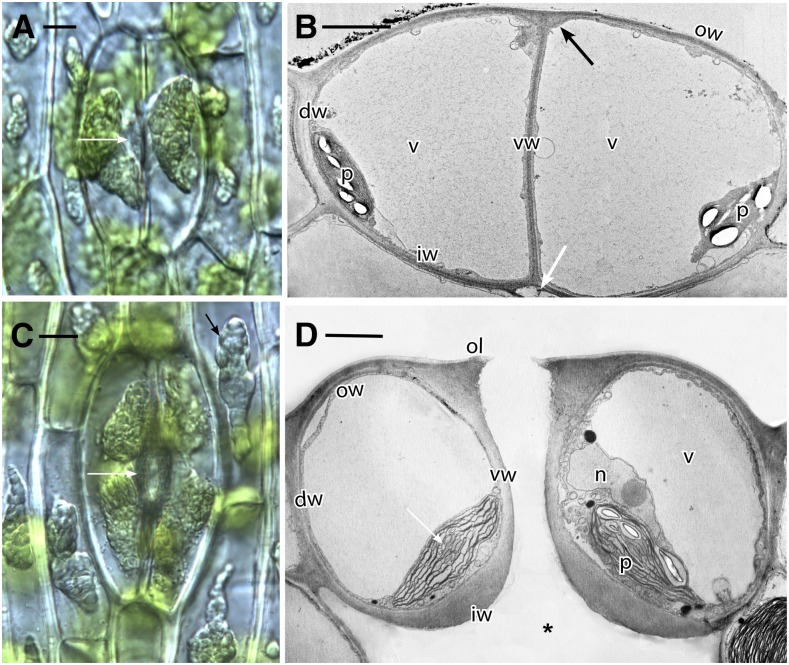
Hornwort stomata originate within the confines of the gametophytic involucre (Fig. 1A), and the pore forms before guard cell and epidermal walls have completed development (Fig. 2, A–C). References to specific guard cell walls are as labeled in cross sections of stomata in Figure 2, B and D. Before opening of the pore, guard cell walls are uniformly thin (Fig. 2B). Thickening of guard cell walls begins at the juncture of outer and ventral walls, where outer ledges will form (Fig. 2, A and B). At this time, the inner walls separate from cortical cells to form substomatal cavities (Fig. 2B). In fully developed stomata, the guard cells are turgid with large vacuoles, and an open aperture connects the outside environment to the schizogenous substomatal cavity (Figs. 1B and 2D). The prominent plastids in guard cells are well developed with abundant starch and pyrenoids Phaeoceros carolinianus (Michx.) Prosk. (Fig. 2).
Figure 2.
Phaeoceros carolinianus. A, Differential interference contrast image showing two new guard cells, each with a large amyloplast and an aperture beginning to form in ventral walls (arrow). B, Transmission electron microscopy (TEM) cross section of young guard cells before forming the pore. Each cell contains a large vacuole (v) and plastid (p) with starch. Dorsal (dw), inner (iw), outer (ow), and ventral (vw) walls of the guard cells are thin. The outer ledge (black arrow) and substomatal cavity (white arrow) are beginning to form. C, Differential interference contrast image of older stoma. Each guard cell contains two large amyloplasts, and the aperture (white arrow) is fully developed. Cell walls are thicker than those in A, and epidermal cells contain large amyloplasts (black arrow). D, TEM cross section of a living, fully developed, open stoma with the pore leading to a substomatal cavity (asterisk). Each guard cell contains a thin outer wall (ow), an outer ledge (ol), dorsal (dw) and ventral (vw) walls, and a thickened inner wall (iw). A large vacuole occupies most of the guard cells with nucleus (n) and plastids (p), with pyrenoids (arrow) toward the inside of the stoma. Bars = 10 μm (A and C) and 5 μm (B and D).
Guard Cell Wall Ultrastructure and Pectin Constituents
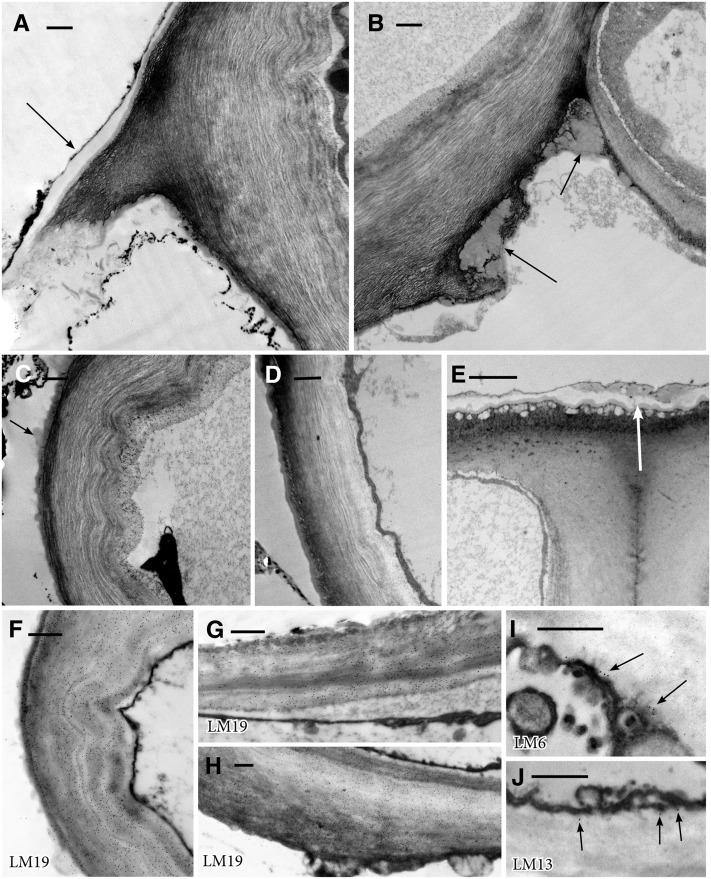
Guard cells in opened stomata (Fig. 1B) are differentially thickened and consist of loose radial fibrils (Figs. 2D and 3, A–D). Outer walls on adjacent epidermal cells are thick, lack radial fibrils, and are covered by a jagged cuticular layer of alternating cutin and branched fibrils (Fig. 3E). Outer guard cell walls, in comparison, are thin, smooth, and covered by a thin cuticle on the outer ledge. The inner wall typically lacks a ledge and has an inner layer of radial fibrils and a thickened outer fibrillar network (Figs. 2D and 3B). Globular waxy deposits are prominent along the substomatal side of the inner wall, especially at cellular junctions (Fig. 3, B and D). The stomatal aperture is lined by adjacent ventral guard cell walls composed of radial fibrils that often buckle along their length (Figs. 2D and 3C). A thin cuticle and cuticular region overlie the outer and ventral guard cell walls (Fig. 3, A, C, and D).
