The activity of LPEAT affects the growth of Arabidopsis and is essential for maintaining an adequate level of PE, LPE, and LPC in the cells.
Abstract
Arabidopsis (Arabidopsis thaliana) contains two enzymes (encoded by the At1g80950 and At2g45670 genes) preferentially acylating lysophosphatidylethanolamine (LPE) with acyl-coenzyme A (CoA), designated LYSOPHOSPHATIDYLETHANOLAMINE ACYLTRANSFERASE1 (LPEAT1) and LPEAT2. The transfer DNA insertion mutant lpeat2 and the double mutant lpeat1 lpeat2 showed impaired growth, smaller leaves, shorter roots, less seed setting, and reduced lipid content per fresh weight in roots and seeds and large increases in LPE and lysophosphatidylcholine (LPC) contents in leaves. Microsomal preparations from leaves of these mutants showed around 70% decrease in acylation activity of LPE with 16:0-CoA compared with wild-type membranes, whereas the acylation with 18:1-CoA was much less affected, demonstrating that other lysophospholipid acyltransferases than the two LPEATs could acylate LPE. The above-mentioned effects were less pronounced in the single lpeat1 mutant. Overexpression of either LPEAT1 or LPEAT2 under the control of the 35S promotor led to morphological changes opposite to what was seen in the transfer DNA mutants. Acyl specificity studies showed that LPEAT1 utilized 16:0-CoA at the highest rate of 11 tested acyl-CoAs, whereas LPEAT2 utilized 20:0-CoA as the best acyl donor. Both LPEATs could acylate either sn position of ether analogs of LPC. The data show that the activities of LPEAT1 and LPEAT2 are, in a complementary way, involved in growth regulation in Arabidopsis. It is shown that LPEAT activity (especially LPEAT2) is essential for maintaining adequate levels of phosphatidylethanolamine, LPE, and LPC in the cells.
Acyl-CoA:lysophospholipid acyltransferases (LPLATs) are important but still inadequately characterized enzymes participating in membrane and storage lipid biosynthesis. The plant genes encoding enzymes with acyl-CoA:lysophosphatidic acid acyltransferase (LPAAT) activity had already been cloned in the 1990s (Brown et al., 1994, 1995; Hanke et al., 1995; Knutzon et al., 1995; Bourgis et al., 1999). However, the first genes (At1g12640 and At1g63050) encoding plant acyl-CoA:lysophosphatidylcholine acyltransferases (LPCAT) were not identified until 2008 (Ståhl et al., 2008). Subsequently, genes encoding enzymes with LPCAT activity also have been cloned from various plant species (Lager et al., 2013). In 2009, two genes encoding proteins with high acyl-CoA:lysophosphatidylethanolamine acyltransferase (LPEAT) activities (At1g80950 and At2g45670) were cloned from Arabidopsis (Arabidopsis thaliana; Stålberg et al., 2009).
To establish the physiological roles of different plant LPLATs, both in vivo and in vitro studies have been performed. The role of LPCAT in acyl editing of plant lipids has been demonstrated through in vivo radiolabeling experiments in developing safflower (Carthamus tinctorius) and sunflower (Helianthus annuus) cotyledons (Griffiths et al., 1988), mature Brassica napus leaves (Williams et al., 2000), expanding pea (Pisum sativum) leaves (Bates et al., 2007), developing soybean (Glycine max) embryos (Bates et al., 2009), and developing Arabidopsis seeds (Bates et al., 2012). In addition to the acylation of lysophospholipids, in vitro experiments have shown that the reverse reaction of LPLATs can transfer acyl groups from phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidic acid into the acyl-CoA pool (Lager et al., 2013; Jasieniecka-Gazarkiewicz et al., 2016). Substrate specificities of LPCAT for ricinoleic acid (12-hydroxy-octadeca-9-enoic acid) differed substantially between the forward and reverse reactions (Lager et al., 2013). Furthermore, the rate in the forward reaction of different LPLATs does not always correlate with the rate of these enzymes in the backward reactions (Jasieniecka-Gazarkiewicz et al., 2016). Convincing evidence that LPCAT also can operate reversibly in vivo was obtained recently in yeast cells (Pan et al., 2015). Thus, LPLATs play a key role in controlling the composition of membrane phospholipid molecular species, which, in turn, play a crucial role for optimal membrane fluidity, vesicle trafficking, and other relevant biological functions (Tasseva et al., 2004).
There are only a few reports on the effects of deletion mutations in plant LPLATs. Wang et al. (2012) showed that disruption of the two Arabidopsis genes (At1g12640 and At1g63050) encoding LPCAT1 and LPCAT2 has no pronounced effect on plant growth and seed development despite the fact that it causes substantially increased turnover of PC. On the other hand, the homozygous Arabidopsis lpaat mutant deficient in plastidial LPAAT dies at an early stage of embryogenesis (Kim and Huang, 2004). Until now, there was no information on the effects of mutations or overexpression of LPEAT genes in plants.
In this study, we investigated the effects on Arabidopsis growth and development of T-DNA insertion mutants of At1g80950 (encoding AtLPEAT1) or At2g45670 (encoding AtLPEAT2) and of the double mutant as well as overexpressors of these genes. The single mutant lpeat2 and the double mutant lpeat1 lpeat2 showed strongly impaired growth and seed setting, whereas overexpression of either LPEAT1 or LPEAT2 led to increased growth and seed setting compared with the wild type. Mutations in LPEATs also led to changes in the membrane lipid composition of leaves and roots. In vitro acyl specificity studies of the LPEATs indicate that LPEAT2 might be involved in the synthesis of rare molecular species of PE involved in growth processes.
RESULTS
Characterization of Gene Expression in the lpeat1 and lpeat2 Single and Double Mutants
Seeds of wild-type Arabidopsis (Columbia-0 [Col-0]) and seeds of T-DNA mutants lpeat1 and lpeat2 were germinated in soil, and genomic DNA from leaves of each line was analyzed by PCR with specific primers for the identification of T-DNA insertions. All tested lines contained the corresponding T-DNA insertions (Supplemental Figs. S1 and S2).
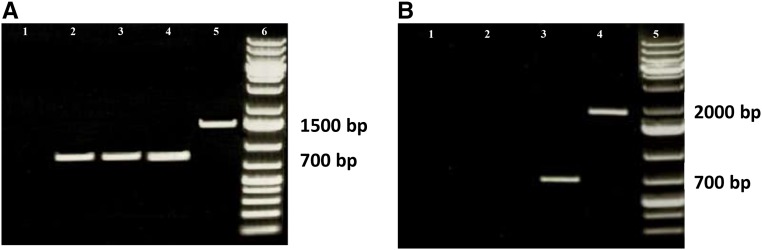
To characterize LPEAT expression in the T-DNA insertion lines of Arabidopsis, reverse transcription (RT)-PCR was used. Three different T-DNA insertion lines of LPEAT1 (SALK_016798C, T-DNA insertion in the third exon; FLAG_217C02, T-DNA insertion in the seventh exon; and GABI_825D08, T-DNA insertion in the seventh exon) were analyzed together with the wild type. Only the GABI_825D08 line (Fig. 1A, lane 1) lacked LPEAT1 in the RT-PCR band; the other tested T-DNA insertion lines (FLAG_217C02 and SALK_016798c) showed a wild-type-like expression of LPEAT1 (Fig. 1A). Thus, these results indicate that only the GABI_825D08 line showed suppressed expression for the At1g80950 gene (AtLPEAT1). For LPEAT2, only one T-DNA insertion line was tested (SALK_107699c; insertion in the sixth intron), and no RT-PCR amplicon for LPEAT2 could be detected (Fig. 1B). Thus, these results confirmed that this line is a knockout for the AtLPEAT2 gene.
Figure 1.
Products of RT-PCR of cDNA of At1g80950 (LPEAT1; A) and At2g45670 (LPEAT2; B) from leaves of Arabidopsis wild-type plants and T-DNA mutants. A, Lane 1, line GABI_825D08; lane 2, FLAG_217C02; lane 3, SALK_016798c; lane 4, the wild type; lane 5, product of the reaction with genomic DNA from leaves of wild-type plants; lane 6, marker GeneRuler. B, Lanes 1 and 2, line SALK_107699c; lane 3, the wild type; lane 4, product of the reaction with genomic DNA from leaves of wild-type plants; lane 5, marker GeneRuler.
GABI_825D08 (lpeat1) and SALK_107699c lines (lpeat2) were used for complementation with constructs harboring the LPEAT genes under the control of the 35S promoter. The expression of RNA of LPEAT1 and LPEAT2 in the homozygous lines was analyzed by RT-PCR (data not shown).
