A single CRISPR-Cas9 target efficiently induces heritable mutations in two rapeseed gene homologs.
Abstract
In polyploid species, altering a trait by random mutagenesis is highly inefficient due to gene redundancy. We have stably transformed tetraploid oilseed rape (Brassica napus) with a CRISPR-Cas9 construct targeting two ALCATRAZ (ALC) homoeologs. ALC is involved in valve margin development and, thus, contributes to seed shattering from mature fruits. Knocking out ALC would increase shatter resistance to avoid seed loss during mechanical harvest. We obtained a transgenic T1 plant with four alc mutant alleles by the use of a single target sequence. All mutations were stably inherited to the T2 progeny. The T2 generation was devoid of any wild-type alleles, proving that the underlying T1 was a nonchimeric double heterozygote. T-DNA and ALC loci were not linked, as indicated by random segregation in the T2 generation. Hence, we could select double mutants lacking the T-DNA already in the first offspring generation. However, whole-genome sequencing data revealed at least five independent insertions of vector backbone sequences. We did not detect any off-target effects in two genome regions homologous to the target sequence. The simultaneous alteration of multiple homoeologs by CRISPR-Cas9 mutagenesis without any background mutations will offer new opportunities for using mutant genotypes in rapeseed breeding.
The primary gene pool of oilseed rape (Brassica napus; 2n = 38, AACC) has a low genetic diversity (Bus et al., 2011). Apart from wide crosses and genetic modification, spontaneous and induced mutations have been used to increase genetic variation. Inducing mutations with a measurable phenotypic effect is complicated by its amphidiploid nature. The nuclear genome consists of two genomes, A and C, originating from a hybridization between Brassica rapa (2n = 20, AA) and Brassica oleracea (2n = 18, CC). Consequently, every ortholog of Arabidopsis (Arabidopsis thaliana) is represented by at least two rapeseed homoeologs with putatively redundant functions. To alter a monogenic trait, therefore, it is necessary to combine mutated homoeologs from both subgenomes (Wells et al., 2014; Emrani et al., 2015).
The acquisition of novel desired mutations has been facilitated by the introduction of the CRISPR-Cas9 system. The Cas9 nuclease can easily be programmed to induce double-strand breaks within a target sequence (Doudna and Charpentier, 2014). Those breaks are quickly mended by the innate repair system via nonhomologous end joining. However, this repair mechanism frequently creates small insertions and deletions, which, if located within a coding sequence, often result in frame-shift mutations. The application of CRISPR-Cas9-targeted mutagenesis in plants has been demonstrated not only in Arabidopsis (Fauser et al., 2014; Feng et al., 2014) but also in crops like wheat (Triticum aestivum), tomato (Solanum lycopersicum), and rice (Oryza sativa; Brooks et al., 2014; Wang et al., 2014; Li et al., 2016a). To our knowledge, a proof of concept in oilseed rape has not been published yet. Lawrenson et al. (2015) reported the successful application of a CRISPR-Cas9 approach in B. oleracea, suggesting that a transfer to oilseed rape also would be feasible. There is one more feature of the CRISPR-Cas9 system that is of particular interest for the application in oilseed rape. The Cas9 protein, when directed to multiple target sites, can induce mutations simultaneously in different (homologous) sequences, as has already been demonstrated in the tetraploid potato (Solanum tuberosum; 2n = 4x = 48) to alter starch synthesis (Andersson et al., 2017).
We applied the CRISPR-Cas9 system for targeted mutagenesis to reduce yield loss in oilseed rape. A major issue of rapeseed production is its natural seed dispersal strategy that involves shattering of dry fruits, the so-called siliques. In extreme cases, preharvest losses of up to 25% have been reported (Price et al., 1996). Numerous studies in the model plant Arabidopsis, recently reviewed by Ballester and Ferrándiz (2017), have unraveled a gene network controlling the development of specialized silique tissues essential for fruit dehiscence. The basic regulators are the transcription factors SHATTERPROOF1 and SHATTERPROOF2, INDEHISCENT, and ALCATRAZ (ALC). SHATTERPROOF1 and SHATTERPROOF2 redundantly induce the expression of INDEHISCENT and ALC. While indehiscent mutants are completely indehiscent due to the absence of both lignified cells and the separation layer at the predetermined breaking point of the silique (Liljegren et al., 2004), alc mutants only lack the separation layer (Rajani and Sundaresan, 2001). We hypothesize that rapeseed plants with knocked out alc function produce siliques with an intermediate level of shatter resistance, which could result in lower seed loss during threshing.
Here, we report the targeted mutagenesis of two BnALC homoeologs by a CRISPR-Cas9 approach. All four alleles were mutated in a single T1 plant using only one target sequence. This demonstrates the potential of CRISPR-Cas9-mediated genome editing for the simultaneous modification of different homoeologous gene copies in a polyploid species.