Figure 3.
TEM images showing wall ultrastructure in guard cell walls of Leiosporoceros dussii. A, Outer ledge with thickened cuticle (arrow). B, Juncture of inner and ventral guard cell walls with wax deposits on cell walls in the substomatal cavity (arrows). C, Thin fibrillar ventral wall with scattered cuticle/waxes (arrow). D, Thin fibrillar outer wall with a thin layer of cuticle. E, Outer thickened wall with the cuticular layer and cuticle (arrow) of an epidermal cell adjacent to a guard cell. F to J, TEM immunogold localization of pectin epitopes in the guard cell walls of L. dussii. Black dots in images are secondary gold labels attached to specific antibodies. Very strong labeling is shown for LM19 in ventral wall (F), outer wall (G), and inner guard cell wall (H). Scarce labeling is shown for LM6 (I) and LM13 (J), both localized toward the inside of the wall at the plasmalemma. Bars = 0.5 μm except for E, where bar = 2 μm.
Guard cell walls Leiosporoceros dussii (Steph.) Hässel abundantly label for unesterified homogalacturonan (LM19; Fig. 3, F–H), but labeling for arabinans (LM6 and LM13) is scarce to none (Fig. 3, I and J). LM19 labeling in ventral (Fig. 3F), outer (Fig. 3G), and inner (Fig. 3H) guard cell walls is very strong and homogenous throughout. When present, LM6 (Fig. 3I) and LM13 (Fig. 3J) labeling is restricted to the inside of the walls at the plasmalemma.
Sporophyte Maturation and Collapse of Guard Cells
Stomata open directly above the involucre (Fig. 1B), where spore mother cells undergo meiosis and tetrads initiate spore wall development. A fluid fills all intercellular spaces in the sporophyte, including the substomatal cavity, the network of schizogenous spaces in the assimilative or cortical tissue, and the sporogenous tissue in this region. The fluid in the sporogenous region is presumed to be mucilage because it labels with pectin epitopes (Supplemental Fig. S1; Macquet et al., 2007). Once the aperture forms by separation of the ventral guard cell walls, the pore at the outer ledges remains open (Fig. 4). Pore opening is followed by the disappearance of liquid in the substomatal cavity and progressively inwardly in intercellular spaces. Newly opened stomata are raised slightly above the epidermal surface (Fig. 4, A and B).
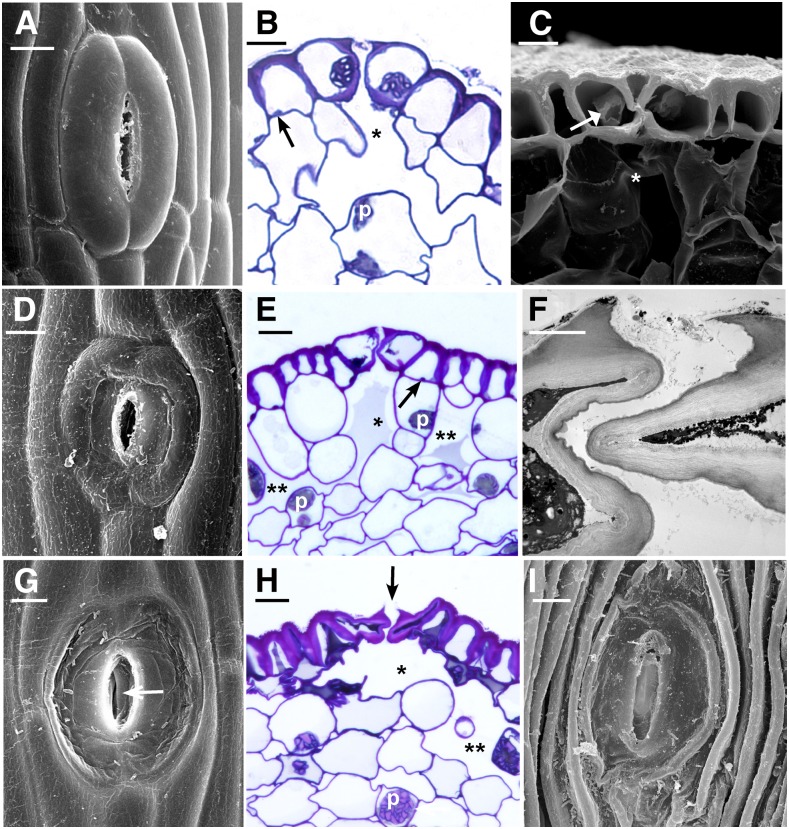
Figure 4.
Stages of senescence and collapse of stomata in three genera of hornworts. The outer aperture remains open and increases in diameter during the drying process. A, P. carolinianus. Scanning electron microscopy (SEM) shows newly opened, slightly raised stoma directly above the involucre. B, L. dussii. Cross section light micrograph of a newly opened stoma shows large starch-filled plastids in guard cells and differentially thickened epidermal and guard cell walls. Small plastids (arrow) occur in epidermal cells, and a substomatal cavity (asterisk) leads to intercellular spaces in the assimilative (cortical) tissue. C, A adscendens Lehm. and Lindenb. SEM cross section shows the epidermis and a stoma with dead collapsing guard cells that contain degenerated protoplasm (arrow). Adjacent epidermal cells have thickened radial walls and are beginning to collapse in the opposite direction from the guard cells. The aperture is wide open superficially, and the thin ventral guard cell walls are buckled. A large substomatal cavity (asterisk) leads to internal air spaces. D, A. adscendens. SEM of stoma shows the onset of guard cell collapse before epidermal cells dry. A thicker cuticle covers epidermal cells compared with guard cells. E, L. dussii. Cross section light micrograph shows dead guard cells with degenerated protoplasm at the onset of collapse of the outer cell wall and while fluid is still within the substomatal cavity (asterisk) and intercellular spaces (double asterisks). Small plastids (arrow) in epidermal cells contrast with large starch-filled plastids (p) in assimilative cells. F, L. dussii. TEM of dead, collapsed stoma shows the coordinated folding of the thin ventral walls of guard cells. The aperture is open from the outside due to the rigid outer ledges. G, A. adscendens. SEM shows completely collapsed guard cells surrounded by hydrated epidermal cells. Stoma diameter is greater than in the precollapsed guard cell in E. The outer aperture is open, and folded ventral walls of guard cells are visible internally (arrow). H, L. dusii. Cross section light micrograph shows collapsed guard cells. The adjacent epidermal cell contains degenerated cytoplasm and has begun to collapse like an accordion in the opposite direction from the guard cells. Assimilative cells begin to die around the substomatal cavity (asterisk) and intercellular space (double asterisks). I, P. carolinianus. SEM shows the epidermis in desiccated and dehisced sporophyte with ridges of collapsed epidermal cell surrounding an enlarged stoma that has a broadened outer aperture. Bars = 5 μm except for F, where bar = 2 μm.
