Analysis of single and double mutants shows an ABA-independent role for ERA1 in stomatal opening.
Abstract
Proper stomatal responses are essential for plant function in an altered environment. The core signaling pathway for abscisic acid (ABA)-induced stomatal closure involves perception of the hormone that leads to the activation of guard cell anion channels by the protein kinase OPEN STOMATA1. Several other regulators are suggested to modulate the ABA signaling pathway, including the protein ENHANCED RESPONSE TO ABA1 (ERA1), that encodes the farnesyl transferase β-subunit. The era1 mutant is hypersensitive to ABA during seed germination and shows a more closed stomata phenotype. Using a genetics approach with the double mutants era1 abi1-1 and era1 ost1, we show that while era1 suppressed the high stomatal conductance of abi1-1 and ost1, the ERA1 function was not required for stomatal closure in response to ABA and environmental factors. Further experiments indicated a role for ERA1 in blue light-induced stomatal opening. In addition, we show that ERA1 function in disease resistance was independent of its role in stomatal regulation. Our results indicate a function for ERA1 in stomatal opening and pathogen immunity.
Plants need to monitor their environment and precisely respond when conditions around them change. At the frontline are plant stomata, formed by a pair of guard cells, which regulate CO2 uptake and simultaneously control water release. Guard cell function is regulated by a multitude of signals, including light, humidity, CO2 concentration, abscisic acid (ABA) and secondary signals, such as reactive oxygen species, nitric oxide, and Ca2+ (Kollist et al., 2014; Sierla et al., 2016).
The plant hormone ABA plays a central role in the regulation of guard cell function. ABA signaling is initiated by binding of the hormone to PYR/RCAR receptors that leads to inactivation of type 2C protein phosphatases (PP2Cs; Ma et al., 2009; Park et al., 2009). This releases SNF1-related protein kinases such as OPEN STOMATA1 (OST1) to activate multiple signaling pathways, including activation of guard cell anion channels that lead to extrusion of water, loss of turgor, and concomitant stomatal closure (Kollist et al., 2014). Regulation of stomatal closure is coordinated with the regulation of stomatal opening. The driving force for stomatal opening is the phosphorylation-dependent activation of plasma membrane H+-ATPases. Blue light-induced stomatal opening is mediated through phototropins PHOT1 and PHOT2, BLUE LIGHT SIGNALING1 kinase, and activation of H+-ATPases (Shimazaki et al., 2007; Takemiya and Shimazaki, 2016). ABA is involved in both stomatal closure and opening, promoting closure and inhibition of opening via OST1 (Hayashi et al., 2011; Yin et al., 2013).
Another regulator of stomatal function is ENHANCED RESPONSE TO ABA1 (ERA1). ERA1 encodes the β-subunit of farnesyl-transferase (Cutler et al., 1996). Farnesylation is a posttranscriptional protein modification where 15-carbon isoprenoid units are attached to target proteins at the sequence CaaX (C = Cys; a = aliphatic amino acid; X = typically Ala, Cys, Gln, Met, or Ser). The addition of farnesyl groups facilitates protein association with membranes (Galichet and Gruissem, 2003). The era1 mutant was initially identified through its hypersensitivity to ABA inhibition of seed germination (Cutler et al., 1996). Furthermore, era1 mutant plants have more closed stomata, enhanced ABA activation of anion channels, and increased drought tolerance (Pei et al., 1998). While around 700 Arabidopsis (Arabidopsis thaliana) proteins were identified as potential targets of ERA1-induced farnesylation, the underlying mechanisms have been characterized only for ALTERED SEED GERMINATION2 (ASG2) and the cytochrome P450 enzyme CYP85A2 that executes the last step in brassinosteroid biosynthesis (Dutilleul et al., 2016; Northey et al., 2016). Consistent with ASG2 being an ERA1 target, the asg2 mutant has a similar ABA-hypersensitive seed germination phenotype as era1 (Dutilleul et al., 2016). CYP85A2 was identified as a potential ERA1 target due to similar developmental phenotypes (including shorter petioles and flowers with protruding carpels) between era1 and cyp85a2 mutants (Northey et al., 2016). In addition to regulation of stomatal responses, seed germination, and developmental responses, ERA1 also regulates pathogen and heat-stress responses (Goritschnig et al., 2008; Wu et al., 2017). The era1 mutant has enhanced susceptibility to the virulent pathogens Pseudomonas syringae pv maculicola and Hyaloperonospora parasitica (Goritschnig et al., 2008). However, despite some progress in decoding the function of ERA1-induced farnesylation in plants, its role in stomatal and immune functions remains an enigma.
