Abstract
Recent progress of the blue light signaling pathway in guard cells highlights its regulation of H+-ATPase activity.
Light-induced stomatal responses were first reported by Darwin (1989). Stomata open in response to light, including blue and red light (Shimazaki et al., 2007). Red light induces stomatal opening via photosynthesis in the mesophyll and guard cell chloroplasts (Mott et al., 2008; Suetsugu et al., 2014). In contrast, blue light as a signal induces stomatal opening. Phototropins expressed in guard cells act as major blue light receptors for stomatal opening (Kinoshita et al., 2001, 2003; Inoue et al., 2008). Blue light-induced stomatal opening is mediated through activation of a plasma membrane (PM) H+ pump, later identified as the PM H+-ATPase, in guard cells (Assmann et al., 1985; Shimazaki et al., 1986; Kinoshita and Shimazaki, 1999). The blue light-activated pump provides driving force for stomatal opening concomitant with ion accumulation and cell volume increase in guard cells (Schroeder et al., 1987; Kinoshita and Hayashi, 2011). Note that stomatal opening in response to weak blue light as a signal requires background red light, indicating that red light has a synergistic effect on the blue light response in guard cells (Shimazaki et al., 2007).
Recent investigations of guard cells with respect to blue light-induced stomatal opening have greatly advanced our understanding. In this review, we focus on the recent progress of the blue light signaling pathway in guard cells and its regulation of the PM H+-ATPase activity.
BLUE LIGHT SIGNALING FOR STOMATAL OPENING
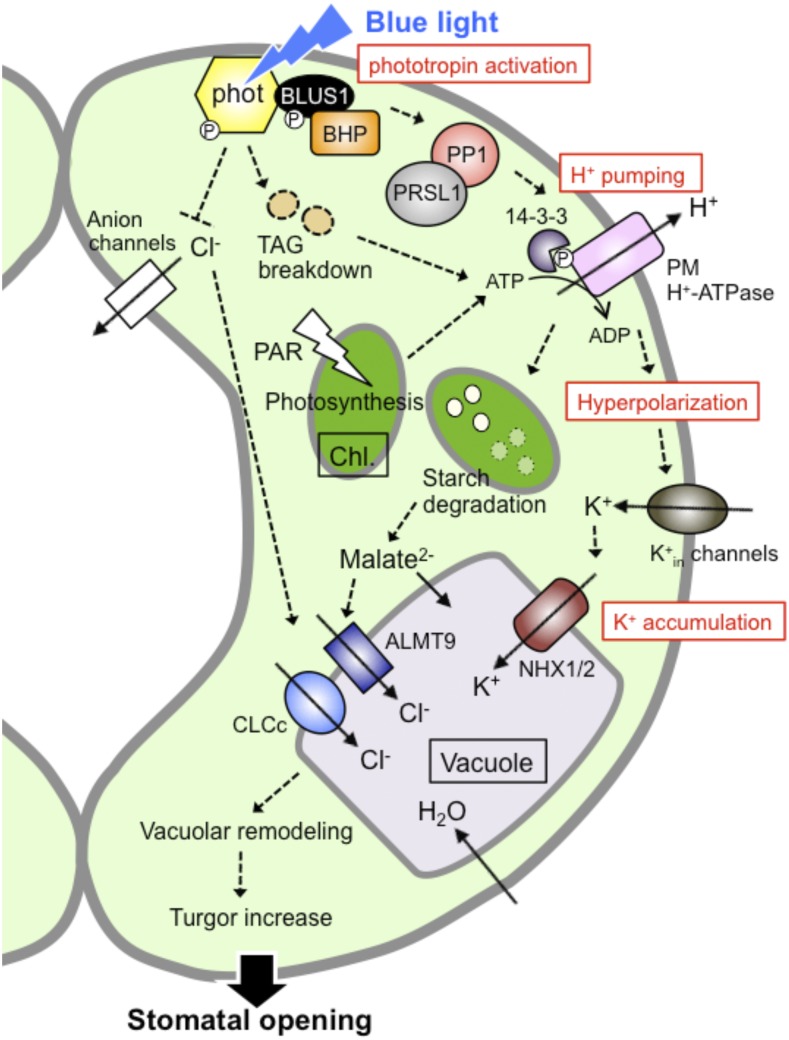
Stomata effectively open in response to blue light, especially under strong red light (Shimazaki et al., 2007; Marten et al., 2010). Our understanding of the signaling model for stomatal opening was mainly constructed from studies of blue light-induced stomatal opening (Fig. 1). A single guard cell possesses all signaling components, from blue light perception to cell volume increase, for stomatal opening. When guard cells are irradiated by blue light, blue light-photoreceptor protein kinases, phototropins, are activated through autophosphorylation and initiate signaling for stomatal opening (Kinoshita et al., 2001; Christie, 2007). Blue light induces autophosphorylation of two Ser residues in the kinase activation-loop of phototropin molecules, and phosphorylation is required for downstream signaling, probably through substrate recognition (Inoue et al., 2008, 2010, 2011). The activated phototropins directly phosphorylate another protein kinase BLUE LIGHT SIGNALING1 (BLUS1), and phosphorylated BLUS1 indirectly transmits the signal to type 1 protein