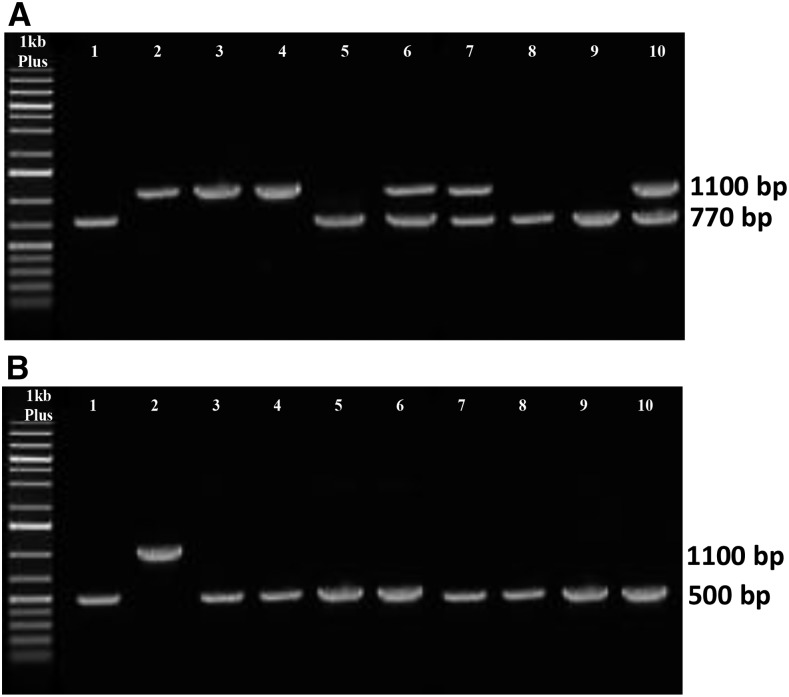
The double mutant plants disrupted in both LPEAT1 and LPEAT2 were obtained by crossing a GABI_825D08 line (lpeat1) with a SALK_107699c line (lpeat2). The seeds of T2 plants were germinated on soil, and genomic DNA from leaves of individual plants was amplified by PCR with primers specific for the identification of T-DNA insertion in the two genes. Double homozygous mutant plants carrying T-DNA insertions in both LPEAT1 and LPEAT2 were identified by PCR (Fig. 2, lanes 5, 8, and 9).
Figure 2.
Products of PCR of genomic DNA isolated from leaves of mutants of the F3 generation obtained by crossing of homozygous line GABI_825D089 (At1g80950) and SALK_107699c (At2g45670) using primers for genotyping SALK_107699c (A) and primers for genotyping GABI_825D089 (B). A, Lane 1, single insertion mutant line SALK_107699c (positive control); lane 2, untransformed plant Col-0; lanes 3 to 10, F3 generation of hybrids. B, Lane 1, single insertion mutant line GABI_825D089 (positive control); lane 2, untransformed plant Col-0; lanes 3 to 10, F3 generation of hybrids.
LPEAT1 and LPEAT2 Are Required for Normal Vegetative Growth and Seed Set
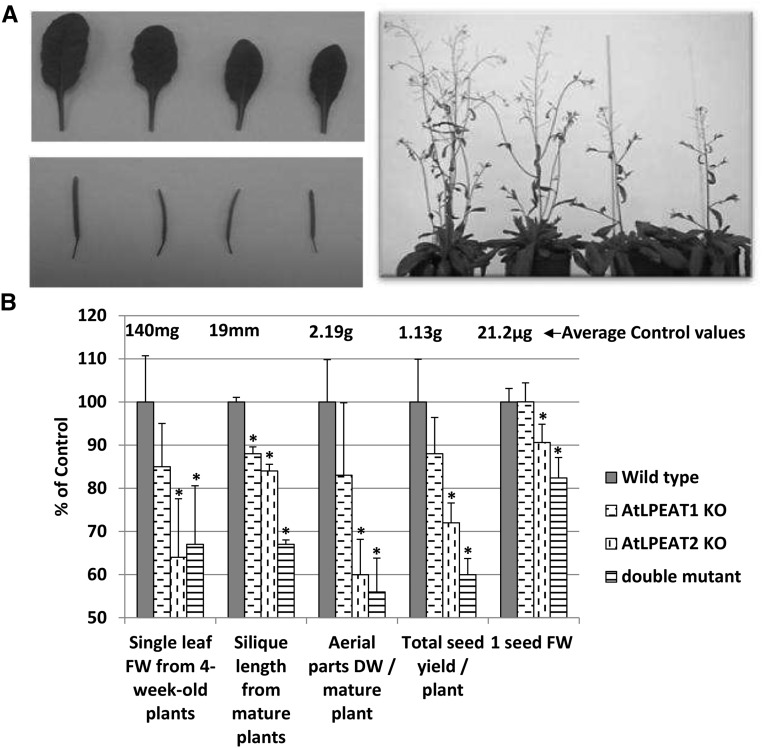
To investigate the physiological functions of AtLPEAT1 and AtLPEAT2, the insertion mutants lpeat1 (GABI_825D08) and lpeat2 (SALK_107699c), which were deficient in the expression of the LPEAT1 and LPEAT2 genes (Fig. 1), respectively, were used. Additionally, the double homozygous line lpeat1 lpeat2 (Fig. 2) was obtained by crossing the two single mutants. Both single lpeat mutants and the double mutant revealed several abnormalities in plant development compared with the wild type, although for lpeat1, the differences were only statistically significant for silique length. The first effects of the mutations on plant growth were observed after 3 to 4 weeks of growth on soil. Germination and early growth of the seedlings did not differ from those of the wild-type plants. The rosette leaves were smaller, from about 15% for the lpeat1 mutant to about 35% for the lpeat2 and double mutants, compared with control leaves (Fig. 3B), but the leaf shape was not altered (Fig. 3A). After 5 to 6 weeks of cultivation, the differences in size of developing plants became clearly visible. The mutants, especially lpeat2 and the double mutant, were much smaller than wild-type plants (Fig. 3A). During this time of development, the differences in silique size also became visible (Fig. 3A). The mature siliques were shorter by around 15% to 16% in the single mutants and by over 30% in double mutant plants compared with the wild type (Fig. 3B). The differences in morphology of the mutants compared with wild-type plants did not disappear with time of cultivation, and after 11 to 12 weeks (when plants started to dry naturally), the dry mass of aerial parts of lpeat1, lpeat2, and double mutants was around 17%, 40%, and 44% lower, respectively, than the dry mass of wild-type plants (Fig. 3B). The mutants also produced lower numbers of seeds than the control plants: the lpeat1 mutant by about 12%, the lpeat2 mutant by about 38%, and the double mutant by about 40% (Fig. 3B). The lpeat2 and double mutant seeds also were smaller than the wild-type seeds by about 10% and 18%, respectively (Fig. 3B).
Figure 3.
Phenotypic analysis of wild-type plants of Arabidopsis (Col-0) and plants of lpeat1 and lpeat2 mutants and double mutants. A, Photographs of 5-week-old leaves and siliques of 7- and 6-week-old plants. From left to right: the wild type, lpeat1 mutant, lpeat2 mutant, and lpeat1 lpeat2 double mutant. B, Mean values and sd of chosen parameters characterizing tested plants presented as percentage of control values (wild-type plants). DW, Dry weight; FW, fresh weight; KO, T-DNA mutant. Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n = 5–8; in the case of 1 seed FW, a replication average weight of 300 seeds was used). The average control values are depicted above the control bars.
To study the effects of the disruption of the LPEAT genes on root growth, the seeds of wild-type plants and the seeds of the mutants were germinated and grown on agar for 2 or 4 weeks in a vertical position in a growth chamber. Alternatively, the seedlings were transferred to liquid culture after 2 weeks and incubated for an additional 2 weeks with gentle shaking under light.
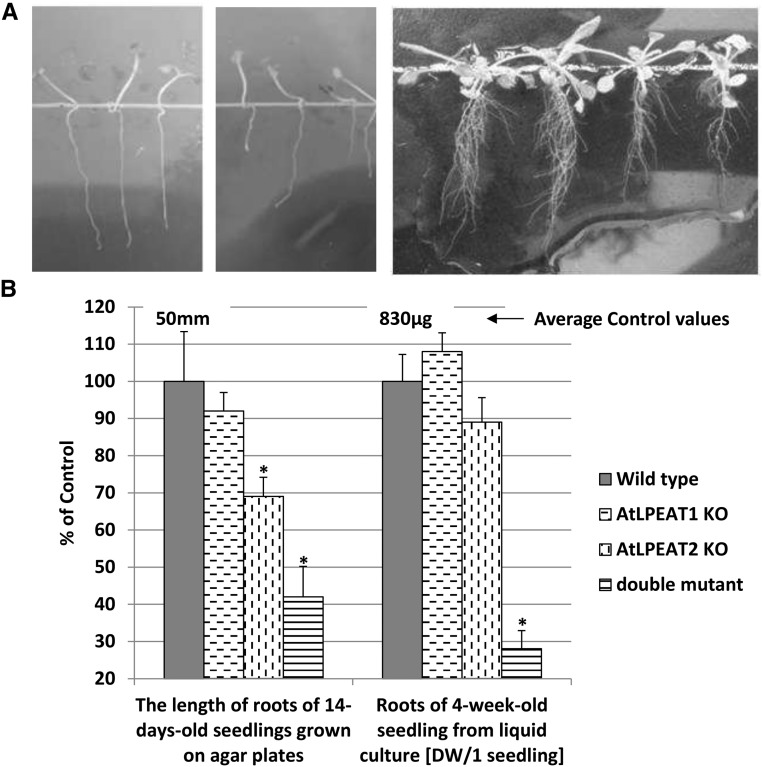
The primary root length of the lpeat1 mutant grown on agar for 14 d was shorter by about 8%, while the roots of lpeat2 and double mutant plants were shorter than the roots of wild-type plants by about 31% and 58%, respectively (Fig. 4). The growth inhibition continued during the next 14 d. The main roots were still shorter than the roots of wild-type plants, and additionally, a lower amount of lateral and additional roots was produced (Fig. 4A). The dry weight of the roots of lpeat1 and lpeat2 single mutants from liquid culture was not statistically different from the dry weight of roots of wild-type plants, whereas the double mutant root weight was about 72% lower (Fig. 4B).