Here, we used double-mutant analysis to better understand the role of ERA1 in stomatal signaling. This revealed that ERA1 function in guard cells is not required for stomatal closure in response to ABA and a change in the environment. Instead, ERA1 is required for proper stomatal opening to blue light and to maintain overall plant stomatal openness. In pathogen infections, ERA1 regulated disease resistance independently from stomatal function. Collectively, our data suggest that guard cell signaling output is the sum of multiple signaling pathways and that ERA1 regulates the basal level of stomatal openness.
RESULTS AND DISCUSSION
Steady-State Stomatal Conductance of era1 Single and Double Mutants
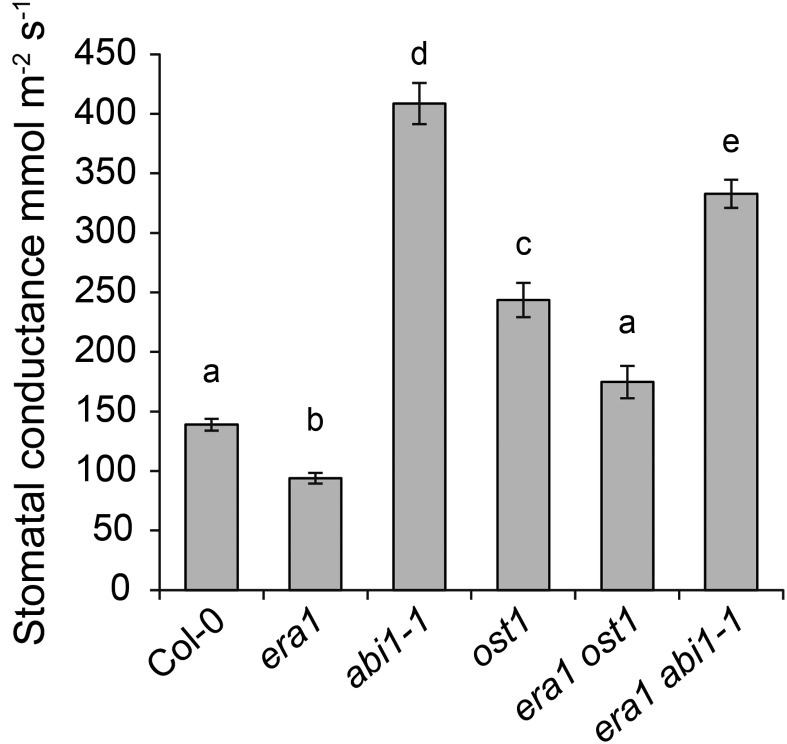
Genetic analysis is a powerful method to identify regulators of signaling pathways. Furthermore, through the use of double mutants it becomes possible to investigate whether a given mutant acts in the same or separate signaling pathways based on epistasis or additive effects between mutations. We crossed era1-2, which has low stomatal conductance, with ost1-3 and abi1-1, which have high stomatal conductance, and measured stomatal conductance and rapid stomatal responses to different abiotic stimuli using a custom-made gas exchange device as described previously (Kollist et al., 2007). Consistent with previous results for era1 abi1 (Pei et al., 1998), the era1 abi1-1 double mutant had lower stomatal conductance compared to the single mutant abi1-1 (Fig. 1). Similarly, the era1 mutation significantly lowered the high stomatal conductance of ost1 in the double mutant era1 ost1 (Fig. 1). One way to explain the steady-state stomatal conductance data would assign a role for ERA1 in the regulation of the ABA signaling pathway, where ABI1 and OST1 are key regulators. However, ABI1, OST1, and also other significant proteins of the ABA signaling pathway, including ABA receptors, ABI2, and the ion channel SLOW ANION CHANNEL1 (SLAC1) do not have the CaaX motif and thus are unlikely direct targets of ERA1. Another option would be that ERA1 functions in a different signaling cascade, which affects stomatal conductance but is not the ABA signaling pathway.
Figure 1.
Whole-plant stomatal conductances of single and double mutants of era1 with ost1 and abi1-1. Stomatal conductance was measured from intact plants (Kollist et al., 2007). Letters denote statistically significant differences (ANOVA with Tukey unequal N HSD post hoc test, P < 0.05; n = 8–15).