phosphatase (PP1) and its regulatory subunit PRSL1 (Takemiya et al., 2006, 2013a, 2013b; Takemiya and Shimazaki, 2016). Note that BLUS1 expression is specific to guard cells and is not involved in the other phototropin-mediated responses, such as phototropism, chloroplasts movements, and leaf flattening (Takemiya et al., 2013a), suggesting that BLUS1 defines signaling specificity of stomatal opening among the phototropin-mediated responses. The signal generated by BLUS1 finally activates the PM H+-ATPase, mainly isoform AHA1, in guard cells through phosphorylation of a penultimate Thr in the C terminus with subsequent binding of a 14-3-3 protein (Shimazaki et al., 2007; Hayashi et al., 2011; Yamauchi et al., 2016).
Figure 1.
Blue light signaling pathway in stomatal guard cells. Arrows and a T-bar represent positive and negative regulation, respectively. The P in the white circles indicates a phosphorylation of each protein. The timescale of each peak of the key signaling events for blue light-induced stomatal opening (∼2 h) is shown as follows: phototropin activation (within 1 min), H+ pumping (∼2.5 min), hyperpolarization (several min), K+ accumulation (between 30 and 60 min). Triacylglycerol breakdown, starch degradation, and vacuolar remodeling are observed within 1 to 2 h after the start of light illumination. phot, Phototropin; 14-3-3, 14-3-3 protein; Chl., chloroplast; PAR, photosynthetically active radiation; TAG, triacylglycerol.
Very recently, a Raf-like protein kinase, BLUE LIGHT-DEPENDENT H+-ATPASE PHOSPHORYLATION (BHP), was reported, to our knowledge, as the novel signaling component in blue light-dependent stomatal opening (Hayashi et al., 2017). BHP was identified through a screening of protein kinase inhibitors that suppress blue light-dependent PM H+-ATPase phosphorylation in guard cells and similarities to the mammalian targets of the inhibitors. BHP does not bind to the PM H+-ATPase but to BLUS1 and forms an early signaling complex with phototropins to mediate phosphorylation of a penultimate Thr of the PM H+-ATPase (Hayashi et al., 2017). Guard cells in bhp mutant exhibited normal phosphorylation of the PM H+-ATPase in response to the PM H+-ATPase activator fusicoccin, suggesting that BHP is not likely to directly phosphorylate the penultimate Thr of PM H+-ATPase. There may be an unidentified protein kinase that directly phosphorylates the PM H+-ATPase in stomatal opening. In addition, whether BLUS1 phosphorylates and activates BHP is unknown at this time. Further analyses are needed to clarify the early signaling for stomatal opening from phototropins to the PM H+-ATPase activation and to identify the endogenous substrates of BLUS1, BHP, and PP1 in guard cells.