RESULTS
Sequence Identification for Multiple Homoeolog Targeting
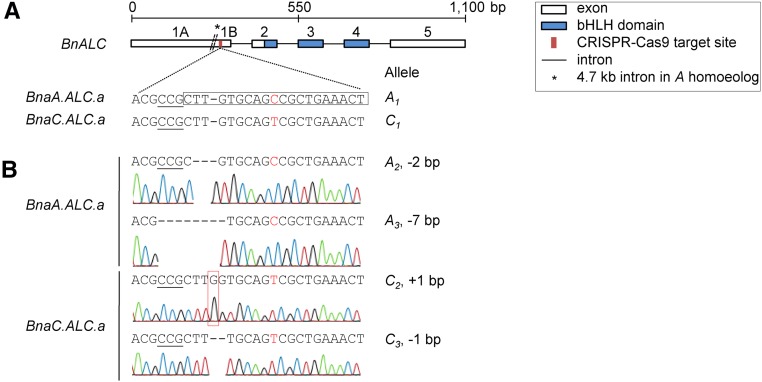
We aimed to knock out two rapeseed ALC homoeologs, BnaA.ALC.a (BnaA07g12110D) and BnaC.ALC.a (BnaC07g16290D), by CRISPR-Cas9-mediated mutations. For this purpose, we searched for sequences with high similarity between both genes. We chose a 20-bp target region from the BnaA.ALC.a homoeolog located in exon 1B upstream of the basic helix-loop-helix (bHLH) transcription factor domain (Fig. 1A). This sequence is highly conserved between both homoeologs and is located adjacent to an NGG PAM, which is an essential targeting component for Cas9. The only difference between the target sites is an SNP in the BnaC.ALC.a homoeolog 10 bp upstream of the PAM sequence (95% identity).
Figure 1.
Nonhomologous end joining-mediated knockout of two BnALC homoeologs. A, CRISPR-Cas9 target upstream of the bHLH domain of BnALC. The protospacer-adjacent motif (PAM) is underlined. The boxed target sequence was based on BnaA.ALC.a and comprised a single-nucleotide polymorphism (SNP) to BnaC.ALC.a at position 10, highlighted in red. B, Four CRISPR-Cas9-induced mutant alleles detected by Sanger sequencing of a single double heterozygous T1 rapeseed plant. The size of each deletion/insertion and the name of the allele are indicated at right. The inserted base of allele C2 is highlighted by the red box.
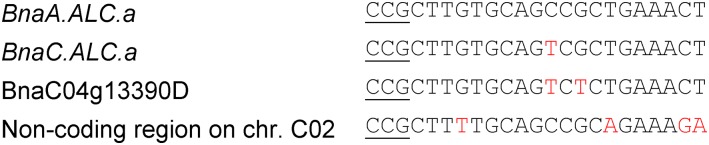
Then, we performed a BLAST search with our target sequence against the rapeseed genome (Darmor-bzh, version 4.1). Apart from the targeted BnALC paralogs, only two sequences were found that are located next to a PAM site (Fig. 2). The first sequence, which is 90% identical to the selected one, belongs to gene model BnaC04g13390D, which is predicted to encode a protein with a bHLH domain. The sequence similarity between the bHLH domains of BnaA.ALC.a and BnaC04g13390D is 81%, whereas the similarity between the overall amino acid sequences is much lower (53%). The second sequence (80% identity) is located on chromosome C02 but without any predicted gene model. Based on these findings, we expected a BnALC gene-specific mutagenesis without off-target effects.
Figure 2.
Alignment of the CRISPR-Cas9 target sequences from BnaA.ALC.a and BnaC.ALC.a in comparison with two potential off-target sites identified in the reference genome of Darmor-bzh by a BLAST search. The PAMs are underlined. SNPs are highlighted in red.
Rapeseed Transformation and T2 Seed Production
We cloned the 20-bp target sequence into the pChimera plasmid (Fauser et al., 2014) upstream of the chimeric single-guide RNA (sgRNA) and under the control of the Arabidopsis ubiquitin 6-26 promoter. Then, the construct was cloned into the final pCas9-TPC plasmid (Fauser et al., 2014), containing a bar resistance cassette and a plant codon-optimized Streptococcus pyogenes Cas9 nuclease under the control of the constitutive Petroselinum crispum ubiquitin 4-2 promoter.
We cocultivated 625 hypocotyl explants of the spring rapeseed cv Haydn with Agrobacterium tumefaciens containing the recombinant pCas9-TPC plasmid. We obtained 370 independent calli, of which 112 initiated shoot regeneration under herbicide selection. Four shoots from four independent events (named CP1–CP4) survived an extended herbicide treatment and were regenerated as rooted plantlets after 9 to 11 months in tissue culture. These four T1 plants were transferred to the greenhouse, where they produced T2 seeds after self-pollination. We used the T1/T2 nomenclature in accordance with inbred populations where the F2 is the first segregating generation.
Identification of CRISPR-Cas9-Induced BnALC Mutations
We performed a PCR test using Cas1_f and Cas1_r primers (Supplemental Table S1) to select T1 plants that carry the transgene insertion. The primers amplify a T-DNA region containing the CRISPR-Cas9 target sequence and the sgRNA. Only one plant (CP1) turned out to be transgenic, which results in a transformation rate of 0.9% as calculated by the number of induced shoots. The rate of false-positive shoots, surviving prolonged herbicide selection but being nontransgenic, was 2.7%.