The senescence of guard cells begins in the green sporophyte region with gradual degradation of the protoplasm and depression of the outer cell wall (Figs. 1C and 4, C and D). The sporophyte is green in this region above the involucre due to chloroplasts in the assimilative region, and the intercellular spaces may have some fluid (Fig. 4E), but they are typically dry by this stage due to contact with the environment. The large amyloplasts (Fig. 2, A and C) in epidermal cells have transformed into numerous small plastids (Fig. 4, B and E). Following senescence, guard cells collapse inwardly until the outer walls rest against the inner walls (Fig. 4, G–I). The thickened inner walls of guard cells suspend the collapsed guard cells over the substomatal cavity, where they remain throughout drying of the intercellular spaces (Fig. 4H). During cell collapse, the ventral guard cell walls fold onto each other, forming a convoluted inner pore (Fig. 4, F and H). This process widens the gap between the outer ledges of guard cells and progressively increases the width of the outer aperture from an average of 1.5 μm (n = 23) in newly opened stomata to 3.3 μm (n = 31) in collapsed stomata. This open configuration is evident from a surface view, but the convoluted ventral guard cell walls surround an irregular inner pore (Fig. 4, F–I).
Following stomatal collapse, continued drying of the sporophyte results in the death of epidermal and assimilative cells and browning of the sporophyte (Fig. 4H; Supplemental Fig. S2). Due to differential thickening along outer and periclinal walls, epidermal cells collapse in a direction that is opposite that of collapsed guard cells, leaving parallel ridges formed by the thickened periclinal walls (Fig. 4I). These dried epidermal cells, together with the differentially thickened guard cell walls, hold the broad stomata in position over intercellular spaces (Figs. 1D and 4, H and I).
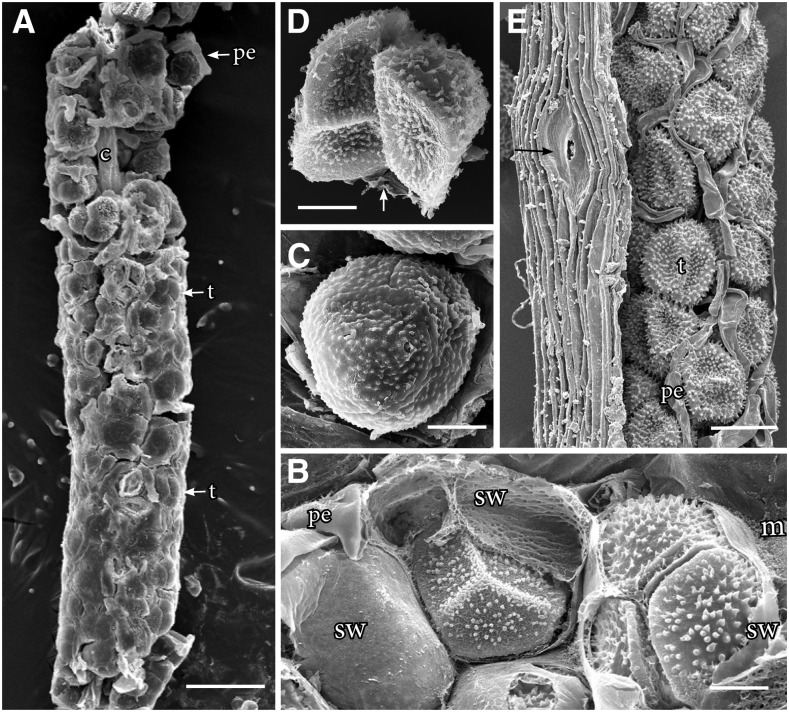
Sporogenous tissue in the spore sac is surrounded by mucilage that dries progressively as spores differentiate (Fig. 5; Supplemental Fig. S2). The rate of mucilage drying is governed by seasonal conditions and is completed where the sporophyte dehisces. Directly above the involucre where stomata collapse, young spores in tetrads develop spore walls but remain surrounded by the spore mother cell wall along most of the length of the sporophyte (Fig. 5). During the drying process, the spore mother cell wall adheres to individual spores, forming a pseudoperine (Fig. 5). Dehiscence of the sporophyte occurs at the tip after the mucilage is dried. The epidermal cells are fully compressed in width at this location (Fig. 5E). Collapsed stomata remain broad and prominent throughout the drying process (Fig. 5E). Dehiscence occurs along two clearly defined sutures that separate the sporophyte into two valves and expose the dried spore mass.
Figure 5.
SEM images of hornwort sporophytes. A to D, P. carolinianus. A, One millimeter of sporogenous tissue extracted from remaining sporophyte shows young tetrads (t) and pseudoelaters (pe) in mucilaginous mass around the columella (c). Stomata collapse at the base where mucilage surrounds tetrads, and through progressive drying of mucilage upwardly, spores and pseudoelaters separate. B, Sporogenous tissue where stomata collapse held together in mucilage (m) showing mature spores of tetrads embedded in the spore mother cell wall (sw) with an imprint of spore wall ornamentation and pseudoelaters. C, Tetrad with spore mother cell wall drying down on the papillate distal wall ornamentation. At this level, stomata are collapsed and internal air spaces are dry within the assimilate tissue. D, Two spores of separated tetrad with a veil of spore mother cell wall adhering to the spore wall. The arrow identifies a spore mother cell wall remnant from a lost spore. E, Large collapsed stoma (arrow) in dried epidermis of a dehiscing Anthoceros cristatus Stephani. sporophyte that contains pseudoelaters separating dried tetrads not surrounded by mucilage or spore mother cell wall. This figure appears courtesy of Silvia Pressel and Jeffrey Duckett. Bars = 100 μm (A), 20 μm (B–D), and = 50 μm (E).