The blue light-activated PM H+-ATPase drives H+ transport across the PM and hyperpolarizes of the membrane (Shimazaki et al., 2007; Marten et al., 2010). This membrane hyperpolarization activates inward-rectifying K+ (K+in) channels and induces an influx of K+ (Lebaudy et al., 2008; Kim et al., 2010), resulting in the accumulation of K+, and the counteranions Cl−, nitrate, and malate2−; K+, and Cl− are immediately transported into the vacuole via the tonoplast-localized K+/H+ exchangers NHX1 and NHX2 and the vacuolar chloride channels aluminum-activated malate transporter 9 (ALMT9) and chloride channel c (CLCc), respectively (Jossier et al., 2010; Chen et al., 2012; De Angeli et al., 2013; Andrés et al., 2014), and K+ accumulation into the vacuole involves dynamic remodeling of vacuolar structure for stomatal opening (Andrés et al., 2014). Accumulation of these ions decreases the water potential of guard cells, which leads to water uptake into the vacuole and turgor increase, leading to stomatal opening (Inoue et al., 2010; Marten et al., 2010). The details of ion transports in guard cells are reviewed in this Focus Issue (Eisenacha and De Angeli, 2017; Jezek and Blatt, 2017). Recently, Santelia and colleagues demonstrated that starch in guard cell chloroplasts is degraded by phototropin-mediated signaling downstream of PM H+-ATPase activity and the degradation contributes to stomatal opening (Horrer et al., 2016), probably through malate synthesis (Shimazaki et al., 2007). It remains an interesting and important question on how the activity of the PM H+-ATPase is linked to the starch degradation pathway in chloroplasts (Santelia and Lunn, 2017).
In addition to the pursuit of signaling components as described above, regulation of the expression and localization of key signaling factors that determines stomatal aperture indirectly have also been investigated recently. For example, the bHLH family transcription factors of ABA-RESPONSIVE KINASE SUBSTRATEs (AKSs) and GARP transcription factors of GOLDEN 2-LIKE1 (GLK1) and GLK2 bind to the promoter of the K+in channel KAT1 gene and increase the K+in channel expression in guard cells (Takahashi et al., 2013; Nagatoshi et al., 2016). GLKs also positively regulate BLUS1 expression (Nagatoshi et al., 2016). These transcriptional regulations contribute to enhance stomatal opening. Moreover, the signaling components for photoperiodic flowering including cryptochromes, GIGANTEA, CONSTANS, EARLY FLOWERING3, FLOWERING LOCUS T (FT), TWIN SISTER OF FT, and SUPPRESSOR OF OVEREXPRESSION OF CO1, are expressed in guard cells and also affect light-induced stomatal opening probably via transcriptional regulations in guard cells, but details of the regulatory targets are unknown (Kinoshita et al., 2011; Ando et al., 2013; Kimura et al., 2015). In addition, the Munc13-like protein PATROL1 may be involved in the enhancement of light-induced stomatal opening by promoting the recruitment of AHA1 to the PM of guard cells (Hashimoto-Sugimoto et al., 2013). Both aminophospholipid ATPase (ALA10) flippase and phospholipase A2β (PL A2β) are involved in light-induced stomatal opening through lysophospholipid generation (Seo et al., 2008; Poulsen et al., 2015). ALA10 promotes phospholipid uptake into guard cells, and PL A2β generates lysophospholipids using phospholipids as a substrate. One of the products, lysophosphatidylcholine, functions as a specific activator of the PM H+-ATPase (Palmgren, 2001), because both ALA10 and PL A2β may have a positive effect on the stomatal opening through enhancement of PM H+-ATPase activity.
Furthermore, photosynthetic processes in guard cell chloroplasts provide fuel (ATP and/or reducing equivalents) for blue light-dependent H+ pumping of the PM H+-ATPase and contribute to stomatal opening (Suetsugu et al., 2014). Correspondingly, guard cells lacking chloroplasts in the crumpled leaf mutant displayed attenuation of both guard cell ATP levels and stomatal opening in response to light (Wang et al., 2014). Stored triacylglycerols in guard cells are broken down in response to light and the catabolic process is also thought to supply ATP for PM H+-ATPase activity in stomatal opening (McLachlan et al., 2016).
CROSS TALK BETWEEN BLUE LIGHT AND ABA SIGNALING IN LIGHT-INDUCED STOMATAL OPENING
The plant hormone ABA synthesized in response to drought stress conditions drastically reduces stomatal aperture to prevent water loss in the presence of light (Bauer et al., 2013; Waadt et al., 2014; Kim et al., 2010; Murata et al., 2015; Osakabe et al., 2014). ABA induces stomatal closure in already open stomata, called “stomatal closure,” and simultaneously inhibits light-induced stomatal opening, called “inhibition of stomatal opening,” and both physiological regulatory mechanisms are required to close stomata efficiently under sunlight. It is known that ABA-signaling in guard cells effectively suppresses blue light-signaling in inhibition of stomatal opening with various ways, as follows.