Then, we sequenced both BnALC homoeologs of the transgenic CP1 plant. We expected that this plant carries at least one mutation in one out of four BnALC alleles. To our surprise, we found mutations in all target sequences, two in BnaA.ALC.a and two in BnaC.ALC.a (Fig. 1B). For ease of understanding, the respective alleles were termed A2, A3, C2, and C3. Thus, CP1 does not contain nonmutated (wild-type) alleles (A1/C1) anymore, at least not in the leaf tissue we analyzed. It could be either a double heterozygote or a chimeric plant. As expected, the CRISPR-Cas9-induced mutations occurred in the vicinity of the PAM sequence. We identified deletions of 1, 2, and 7 bp and a 1-bp insertion, respectively. Those frame-shift mutations will most likely result in nonfunctional proteins. Moreover, in silico analysis revealed that the mutations gave rise to premature stop codons upstream of the bHLH domain. In conclusion, we reason that CP1 has no functional BnALC gene anymore.
Inheritance of BnALC Mutations
We wanted to know how the mutations and the transgene are inherited. If CP1 was not a chimera, we expected a digenic segregation pattern. Moreover, we expected that the BnALC loci and the transgene locus are not linked. The mutant plant CP1 yielded 858 T2 seeds. First, we screened 36 T2 plants by PCR using the Cas1_f and Cas1_r primers, which bind to the multiple cloning site of the pCas-TPC vector (Supplemental Fig. S1). We found 27 transgenic and nine nontransgenic plants, which perfectly matches a Mendelian segregation for a single gene (Table I). Thus, CP1 carries a single Cas9 insertion.
Table I. Inheritance of CRISPR-Cas9-induced BnALC mutations.
Thirty-six T2 plants were tested for the presence of the transgene by PCR. Both BnALC homoeologs were sequenced to identify the mutated alleles. E, Expected number of plants; O, observed number of plants.
| Plants | Transgene Genotypes |
alc Genotypes |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transgenic | Nontransgenic | χ2 Testa | A2A2/C2C2 | A2A2/C2C3 | A2A2/C3C3 | A2A3/C2C2 | A2A3/C2C3 | A2A3/C3C3 | A3A3/C2C2 | A3A3/C2C3 | A3A3/C3C3 | χ2 Testb | |
| O | 27 | 9 | 0 | 3 | 4 | 0 | 3 | 14 | 2 | 1 | 6 | 3 | 8.52 |
| Ec | 27 | 9 | 2.25 | 4.25 | 2.25 | 4.25 | 9 | 4.25 | 2.25 | 4.25 | 2.25 | ||
3:1 segregation, χ2(0.999;2) = 13.82.
1:2:1:2:4:2:1:2:1 segregation, χ2(0.999; 8) = 26.12.
Under the assumption that the T1 parent CP1 was nonchimeric (A2A3/C2C3).
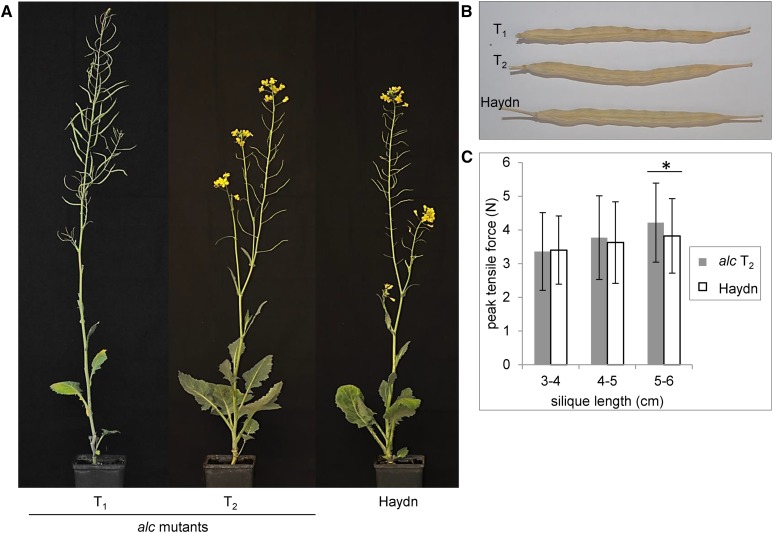
Then, both BnALC homoeologs from all T2 plants were sequenced. All plants carried the mutated alleles, proving that CP1 was a nonchimeric double heterozygote (A2A3/C2C3). Furthermore, the segregation pattern was in accordance with a digenic inheritance and random segregation between genes. The transgene insertion and the mutations were not linked, as we could find nontransgenic plants with all four mutant alleles. Altogether, we found eight out of nine genotypes expected for random segregation. Only A2A2/C3C3 was missing from the T2 plants investigated, which is not surprising because the expected frequency is only 6.25%. A phenotypical inspection of the T2 plants did not reveal any visible differences from cv Haydn. The plants displayed the same architecture, and their fertility was not different from that of the wild type (Fig. 3). Taken together, we have produced in one step a nonchimeric double mutant devoid of any wild-type allele but carrying mutations in all BnALC homoeologs.