Figure 4.
Effects of the lpeat mutations on root growth of Arabidopsis plants. A, Images of 14-d-old roots of wild-type plants (left), 14-d-old lpeat2 mutant roots (middle), and 4-week-old roots (right); from left to right: the wild type, lpeat1 mutant, lpeat2 mutant, and lpeat1 lpeat2 double mutant. Roots were grown on agar plates. B, Mean values and sd of chosen parameters characterizing roots of tested plants presented as percentage of control values (wild-type plants). DW, Dry weight; KO, T-DNA mutant. Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n ≥ 5 for dry weight and n ≥ 10 for root length). The average control values from wild-type plants are depicted above the control bars.
To confirm that the abnormalities in growth and development of the lpeat mutants described above were caused by the deficiency in LPEAT gene function, complementation experiments of the lpeat1 mutant with a 35S::At1g80950 construct and of the lpeat2 mutant with a 35S::At2g45670 construct were performed. In the transformed mutants (T3, homozygous), all defects in development observed in the T-DNA insertion mutants were complemented (Supplemental Fig. S3), and most transformed plants had improved growth compared with the wild type and morphologically resembled overexpressors of the LPEAT genes in wild-type plants (see below). Since the latter plants were generated before the complemented plants, only the overexpression plants in the wild-type background were characterized further.
Since the disruption of the LPEAT genes was expected to result in an increased lysophosphatidylethanolamine (LPE) concentration, we tested the effect of adding LPE to wild-type seedlings. The addition of exogenous LPE in a low concentration of 5 nmol mL−1 to the agar-solidified growth medium resulted in symptoms similar to those observed in the lpeat mutant plants. The roots of wild-type plants grown on the agar plates with the addition of LPE produced smaller amounts of lateral roots compared with roots grown without the addition of LPE. Higher concentrations of LPE caused additional disturbances of root growth (Supplemental Fig. S4; data not shown).
The Alteration in Morphology Caused by Disruption of LPEAT Genes Correlates with Changes in PE, Lysophosphatidylethanolamine, and Lysophosphatidylcholine Concentrations
To investigate the correlation between the retardation of plant growth and lipid metabolism in Arabidopsis lpeat1 and lpeat2 mutant plants, lipid content and composition were analyzed in leaves of 4-week-old plants, roots of 4-week-old seedlings from liquid cultures, and mature seeds.
The gas chromatographic analysis demonstrated that the total acyl-lipid content in roots of the lpeat2 mutant and of the lpeat1 lpeat2 double mutant was reduced by about 14% and 30%, respectively (Fig. 5). A statistically significant reduction in lipid content also occurred in seeds of lpeat2 and of the double mutant. The acyl-lipid content in young rosette leaves was not affected by the mutations (Fig. 5).
Figure 5.
Effects of lpeat mutations on acyl-lipid content (presented as the sum of fatty acids of acyl lipids) in leaves of 4-week-old seedlings grown in soil, roots of 4-week-old seedlings from liquid cultures, and mature seeds of Arabidopsis. Mean values and sd are presented as percentage of control values (the wild type). FW, Fresh weight; KO, T-DNA mutant. Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n ≥ 3). The average control values from wild-type plants are depicted above the control bars.
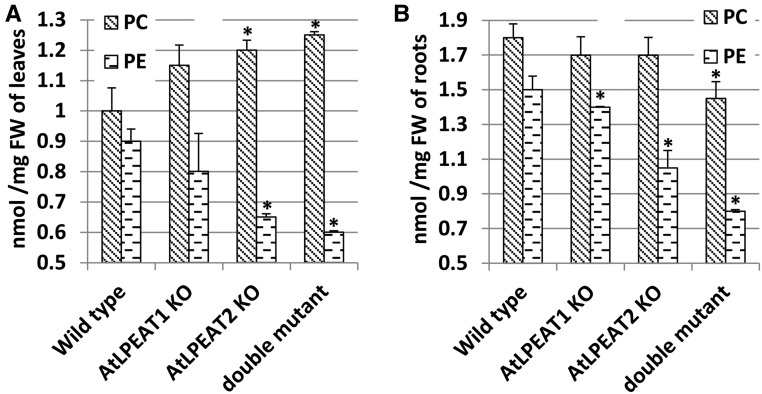
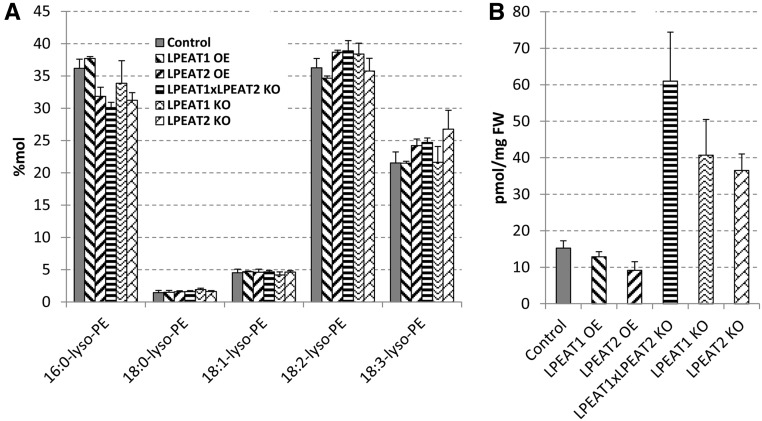
Quantification of major polar lipid classes present in leaves and roots of the mutants revealed that the plants displaying the most severe phenotype (lpeat2 and lpeat1 lpeat2 double mutant) showed significantly decreased relative (mol %) and absolute (nmol mg−1 fresh weight) amounts of PE in leaves and roots and increased relative amounts of PC, with a slight increase in absolute amounts of this lipid in leaves and a slight decrease in roots (Fig. 6; Supplemental Tables S2 and S3). The relative contents of the other lipids were not affected significantly (Supplemental Tables S2 and S3), except for LPE and lysophosphatidylcholine (LPC; Figs. 7 and 8), which normally constitute very minor polar lipid classes. The levels of these two lysolipids in leaves of lpeat mutants were strongly elevated, whereas in the overexpressors (LPEAT1 OE and LPEAT2 OE), the levels of these lysolipids were similar to control levels. In the lpeat mutants, the concentration of LPE was elevated 3 to 4 times, whereas the LPC concentration increased 10 to 15 times compared with wild-type leaves. The major species of LPE were 16:0-LPE and 18:2-LPE followed by 18:3-LPE (Fig. 7). For LPC, the major molecular species were 18:3-LPC and 18:2-LPC followed by 16:0-LPC (Fig. 8). The molecular species composition of the lysolipids was similar between the wild type, mutants, and overexpressors.
Figure 6.
Effects of lpeat mutations on PC and PE levels in 4-week-old leaves of Arabidopsis plants growing in soil (A) and in 4-week-old roots of plants from liquid culture (B). Mean values and sd are presented. FW, Fresh weight; KO, T-DNA mutant. Asterisks indicate significant differences compared with wild-type plants in a mean difference two-sided test at α = 0.05 (n ≥ 3).
Figure 7.
The mol % of molecular species of LPE (A) and the nmol sum of all LPE species per mg fresh weight (B) in Arabidopsis leaves of wild-type plants, lpeat mutants, and LPEAT overexpressors grown in soil for 4 weeks. Other molecular species of LPE than are presented were below the detection level or were not present. Mean values and sd are presented. FW, Fresh weight; KO, T-DNA mutant; OE, overexpressor.
Figure 8.
The mol % of molecular species of LPC (A) and the nmol sum of all LPC species per mg fresh weight (B) in Arabidopsis leaves of wild-type plants, lpeat mutants, and LPEAT overexpressors grown in soil for 4 weeks. Other molecular species of LPC than are presented were below the detection level or were not present. Mean values and sd are presented. FW, Fresh weight; KO, T-DNA mutant; OE, overexpressor.