Guard Cell Sizes during Sporophyte Development
Guard cell dimensions as viewed in surface section increase following their collapse due to differential wall thickenings of guard cells and adjacent epidermal cells (Fig. 4, D and G). This phenomenon was observed in all genera and illustrated quantitatively in Anthoceros agrestis Paton. (Table I). The size increase is greatest immediately following the collapse of guard cells (green-brown zone in Fig. 1C), which remain larger than newly formed stomata (green zone in Fig. 1C) even after the entire epidermis dries and the sporophyte splits open (brown zone in Fig. 1D).
Table I. Guard cell size at developmental regions of an A. agrestis sporophyte.
| Region of the Sporophyte | Length |
Width |
||
|---|---|---|---|---|
| Mean ± se | Range | Mean ± se | Range | |
| µm | ||||
| Green zone (n = 50) | 38.4 ± 0.5 | 29.2–45.2 | 9.3 ± 0.3 | 5.3–13.0 |
| Green to brown zone (n = 49) | 48.7 ± 0.8 | 39.0–58.9 | 10.9 ± 0.4 | 4.9–16.6 |
| Brown zone (n = 50) | 43.33 ± 0.6 | 33.0–51.1 | 8.9 ± 0.2 | 5.9–13.2 |
Guard Cell Size versus Genome Size
Average guard cell lengths, measured in green stomata, across all seven hornwort genera with stomata range from 51 to 81 μm, and genome sizes of these same species vary from 159 to 269 Mb (Supplemental Table S1). The largest guard cells among the 16 hornwort species are those in Phymatoceros bulbiculosus (Brot.) Stotler et al. (66.8 μm), Leiosporoceros dussii (76.5 μm), and Phaeoceros engelii Cargill and Fuhrer. (81.3 μm; Supplemental Table S1). The smallest guard cells are those in Anthoceros fusiformis Aust. (51.6 μm), Anthoceros lamellatus Steph. (54.8 μm), and Paraphymatoceros proskauerii (Stotler, Crand.-Stotl. and W.T.Doyle) J.C.Villarreal and Cargill (54.8 μm). A. agrestis and L. dussii have the smallest genome sizes among hornworts (0.085 and 0.16 pg, respectively [1 pg = 0.978 × 109 bp; Dolezel et al., 2003]), while the largest genome size is found in P. bulbiculosus (0.28 pg). There is no correlation between the mean guard cell length and genome size in hornworts (multiple r2 = 0.03607, adjusted r2 = −0.02819; F statistic = 0.5613, P = 0.4653).
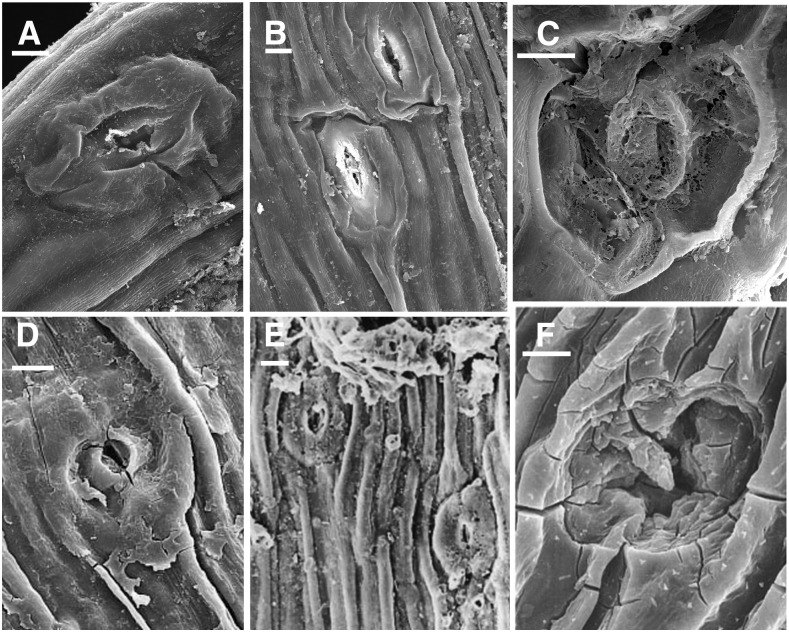
The 51- to 81-μm range of guard cell length in hornworts is on the higher end of lengths observed in fossil Devonian plants, which range from 21.5 to 85 μm (Lomax et al., 2014; Supplemental Table S2). Particularly notable are the guard cells of fossils from the Early Devonian, which are over 80 μm in length in Horneophyton lignieri and Aglaophyton major. The stomata in Figure 6 from a Silurian fossil are similar in size to those of hornworts.
Figure 6.
SEM images of hornwort stomata compared with fossil stomata. A to C, Extant hornwort stomata. A and B, L. dussii. C, P. carolinianus. D to F, Fossil stomata reproduced with permission from Edwards et al. (1998). D, Silurian stoma NMW97.37G.3 with no evidence of two guard cells as in A. The circular pore formed by the outer ledges opens to a constricted aperture below as in B. Epidermal cells are identical to hornwort epidermal cells. E, Early Devonian fossil stoma at the base of terminal sporangium of Sporogonites NMW96.5G.3. Epidermal cells are identical to dried hornwort epidermal cells. F, Silurian stoma NMW94.60G.2 with degenerated outer walls similar to C. Bars = 10 μm.
The Earliest Fossil Stomata
Stomata on the earliest fossil land plants share remarkable similarities with hornwort stomata and sporophyte surfaces (Fig. 6). In many of these fossils, the epidermal cells are elongated and the outer walls of guard cells are collapsed or entirely missing, as they are in hornworts (Fig. 6, C and D). The surrounding epidermal cells also are similar in width and appearance to those in dried hornwort sporophytes (Fig. 6, D and E). Moreover, a low frequency of stomata and large size of guard cells (Supplemental Table S2) are shared by hornworts and early fossil plants. Fossil stomata occur on sporangia as they do in bryophytes, and many occur on leafless axes that bear terminal sporangia (Edwards et al., 1998).
DISCUSSION
The development and wall architecture of hornwort stomata are intricately associated with spore and sporophyte differentiation. Following maturation, stomata die and collapse, while the surrounding cells remain alive. Due to differential wall thickenings on epidermal and guard cell walls, guard cells remain perched in position over the substomatal cavity, expanding the surface area in contact with the environment, including the width of the outer aperture. The thin ventral walls of guard cells form a folded convoluted inner pore that reduces the passage way for pathogens to enter (Lee and Luan, 2012) as the sporophyte differentiates. Beginning with pore formation in the young stoma, the internal network of intercellular spaces that are fluid filled gradually dry from the substomatal cavity inwardly until mucilage in the spore sac is progressively and incrementally dried down on spores. During much of this process, stomata are collapsed. Spores develop their thickened walls while still enclosed in the spore mother cell wall, remaining in tetrads until spores separate where they are dispersed at the sporophyte tip. This condition of spores remaining together in a common wall brings to mind the envelope-enclosed cryptospore tetrads from the Ordovician and Silurian (Edwards et al., 2014).