First, ABA accelerates the release of ions from guard cells by activating S- and R-type anion channels, outward-rectifying K+ channels, and K+ uptake transporters in stomatal closure (Negi et al., 2008; Vahisalu et al., 2008; Kim et al., 2010; Osakabe et al., 2013). ABA suppresses these processes via ABA-receptor components PYR/PYL/RCAR-PP2Cs-SnRK2s in guard cells (Fujii et al., 2009; Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009; Vlad et al., 2009; Jezek and Blatt, 2017) with subsequent second messengers H2O2, H2S, NO, phosphatidic acid (PA), and cytosolic Ca2+ (Inoue et al., 2010; Scuffi et al., 2014). It has been reported that H2S also functions in parallel to ABA signaling events (Papanatsiou et al., 2015). Simultaneously, in the inhibition of stomatal opening, ABA suppresses blue light-signaling of the activation of PM H+-ATPase via ABA receptor components and H2O2, NO, PA, and Ca2+ (Shimazaki et al., 2007; Zhang et al., 2007; Inoue et al., 2010; Kim et al., 2010; Takemiya and Shimazaki, 2010; Hayashi et al., 2011; Hayashi and Kinoshita, 2011). PA directly inhibits PP1 catalytic activity and blocks blue light-signaling between phototropins and the PM H+-ATPase (Takemiya and Shimazaki, 2010). In addition, ABA inhibits K+in channel activity and many ABA-signaling components affect this inhibition (Kim et al., 2010; Jezek and Blatt, 2017). OPEN STOMATA1 (OST1), an ABA-activated protein kinase that operates downstream of the ABA-receptor components, suppresses K+in channel KAT1 activity through direct phosphorylation (Sato et al., 2009). The second messengers, such as Ca2+, and G-proteins Gα and Gβ, are also involved in ABA-induced K+in channel inhibition (Fan et al., 2008; Kim et al., 2010). Moreover, the S-type anion channels SLAC1 and SLAH3, which drive stomatal closure, are strongly upregulated by drought stress in guard cells and inhibit KAT1 activity by direct binding (Zhang et al., 2016).
Second, ABA decreases the expression of K+in channel genes via inactivation of AKS transcription factors through phosphorylation by OST1 in guard cells (Takahashi et al., 2013, 2016).
Third, ABA promotes internalization of KAT1 from the PM into endomembrane compartments by endocytosis, thereby reducing the amount of K+in channels functioning at the PM (Sutter et al., 2007).
Conversely, when plants are grown under well-watered conditions, blue light suppresses signaling of ABA-induced stomatal closure to promote stomatal opening. Blue light receptor cryptochromes reduce ABA content in the plant body, and this process is thought to affect ABA signaling in guard cells (Boccalandro et al., 2012). More directly, blue light also stimulates stomatal opening by suppressing anion release from guard cells. This process involves light-dependent inhibition of S-type anion channels in a phototropin-dependent manner (Marten et al., 2007). It was also reported that light-produced phosphatidylinositol 4,5-bisphosphate inhibits anion channel activity in guard cells (Lee et al., 2007). Consistent with these results, stomata in the slac1-1 mutant are slightly open under dark-adapted conditions and open larger than those in wild type in response to light (Wang et al., 2012).