Figure 3.
Growth types of CRISPR-Cas9 alc mutants resemble the wild type, while siliques are more shatter resistant. A, T1 and T2 rapeseed plants carrying CRISPR-Cas9-mutated BnALC alleles next to cv Haydn, which had been used for transformation. Plants were grown in 11- × 11-cm pots in the greenhouse. B, alc mutations show no visible effect on mature siliques of T1 and T2 mutants compared with cv Haydn. C, Results of shatter resistance measurements of alc T2 in comparison with cv Haydn. Peak tensile forces are displayed as means of siliques grouped according to their length. Error bars represent sd. A significant difference is indicated by the asterisk (Student’s t test, P < 0.05).
Searching for Putative Off-Target Effects
We searched in our mutant plant CP1 for possible off-target activities of the Cas9 endonuclease. We assumed that if any of these activities had occurred, mutations are to be expected within the two sequences with high similarity to our BnALC genes (BnaC04g13390D and the noncoding sequence on C02). We designed PCR primers that bind to flanking sequences of the potential off-target sites (Supplemental Table S1). We sequenced the resulting PCR products from CP1 and from five T2 offspring. As we did not find any sequence variations compared with the Darmor-bzh reference genome, we reason that the plants probably do not carry off-target mutations in these regions.
Analysis of Whole-Genome Sequence Regarding T-DNA Insertions
We wanted to know whether CP1 houses any other T-DNA vector sequences apart from the expected T-DNA insertion. This information is important for field cultivation as a non-GMO plant. Whole-genome sequence data of the T1 plant CP1 was produced by Illumina sequencing. A total of 412 million raw data paired-end reads were produced. After quality trimming, the genome coverage was, on average, 20×.
The reads were mapped against the sequence of the transformation vector to validate the assumption of a single T-DNA insertion, which was based on segregation analysis. The coverage of T-DNA reads was 20×, like the average genome coverage, thus confirming a single-copy locus (Supplemental Fig. S2).
Unexpectedly, the whole-genome data mapped not only against the T-DNA but also against a 700-bp vector backbone region. This region encodes a bacterial origin of replication (pUC19 ori) and was strongly enriched with greater than 100× coverage. Furthermore, Illumina sequence reads anchored the pUC19 ori to five different rapeseed genomic sequences, suggesting at least five insertions of the vector fragment in the plant genome.
Silique Shatter Resistance
We assessed the shatter resistance of T2 alc mutants in comparison with cv Haydn by disrupting single siliques that we attached to a force meter. Maximum tensile forces were measured as the silique walls were torn away from the replum. We grouped siliques according to their lengths into three size classes of 3 to 4 cm, 4 to 5 cm, and 5 to 6 cm. Longer siliques tended to be more robust than short siliques, indicating a correlation of silique length and shatter resistance (Fig. 3C). Regarding 3- to 4-cm-long and 4- to 5-cm-long siliques, no difference in silique robustness was observed between the two genotypes. However, 5- to 6-cm-long siliques of the alc mutants were more shatter resistant than same-sized siliques of the cultivar.
DISCUSSION
We have demonstrated the potential of CRISPR-Cas9-targeted mutagenesis in the rapeseed genome. The main findings can be summarized as follows. (1) Frame-shift mutations have been induced within the target sequence of BnaA.ALC.a. (2) Moreover, mutations also were induced within the second BnALC homoeolog, BnaC.ALC.a, although it differs from the sgRNA target by one SNP. (3) The mutation efficiency was 100% and the T1 plant was nonchimeric; all T2 offspring were mutants. (4) The lack of mutations within two potential off-target sites with high homology to the sgRNA target sequence indicates that CRISPR-Cas9-targeted mutagenesis in rapeseed could be very precise. (5) We recovered T2 plants with four mutated BnALC alleles that did not contain any T-DNA sequences. (6) Whole-genome sequencing data revealed the integration of vector backbone sequences into the rapeseed genome. (7) Five- to 6-cm-long siliques of alc mutants were more shatter resistant than siliques of cv Haydn.
Specificity of the Cas9 System
We had designed an sgRNA that is identical to the BnaA.ALC.a target region, whereas it differs from BnaC.ALC.a by an SNP 10 nucleotides upstream of the PAM site. Finding induced mutations also in BnaC.ALC.a is in line with previous reports that Cas9 tolerates mismatches within the target site (Hsu et al., 2013; Endo et al., 2015; Lawrenson et al., 2015). Regarding polyploid species like rapeseed, this opens up new opportunities for a one-step modification of whole gene families. Simultaneous editing of three homoalleles in hexaploid bread wheat was reported previously by the use of a transcription activator-like effector nuclease (Wang et al., 2014). Meanwhile, mutating three to four closely related genes with a single CRISPR-Cas9 target has been achieved for Arabidopsis and tetraploid potato (Yan et al., 2016; Andersson et al., 2017).