Chloroplast ultrastructure and sporophyte anatomy in hornworts support an early role of stomata in gas exchange, including CO2 acquisition for photosynthesis and water evaporation as the fluid disappears from intercellular spaces (Villarreal and Renzaglia, 2015). Chloroplasts are large and prominent in assimilative cells throughout sporophyte maturation and until cells dry and die. There are usually two chloroplasts in each guard cell that are substantially bigger and with more starch and thylakoids than chloroplasts in epidermal cells. Guard cells are the first epidermal cells to dry. It is possible that the well-developed chloroplast in guard cells may play a role in the perception of environmental cues and perhaps signals the onset of senescence. The role of chloroplasts in signaling to the nucleus and cross talk with other organelles is increasingly apparent. Chloroplasts have been shown to perceive abiotic and biotic stimuli to bring about a range of responses, including the initiation of senescence and programmed cell death (Spetea et al., 2014).
To our knowledge, there are no other stomata in extant plants that have the structure and developmental fate of those in hornworts. Guard cell walls, especially outer walls, in tracheophytes and true mosses are dense, thickened, and do not normally collapse (Sack, 1987; Ziegler, 1987; Everet, 2006; Merced and Renzaglia, 2013). Similar epidermal walls are unparalleled in extant sporangia but are found in Sporogonites and Tortilicaulis from the lower Devonian. Referring to Silurian stomata from unknown plants, Edwards et al. (1998) remarked, “In many cases the outer periclinal walls are incomplete or even absent suggesting that they and/or the overlying cuticle were thinner than on the surrounding epidermal cells.” This is precisely the condition of hornwort stomata.
Both the large stomatal size and pectin composition are counterindicators of active opening and closing of hornwort stomata, even in green portions of the sporophyte. Hornwort stomata open once and remain open throughout development. Lucas and Renzaglia (2002) demonstrated an increase in ionic concentration in newly developed guard cells, suggesting that, in addition to cell wall development, increased turgor may contribute to pore formation. When developed, guard cell walls are rich in unesterified homogalacturonans similar to mosses (Merced and Renzaglia, 2013, 2014; Merced, 2015a) and Arabidopsis (Arabidopsis thaliana (L.) Heynh.; Merced, 2015b; Amsbury et al., 2016). However, arabinan-rich pectins that are essential for the opening and closing of guard cells and the resilience of walls in tracheophytes (Jones et al., 2003, 2005; Moore et al., 2013) are not wall constituents of hornwort stomata. The scarce labeling with antibodies to arabinan-containing carbohydrate epitopes seen in this study was restricted to the plasmalemma, supporting the presence of arabinogalactan proteins and not wall pectins, as this antibody labels epitopes from either pectins or arabinogalactan proteins (Caffall and Mohnen, 2009).
The large size of hornwort stomata is shared with the earliest fossil stomata and is counter to the documented correlation between guard cell length and genome size (Beaulieu et al., 2008; Hodgson et al., 2010). Hornwort genomes are among the smallest of all land plants (Renzaglia et al., 1995). Lomax et al. (2014) noted the inconsistency in guard cell length of fossil stomata vis-a-vis a predicted increase in genome size from the earliest plants through geologic time. They argued that high levels of atmospheric CO2 as demonstrated in vitro for angiosperms (Edwards, 2003; Franks et al., 2012; Lomax et al., 2012) and paleopolyploidy may have resulted in exceptionally long guard cells. However, hornwort stomata size and number do not vary in response to CO2 concentration (Field et al., 2015). We suggest an alternative explanation in which selection in hornworts and early plants favored larger stomata due to a role in desiccation or sporangial maturation/dehiscence. Stomata of tracheophytes do not facilitate gas exchange to accelerate internal water loss; on the contrary, stomata open to increase CO2 acquisition for photosynthesis and close when leaf water status declines to hydraulically threatening levels due to increased evaporation. Without a rapid osmotic control of pore opening and closing, the constraints of guard cell size that suggest that small is faster do not exist (Raven, 2014). Guard cells of hornworts are similar in length to those of Psilotum (72.7 μm) and Ophioglossum (65.6 μm; Obermayer et al., 2002), both of which have genome sizes 300 times that of the largest hornwort genome. In contrast, Arabidopsis has a comparable genome size (0.16 pg) to Leiosporoceros, P. carolinianus, and Anthoceros punctatus L. but produces much smaller guard cells that are approximately 25 × 7.5 μm (Lomax et al., 2009).
The role of stomata in facilitating sporangial drying/dehiscence is supported by experiments involving the moss Physcomitrella patens, in which mutation of basic helix-loop-helix transcription factors, orthologous to those governing stomata development in Arabidopsis, resulted in stomataless capsules that were delayed in dehiscence compared with wild-type capsules (Chater et al., 2016). As in Physcomitrella and the hornworts, the pseudostomata of Sphagnum also are implicated in sporangial drying (Duckett et al., 2009). Indeed, the thin outer walls and collapse of ventral walls in hornwort guard cells are strikingly similar to those of Sphagnum pseudostomata (Merced, 2015a). Hornwort stomata, however, differ from Sphagnum pseudostomata in that the former do not form a complete pore to an internal gas-exchange system.
To date, there are no experimental studies involving stomatal development genes in hornworts. Chater et al. (2017) identified othologs of SPCH/MUTE/FAMA (SMF), ICE/SCREAM (SCRM), and EPIDERMAL PATTERNING FACTOR (EPF), genes required for stomatal development, in the draft genome of the hornwort Anthoceros punctatus. Further phylogenetic analysis revealed that the Anthoceros ApSMF1 and ApSCRM1 are closely related to the respective genes of Physcomitrella, PpSMF1 and PpSCRM1, and that the peptide sequences share high degrees of homology across all plants. Because stomatal genes are conserved across land plants with stomata (MacAlister and Bergmann, 2011), we anticipate that hornworts SMF, SCRM, and EPF orthologs will have a similar role in stomata development of hornworts as in other plants.