INVOLVEMENT OF PM H+-ATPASE IN STOMATAL OPENING
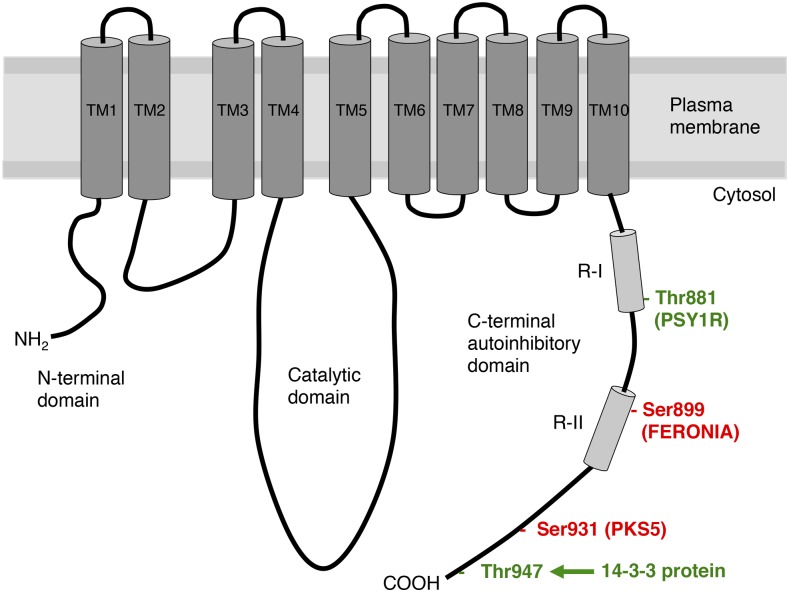
PM H+-ATPases, a family of P-type ATPases, consist of a functional polypeptide with 10 transmembrane domains and three cytosolic domains, including the N-terminal domain, catalytic domain, and C-terminal autoinhibitory domain (Fig. 2). In Arabidopsis, 11 genes encode PM H+-ATPases (AHA1 to AHA11), and a double-knockout mutant of AHA1 and AHA2, which are predominantly expressed in all cell types, displays an embryonic lethal phenotype (Haruta et al., 2010). The membrane potential and proton gradient created by PM H+-ATPases energize multiple ion channels and various H+-coupled transporters in the PM for diverse physiological responses including stomatal movement, phloem loading and unloading, xylem loading and unloading, seed germination, solute uptake in roots, leaf movement, tip growth, and cell expansion (Haruta et al., 2015; Wang et al., 2014b; Takahashi and Kinoshita, 2016). In addition, proton transport through PM H+-ATPases controls cytosolic pH homeostasis and apoplastic pH (Falhof et al., 2016). It is worthy of note that modeling analyses of ion transport in the stomatal guard cells reveal the importance of PM H+-ATPase activity not only in proton gradient formation and pH control, but also in driving K+ Ca2+, anion transport and metabolism in guard cells (Chen et al., 2012; Hills et al., 2012; Minguet-Parramona et al., 2016), demonstrating that PM H+-ATPase plays a pivotal role in guard cells physiology.
Figure 2.
Schematic structure of PM H+-ATPases. PM H+-ATPases possess 10 transmembrane domains (TM1 to TM10) and three cytosolic domains, including the N-terminal domain, catalytic domain, and C-terminal autoinhibitory domain containing the R-I and R-II regions (Palmgren, 2001). There are several phosphorylation sites in the C-terminal domain (Thr-881, Ser-899, Thr-924, Ser-931, and Thr-947). Thr-881, Ser-899, and Ser-931 are phosphorylated by PSY1R, FERONIA, and PKS5, respectively. The 14-3-3 protein binds to the phosphorylated penultimate Thr (Thr-947). The numbering of the amino acid residues corresponds to Arabidopsis H+-ATPase2.
Since the 1970s, it has been known that light induces proton extrusion from stomatal guard cells, K+ uptake, and swelling of guard cells. Physiological and electrophysiological analyses revealed the properties of the blue light-activated proton pump using fava bean (Vicia faba) guard cell protoplasts (Assmann et al., 1985; Shimazaki et al., 1986). Later, conclusive evidence was obtained by biochemical analysis. PM H+-ATPase is activated and phosphorylated in response to blue light on a penultimate Thr residue in the C terminus and 14-3-3 protein binds to the phosphorylated C terminus region (Kinoshita and Shimazaki, 1999, 2002).
In addition to biochemical evidence, genetic evidence has also been obtained. Dominant mutants of AHA1, a major H+-ATPase isoform in Arabidopsis, ost2-1D and ost2-2D, displayed constitutively open stomatal phenotypes, because dominant mutations cause constitutive activity of H+-ATPase (Merlot et al., 2007). More recently, it was reported that loss-of-function mutants of AHA1 showed reduced blue light-induced stomatal opening (Yamauchi et al., 2016) and a closed stomatal phenotype (Osakabe et al., 2016). Furthermore, a loss-of-function mutant of OSA7, a major PM H+-ATPase isoform in rice, also showed reduced blue light-induced increase of stomatal conductance (Toda et al., 2016). Overexpression of AHA2 using a strong guard cell promoter enhanced light-induced stomatal opening, leading to increased photosynthetic activity and plant biomass (Yang et al., 2008; Wang et al., 2014a). These biochemical and genetic findings clearly demonstrate that the PM H+-ATPases act as H+ pumps in the PM and are vital for stomatal opening.