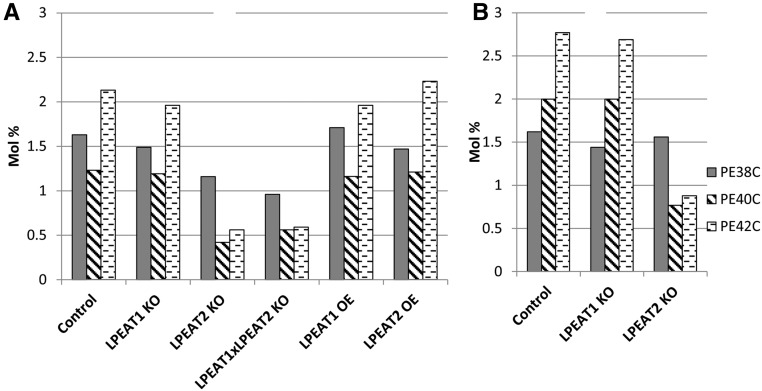
The mutations of LPEAT genes only slightly affected the fatty acid composition of both roots and leaf polar lipids, and the most pronounced differences were observed in the lpeat1 lpeat2 double mutant. In leaves, statistically proven decrease of the relative amount of 18:1 and increase of 18:3 level occurred in PC, whereas a decrease of 18:3 was seen in PE (Supplemental Table S4). In roots, significant changes of fatty acid composition occurred only in PE, with an increase in 18:1 (Supplemental Table S5). It is pertinent to note that mass spectrometry analyses showed that the lpeat2 mutation (in lines lpeat2 and lpeat1 lpeat2) resulted in a significant reduction in PE species with very-long-chain fatty acids in roots and leaves, especially of PE-40C and PE-42C (up to one-third of the wild-type level), and also in leaves of PE-38C (Fig. 9; Supplemental Tables S6 and S7).
Figure 9.
Very-long-chain molecular species of PE in Arabidopsis leaves (A) of wild-type plants (control), lpeat mutants, and LPEAT overexpressors grown in soil for 4 weeks and in roots (B) of wild-type plants (control) and lpeat mutants grown in liquid culture. The sums of all molecular species of PE with the same number of carbons in the acyl chains are presented (for individual values, see Supplemental Tables S6 and S7). KO, T-DNA mutant; OE, overexpressor.
Overexpression of AtLPEAT1 or AtLPEAT2 Leads to Increased Plant Growth and Seed Setting and Increased Lipid Content in the Roots
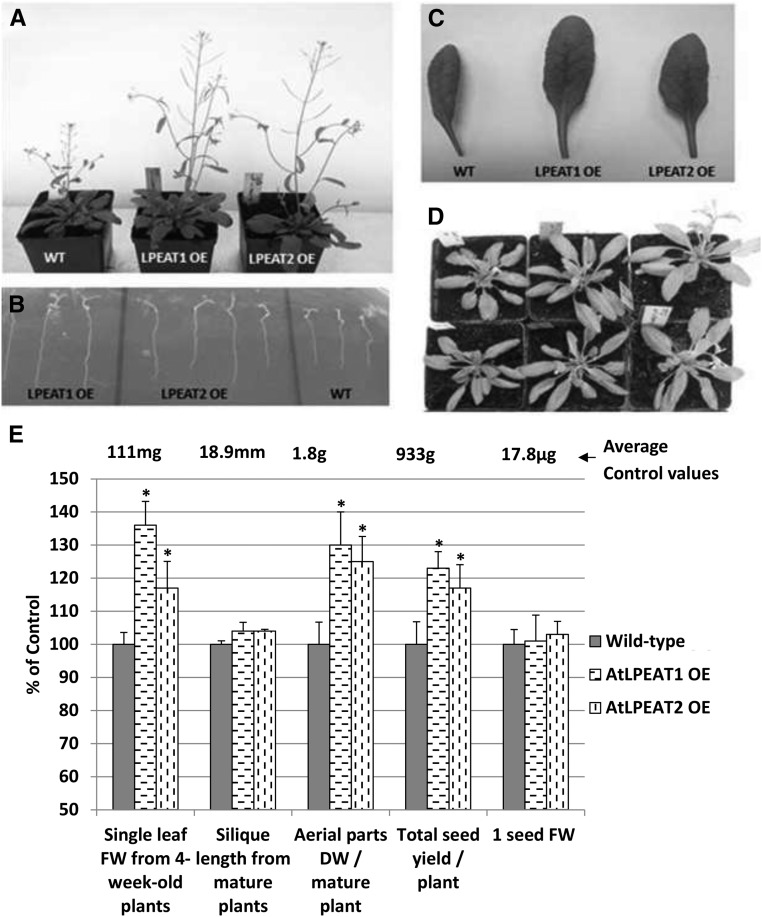
To investigate the effect of LPEAT overexpression on plant morphology and lipid metabolism, we transformed wild-type plants of Arabidopsis (Col-0) with constructs harboring 35S::At1g80950 (LPEAT1) or 35S::At2g45670 (LPEAT2). The transformation was confirmed by selection on plates containing kanamycin. T2 plants produced seeds of which 100% germinated on the kanamycin plate were identified as homozygous plants. Only the seeds of homozygous plants were used for further study. Most of the homozygous plants showed stronger growth than the wild type. One line of each of the transgenic plants overexpressing LPEAT1 or LPEAT2 showing the most vigorous growth was selected for further investigation. Both LPEAT1 and LPEAT2 overexpression stimulated the growth of aerial parts of plants to a similar extent. Already after 4 weeks of growth on soil, the overexpressors (both LPEAT1 and LPEAT2) produced rosettes bigger than the control plants, and after 5 to 6 weeks of cultivation, the stalk sizes were much bigger compared with the control (Fig. 10).
Figure 10.
Phenotypic analysis of wild-type plants of Arabidopsis (Col-0) and plants overexpressing LPEAT1 or LPEAT2 (OE). A to D, Images of 5-week-old plants (A), 2-week-old roots grown on an agar plate (B), 5-week-old leaves (C), and 4-week-old plants (D). From left to right: the wild type (WT), LPEAT1 OE, and LPEAT2 OE (except for B). E, Mean values and sd of the chosen parameters characterizing tested plants presented as percentage of control values (wild-type plants). DW, Dry weight; FW, fresh weight. Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n = 5–8; in the case of 1 seed FW, a replication average weight of 300 seeds was used). The average control values from wild-type plants are depicted above the control bars.
The dry weight of the aerial part of mature plants of studied overexpressors was on average 25% to 30% higher than the dry weight of wild-type plants (Fig. 10). Both overexpressors produced significantly more seeds (up to 23%) than wild-type plants; however, the single seed weight did not differ compared with the control (Fig. 10).
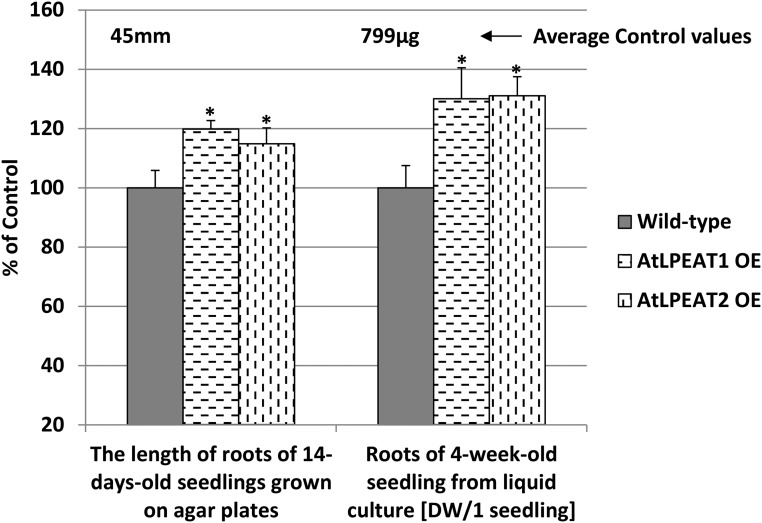
Overexpression of both LPEAT1 and LPEAT2 also affected root growth. The primary roots of the two overexpressors were about 15% to 20% longer than the roots of wild-type plants after 14 d of growth on agar, and the dry weight of the roots transferred to liquid culture after 14 d for an additional 14 d was about 30% higher than the dry weight of roots of wild-type plants (Fig. 11).
Figure 11.
Effects of LPEAT1 or LPEAT2 overexpression (OE) on root growth of Arabidopsis plants. Mean values and sd are presented as percentage of control values (wild-type plants). DW, Dry weight. Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n ≥ 5 for dry weight and n ≥ 10 for root length). The average control values are depicted above the control bars.
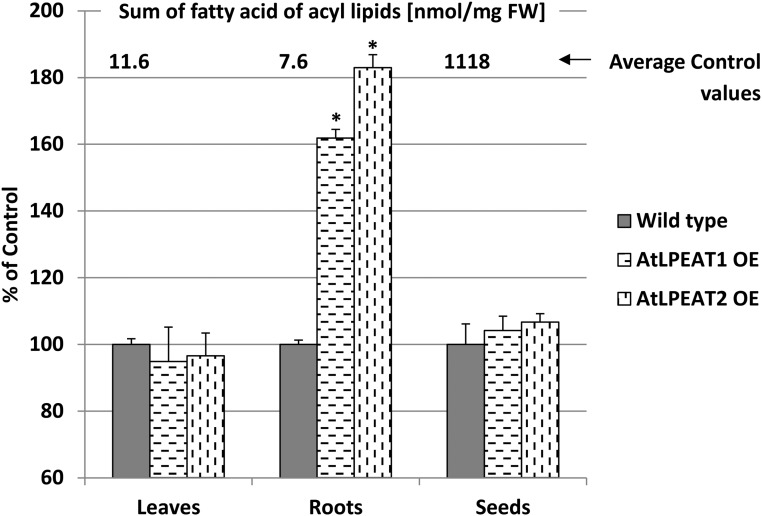
The observed changes in plant growth rate of the overexpressors were not connected with any significant alteration of lipid profiles or fatty acid composition (data not shown). The only noticed lipid effect of the overexpression of LPEAT-encoding genes concerned the acyl-lipid content in roots. The overexpressors contained 1.6 to 1.8 more acyl lipids (expressed as the sum of fatty acids per fresh weight) compared with roots of wild-type plants (Fig. 12).