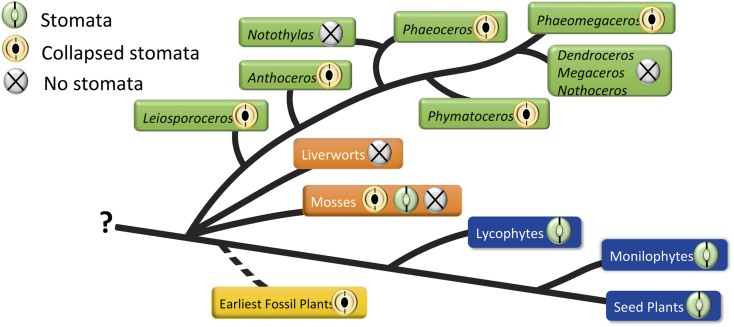
When the evolution of stomata is considered across land plants, several conclusions emerge (Fig. 7). First, stomata on sporangia, as occur in hornworts and mosses, are expendable. They were lost twice in hornworts and multiple times in mosses. This is not the case in tracheophytes, where they are ubiquitous on leaves or vegetative stems, except in submerged organs and isolated amphibious taxa such as Isoetes. Second, similarities in pseudostomata of Sphagnum and those in hornworts (e.g. collapsed guard cells and the scattered distribution along the sporangium) leave open the possibility of a common origin, as suggested by Merced (2015a). In most true mosses, stomata are restricted to the apophysis, where they are hypothesized to function in drying and dehiscence, as they are in Sphagnum and hornworts. The complete absence of stomata in liverworts may be interpreted either as a loss or a pleisiomorphy, depending on whether hornworts or liverworts are sister to land plants (Villarreal and Renzaglia, 2015).
Figure 7.
Presence and loss of collapsed stomata in hornworts (green tags). Stomata are plesiomorphic in hornworts, with stomata lost in two clades, Notothylas and the crown group Megaceros/Nothoceros/Dendroceros. The earliest fossil stomata from the Silurian (yellow tag) exhibit the collapsed condition. Among other bryophytes (orange tags), liverworts lack stomata and mosses exhibit all three conditions; Sphagnum has collapsed stomata, and other mosses either possess or have lost stomata. All tracheophytes (blue tags) have green, living stomata. Without a resolution of bryophyte relationships, represented here as a polytomy, it is impossible to determine if stomata are plesiomorphic in embryophytes.
In hornworts, stomata are plesiomorphic, as evidenced by their occurrence in Leiosporoceros and Anthoceros. The loss of stomata in Notothylas, the sister taxon to Phaeoceros, can be explained by their highly reduced sporophytes that are often cleistocarpic and remain within the involucre throughout development (Renzaglia, 1978). The loss of stomata in the hornwort crown group that includes Nothoceros, Megaceros, and Dendroceros may be a function of their life history traits. All three taxa are tropical and produce highly elongated involucres and spiraled pseudoelaters. Dehiscence in the epiphytic Dendroceros is irregular and appears to be influenced by the continued growth and expansion of the precocious, multicellular spores (Renzaglia, 1978; Schuette and Renzaglia, 2010). It is difficult to test the impact of character loss on organisms, but hornworts do present a clear case of the loss of stomata in well-defined genera with specific life history strategies. Loss of stomata in moss species is much more complicated and remains to be analyzed (Paton and Pearce, 1957; Merced, 2015b).
Based on the evidence presented here, we hypothesize that hornworts have retained ancestral features of stomata that occurred on axes with solitary terminal sporangia in the earliest land plants. Open pores of stomata provide a larger area for gas exchange and allow the assimilative tissue to be thicker, consequently increasing the self-sufficiency of sporophytes while developing spores. Given the preponderance of collapsed and thin outer walls of guard cells from Silurian and Early Devonian fossils, we suggest that at least some of these earliest stomata were involved in drying of the tissue as in hornworts. Whether at the base of the sporangium as in Sporogonites (Croft and Lang, 1942) or on the sporangium as in some Cooksonia (Edwards et al., 1998), stomata were likely positioned to enhance this process. The fact that some epidermal cells surrounding the earliest fossil stomata have the identical shape and the appearance of walls as in dehydrated hornwort sporophytes supports a role in axis drying.
Our demonstration of the systematic death and collapse of hornwort stomata as soon as they are produced is consistent with the findings of Field et al. (2015) that CO2 levels are inconsequential to guard cell development. Brodribb and McAdam (2011) suggested that the physiologically complex, regulatory role in water loss and gas exchange evolved in the Mid-Devonian, well after stomata first appeared in the fossil record. The CO2 sensitivity of stomata evolved by the time modern tracheophytes radiated, as this physiological response is found in ferns (Franks and Britton-Harper, 2016). However, stomata in ferns are found on leaves, organs that are not found in bryophyte sporophytes and that did not exist in the earliest fossil plants. Stomata in hornworts occur on sporangia that are fluid filled and lack water-conducting cells. Drying and dehiscence in this system are essential for spore maturation, sporophyte dehiscence, and spore release. It follows that stomata are intricately involved in these processes. We suggest that the striking similarities between stomata on hornwort sporophytes and on some of the oldest fossil land plants indicate an ancient origin and point to a common function of stomata on fertile, leafless axes (Ligrone et al., 2012b). Examination of more early fossil stomata on or near sporangia is necessary to test these inferences.
CONCLUSION
Our findings on hornwort stomata shed new light on stomatal evolution in three realms. First, a major finding in the stomatal development of hornworts is that pore formation is followed by the production of differentially thickened cell walls, then the death and collapse of guard cells. During this process, the surface area of the guard cells and the outer aperture width actually expand, and following collapse, the remaining epidermal cells, assimilative cells (cortical cells), and internal fluid progressively dry down from the substomatal cavity inwardly. Spores form walls early in development but remain bathed in mucilage as the sporophyte dries until dehiscence. Second, guard cell walls in hornworts are different from those of other plants in that they are devoid of arabinan-containing pectins, supporting an inability to open and close. Finally, we demonstrate the lack of correlation between genome size and guard cell length within hornworts, the first group of land plants that do not conform to this axiom (Beaulieu et al., 2008; Lomax et al., 2009). These seemingly disparate approaches to the study of guard cells come together with the oldest fossil stomata to provide an understanding of the role and evolution of stomata in hornworts and the first land plants.
MATERIALS AND METHODS
Microscopic studies focused on four hornwort genera with stomata. Species examined were Leiosporoceros dussii (Steph.) Hässel, the sister taxon to all remaining hornworts, collected in Panama, Anthoceros adscendens from Florida, Phaeoceros carolinianus (Michx.) Prosk. from Puerto Rico and Makanda, Illinois, and Anthoceros agrestis Paton from Makanda, Illinois.