REGULATION OF PM H+-ATPASE BY REVERSIBLE PHOSPHORYLATION
It has been demonstrated that PM H+-ATPase activity is regulated by phosphorylation of several sites (Haruta et al., 2015; Falhof et al., 2016). To our knowledge, the first reported and most studied phosphorylation site is the penultimate Thr in the C terminus (Palmgren, 2001; Wang et al., 2014b). Blue light activates PM H+-ATPase via the guard cell-specific signaling pathway, leading to phosphorylation of the penultimate Thr in guard cells (Shimazaki et al., 2007). In addition, recent investigations revealed that phosphorylation level of the penultimate Thr in PM H+-ATPase were modulated in response to physiological and environmental signals, such as light, salt, Suc, auxin, gibberellin, and ABA in several tissues and cell types besides guard cells (Niittylä et al., 2007; Chen et al., 2010; Okumura et al., 2012, 2016; Takahashi et al., 2012; Hayashi et al., 2014; Inoue et al., 2016). These results indicate that there are several unique signaling pathways in each tissue and cell type, but a final regulatory mechanism, that is, phosphorylation of the penultimate Thr at the C terminus of the H+-ATPase, is common in these responses.
The protein kinase responsible for the phosphorylation of the penultimate Thr in PM H+-ATPase has not been identified, despite strenuous efforts, although in vitro protein kinase activity for the penultimate Thr of PM H+-ATPase was found in the plasma membrane isolated from spinach leaves, the microsomes from guard cell protoplasts of fava bean, and the plasma membrane from etiolated seedlings of Arabidopsis (Kinoshita and Hayashi 2011). On the other hand, it was suggested that dephosphorylation of the phosphorylated penultimate Thr of PM H+-ATPases is mediated by the membrane-localized Mg2+/Mn2+-dependent protein phosphatase 2C (PP2C)-like activity in fava bean guard cells and Arabidopsis (Arabidopsis thaliana) etiolated seedlings (Hayashi et al., 2010). Eventually, D-clade PP2Cs were shown to be involved in the dephosphorylation of PM H+-ATPase in etiolated seedlings (Schweighofer et al., 2004; Spartz et al., 2014; Ren and Gray, 2015). In addition, SMALL AUXIN-UP RNAs (SAURs), a large multigene family of early auxin-responsive genes, inhibit D-clade PP2C activity through physical interaction (Spartz et al., 2014; Sun et al., 2016). It is noteworthy that SAUR19-overexpressing plants displayed enhanced water loss in detached leaves, wilted faster than wild type upon cessation of watering, and exhibited delayed stomatal closure (Spartz et al., 2014, 2017), and that many SAUR genes are repressed by ABA, which certainly reduces stomatal aperture (Nemhauser et al., 2006; Kodaira et al., 2011). Taken together, these biochemical and genetic data strongly suggest that clade D of PP2C and SAURs are involved in the regulation of PM H+-ATPase in stomatal guard cells. However, it is still unknown how SAURs are regulated in response to blue light in guard cells.
In addition to the penultimate Thr, three residues were demonstrated to regulate PM H+-ATPase activity using nonguard cells (Haruta et al., 2015; Rudashevskaya et al., 2012; Fig. 2). A receptor kinase, FERONIA, phosphorylates a Ser residue (Ser-899 in AHA2) in the C-terminal autoinhibitory domain of PM H+-ATPase and this phosphorylation suppresses proton efflux by PM H+-ATPase (Haruta et al., 2014). Moreover, the phosphorylation of a Thr residue (Thr-881 in AHA2) in the C-terminal autoinhibitory domain of PM H+-ATPase is induced by a receptor kinase, PSY1R, and application of the ligand peptide for PSY1R, PSY1, increased proton efflux, suggesting that phosphorylation of Thr-881 activates PM H+-ATPase activity (Fuglsang et al., 2014). In addition, the Ser residue (Ser-931 in AHA2) in the C-terminal autoinhibitory domain of the PM H+-ATPase is phosphorylated by PKS5, a Ser/Thr protein kinase. Phosphorylation of Ser-931 inhibits interaction of the PM H+-ATPase with the 14-3-3 protein and decreases PM H+-ATPase activity (Fuglsang et al., 2007). The physical interaction of chaperone J3 with PKS5 induces the activation of PM H+-ATPase by repressing PKS5 activity (Yang et al., 2010). In addition, it has been reported that type 2A protein phosphatase scaffolding subunit A interacts with the C terminus region of PM H+-ATPase (Fuglsang et al., 2006), and that a negative regulator of plant immunity RIN4 activates PM H+-ATPase activity and the rin4 mutant shows reduced stomatal aperture (Liu et al., 2009). Thus, posttranslational modifications, such as phosphorylation of multiple sites in the C-terminal domain and/or protein-protein interactions, regulate PM H+-ATPase activity. Blue light induces phosphorylation on multiple Ser and Thr residues in the C terminus of the PM H+-ATPase in fava bean guard cells (Kinoshita and Shimazaki, 1999). However, it is still unclear whether other phosphorylation sites, such as Thr-881, Ser-899, and Ser-931, are involved in the regulation of PM H+-ATPase in guard cells in response to blue light. Further investigations will be needed to clarify this.