We did not observe any off-target effects in two sequences that are homologous to the intended BnALC target. The number and location of SNPs between the target sequence and the potential off-target site are likely to play a role, as was described already for an extensive off-target study in human cells, with more than 700 sgRNA variants tested (Hsu et al., 2013). In our study, the off-target sites contained either two SNPs at positions 10 and 12 upstream of the PAM or four SNPs at positions 4, 14, 19, and 20 (Fig. 2). This minimizes the probability for off-target mutations to occur in any other sequence of the genome with an even lower similarity to the sgRNA.
Integration of Vector Backbone Fragments
After A. tumefaciens-mediated transformation, we expected to find only T-DNA insertions of the region flanked by left and right borders into the plant genome. However, we also detected sequences of the pUC19 origin of replication in our T1 plant. The observation of integrated vector backbone fragments was reported recently for transgenic Arabidopsis, wheat, and rice (Li et al., 2016b; Wang et al., 2016; Schouten et al., 2017). Thus, backbone insertions seem to be common across plant species.
To some extent, those sequence insertions are a shortcoming of A. tumefaciens-mediated CRISPR-Cas9 transformations. We will establish a PCR-based protocol to select homozygous mutants lacking any vector sequences. This will allow us to perform field trials even under strict European GMO regulations. In the future, this problem could be avoided by using DNA-free transformation techniques like in vitro preassembled Cas9-sgRNA ribonucleoproteins. Successful induction of CRISPR-Cas9 mutations by ribonucleoproteins has been demonstrated for Arabidopsis, tobacco (Nicotiana tabacum), rice, lettuce (Lactuca sativa), and maize (Zea mays; Woo et al., 2015; Svitashev et al., 2016), although a routine application in crops remains to be demonstrated.
The Efficiency of the CRISPR-Cas9 Mutation System in Rapeseed in Comparison with EMS Mutagenesis
The 100% success rate regarding mutations in all BnALC alleles suggests high Cas9 activity in early stages of tissue culture, giving rise to nonchimeric plants. Lawrenson et al. (2015) reported lower mutation frequencies in first-generation transgenic barley (Hordeum vulgare) and B. oleracea plants of 23% and 10%, respectively. The frequency of potato lines with multiple mutated alleles ranged between 20% and 67% (Andersson et al., 2017). In rice, the expression level of Cas9 and sgRNA as well as the extent of the culture period of the transgenic callus impact the frequency of CRISPR-Cas9-induced mutations. Mikami et al. (2015) reported that high expression levels and long culture periods increased the number of mutations. In our experiment, we did not measure the expression rate of the transgenic sequences in T1 plants because we assume a strong expression under the control of the constitutive ubiquitin promoters. Moreover, their high transcriptional activity was confirmed by the mutations in the target sequences. In conclusion, we do not see a reason to modify the expression cassettes for future CRISPR-Cas9 experiments with rapeseed.
Conventionally, novel genetic variation has been induced into breeding programs by random mutagenesis through irradiation or treatment with chemicals like EMS. A well-established method is the identification of mutations from randomly mutagenized plant populations by TILLING (Till et al., 2006). However, the huge background mutation load is a severe drawback of randomly induced mutations. For rapeseed, after an EMS mutation experiment, the number of background mutations was estimated to be around 130,000 mutations per plant (Harloff et al., 2012). They can have a negative impact on various characters. We have observed stunted growth, abnormal inflorescence, and reduced seed yield in the M2 generation (J.B., unpublished data).
Another problem arises mainly in polyploids if several homoeologs contribute independently to a phenotype. Chemical and irradiation mutagenesis only yields plants with single mutations in one or another homoeolog. The probability for double mutations to occur is extremely low. However, in many cases, single mutations do not have the desired effect. As a consequence, different mutations must be combined in one genotype by time-consuming crossing and backcrossing procedures, which can take many years. This was demonstrated recently for genes that are involved in the biosynthesis of sinapine. Knockout of only one homoeolog had no measurable effect, whereas a double mutant obtained after crossing two single mutants showed significantly reduced sinapine contents in the seed (Emrani et al., 2015).
In conclusion, the CRISPR-Cas9 system is clearly superior to classical mutagenesis. We propose that, in the future, all members of a given gene family can be knocked out by a single CRISPR-Cas9 experiment and without off-target effects. Thus, targeted mutation induction will accelerate the introduction of mutants into breeding programs.
Silique Measurements Imply Increased Shatter Resistance Due to alc Mutations
We assessed the shatter resistance of alc mutants and cv Haydn by measuring the peak tensile force necessary to tear silique walls apart from the replum. While the genotypes had no effect on the shatter resistance of short siliques, we observed an increased shatter resistance of alc siliques longer than 5 cm. This finding is promising, because field-grown rapeseed usually produces siliques greater than 5 cm. Therefore, we expect to find stronger effects in the field as a verification of our greenhouse observations.