ERA1 Does Not Affect Fast Stomatal Closure in Response to External ABA or Environmental Stimuli
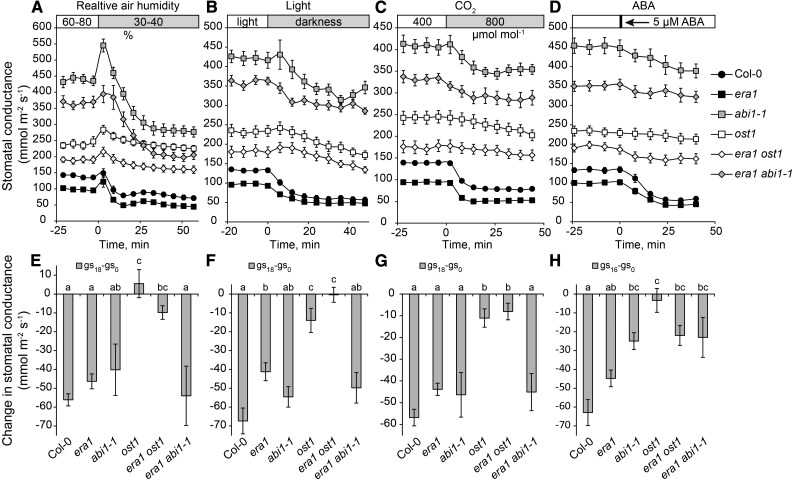
Several factors induce fast stomatal closure, including external ABA application, decreased air humidity, darkness, and elevated CO2 concentration. All these treatments require OST1 for normal stomatal closure to take place (Mustilli et al., 2002; Merilo et al., 2013, 2015). Since era1 suppressed the high stomatal conductance in ost1 (Fig. 1), we tested the response of era1 ost1 to these stimuli (Fig. 2). The era1 ost1 double mutant behaved similarly to the single ost1 mutant and showed reduced stimuli-induced stomatal closures (Fig. 2, A–D), with the exception of small nonsignificant responsiveness to ABA regained in era1 ost1.
Figure 2.
Stomatal responses of single and double mutants of era1 with ost1 and abi1-1. A to D, Time courses of stomatal conductances in response to reduced air humidity, darkness, elevated CO2, and ABA treatment, respectively. E to H, Changes in stomatal conductance during the first 18 min (except for ABA, where 16 min was measured). Letters denote statistically significant differences between the studied genotypes (ANOVA with Tukey unequal N HSD post hoc test, P < 0.05; n = 6–15).
ABI1 belongs to the PP2Cs that inhibit OST1 function (Fujii et al., 2009). While the abi1-1 mutation led to very high stomatal conductance (Fig. 1) and reduced response to ABA (Fig. 2, D and H), the initial changes in stomatal conductance induced by reduced air humidity, darkness, and elevated CO2 were similar in abi1-1 and Col-0 due to nearly three times higher conductance of abi1-1. The era1 abi1-1 double mutant behaved similarly to the single abi1-1 mutant and showed reduced ABA-induced stomatal closure (Fig. 2). No major differences between era1 and Col-0 to the applied treatments were detected, suggesting that ERA1-dependent farnesylation does not regulate fast stomatal closure. We conclude that while era1 can suppress the high stomatal conductance of abi1-1 and ost1, the function of ERA1 is not related to stomatal closure in response to ABA and abiotic factors. Recently, it was demonstrated that protein farnesylation by ERA1 plays an important role in the regulation of plant heat-stress responses in an ABA-independent manner (Wu et al., 2017). These results further support that ERA1-dependent protein farnesylation also functions outside of ABA signaling.
Taken together, OST1 was required for fast stomatal closure, while ERA1 was more important for the basal openness of the stomata. One challenge in building a proper model of stomatal behavior is the heterogeneity of assays used to investigate stomatal function. One of the most popular assays to study guard cell function is to measure stomatal aperture in epidermal peels or from leaf photos after a treatment (e.g. ABA), which is frequently done only at a single time point rather late after the treatment (Pei et al., 1998; Mustilli et al., 2002; Acharya et al., 2013; Yin et al., 2013). This type of assay is likely to miss the early dynamics of the stomatal response. While characterizing the function of a particular stomatal regulator, it is thus important to address its role in fast responses triggering stomatal movements as well as the role for overall stomatal opening.