CONCLUSION
In this review, we described recent advances in the blue light signaling pathway in stomatal guard cells and the regulatory mechanisms of PM H+-ATPase. Stomata open in response to blue light to facilitate gas exchange between the plant and the atmosphere. This response is key to terrestrial plant life, as gas exchange is necessary not only for photosynthesis but also for water uptake from the roots. So far, major signaling components involved in the blue light signaling pathway in stomatal guard cells have been identified, such as phototropin, BLUS1, BHP, PP1, and PM H+-ATPase; however, the signaling mechanism is not fully understood (see Outstanding Questions). For example, how do blue light signals induce phosphorylation of the penultimate Thr of PM H+-ATPase, which is a key enzyme for stomatal opening? Whether blue light signal induces the phosphorylation of PM H+-ATPase or suppresses dephosphorylation of PM H+-ATPase, or both, is still unknown. Elucidation of this mechanism and identification of the kinase and phosphatase for PM H+-ATPase in guard cells will provide, to our knowledge, novel insights into both the blue light signaling pathway through phototropins and the regulation of H+-ATPase in plant cells. Given the importance of stomatal regulation, future investigations will not only improve our understanding of the molecular mechanisms of signaling pathways in plants, but also provide important clues for agricultural strategies to improve photosynthetic or water use efficiency, leading to an increase in the biomass and harvest of crops.
Footnotes
This work was supported by KAKENHI from the Japan Society for the Promotion of Science (grant nos. 25840105 and 15K07101 to S.I. and grant nos. JP15H05956 and 15H04386 to T.K.).
Articles can be viewed without a subscription.
References
- Ando E, Ohnishi M, Wang Y, Matsushita T, Watanabe A, Hayashi Y, Fujii M, Ma JF, Inoue S, Kinoshita T (2013) TWIN SISTER OF FT, GIGANTEA, and CONSTANS have a positive but indirect effect on blue light-induced stomatal opening in Arabidopsis. Plant Physiol 162: 1529–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, Pardo JM (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci USA 111: E1806–E1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Simoncini L, Schroeder JI (1985) Blue-light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318: 285–287 [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Boccalandro HE, Giordano CV, Ploschuk EL, Piccoli PN, Bottini R, Casal JJ (2012) Phototropins but not cryptochromes mediate the blue light-specific promotion of stomatal conductance, while both enhance photosynthesis and transpiration under full sunlight. Plant Physiol 158: 1475–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hoehenwarter W, Weckwerth W (2010) Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. Plant J 63: 1–17 [DOI] [PubMed] [Google Scholar]
- Chen ZH, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM. (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58: 21–45 [DOI] [PubMed] [Google Scholar]
- Darwin F. (1989) Observations on stomata. Philos Trans R Soc Lond B Biol Sci 190: 531–621 [Google Scholar]
- De Angeli A, Zhang J, Meyer S, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4: 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, De Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falhof J, Pedersen JT, Fuglsang AT, Palmgren M (2016) Plasma membrane H+-ATPase regulation in the center of plant physiology. Mol Plant 9: 323–337 [DOI] [PubMed] [Google Scholar]
- Fan LM, Zhang W, Chen JG, Taylor JP, Jones AM, Assmann SM (2008) Abscisic acid regulation of guard-cell K+ and anion channels in Gβ- and RGS-deficient Arabidopsis lines. Proc Natl Acad Sci USA 105: 8476–8481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu JK (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19: 1617–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang AT, Kristensen A, Cuin TA, Schulze WX, Persson J, Thuesen KH, Ytting CK, Oehlenschlæger CB, Mahmood K, Sondergaard TE, Shabala S, Palmgren MG (2014) Receptor kinase-mediated control of primary active proton pumping at the plasma membrane. Plant J 80: 951–964 [DOI] [PubMed] [Google Scholar]