Compared with other rapeseed cultivars, cv Haydn has an elevated shatter resistance (data not shown). We had chosen this genotype for our experiments because of its high transformation efficiency (Boszoradova et al., 2011). However, cv Haydn is considered an old variety because it was released in the year 2000. The mutations can easily be introduced into current elite material by marker-assisted selection to breed new varieties with a high shatter resistance.
CONCLUSION
We demonstrate the power of the CRISPR-Cas9 system for targeted mutation induction in rapeseed. This technique opens new possibilities to precisely alter the function of genes, avoiding the obstacles of random mutagenesis. Simultaneous modification of several homoeologs is of key interest to create new genetic variation in a polyploid species. Although the legal situation in Europe is not yet clear, we expect that CRISPR-Cas9-induced single-nucleotide (or oligonucleotide) mutations will increase the genetic basis for rapeseed breeding in the future. Moreover, we demonstrated the power of whole-genome sequencing to detect transformation vector backbone fragments in the recipient genome, which must be eliminated from segregating offspring by marker-assisted selection. The next efforts of our research group regarding targeted mutagenesis will concern genes controlling the biosynthesis of secondary metabolites such as glucosinolates or phytic acid, which negatively impact the seed quality.
MATERIALS AND METHODS
Plant Material
We used the spring rapeseed (Brassica napus ‘Haydn’) for hypocotyl transformation. Seeds were obtained from the seed company Norddeutsche Pflanzenzucht Hans-Georg Lembke.
For hypocotyl transformation, seeds were sterilized and plants were grown for 7 d on germination medium in complete darkness. Etiolated hypocotyls were cut into 1-cm pieces.
T1 and T2 plants were grown in 11- × 11-cm pots in the greenhouse (16 h of light/8 h of dark, 20°C–23°C). We mounted selfing bags at the beginning of flowering to control pollination.
BnALC Sequences and Putative Off-Target Sites
Genomic sequences of the two BnALC homoeologs BnaA.ALC.a and BnaC.ALC.a were obtained from Hua et al. (2009). We performed a BLAST search of the sequences against the rapeseed reference genome (Darmor-bzh version 4.1) and identified them to correspond to gene models BnaA07g12110D and BnaC07g16290D (http://www.genoscope.cns.fr/brassicanapus/), located on chromosomes A07 and C07. The coding sequences span 657 and 651 bp and are organized in five exons. BnaA.ALC.a contains an additional intron of about 4.7 kb, splitting exon 1 into exon 1 A and B. A conserved bHLH domain spans exons 2 to 4.
Putative off-target sites were identified by performing a BLAST search of the target sequence against the reference genome. We designed primers for the amplification of off-target regions (Supplemental Table S1). PCR amplicons from T1 and T2 plants were Sanger sequenced.
Vector Construction and Plant Transformation
For targeted mutagenesis, we used the binary vector system pChimera and pCas9-TPC (Fauser et al., 2014). The transformation plasmid pCas9-TPC contains a bar cassette for herbicide selection in plants. A 20-bp target sequence neighboring a 5′-NGG PAM was selected in an exonic region upstream of the bHLH domain in BnALC (Fig. 1A) and was cloned into the respective plasmid (Supplemental Fig. S1). The Agrobacterium tumefaciens strain GV3101 pMP90RK was used for plant transformation.
For rapeseed hypocotyl transformation, we followed the protocol described by Zarhloul et al. (2006) with modifications regarding the selection. We applied 500 mg L−1 carbenicillin to deplete A. tumefaciens and 6 mg L−1 phosphinothricin to select transgenic plants.
Mutant Identification
Genomic DNA was isolated from leaf samples by a standard CTAB method. The presence of the transformation cassette was tested by PCR using primers Cas1_f and Cas1_r (Supplemental Table S1; Supplemental Fig. S1).
CRISPR-Cas9-targeted mutations were identified by Sanger sequencing of PCR amplicons using primer combinations ALC33/ALC16 and ALC13/ALC12 (Supplemental Table S1). In addition, PCR fragments of the T1 plant were cloned into the pGEM-T Easy vector system (Promega) and transformed into Escherichia coli. Single colonies were picked for PCR, and the amplicons were sequenced in the same way.
Illumina Sequencing and Sequence Analysis
Genomic DNA isolated from leaf material of the T1 plant CP1 was used for Illumina sequencing. A whole-genome shotgun library was constructed using standard procedures (TruSeq DNA; Illumina) and provided with Illumina adapter indices (AD002, AD004, and AD007). The library was quantified using real-time PCR (Mascher et al., 2013). Cluster formation using the cBot device and paired-end sequencing (HiSeq2500, 2 × 101 cycles, index read, rapid run modus) were performed according to the manufacturer’s instructions (Illumina).