ERA1 Targets ASG2 and CYP85A2 Do Not Regulate Stomatal Closure
ERA1 mediates farnesylation of target proteins, of which the best characterized are ASG2 (Dutilleul et al., 2016) and CYP85A2 (Northey et al., 2016). Furthermore, the small GTPase ROP11 is a proposed regulator of ABA signaling downstream from the receptor kinase FERONIA (Li and Liu, 2012; Li et al., 2012; Yu et al., 2012), and ROP10 is proposed to be farnesylated by ERA1 (Zheng et al., 2002). We tested asg2 and cyp85a2 responses to various treatments that lead to stomatal closure (Supplemental Fig. S1). Steady-state stomatal conductance and stomatal responsiveness to stimuli of asg2 and cyp85a2 were completely wild type-like. As a next step, we tested stomatal responses of a rop10 rop11 double mutant, which were also similar to the wild type (Supplemental Fig. S1). Further mutant analysis could lead to more ERA1 targets identified, but this might be hampered by genetic redundancy. A protein purification strategy, similar to the one used by Dutilleul et al. (2016) but starting from isolated guard cells, might more directly identify the relevant proteins farnesylated by ERA1 in guard cells.
Gene Expression Analysis in era1
One potential explanation for era1 phenotypes could be a higher accumulation of ABA in this mutant. However, direct ABA measurements in Col-0 and era1-2 showed that ERA1 does not regulate the ABA concentration (Ghassemian et al., 2000). Altered guard cell expression levels of key genes in ABA biosynthesis, ABA catabolism, ABA signaling, or stomatal signaling could be another explanation for ERA1-dependent stomatal phenotypes. We isolated RNA from guard cell-enriched epidermal fragments, obtained with the ice-blender method (Bauer et al., 2013). Comparing the guard cell RNA and corresponding whole-leaf RNA samples for two guard cell-expressed genes (HIGH LEAF TEMPERATURE1, GATED OUTWARDLY-RECTIFYING K+ CHANNEL), at least 4-fold enrichment of guard cell gene expression was detected (Supplemental Fig. S2). We tested the expression of 12 genes representing different steps of ABA homeostasis, ABA signaling, and key stomatal ion transporters. No significant differences compared to Col-0 were observed except for slightly increased expression of ABSCISIC ALDEHYDE OXIDASE3, GATED OUTWARDLY-RECTIFYING K+ CHANNEL, HYPERSENSITIVE TO ABA1, HIGHLY ABA-INDUCED PP2C GENE1, and SLAC1 in the ost1 background (Supplemental Fig. S2). Thus, ERA1 is unlikely to be a regulator of ABA-related gene expression in guard cells.
The Role of ERA1 in Stomatal Opening
The stimuli studied above, decreased air humidity, darkness, increased CO2, and ABA, all induce stomatal closure. While traditionally signaling pathways in guard cells are broadly divided into the closure and opening pathways (Kollist et al., 2014), these pathways have extensive interactions (Lawson and Blatt, 2014). As previously mentioned, the ABA signaling pathway participates in both stomatal closure and opening. Another example is the ion channel SLAC1, whose loss-of-function mutant slac1 is not only impaired in stomatal closure, but also stomatal opening through a feedback change in pH, cytosolic [Ca2+], and the activity of K+ channels (Wang et al., 2012; Laanemets et al., 2013). Since the era1 mutation did not have any influence on fast stomatal closure either in single or double mutants, we tested whether ERA1 might be part of the stomatal opening pathway. Plants were first kept in darkness for 90 min, which ensured that stomatal conductances of era1 and Col-0 were similar. After application of white light, the initial stomatal opening kinetics of dark-adapted era1 was similar to that of Col-0; however, after 20 min in light, the stomatal conductances of wild type and era1 plants departed (Fig. 3). As a result, the era1 stomatal conductance was significantly lower than in Col-0 at the end of the opening experiment (Fig. 3A). ABA can also inhibit light-induced stomatal opening. This response was similar in Col-0 and era1, though stomatal conductance was again lower in era1 at the end of the experiment (Fig. 3A).
Figure 3.