Figure 12.
Effects of LPEAT1 or LPEAT2 overexpression (OE) on lipid content in leaves, roots, and seeds of Arabidopsis plants. Mean values and sd are presented as percentage of control values (wild-type plants). Asterisks indicate significant differences compared with the control in a mean difference two-sided test at α = 0.05 (n ≥ 3). The average control values are depicted above the control bars.
LPEAT1 and LPEAT2 Show High Activity toward Saturated Acyl-CoAs, and LPEAT2 Has High Activity toward Very-Long-Chain Fatty Acyl-CoAs
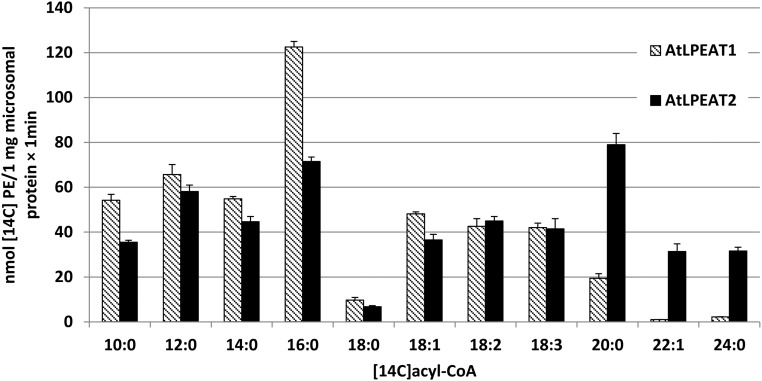
Stålberg et al. (2009) showed that LPEAT1 and LPEAT2 had the highest activities toward 16:0-CoA during the acylation of sn-1-LPE, which is surprising considering that the levels of saturated fatty acids are generally very low in position sn-2 of eukaryote types of phospholipids (Lightner, Wu, Browse, 1994). We assayed the acyl specificity of LPEAT1 and LPEAT2 in microsomal fractions of the yeast ale1 strain expressing either of the two genes and confirmed that 16:0-CoA was a better acyl donor than 18:1-CoA for both enzymes using 18:1-LPE as the acyl acceptor (Fig. 13). Among 11 different acyl-CoA species tested, 16:0 was the best acyl donor for LPEAT1. However, 20:0-CoA was the best acyl donor for LPEAT2, and substantial activity also was found for 22:1Δ13-CoA and 24:0-CoA, but these two latter substrates were virtually inactive in LPEAT1 assays (Fig. 13).
Figure 13.
Substrate specificity of LPEATs of Arabidopsis toward different acyl-CoAs. The enzyme assays were performed with microsomal preparations from the yeast ale1 strain expressing the LPEAT genes with 18:1-LPE as acyl acceptor. Mean values and sd are presented (n ≥ 3).
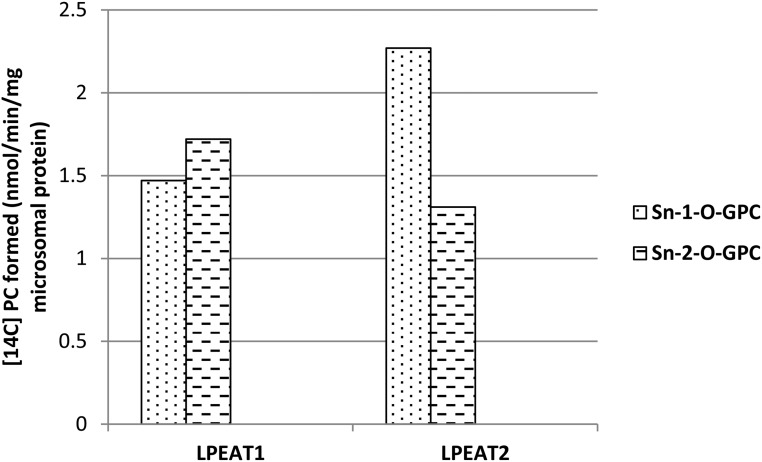
LPEAT enzymes can acylate not only LPE but also to some extent LPC (Stålberg et al., 2009; Jasieniecka-Gazarkiewicz et al., 2016). Using an ether analog to sn-1-18:1-LPC and to sn-2-18:1-LPC as acyl acceptor and 16:0-CoA as acyl donor, we showed that both LPEAT1 and LPEAT2 can acylate either sn-1 or sn-2 position of LPC. LPEAT1 showed a somewhat higher specificity toward the sn-1 position, and LPEAT2 showed a somewhat higher specificity toward the sn-2 position (Fig. 14).
Figure 14.
Activity of Arabidopsis LPEAT1 and LPEAT2 toward the ether analog of sn-1-18:1-LPC (sn-1-O-GPC) and the ether analog of sn-2-18:1-LPC (sn-2-O-GPC) with [14C]16:0-CoA as acyl donor. Values shown are differences of average activities of microsomal fractions of yeast ale1 expressing LPEATs and average activities of control yeast (ale1) harboring an empty vector.
The LPEAT Activity in Leaves of Wild-Type Plants Differs from the LPEAT Activity in Leaves of LPEAT Mutants and Overexpressors
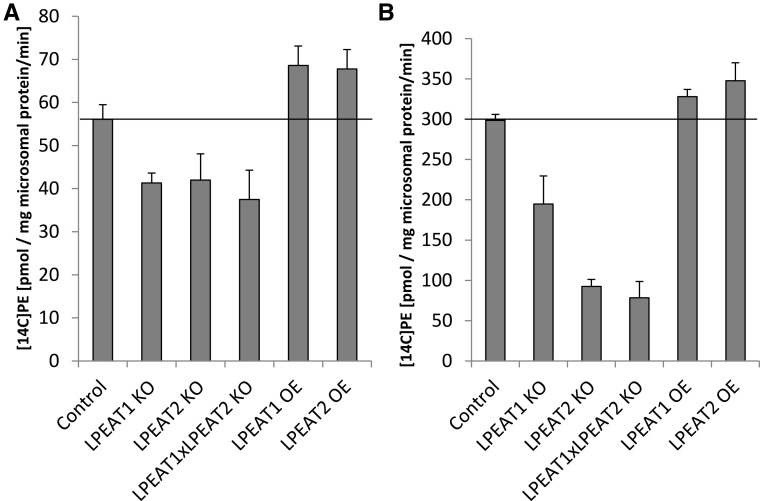
We assayed LPEAT activity in microsomal fractions of Arabidopsis leaves of wild-type plants, lpeat mutants, and overexpressors (grown in soil for 4 weeks) using 18:1-LPE as acyl acceptor and either 18:1-CoA or 16:0-CoA as acyl donor. The assays with 16:0-CoA showed that lpeat mutants (especially lpeat2) had significantly reduced LPEAT activity (up to around 73%) compared with that observed in wild-type plants. In assays with 18:1-CoA, the difference between wild-type plants and the mutants was less pronounced. The high background activity was probably caused by other types of LPLAT enzymes, in particular the LPCATs (Lager et al., 2013). The activities of LPEAT in microsomal fractions of leaves of LPEAT1 OE and LPEAT2 OE were somewhat higher with both 18:1-CoA and 16:0-CoA than in the membranes from wild-type plants (Fig. 15).
Figure 15.
Activity of LPEATs in microsomal fractions of Arabidopsis leaves of wild-type plants, lpeat mutants, and LPEAT overexpressors grown in soil for 4 weeks. Substrates were 5 nmol of sn1-18:1-LPE + 5 nmol of [14C]18:1-CoA (A) and 5 nmol of sn1-18:1-LPE + 5 nmol of [14C]16:0-CoA (B). Mean values and sd are presented (n ≥ 3). KO, T-DNA mutant; OE, overexpressor.