Sequence reads of CP1 and a negative control cultivar, Grossluesewitzer (Schmutzer et al., 2015), were aligned to a reference consisting of the rapeseed reference genome (Chalhoub et al., 2014) and the vector sequence with BWA mem version 0.7.15 (Li, 2013). The resultant SAM file was converted to BAM format with SAMtools (Li et al., 2009) and sorted by reference position using Novosort (http://www.novocraft.com/products/novosort/). Read depth was calculated with the command samtools depth using only uniquely aligned reads with a mapping quality of 20 or greater and plotted with standard R functions (R Core Team, 2015).
Shatter Resistance Measurements
Siliques were harvested manually at maturity and stored in paper bags. The samples were equilibrated for moisture content in a climate control cabinet (VB0714; Vötsch Industrietechnik) at 25°C and 40% relative humidity for at least 3 d prior to measurement. Equilibration conditions were derived from Bruce et al. (2002).
To determine the force necessary to tear the valves of a silique apart from the replum, siliques were fixed with two alligator clamps attached to a Newton meter (type FH10) on a manual test stand (model TVL; Sauter). Maximum peak tensile forces were measured. Silique length was recorded for every data point. The beak of the silique was excluded from the length measurement. A total of 150 siliques pooled from five to seven individual plants were measured per genotype. Siliques were grouped according to their lengths to calculate mean peak tensile forces of three size classes (3–4 cm, 4–5 cm, and 5–6 cm; Supplemental Table S2). Student’s t tests were run to assess the significance of the observed differences.
Accession Numbers
The WGS sequence of T1 plant CP1 can be found in the EMBL European Nucleotide Archive (ENA) under accession number PRJEB20660.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Vector map of the recombinant pCas9-TPC plasmid used for this study.
Supplemental Figure S2. Mapping results for genome sequences of the transgenic T1 plant CP1 and the negative control cv Grossluesewitzer against the transformation vector sequence.
Supplemental Table S1. Primers used in this study.
Supplemental Table S2. Results of silique shatter trials.
Supplementary Material
Acknowledgments
We thank Monika Bruisch and Hilke Jensen for technical assistance; Dr. José Orsini (formerly Saaten-Union Biotec) for valuable discussion regarding hypocotyl transformation of rapeseed; the breeding company Norddeutsche Pflanzenzucht Hans-Georg Lembke for providing cv Haydn seeds and Dr. Holger Puchta (Karlsruhe Institute of Technology) for supplying pChimera and pCas9-TPC vectors; the Institute of Clinical Molecular Biology for Sanger sequencing; and the next-generation sequencing laboratory of the Leibniz Institute of Plant Genetics and Crop Plant Research, in particular Sandra Driesslein, for Illumina library preparation.
Glossary
- bHLH
basic helix-loop-helix
- PAM
protospacer-adjacent motif
- SNP
single-nucleotide polymorphism
- sgRNA
single-guide RNA
Footnotes
This work was supported by the Stiftung Schleswig-Holsteinische Landschaft (project no. 2013/69).
References
- Andersson M, Turesson H, Nicolia A, Fält AS, Samuelsson M, Hofvander P (2017) Efficient targeted multiallelic mutagenesis in tetraploid potato (Solanum tuberosum) by transient CRISPR-Cas9 expression in protoplasts. Plant Cell Rep 36: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester P, Ferrándiz C (2017) Shattering fruits: variations on a dehiscent theme. Curr Opin Plant Biol 35: 68–75 [DOI] [PubMed] [Google Scholar]
- Boszoradova E, Libantova J, Matusikova I, Poloniova Z, Jopcik M, Berenyi M, Moravcikova J (2011) Agrobacterium-mediated genetic transformation of economically important oilseed rape cultivars. Plant Cell Tiss Org 107: 317–323 [Google Scholar]
- Brooks C, Nekrasov V, Lippman ZB, Van Eck J (2014) Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-associated9 system. Plant Physiol 166: 1292–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce DM, Farrent JW, Morgan CL, Child RD (2002) Determining the oilseed rape pod strength needed to reduce seed loss due to pod shatter. Biosyst Eng 81: 179–184 [Google Scholar]
- Bus A, Körber N, Snowdon RJ, Stich B (2011) Patterns of molecular variation in a species-wide germplasm set of Brassica napus. Theor Appl Genet 123: 1413–1423 [DOI] [PubMed] [Google Scholar]
- Chalhoub B, Denoeud F, Liu S, Parkin IAP, Tang H, Wang X, Chiquet J, Belcram H, Tong C, Samans B, et al. (2014) Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 345: 950–953 [DOI] [PubMed] [Google Scholar]
- Doudna JA, Charpentier E (2014) Genome editing: the new frontier of genome engineering with CRISPR-Cas9. Science 346: 1258096. [DOI] [PubMed] [Google Scholar]
- Emrani N, Harloff HJ, Gudi O, Kopisch-Obuch F, Jung C (2015) Reduction in sinapine content in rapeseed (Brassica napus L.) by induced mutations in sinapine biosynthesis genes. Mol Breed 35: 37 [Google Scholar]