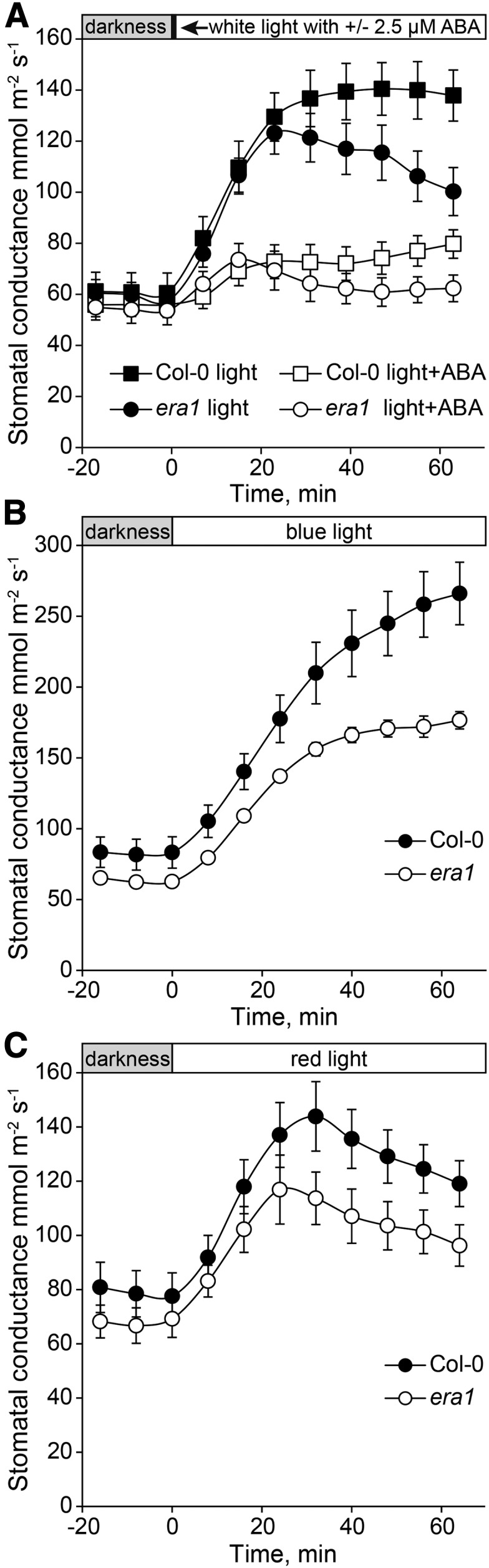
Stomatal opening of dark-adapted Col-0 and era1 plants in response to (A) white light added as a single factor or simultaneously with 2.5 μm ABA (n = 8), (B) blue, and (C) red light (n = 7–8). Before application of light, plants were adapted to full darkness for 90 min.
Stomatal opening in response to light is largely driven by blue light (Hayashi et al., 2011). Next, we compared the stomatal opening induced by blue and red light (Fig. 3, B and C). This revealed that stomatal opening induced by blue light was impaired in era1 plants and suggests a potential function for ERA1 farnesylation in a biological process related to stomatal opening under blue light. PROTON ATPase TRANSLOCATION CONTROL1 (PATROL1) regulates intracellular membrane traffic, including the transport of the H+-ATPase AHA1 to the plasma membrane (Hashimoto-Sugimoto et al., 2013). The patrol1 mutant is impaired in light-induced stomatal opening, similar to era1 (Fig. 3; Hashimoto-Sugimoto et al., 2013); however, PATROL1 does not have the CaaX motif, and thus it is unlikely that this stomatal regulator is a direct target of ERA1. The vesicle-trafficking protein SYP121 is another regulator of stomatal opening and transport of ion channels, especially K+ channels (Eisenach et al., 2012). Possibly, the protein farnesylated by ERA1 is associated with some aspect, for example vesicle transport, of the proper translocation of H+-ATPases or other ion channels involved in stomatal opening to the plasma membrane.