For TEM, sporophytes were harvested, cut into sections at 2-mm intervals from the gametophyte upward, and fixed in 2% glutaraldehyde in 0.05 m sodium phosphate buffer for 1 h at room temperature, then overnight at 20°C. Specimens were rinsed three times in 0.05 m NaPO4 buffer 30 min each and postfixed 20 min in 1% OsO4 in 0.05 m NaPO4 buffer, followed by three rinses in distilled water 10 min each, and then dehydrated in a graded ethanol series ending with 3× 100% ethanol. Specimens were infiltrated in LR White resin (London Resin) by increasing the percentage of resin to ethanol over 4 d. After two changes in 100% resin, the material was place in molds with fresh resin and cured for 2 d at 65°C. Semithin sections (250–750 nm) were mounted on glass slides and stained with 1.5% Toluidine Blue in distilled water to monitor for stomata using light microscopy. Thin sections (60–90 nm) were collected on nickel grids and dried for 1 to 3 h at room temperature.
SEM preparation followed that described by Merced and Renzaglia (2013). Briefly, sporophytes were processed as for TEM up to 3× 100% ethanol. Specimens were critical point dried using CO2 as the transitional fluid, mounted on stubs, sputter coated for 230 s with palladium-gold, and viewed using a FEI 450 scanning electron microscope.
Sporophytes of L. dussii were examined using immunogold labeling to identify pectin epitopes in guard cell walls and intercellular spaces. Mature stomata were examined using three primary monoclonal antibodies: LM19 (unesterified homogalacturonan), LM6 (arabinan rhamnogalacturonan I), and LM13 (linear arabinan rhamnogalacturonan I; Plant Probes, University of Leeds). One control that excluded incubation of the primary antibody and two treatments were made for each antibody on three to five individual stomata. Grids were placed in 2% BSA in 0.02 mol L−1 PBS solution, pH 7.2 (PBS), overnight at 4°C in a humid chamber. Treatments were transferred to primary antibody (diluted 1:20 in 2% BSA/PBS) for 3 h while controls were left in buffer. Treatment and control grids were rinsed in 2% BSA/PBS four times for 3 min each, then incubated for 30 min in gold-conjugated (10 nm) IgG anti-rat secondary antibody (Sigma-Aldrich) diluted 1:20 in 2% BSA/PBS. Grids were then rinsed four times with PBS for 3 to 5 min each, followed by distilled/deionized autoclaved filtered water, and dried at room temperature. Grids were observed unstained with a Hitachi H7650 transmission electron microscope at 60 kV.
To determine any developmental changes in guard cell size, we measured guard cell length and width in surface sections of A. agrestis sporophytes along three regions of the axis (green zone, green-brown zone, and brown zone). Guard cell lengths of 16 hornwort species, representing approximately 9% of all hornwort species and 16% of those with stomata, were measured, and their means were compared with published genome size data (Bainard and Villarreal, 2013) using a correlation implemented in the R package. For consistency, all guard cell measurements that were correlated with genome sizes were made on green parts of sporophytes. Out of the 24 hornwort species with available genome sizes (Bainard and Villarreal, 2013), eight lack stomata; thus, only 16 taxa were used in our correlation.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. TEM immunogold localization of LM19 pectin epitopes in mucilage in spore sacs of Leiosporoceros sporophytes.
Supplemental Figure S2. Cross-section light micrograph of an L. dusii sporophyte with a large collapsed stoma over a substomatal cavity that connects to a system of intercellular air-filled spaces.
Supplemental Table S1. Average genome sizes (Bainard and Villarreal, 2013) and stomatal guard cell length from mature guard cells in sixteen hornwort species.
Supplemental Table S2. Stomatal guard cell length from selected early Devonian fossils of rhyniophytes, zosterophllyloids, aglaophytes, and lycophytes taken from Lomax et al. (2013).
Supplementary Material
Acknowledgments
We thank Nicholas Flowers for technical assistance.
Glossary
- ABA
abscisic acid
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
Footnotes
This work was supported by the National Science Foundation (grant no. DUE 1136414) and National Institutes of Health (grant no. 5 R25 GM107760-05).
References
- Amsbury S, Hunt L, Elhaddad N, Baillie A, Lundgren M, Verhertbruggen Y, Scheller HV, Knox JP, Fleming AJ, Gray JE (2016) Stomatal function requires pectin de-methyl-esterification of the guard cell wall. Curr Biol 26: 2899–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainard JD, Villarreal JC (2013) Genome size increases in recently diverged hornwort clades. Genome 56: 431–435 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179: 975–986 [DOI] [PubMed] [Google Scholar]
- Berry JA, Beerling DJ, Franks PJ (2010) Stomata: key players in the earth system, past and present. Curr Opin Plant Biol 13: 233–240 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344: 1879–1900 [DOI] [PubMed] [Google Scholar]
- Cai S, Chen G, Wang Y, Huang Y, Marchant B, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, et al. (2017) Evolutionary conservation of ABA signaling for stomatal closure in ferns. Plant Physiol 23: in press http://doi.org/10.1104/pp.16.01848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Gray JE, Beerling DJ (2013) Early evolutionary acquisition of stomatal control and development gene signalling networks. Curr Opin Plant Biol 16: 638–646 [DOI] [PubMed] [Google Scholar]
- Chater C, Caine RS, Fleming AJ, Gray JE (2017) Origins and evolution of stomatal development. Plant Physiology 174: 624–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ (2011) Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Curr Biol 21: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Chater CC, Caine RS, Tomek M, Wallace S, Kamisugi Y, Cuming AC, Lang D, MacAlister CA, Casson S, Bergmann DC, et al. (2016) Origin and function of stomata in the moss Physcomitrella patens. Nat Plants 2: 16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft WN, Lang WH (1942) The Lower Devonian flora of the Senni Beds of Monmouthshire and Breconshire. Philos Trans B 231: 131–168 [Google Scholar]
- Dolezel J, Bartoš J, Voglmayr H, Greilhuber J (2003) Nuclear DNA content and genome size of trout and human. Cytometry A 51: 127–128, author reply 129 [DOI] [PubMed] [Google Scholar]
- Duckett JG, Pressel S, P’ng KM, Renzaglia KS (2009) Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytol 183: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Edwards D. (2003) Embryophytic sporophytes in the Rhynie and Windyfield cherts. Trans R Soc Edinb 94: 397–410 [Google Scholar]
- Edwards D, Kerp H, Hass H (1998) Stomata in early land plants: an anatomical and ecophysiological approach. J Exp Bot 49: 255–278 [Google Scholar]