DISCUSSION
Plants of the lpeat2 mutant show a clear phenotype with smaller plants, fewer and smaller seeds, and shorter primary roots than wild-type plants. In the double knockout mutant lpeat1 lpeat2, the phenotypic disturbances seen in the lpeat2 mutant were further augmented. Disruption of the LPEAT1 gene had much milder effects, because, of all traits studied, only the siliques were significantly shorter compared with wild-type plants. The lipid content, measured as the amount of acyl groups per fresh weight, was reduced substantially in roots and seeds of lpeat2 and lpeat1 lepeat2 mutants. The mol % distribution among major polar lipids was unaffected by the mutations, except for a decrease in PE in leaves and roots and an increase in PC in roots in lpeat2 and lpeat1 lpeat2 mutants. The absolute amount (nmol mg−1 fresh weight) of PE also was severely lowered in leaves and roots, whereas LPE and LPC were shown to be substantially increased in leaves of the tested mutants. The effects of lpeat mutations (especially lpeat2) were somewhat similar to those observed for mutations or RNA interference silencing of aminoalcoholphosphotransferases (aapt1 and especially aapt2). The AAPT1 and AAPT2 enzymes also are involved in PE and PC synthesis. These Arabidopsis mutants showed aberrant growth and decreased levels of PE but not PC (Liu et al., 2015). Dysfunction of CTP:phosphorylethanolamine cytidyltransferase (PECT1) also reduced PE levels in leaves of the Arabidopsis mutant lines, but to a lower extent than in the above-mentioned mutations. The pect1 mutant plants displayed dwarfism at low temperatures (Mizoi et al., 2006). Thus, the growth inhibition of plants with the dysfunction in mechanisms responsible for PE homeostasis in plants seems to be a general phenomenon. However, our further investigations show that LPEAT plays a much more intricate role in regulating growth than just maintaining PE homeostasis. In particular, the observation that overexpression of either LPEAT1 or LPEAT2 under the control of the 35S promotor led to morphological changes opposite to what was seen in lpeat2 and lpeat1 lpeat2 mutants, with bigger plants, higher dry weight, higher seed yield, longer roots, and an almost doubling in acyl groups in roots per fresh weight compared with wild-type plants. These results cannot easily be explained by effects on lipid homeostasis.
The strong effect of lpeat2 and lpeat1 lpeat2 mutants is in contrast to what is reported for mutations in other LPLAT genes. Arabidopsis lpcat1 and lpcat2 single and double mutants showed normal growth and development despite the fact that the mutations caused a substantially increased synthesis and catabolism of PC (Wang et al., 2012).
The results obtained in this study indicate that the activity of LPEAT-type enzymes, especially LPEAT2, is absolutely essential for maintaining an adequate level of PE in the cells, since the reduction of the absolute amount of PE in the mutants ranged from 30% to 47% compared with wild-type plants. It should be noted, however, that LPEAT cannot contribute to any net synthesis of PE but only to a turnover of acyl groups at position sn-2 or sn-1, either by removal of an acyl group by a phospholipase and then reacylation of the LPE formed by acyl groups from acyl-CoA by the LPEAT (i.e. the so-called Lands cycle; Lands WE, 1965) or by the combined reverse and forward reactions of LPEAT (Jasieniecka-Gazarkiewicz et al., 2016). The substantial increases seen in the LPE content in the lpeat mutants indicate an inefficient reacylation of PE during remodeling of the PE molecule. In addition to LPEAT, LPCATs also can acylate LPE, although with lower efficiency compared with LPC (Ståhl et al., 2008; Jasieniecka-Gazarkiewicz et al., 2016). However, our results clearly show that LPCAT cannot completely replace the LPEAT function. In fact, the LPC content in the lpeat mutants is much more elevated compared with wild-type plants (up to 15 times compared with the control) as compared with the LPE content. With the present knowledge, we cannot give any explanation for this effect except to note that LPEAT activity is essential for controlling the levels of other lysophospholipids than LPE. Liu et al. (2015) suggested that LPE could be methylated and converted to LPC. However, such a pathway has not yet been experimentally proven. It should be noted, however, that the different lysophospholipid acyltransferases have quite broad specificities for different lysophospholipids (Jasieniecka-Gazarkiewicz et al., 2016). The differences in in vitro activities shown toward different lysophospholipids might not reflect their in vivo contribution to the acylation of a particular lysolipid, which could depend on other factors such as their subcellular localizations. Therefore, we cannot exclude the possibility that LPEATs play a major role also in acylating LPC in Arabidopsis leaves, despite its much lower in vitro activity than LPCAT1 and LPCAT2 toward this acyl acceptor.
The substrate specificities of LPEATs differ strongly from LPCAT in that the highest activities are seen with 16:0-CoA (Stålberg et al., 2009). Here, we show that, although both LPEATs acylate 16:0-CoA to LPE with high rates compared with other acyl-CoAs, 20:0-CoA is an even better acyl donor with LPEAT2, and LPEAT2 also acylates 24:0-CoA at a substantial rate, a substrate that is virtually ineffective with LPEAT1. In this context, it is of particular interest that, in lpeat2 and lpeat1 lpeat2 mutant plants, the molecular species of PE with very-long-chain fatty acids (especially PE-40C and PE-42C) were reduced to about one-third of the levels of these PE species in the wild-type plants. Therefore, it is tempting to speculate that Arabidopsis LPEAT2 is involved in the formation of specific and rare PE molecular species that might be involved in a global network regulating cell growth.
The sn-2 lysophospholipids (sn-2-LPL) rapidly isomerize to sn-1-LPL, and for LPC, the equilibrium between sn-1 and sn-2 isomers is 9:1 (Lager et al., 2015). Both sn-1 and sn-2 phospholipases are present in Arabidopsis (Noiriel at al., 2004; Chen et al., 2012); thus, both types of LPC and LPE can be generated. In contrast to LPCATs, which primarily acylate the sn-2 position of the lysophospholipid (Lager et al., 2013), we show here that the LPEATs can acylate both positions of an ether analog of LPC.
Several studies have examined the effect of exogenous LPE on different aspects of plant physiology. They demonstrated that the treatment with LPE delayed, for instance, senescence of the leaves, lowered respiration rates and ethylene production, or enhanced the plant’s ability to recover from water stress. They also showed that, in some cases, exogenous LPE could have opposite effects, such as the stimulation of ethylene production (Almeida, 2016). In this study, we have established that exogenous LPE could have an inhibitory effect on the growth of the roots of Arabidopsis seedlings. Taken together, it is difficult to predict the extent to which (if at all) elevated levels of LPE and/or LPC contribute to the growth inhibition of lpeat mutant plants. Again, the fact that overexpression of LPEAT causes a more vigorous growth compared with wild-type plants, whereas lysophospholipid levels remain the same, indicates that LPEATs have a particular role in growth regulation beyond the regulation of the content of LPE in the tissue.
The differences in acyl-CoA preferences, in expression pattern, and in predicted cellular localization of Arabidopsis LPEAT1 and LPEAT2 (Stålberg et al., 2009), coupled to the much stronger growth phenotype of lpeat2 as compared with lpeat1 mutants, suggest that these enzymes are not redundant but play somewhat different roles in growth regulation. At this stage, we cannot precisely determine the nature of these roles. In addition to the points presented above, we should also note that initiation of autophagy, a cell-recycling system conserved throughout eukaryotic organisms, requires the conjugation of a PE molecule to the autophagy protein ATG8 (Kaufmann et al., 2014).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana ecotype Col-0), LPEAT1 (SALK_016798c, FLAG_217C02, and GABI_825D08), and LPEAT2 (SALK_107699c) T-DNA insertion mutant lines (all in the Col-0 background) were obtained from the Nottingham Arabidopsis Stock Centre (University of Nottingham). Homozygous T-DNA insertion lines were identified by PCR screening using primers SALK_016798LP, SALK_016798RP, T-DNA LB1.3. for LPEAT1 and SALK_107699LP, SALK_107699RP, T-DNA LB1.3. for LPEAT2 and FLAG_217C02_RB, FLAG_217C02_LB, T-DNA_LB4 for LPEAT1 and GABI_825D08_RB, GABI_825D08_LB, T-DNA 08474 for LPEAT1 (Supplemental Table S1).
The lpeat1 lpeat2 double mutant was generated using lpeat1 (GABI_825D08) as female and as pollen donor lpeat2 (SALK_107699c) T-DNA insertion mutation lines. These lines also were used for complementation experiments. To generate LPEAT1 and LPEAT2 overexpressors, a wild-type Arabidopsis (ecotype Col-0) background was used. Homozygous double mutant plants as well as lines with complemented genes were identified by PCR genotyping using the primers listed in Supplemental Table S1.
Total DNA for PCR was extracted from the leaves of tested lines according to the phenol extraction method with modifications (Sambrook et al., 1989).
T-DNA insertion mutants, double mutant plants, as well as the LPEAT1 and LPEAT2 overexpressors and the lines with complemented genes were always grown along with the wild type. All plants were grown in a growth chamber at 23°C with a photoperiod of 16 h of light (120 µmol photons m−2 s−1)/8 h of dark and relative humidity of 60%.
To obtain the roots for fresh and dry weight measurement and for lipid analysis, Arabidopsis seeds of the tested lines were surface sterilized by immersion in 70% ethanol for 2 min, followed by immersion in 4% calcium hypochlorite for 10 min. Seeds were then washed four times with distilled water and sown on 1% agar containing 0.5× Murashige and Skoog (MS) medium and 1% Suc. After 14 d, the seedlings were transferred into flasks with liquid 0.5× MS medium containing 1% Suc and grown for an additional 14 d at 23°C, under constant light (35 µmol photon m−2 s−1) and with gentle shaking (80 rpm). To measure the effect on root length, the seeds were sterilized and sown on 1% agar plates containing 0.5× MS medium and 1% Suc as described above with the following modification: the seeds were placed only in a line of the upper part of the plates, and the plates were kept vertically in the growth chamber. A similar modification also was applied for the evaluation of the effect of the exogenous LPE level on root development. In these experiments, the agar-solidified 0.5× MS medium did not contain sugar but LPE from soybean (Glycine max; Larodan) at two different concentrations: 5 and 500 nmol mL−1.