ERA1 Regulates Pathogen Responses Independently of Its Stomatal Function
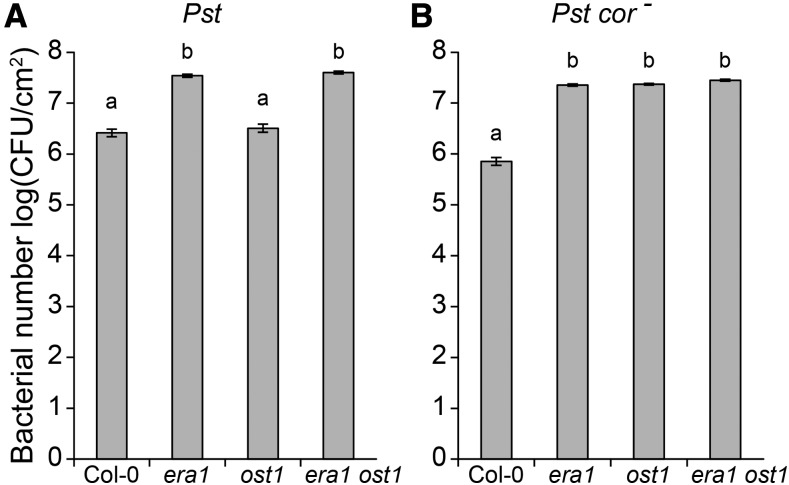
The likely role of ERA1 farnesylation of target proteins in multiple biological processes makes it a challenge to pinpoint the precise function of ERA1 in any of the many phenotypes attributed to era1. Stomata also regulate entry of pathogens into leaves (Melotto et al., 2006). To investigate whether the ERA1 stomatal function is related to its role in basal pathogen resistance, we inoculated Col-0, era1, ost1, and era1 ost1 with virulent P. syringae pv tomato DC3000 (Pst) and the coronatine-deficient strain Pst DC3118 (Pst cor−; Fig. 4). In pathogen and stomatal responses, coronatine activates JA signaling to suppress salicylic acid-mediated defenses (Brooks et al., 2005) and reopens closed stomata during bacterial infection (Melotto et al., 2006).
Figure 4.
Bacterial growth in Col-0, era1, ost1, and era1 ost1 plants. Pst titers were evaluated at 2 d postinoculation. Five-week-old plants were dip-inoculated with 106 CFU/mL Pst DC3000 (A) or 107 CFU/mL Pst DC3118 (cor− ; B). Results are average ± sem of three biological replicates each consisting of nine leaf discs. Letters denote statistically significant differences (ANOVA with Tukey HSD post hoc test, P < 0.05).
For the pathogen assays, dip inoculation was used to favor the entry of Pst bacteria into leaves through stomata, thus allowing stomatal immunity to take place (Melotto et al., 2006). Importantly, the coronatine-deficient strain, Pst cor−, is less virulent than Pst when surface inoculation is used, as this strain cannot reopen stomata upon bacterial infection (Brooks et al., 2005; Melotto et al., 2006). Consistent with previous results, the ost1 mutant was susceptible to Pst cor− infection (Melotto et al., 2006). Both Pst and Pst cor− were strongly virulent in the era1 single mutant. This implies that the ERA1 function in stomata and its role in basal immunity are not related, since era1 has constitutively closed stomata (Fig. 1), predicted to provide some level of resistance. Similarly, the era1 ost1 double mutant was susceptible to pathogen infection to the same level as era1, further suggesting that ERA1 regulation of disease resistance is not directly associated with its stomatal function (Fig. 4). However, since the exact target of ERA1 farnesylation in pathogen responses is currently not known (Goritschnig et al., 2008), further research is required to entangle the role of ERA1 and stomatal function in the response to pathogens.
CONCLUSION
Proper timing of stomatal movements is crucial to maintain overall plant water status. In this study, we are able to dissect the kinetics of stomatal conductance following a sudden change in the surrounding environment (Fig. 2). This made it clear that OST1 is required for fast responses, while ERA1 controls basal whole-plant stomatal conductance. Further phenotypic characterization suggests that ERA1 function in stomatal signaling is related to blue light-induced opening, although the exact target protein that gets farnesylated by ERA1 remains elusive.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Col-0, era1-2, ost1-3 (srk2e, SALK_008068), rop10 (SALK_018747), rop11 (SALK_063154C), cyp85a2-2 (SALK_ 129352), asg2-1 (SALK_040151), and asg2-2 (SALK_113565) were from the European Arabidopsis Stock Centre (www.arabidopsis.info). The abi1-1 allele used was in the Col-0 accession and was a gift from Julian Schroeder. Double mutants and other crosses were made through standard techniques and genotyped with PCR-based markers (Supplemental Table S1).
Plants for gas-exchange measurements were sown into 2:1 (v:v) peat:vermiculite mixture and grown through a hole in a glass plate covering the pot as described previously (Kollist et al., 2007). Plants were grown in growth chambers (AR-66LX; Percival Scientific and Snijders Scientific) with 12-h photoperiod, 23°C/18°C day/night temperature, 100 to 150 μmol m−2 s−1 light, and 70% relative humidity. Plants were 24 to 32 d old during gas-exchange experiments. For guard cell isolation, seeds were sown into 8 × 8-cm pots with four plants per pot and grown in the conditions described above.