- Edwards D, Morris JL, Richardson JB, Kenrick P (2014) Cryptospores and cryptophytes reveal hidden diversity in early land floras. New Phytol 202: 50–78 [DOI] [PubMed] [Google Scholar]
- Everet RF. (2006) Esau’s Plant Anatomy: Meristems, Cells and Tissues of the plant Body—Their Structure, Function and Development. Wiley, Hoboken, NJ [Google Scholar]
- Field KJ, Duckett JG, Cameron DD, Pressel S (2015) Stomatal density and aperture in non-vascular land plants are non-responsive to above-ambient atmospheric CO2 concentrations. Ann Bot (Lond) 115: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Britton-Harper ZJ (2016) No evidence of general CO2 insensitivity in ferns: one stomatal control mechanism for all land plants? New Phytol 211: 819–827 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Leitch IJ, Ruszala EM, Hetherington AM, Beerling DJ (2012) Physiological framework for adaptation of stomata to CO2 from glacial to future concentrations. Philos Trans R Soc Lond B Biol Sci 367: 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Sharafi M, Jalili A, Díaz S, Montserrat-Martí G, Palmer C, Cerabolini B, Pierce S, Hamzehee B, Asri Y, et al. (2010) Stomatal vs. genome size in angiosperms: the somatic tail wagging the genomic dog? Ann Bot (Lond) 105: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ (2005) A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta 221: 255–264 [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci USA 100: 11783–11788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant Cell Environ 35: 53–60 [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS (2012a) Major transitions in the evolution of early land plants: a bryological perspective. Ann Bot (Lond) 109: 851–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS (2012b) The origin of the sporophyte shoot in land plants: a bryological perspective. Ann Bot (Lond) 110: 935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax BH, Hilton J, Bateman RM, Upchurch GR, Lake JA, Leitch IJ, Cromwell A, Knight CA (2014) Reconstructing relative genome size of vascular plants through geological time. New Phytol 201: 636–644 [DOI] [PubMed] [Google Scholar]
- Lomax BH, Knight CA, Lake JA (2012) An experimental evaluation of the use of C3 δ13C plant tissue as a proxy for the paleoatmospheric δ13CO2 signature of air. G3 13: Q0AI03 [Google Scholar]
- Lomax BH, Woodward FI, Leitch IJ, Knight CA, Lake JA (2009) Genome size as a predictor of guard cell length in Arabidopsis thaliana is independent of environmental conditions. New Phytol 181: 311–314 [DOI] [PubMed] [Google Scholar]
- Lucas JR, Renzaglia KS (2002) Structure and function of hornwort stomata. Microsc Microanal 8: 1090–1091 [Google Scholar]
- MacAlister CA, Bergmann DC (2011) Sequence and function of basic helix-loop-helix proteins required for stomatal development in Arabidopsis are deeply conserved in land plants. Evol Dev 13: 182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM (2007) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48: 984–999 [DOI] [PubMed] [Google Scholar]
- McAdam SA, Brodribb TJ (2013) Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol 198: 429–441 [DOI] [PubMed] [Google Scholar]
- McAdam SA, Brodribb TJ, Banks JA, Hedrich R, Atallah NM, Cai C, Geringer MA, Lind C, Nichols DS, Stachowski K, et al. (2016) Abscisic acid controlled sex before transpiration in vascular plants. Proc Natl Acad Sci USA 113: 12862–12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merced A. (2015a) Novel insights on the structure and composition of pseudostomata of Sphagnum. Am J Bot 102: 329–335 [DOI] [PubMed] [Google Scholar]
- Merced A. (2015b) Evolution of stomata in mosses (Bryophyta): from molecules to form and function. PhD dissertation. Southern Illinois University, Carbondale, IL [Google Scholar]
- Merced A, Renzaglia KS (2013) Moss stomata in highly elaborated Oedipodium (Oedipodiaceae) and highly reduced Ephemerum (Pottiaceae) sporophytes are remarkably similar. Am J Bot 100: 2318–2327 [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia K (2014) Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Ann Bot (Lond) 114: 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Nguema-Ona EE, Vicré-Gibouin M, Sørensen I, Willats WG, Driouich A, Farrant JM (2013) Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237: 739–754 [DOI] [PubMed] [Google Scholar]
- Obermayer R, Leitch IJ, Hanson L, Bennett MD (2002) Nuclear DNA C-values in 30 species double the familial representation in pteridophytes. Ann Bot (Lond) 90: 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Pearce JV (1957) The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society 3: 228–259 [Google Scholar]
- Pressel S, Goral T, Duckett JG (2014) Stomatal differentiation and abnormal stomata in hornworts. J Bryol 36: 87–103 [Google Scholar]
- Raven JA. (2014) Speedy small stomata? J Exp Bot 65: 1415–1424 [DOI] [PubMed] [Google Scholar]
- Renzaglia KS. (1978) A comparative morphology and developmental anatomy of the Anthocerotophyta. J Hattori Bot Lab 44: 31–90 [Google Scholar]
- Renzaglia KS, Duff RJT, Nickrent DL, Garbary DJ (2000) Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philos Trans R Soc Lond B Biol Sci 355: 769–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Rasch EM, Pike LM (1995) Estimates of nuclear DNA content in bryophyte sperm cells: phylogenetic considerations. Am J Bot 82: 18–25 [Google Scholar]
- Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG (2007) Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist 110: 179–213 [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Sack FD. (1987) The development and structure of stomata. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 59–89 [Google Scholar]
- Schuette S, Renzaglia KS (2010) Development of multicellular spores in the hornwort genus Dendroceros (Dendrocerotaceae, Anthocerotophyta) and the occurrence of endospory in bryophytes. Nova Hedwigia 91: 301–316 [Google Scholar]
- Spetea C, Rintamäki E, Schoefs B (2014) Changing the light environment: chloroplast signaling and response mechanisms. Philos Trans R Soc Lond B Biol Sci 369: 20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SA (2017) What are the evolutionary origins of stomatal responses to abscisic acid in land plants? J Integr Plant Biol 59: 240–260 [DOI] [PubMed] [Google Scholar]
- Villarreal JC, Renzaglia KS (2006) Sporophyte structure in the neotropical hornwort Phaeomegaceros fimbriatus: implications for phylogeny, taxonomy, and character evolution. Int J Plant Sci 167: 413–427 [Google Scholar]
- Villarreal JC, Renzaglia KS (2015) The hornworts: important advancements in early land plant evolution. J Bryol 37: 157–170 [Google Scholar]
- Ziegler H. (1987) The evolution of stomata. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 29–57 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.