Morphological Analysis
The morphology of the mutants, overexpressors, and wild-type plants was recorded simultaneously during their development. The fresh weight of the leaves was determined for the second true leaves of 4-week-old plants. Primary root length was recorded for 14-d-old seedlings growing on vertical agar plates containing 0.5× MS medium supplemented with 1% Suc. Four-week-old roots (growing first on agar plates for 14 d and then in beakers containing 0.5× MS medium plus 1% Suc) were dried at 80°C for 24 h, and the tissue weight was determined. The length of the siliques, the total seed yield, and the average weight of aerial dry seed (from samples of 300 seeds) were measured using mature plants. The seeds were harvested from the plants, scaled, and used for lipid content analysis. The remaining aerial parts of the plants were then dried at 80°C for 24 h and used for dry weight measurement. Measurements were made on six to eight different plants (for each genotype), grown during the same time under controlled conditions.
Transcript Analyses of lpeat1 and lpeat2 Mutant Lines
Total RNA was isolated from developing rosette leaves from wild-type plants and SALK_107699, SALK_016798, GABI_825D08, and FLAG_217C02 T-DNA insertion mutation plants using CONCERT Plant RNA Reagent (Invitrogen). The RNA was DNase treated with the Turbo DNA-free Kit (Ambion). The synthesis of single-stranded cDNA was performed using the Maxima First Strand cDNA Synthesis Kit (Fermentas). The resulting cDNA was amplified by PCR using Taq DNA polymerase (Invitrogen).
Plasmid Construction
The At1g80950 gene encoding LPEAT1 was subcloned from the parent vector pYES2 (kindly provided by Dr. Ulf Ståhl), whereas the At2g45670 gene encoding LPEAT2 was amplified by PCR using Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Scientific). The primers used (LPEAT2 sense and LPEAT2 antisense; Supplemental Table S1) contained restriction sites for further cloning into a binary vector. The amplified fragments were ligated into pJET1.2 and sequenced. Both LPEAT genes (At1g80950 and At2g45670) were cloned into the binary vector pART27 (Gleave, 1992) and expressed under the control of the 35S promoter.
The binary vectors harboring either AtLPEAT1 or AtLPEAT2 were introduced into Agrobacterium tumefaciens strain GV3101::pMP90 by electroporation.
Arabidopsis Transformation and Transgene Selection
Five-week-old Arabidopsis plants (Col-0) were transformed with a construct harboring 35S::At1g80950 (AtLPEAT1) or 35S::At2g45670 (AtLPEAT2) using A. tumefaciens and following the floral dip method (Clough and Bent, 1998).
The transformed plants were grown in a growth chamber until the siliques were completely dried. For selection, T1 seeds were germinated on plates using kanamycin as a selection marker (50 µg mL−1). After about 2 weeks, the transgenic plants were transferred to soil and grown in a growth chamber to obtain T2 seeds. Segregation analysis was carried out on the T2 generation. Seedlings (T3) derived from the T2 plants were scored for the number of resistant versus sensitive seedlings. Lines where 100% of seedlings survived on plates containing kanamycin were kept for further analysis. It was noticed that nearly all transformed plants were bigger than wild-type plants, and the lines expressing the LPEATs that showed the biggest growth stimulation were selected for further studies.
Lipid Analyses
Lipids were extracted according to a modified method originally described by Bligh and Dyer (1959). Roots or leaves were homogenized in 3.75 mL of chloroform:methanol (1:2, v/v) followed by the addition of 1.25 mL of water, 1.25 mL of chloroform, and 1.25 mL of 0.15 m acetic acid. The chloroform fractions containing lipids were dried under a stream of N2 and dissolved in 2 mL of chloroform. For total lipid analysis, aliquots of these fractions were evaporated and methylated as described below. In order to investigate the lipid profile in different plant tissues, the chloroform fractions were separated by thin-layer chromatography (Merck) in chloroform:methanol:acetic acid:water (90:15:10:3, v/v/v/v). Lipid classes were visualized by spraying the plate with 0.05% primuline (White et al., 1998) in acetone, and silica gel from areas corresponding to the various lipids was removed from the plate. The lipids were methylated in situ on the gel with 2% H2SO4 in dry methanol (60 min at 90°C). After incubation, methyl-heptadecanoate (internal standard) was added to the methylation mixtures. The fatty acid methyl esters were extracted with hexane and analyzed by gas-liquid chromatography (Shimadzu; GC-2010) on a device equipped with a flame ionization detector and a 60-m × 0.25-mm CP-WAX 58-CB fused-silica column (Agilent Technologies).
For the determination of the molecular species composition of PE in leaves and roots and the total amount of LPE and LPC in leaves of wild-type Arabidopsis plants, lpeat mutants, and LPEAT overexpressors, lipids were isolated from rosettes of 4-week-old plants grown in soil or from roots of plants grown in liquid culture as described previously (Gasulla et al., 2013). Aliquots of extracts corresponding to 100 mg fresh weight were used for lipid analyses via quadrupole time-of-flight mass spectrometry (Q-TOF MS). PE was measured using nanospray direct-infusion Q-TOF MS as described previously (Gasulla et al., 2013).
LPE and LPC were further purified from a total lipid extract via solid-phase extraction. Aliquots of extracts corresponding to 100 mg fresh weight were evaporated to dryness, dissolved in 0.2 mL of chloroform, and separated on self-made silica columns (Pasteur pipette plugged with deactivated glass wool and filled with 100 mg of Silica gel 60, 0.04–0.063 mm; Merck). The columns were flushed first with 1.5 mL of chloroform to elute nonpolar lipids followed by 3 mL of acetone:isopropanol (1:1, v/v) to elute glycolipids. Phospholipids and lysophospholipids were eluted with 1.2 mL of pure methanol followed by 1.2 mL of water:methanol (1:1, v/v). The combined fractions were evaporated to dryness under nitrogen. Afterward, the lipids were dissolved in 100 µL of methanol, and internal standards were added (0.5 nmol each of 14:0-LPE and 14:0-LPC). LPE and LPC were separated on a Eurospher II C8 column (150 mm × 3 mm, 3 µm; Knauer) using a gradient of water + 0.1% (v/v) formic acid (solvent A) and methanol + 0.1% (v/v) formic acid (solvent B). Solvent B was increased from 90% to 100% in 15 min, this was held for 10 min, followed by an equilibration at 90% solvent B for 5 min. Lipids were ionized in the positive ion mode using an Agilent Jet Stream ion source and analyzed on an Agilent 6530 Accurate Mass Q-TOF MS instrument. After collision-induced dissociation, LPE and LPC were detected by a neutral loss scan (NL 141.0191) or precursor ion scan (EIC 184.0739), respectively.
Enzyme Assays
LPEAT activities were measured in microsomal preparations from yeast ale1 cells harboring the empty pYES2 plasmid or plasmids with At1g80950 (AtLPEAT1) or At2g45670 (AtLPEAT2) according to Jasieniecka-Gazarkiewicz et al. (2016). To determine the substrate specificities of the acyltransferases toward different acyl-CoAs, 10 and 5 µg of microsomal protein of AtLPEAT1 and AtLPEAT2, respectively, were used, whereas in assays destined to measure substrate specificity toward sn-1- and sn-2-lysolipid acceptors, 8.8 µg of microsomal protein was used. Oleoyl-LPE (10 nmol; Sigma) as an acyl acceptor and various [14C]acyl-CoAs (5 nmol; synthesized by the method of Sanchez et al. [1973]) were used to measure substrate specificity toward different acyl-CoAs. The ether analog of sn-1-18:1-LPC (sn-1-O-GPC) and the ether analog of sn-2-18:1-LPC (sn-2-O-GPC) were used together with [14C]16:0-CoA (5 nmol of each substrate per assay) in reactions measuring positional specificity of the acyl acceptor. The activities of LPEATs also were measured in microsomal fractions (8.8 µg of microsomal protein per assay) from 4-week-old leaves of wild-type, mutant (lpeat1, lpeat2, and lpeat1 lpeat2), and overexpressor (LPEAT1 OE and LPEAT2 OE) Arabidopsis plants. In these assays, oleoyl-LPE together with [14C]18:1-CoA or [14C]16:0-CoA were used (5 nmol of each substrate per assay). Assays were performed in 100 μL of 40 mm potassium phosphate buffer (pH 7.2) for 4 min (measurement of substrate specificity toward acyl-CoAs) or 30 min (all other assays) at 30°C (with shaking at 1,250 rpm). Lipid extraction from the assays and quantification of the formed [14C]PE or [14C]PC were performed as described by Jasieniecka-Gazarkiewicz et al. (2016).
In order to prepare microsomal fractions from leaves, the leaf tissues were homogenized in a glass homogenizer with 10 parts (v/w) of 0.1 m potassium phosphate buffer (pH 7.2) containing 0.33 m Suc, 1 mg mL−1 BSA (fatty acid free), and catalase (1,000 units mL−1). The homogenates were filtered through two layers of Miracloth, diluted to 20 mL with homogenizing medium, and centrifuged at 20,000g for 12 min. The supernatants were filtered through Miracloth and centrifuged at 100,000g for 90 min. The resulting microsomal pellets were resuspended in 0.1 m potassium phosphate buffer (pH 7.2) and stored at −80°C until used.