Gas-Exchange Measurements
Stomatal conductance of intact plants was measured using a rapid-response gas-exchange measurement device consisting of eight flow-through whole-rosette cuvettes (Kollist et al., 2014). Representative photos of plants used for gas-exchange measurements are presented in Supplemental Figure S3. Plants were inserted into measurement chambers, and after stomatal conductance had stabilized, the following stimuli were applied: reduction in air humidity (decreased from 60–80% to 30–40%), darkness, CO2 (increase from 400–800 µmol mol−1) and ABA. ABA-induced stomatal closure experiments were carried out as described previously (Merilo et al., 2015). Initial changes in stomatal conductance were calculated as gs18 − gs0 (stomatal conductance value 18 min after factor application; 16 min in case of ABA spraying). Opening experiments were performed with the application of either white light, blue light, red light, or white light + ABA on dark-adapted plants kept in the measurement cuvettes at 0 light for at least 90 min. At time point 0, different light bulbs were switched on, light intensities were adjusted so that they were around 150 µmol m−2 s−1 irrespective of light spectral characteristics. ABA-induced inhibition of stomatal opening experiments were carried out as described previously (Hõrak et al., 2016).
RNA Isolation and Quantitative PCR
Samples enriched with guard cells were isolated from 5- to 7-week-old plants, starting from 17 or 18 plants and 4 or 5 leaves per plant, using the ice-blender method (Bauer et al., 2013). RNA was extracted with the Spectrum Plant RNA isolation kit (Sigma-Aldrich). Total RNA was DNAseI treated, and cDNA was synthesized with Maxima H Minus reverse Transcriptase (Thermo Fischer Scientific). qPCR was performed in triplicate with 5× HOT FIREPol EvaGreen qPCR Mix Plus ROX (Soils Biodyne) on an Applied Biosystems 7900HT Fast real-time PCR system. Primer sequences and primer efficiencies are listed in Supplemental Table S1. Analysis of the quantitative PCR data were performed with qBase+ 3.0 (Biogazelle). The reference genes used for normalization were SAND, TIP41, and YLS8. Statistical analysis was performed on log2-transformed data.
Pathogen Assays
Plants were grown in commercial potting soil/perlite (3:2) at 22°C to 24°C day and 17°C to 19°C night temperature under a 9-h-light/15-h-dark photoperiod. The lighting was supplied at an intensity of ∼100 μE m−2 s−1 by fluorescence tubes. Bacterial strains Pst DC3000 and Pst DC3118 (cor−) were provided by Barbara Kunkel (WA University, St. Louis, MO). Bacteria were cultivated at 28°C and 220 rpm in King’s B medium containing 50 mg/mL rifampicin (DC3000) or rifampicin/kanamycin/spectinomycin (DC3118).
Five-week-old Arabidopsis (Arabidopsis thaliana) plants were dipped in a bacterial suspension of 106 colony-forming units (CFU)/mL Pst DC3000 and 107 CFU/mL Pst DC3118 in 10 mm MgSO4 containing 0.01% Silwet L-77 (Lehle Seeds) for 15 min. After dipping, plants were kept at 100% relative humidity overnight. For bacterial titers, leaf discs collected at 2 d postinoculation were washed twice with sterile water and homogenized in 10 mm MgSO4. Quantification of bacterial growth was done as previously described (Zimmerli et al., 2000). Each biological repeat represents nine leaf discs from three different plants.
Statistical Analysis
Statistical analyses were performed with Statistica v. 7.1 (StatSoft). ANOVA with Dunnett’s, Tukey, or Tukey unequal N HSD post hoc tests were used as indicated in figure legends. All effects were considered significant at P < 0.05.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Stomatal responses of asg2-1, asg2-2, cyp85a2-2, and rop10 rop11.
Supplemental Figure S2. Gene expression in guard cell-enriched samples.
Supplemental Figure S3. Representative photos of mutants and Col-0 wild type used for whole-plant gas-exchange experiments.
Supplemental Table S1. Primers used for genotyping and qPCR.
Supplementary Material
Acknowledgments
We thank Irina Rasulova for technical assistance.
Footnotes
This work was funded by the Estonian Ministry of Science and Education (IUT2-21 to H.K. and PUT-1133 to E.M.), the European Regional Development Fund (Center of Excellence in Molecular Cell Engineering to H.K.), the Academy of Finland (grant no. 307335, Center of Excellence in Primary Producers 2014–2019 to M.B.), and by the National Science Council of Taiwan (grant no. 105-2311-B-002-032-MY3 to L.Z.).