- Endo M, Mikami M, Toki S (2015) Multigene knockout utilizing off-target mutations of the CRISPR/Cas9 system in rice. Plant Cell Physiol 56: 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser F, Schiml S, Puchta H (2014) Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J 79: 348–359 [DOI] [PubMed] [Google Scholar]
- Feng Z, Mao Y, Xu N, Zhang B, Wei P, Yang DL, Wang Z, Zhang Z, Zheng R, Yang L, et al. (2014) Multigeneration analysis reveals the inheritance, specificity, and patterns of CRISPR/Cas-induced gene modifications in Arabidopsis. Proc Natl Acad Sci USA 111: 4632–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harloff HJ, Lemcke S, Mittasch J, Frolov A, Wu JG, Dreyer F, Leckband G, Jung C (2012) A mutation screening platform for rapeseed (Brassica napus L.) and the detection of sinapine biosynthesis mutants. Theor Appl Genet 124: 957–969 [DOI] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol 31: 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, Shamsi IH, Guo Y, Pak H, Chen M, Shi C, Meng H, Jiang L (2009) Sequence, expression divergence, and complementation of homologous ALCATRAZ loci in Brassica napus. Planta 230: 493–503 [DOI] [PubMed] [Google Scholar]
- Lawrenson T, Shorinola O, Stacey N, Li C, Østergaard L, Patron N, Uauy C, Harwood W (2015) Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA-guided Cas9 nuclease. Genome Biol 16: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 1303.3997
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yaokui L, Dan Z, Bigang M, Qiming L, Yuanyi H, Ye S, Yan P, Binran Z, Shitou X (2016a) Characteristic and inheritance analysis of targeted mutagenesis mediated by genome editing in rice. Yi Chuan 38: 746–755 [DOI] [PubMed] [Google Scholar]
- Li WX, Wu SL, Liu YH, Jin GL, Zhao HJ, Fan LJ, Shu QY (2016b) Genome-wide profiling of genetic variation in Agrobacterium-transformed rice plants. J Zhejiang Univ Sci B 17: 992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Roeder AHK, Kempin SA, Gremski K, Østergaard L, Guimil S, Reyes DK, Yanofsky MF (2004) Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 116: 843–853 [DOI] [PubMed] [Google Scholar]
- Mascher M, Richmond TA, Gerhardt DJ, Himmelbach A, Clissold L, Sampath D, Ayling S, Steuernagel B, Pfeifer M, D’Ascenzo M, et al. (2013) Barley whole exome capture: a tool for genomic research in the genus Hordeum and beyond. Plant J 76: 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami M, Toki S, Endo M (2015) Parameters affecting frequency of CRISPR/Cas9 mediated targeted mutagenesis in rice. Plant Cell Rep 34: 1807–1815 [DOI] [PubMed] [Google Scholar]
- Price JS, Hobson RN, Neale MA, Bruce DM (1996) Seed losses in commercial harvesting of oilseed rape. J Agric Eng Res 65: 183–191 [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org [Google Scholar]
- Rajani S, Sundaresan V (2001) The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr Biol 11: 1914–1922 [DOI] [PubMed] [Google Scholar]
- Schmutzer T, Samans B, Dyrszka E, Ulpinnis C, Weise S, Stengel D, Colmsee C, Lespinasse D, Micic Z, Abel S, et al. (2015) Species-wide genome sequence and nucleotide polymorphisms from the model allopolyploid plant Brassica napus. Sci Data 2: 150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten HJ, Vande Geest H, Papadimitriou S, Bemer M, Schaart JG, Smulders MJ, Perez GS, Schijlen E (2017) Re-sequencing transgenic plants revealed rearrangements at T-DNA inserts, and integration of a short T-DNA fragment, but no increase of small mutations elsewhere. Plant Cell Rep 36: 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitashev S, Schwartz C, Lenderts B, Young JK, Cigan AM (2016) Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat Commun 7: 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BJ, Zerr T, Comai L, Henikoff S (2006) A protocol for TILLING and Ecotilling in plants and animals. Nat Protoc 1: 2465–2477 [DOI] [PubMed] [Google Scholar]
- Wang GP, Yu XD, Sun YW, Jones HD, Xia LQ (2016) Generation of marker- and/or backbone-free transgenic wheat plants via Agrobacterium-mediated transformation. Front Plant Sci 7: 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu JL (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32: 947–951 [DOI] [PubMed] [Google Scholar]
- Wells R, Trick M, Soumpourou E, Clissold L, Morgan C, Werner P, Gibbard C, Clarke M, Jennaway R, Bancroft I (2014) The control of seed oil polyunsaturate content in the polyploid crop species Brassica napus. Mol Breed 33: 349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JW, Kim J, Kwon SI, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Yan W, Chen D, Kaufmann K (2016) Efficient multiplex mutagenesis by RNA-guided Cas9 and its use in the characterization of regulatory elements in the AGAMOUS gene. Plant Methods 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarhloul KM, Stoll C, Lühs W, Syring-Ehemann A, Hausmann L, Töpfer R, Friedt W (2006) Breeding high-stearic oilseed rape (Brassica napus) with high- and low-erucic background using optimised promoter-gene constructs. Mol Breed 18: 241–251 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.