Accession Numbers
The accession numbers for this article can be found on the NCBI/PubMed homepage and are LPEAT1: AY128312.1 and LPEAT2: AY035098.1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Products of PCR of genomic DNA from leaves of Arabidopsis control plants and T-DNA insertion mutants: lines FLAG_217C02 and GABI_825D08.
Supplemental Figure S2. Products of PCR of genomic DNA from leaves of Arabidopsis control plants and T-DNA insertion mutants: lines SALK_107699c and SALK_016798c.
Supplemental Figure S3. Arabidopsis: photographs of 4- and 6-week-old plants.
Supplemental Figure S4. Arabidopsis: photographs of 3-week-old seedlings of the control line grown on agar plate without and with addition of LPE.
Supplemental Table S1. A list of primers for mutant identification and construct generation.
Supplemental Table S2. The effect of lpeat mutations on lipid composition of 4-week-old leaves of Arabidopsis plants.
Supplemental Table S3. The effect of lpeat mutations on lipid composition of 4-week-old roots of Arabidopsis plants from liquid culture.
Supplemental Table S4. The effect of lpeat mutations on fatty acid composition of selected polar lipids of 4-week-old leaves of Arabidopsis plants.
Supplemental Table S5. The effect of lpeat mutations on fatty acid composition of selected polar lipids of 4-week-old roots of Arabidopsis plants from liquid culture.
Supplemental Table S6. Molecular species of PE of Arabidopsis roots of control (wild-type) plants and lpeat mutants grown in liquid culture for 4 weeks.
Supplemental Table S7. Molecular species of PE of Arabidopsis leaves of control (wild-type) plants and lpeat mutants grown in soil for 4 weeks.
Supplementary Material
Acknowledgments
We thank M. Miklaszewska and J. Ichnatowicz from Gdansk University for help with T-DNA mutant selection.
Glossary
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- Col-0
Columbia-0
- RT
reverse transcription
- LPE
lysophosphatidylethanolamine
- LPC
lysophosphatidylcholine
- MS
Murashige and Skoog
- Q-TOF MS
quadrupole time-of-flight mass spectrometry
Footnotes
This work was supported by the Strategic Research Area project Trees and Crops for the Future and by the Foundation for Strategic Research project Oil Crops for the Future.
References
- Almeida DPF. (2016) Lysophospholipids and postharvest quality of fruits, vegetables, and cut flowers. In Pareek S, ed, Postharvest Ripening Physiology of Crops. Chapter 8, pp. 273–254 CRC Press, Boca Raton, FL [Google Scholar]
- Bates PD, Durrett TP, Ohlrogge JB, Pollard M (2009) Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol 150: 55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282: 31206–31216 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bourgis F, Kader JC, Barret P, Renard M, Robinson D, Robinson C, Delseny M, Roscoe TJ (1999) A plastidial lysophosphatidic acid acyltransferase from oilseed rape. Plant Physiol 120: 913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AP, Brough CL, Kroon JTM, Slabas AR (1995) Identification of a cDNA that encodes a 1-acyl-sn-glycerol-3-phosphate acyltransferase from Limnanthes douglasii. Plant Mol Biol 29: 267–278 [DOI] [PubMed] [Google Scholar]
- Brown AP, Coleman J, Tommey AM, Watson MD, Slabas AR (1994) Isolation and characterisation of a maize cDNA that complements a 1-acyl sn-glycerol-3-phosphate acyltransferase mutant of Escherichia coli and encodes a protein which has similarities to other acyltransferases. Plant Mol Biol 26: 211–223 [DOI] [PubMed] [Google Scholar]
- Chen G, Greer MS, Lager I, Yilmaz JL, Mietkiewska E, Carlsson AS, Stymne S, Weselake RJ (2012) Identification and characterization of an LCAT-like Arabidopsis thaliana gene encoding a novel phospholipase A. FEBS Lett 586: 373–377 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Gasulla F, Vom Dorp K, Dombrink I, Zähringer U, Gisch N, Dörmann P, Bartels D (2013) The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: a comparative approach. Plant J 75: 726–741 [DOI] [PubMed] [Google Scholar]
- Gleave AP. (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207 [DOI] [PubMed] [Google Scholar]
- Griffiths G, Stymne S, Stobart AK (1988) The utilisation of fatty-acid substrates in triacylglycerol biosynthesis by tissue-slices of developing safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) cotyledons. Planta 173: 309–316 [DOI] [PubMed] [Google Scholar]
- Hanke C, Wolter FP, Coleman J, Peterek G, Frentzen M (1995) A plant acyltransferase involved in triacylglycerol biosynthesis complements an Escherichia coli sn-1-acylglycerol-3-phosphate acyltransferase mutant. Eur J Biochem 232: 806–810 [PubMed] [Google Scholar]
- Jasieniecka-Gazarkiewicz K, Demski K, Lager I, Stymne S, Banaś A (2016) Possible role of different yeast and plant lysophospholipid:acyl-CoA acyltransferases (LPLATs) in acyl remodelling of phospholipids. Lipids 51: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Beier V, Franquelim HG, Wollert T (2014) Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell 156: 469–481 [DOI] [PubMed] [Google Scholar]
- Kim HU, Huang AHC (2004) Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis. Plant Physiol 134: 1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutzon DS, Lardizabal KD, Nelsen JS, Bleibaum JL, Davies HM, Metz JG (1995) Cloning of a coconut endosperm cDNA encoding a 1-acyl-sn-glycerol-3-phosphate acyltransferase that accepts medium-chain-length substrates. Plant Physiol 109: 999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager I, Glab B, Eriksson L, Chen G, Banaś A, Stymne S (2015) Novel reactions in acyl editing of phosphatidylcholine by lysophosphatidylcholine transacylase (LPCT) and acyl-CoA:glycerophosphocholine acyltransferase (GPCAT) activities in microsomal preparations of plant tissues. Planta 241: 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lager I, Yilmaz JL, Zhou XR, Jasieniecka K, Kazachkov M, Wang P, Zou J, Weselake R, Smith MA, Bayon S, et al. (2013) Plant acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) have different specificities in their forward and reverse reactions. J Biol Chem 288: 36902–36914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands WE. (1965) Lipid metabolism. Annu Rev Biochem 34: 313–346 [DOI] [PubMed] [Google Scholar]
- Lightner J, Wu J, Browse J (1994) A mutant of Arabidopsis with increased levels of stearic acid. Plant Physiol 106: 1443–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang G, Wang X (2015) Role of aminoalcoholphosphotransferases 1 and 2 in phospholipid homeostasis in Arabidopsis. Plant Cell 27: 1512–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi J, Nakamura M, Nishida I (2006) Defects in CTP:PHOSPHORYLETHANOLAMINE CYTIDYLYLTRANSFERASE affect embryonic and postembryonic development in Arabidopsis. Plant Cell 18: 3370–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiriel A, Benveniste P, Banaś A, Stymne S, Bouvier-Navé P (2004) Expression in yeast of a novel phospholipase A1 cDNA from Arabidopsis thaliana. Eur J Biochem 271: 3752–3764 [DOI] [PubMed] [Google Scholar]
- Pan X, Chen G, Kazachkov M, Greer MS, Caldo KM, Zou J, Weselake RJ (2015) In vivo and in vitro evidence for biochemical coupling of reactions catalyzed by lysophosphatidylcholine acyltransferase and diacylglycerol acyltransferase. J Biol Chem 290: 18068–18078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EE, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2 Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY [Google Scholar]
- Sanchez M, Nicholls DG, Brindley DN (1973) The relationship between palmitoyl-coenzyme A synthase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria. Biochem J 132: 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl U, Stålberg K, Stymne S, Ronne H (2008) A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett 582: 305–309 [DOI] [PubMed] [Google Scholar]
- Stålberg K, Ståhl U, Stymne S, Ohlrogge J (2009) Characterization of two Arabidopsis thaliana acyltransferases with preference for lysophosphatidylethanolamine. BMC Plant Biol 9: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasseva G, Richard L, Zachowski A (2004) Regulation of phosphatidylcholine biosynthesis under salt stress involves choline kinases in Arabidopsis thaliana. FEBS Lett 566: 115–120 [DOI] [PubMed] [Google Scholar]
- Wang L, Shen W, Kazachkov M, Chen G, Chen Q, Carlsson AS, Stymne S, Weselake RJ, Zou J (2012) Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 24: 4652–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Bursten S, Federighi D, Lewis RA, Nudelman E (1998) High-resolution separation and quantification of neutral lipid and phospholipid species in mammalian cells and sera by multi-one-dimensional thin-layer chromatography. Anal Biochem 258: 109–117 [DOI] [PubMed] [Google Scholar]
- Williams JP, Imperial V, Khan MU, Hodson JN (2000) The role of phosphatidylcholine in fatty acid exchange and desaturation in Brassica napus L. leaves. Biochem J 349: 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.