Articles can be viewed without a subscription.
References
- Acharya BR, Jeon BW, Zhang W, Assmann SM (2013) Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 200: 1049–1063 [DOI] [PubMed] [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Brooks DM, Bender CL, Kunkel BN (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol 6: 629–639 [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273: 1239–1241 [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Ribeiro I, Blanc N, Nezames CD, Deng XW, Zglobicki P, Palacio Barrera AM, Atehortùa L, Courtois M, Labas V, et al. (2016) ASG2 is a farnesylated DWD protein that acts as ABA negative regulator in Arabidopsis. Plant Cell Environ 39: 185–198 [DOI] [PubMed] [Google Scholar]
- Eisenach C, Chen ZH, Grefen C, Blatt MR (2012) The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K+ channel activity with vegetative growth. Plant J 69: 241–251 [DOI] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galichet A, Gruissem W (2003) Protein farnesylation in plants—conserved mechanisms but different targets. Curr Opin Plant Biol 6: 530–535 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritschnig S, Weihmann T, Zhang Y, Fobert P, McCourt P, Li X (2008) A novel role for protein farnesylation in plant innate immunity. Plant Physiol 148: 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M, Higaki T, Yaeno T, Nagami A, Irie M, Fujimi M, Miyamoto M, Akita K, Negi J, Shirasu K, et al. (2013) A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun 4: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Inoue S, Takahashi K, Kinoshita T (2011) Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol 52: 1238–1248 [DOI] [PubMed] [Google Scholar]
- Hõrak H, Sierla M, Tõldsepp K, Wang C, Wang YS, Nuhkat M, Valk E, Pechter P, Merilo E, Salojärvi J, et al. (2016) A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28: 2493–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Ramma H, Huve K, Jaspers P, Kangasjarvi J, Kollist H (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: Linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Laanemets K, Wang YF, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. (2013) Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol 197: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Blatt MR (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol 164: 1556–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Kang J, Sui N, Liu D (2012) ROP11 GTPase is a negative regulator of multiple ABA responses in Arabidopsis. J Integr Plant Biol 54: 169–179 [DOI] [PubMed] [Google Scholar]
- Li Z, Liu D (2012) ROPGEF1 and ROPGEF4 are functional regulators of ROP11 GTPase in ABA-mediated stomatal closure in Arabidopsis. FEBS Lett 586: 1253–1258 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Merilo E, Jalakas P, Kollist H, Brosché M (2015) The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2, and exogenous ABA. Mol Plant 8: 657–659 [DOI] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162: 1652–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JGB, Liang S, Jamshed M, Deb S, Foo E, Reid JB, McCourt P, Samuel MA (2016) Farnesylation mediates brassinosteroid biosynthesis to regulate abscisic acid responses. Nat Plants 2: 16114. [DOI] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TFF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Sierla M, Waszczak C, Vahisalu T, Kangasjärvi J (2016) Reactive oxygen species in the regulation of stomatal movements. Plant Physiol 171: 1569–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Shimazaki K (2016) Arabidopsis phot1 and phot2 phosphorylate BLUS1 kinase with different efficiencies in stomatal opening. J Plant Res 129: 167–174 [DOI] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JR, Wang LC, Lin YR, Weng CP, Yeh CH, Wu SJ (2017) The Arabidopsis heat-intolerant 5 (hit5)/enhanced response to aba 1 (era1) mutant reveals the crucial role of protein farnesylation in plant responses to heat stress. New Phytol 213: 1181–1193 [DOI] [PubMed] [Google Scholar]
- Yin Y, Adachi Y, Ye W, Hayashi M, Nakamura Y, Kinoshita T, Mori IC, Murata Y (2013) Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol 163: 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Qian L, Nibau C, Duan Q, Kita D, Levasseur K, Li X, Lu C, Li H, Hou C, et al. (2012) FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc Natl Acad Sci USA 109: 14693–14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZL, Nafisi M, Tam A, Li H, Crowell DN, Chary SN, Schroeder JI, Shen J, Yang Z (2002) Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14: 2787–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Jakab G, Metraux JP, Mauch-Mani B (2000) Potentiation of pathogen-specific defense mechanisms in Arabidopsis by beta-aminobutyric acid. Proc Natl Acad Sci USA 97: 12920–12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.