- Fuglsang AT, Tulinius G, Cui N, Palmgren MG (2006) Protein phosphatase 2A scaffolding subunit A interacts with plasma membrane H+-ATPase C-terminus in the same region as 14-3-3 protein. Physiol Plant 128: 334–340 [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Burch HL, Nelson RB, Barrett-Wilt G, Kline KG, Mohsin SB, Young JC, Otegui MS, Sussman MR (2010) Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J Biol Chem 285: 17918–17929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Gray WM, Sussman MR (2015) Regulation of the plasma membrane proton pump (H+-ATPase) by phosphorylation. Curr Opin Plant Biol 28: 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Sugimoto M, Higaki T, Yaeno T, Nagami A, Irie M, Fujimi M, Miyamoto M, Akita K, Negi J, Shirasu K, Hasezawa S, Iba K (2013) A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun 4: 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Inoue S, Takahashi K, Kinoshita T (2011) Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol 52: 1238–1248 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Inoue SI, Ueno Y, Kinoshita T (2017) A Raf-like protein kinase BHP mediates blue light-dependent stomatal opening. Sci Rep 7: 45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Kinoshita T (2011) Crosstalk between blue-light- and ABA-signaling pathways in stomatal guard cells. Plant Signal Behav 6: 1662–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol 51: 1186–1196 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Takahashi K, Inoue S, Kinoshita T (2014) Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H+-ATPase in Arabidopsis thaliana. Plant Cell Physiol 55: 845–853 [DOI] [PubMed] [Google Scholar]
- Hills A, Chen ZH, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrer D, Flütsch S, Pazmino D, Matthews JS, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol 26: 362–370 [DOI] [PubMed] [Google Scholar]
- Inoue S, Kinoshita T, Matsumoto M, Nakayama KI, Doi M, Shimazaki K (2008) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA 105: 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Matsushita T, Tomokiyo Y, Matsumoto M, Nakayama KI, Kinoshita T, Shimazaki K (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol 156: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Takahashi K, Okumura-Noda H, Kinoshita T (2016) Auxin influx carrier AUX1 confers acid resistance for Arabidopsis root elongation through the regulation of plasma membrane H+-ATPase. Plant Cell Physiol 57: 2194–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Takemiya A, Shimazaki K (2010) Phototropin signaling and stomatal opening as a model case. Curr Opin Plant Biol 13: 587–593 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossier M, Kroniewicz L, Dalmas F, Le Thiec D, Ephritikhine G, Thomine S, Barbier-Brygoo H, Vavasseur A, Filleur S, Leonhardt N (2010) The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J 64: 563–576 [DOI] [PubMed] [Google Scholar]
- Kim TH, Böhmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Aoki S, Ando E, Kitatsuji A, Watanabe A, Ohnishi M, Takahashi K, Inoue S, Nakamichi N, Tamada Y, Kinoshita T (2015) A flowering integrator, SOC1, affects stomatal opening in Arabidopsis thaliana. Plant Cell Physiol 56: 640–649 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Emi T, Tominaga M, Sakamoto K, Shigenaga A, Doi M, Shimazaki K (2003) Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomatal guard cells of broad bean. Plant Physiol 133: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Hayashi Y (2011) New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. Int Rev Cell Mol Biol 289: 89–115 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y, Ohnishi M, Nakano T, Inoue S, Shimazaki K (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (2002) Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol 43: 1359–1365 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira KS, Qin F, Tran LS, Maruyama K, Kidokoro S, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K (2011) Arabidopsis Cys2/His2 zinc-finger proteins AZF1 and AZF2 negatively regulate abscisic acid-repressive and auxin-inducible genes under abiotic stress conditions. Plant Physiol 157: 742–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Hosy E, Simonneau T, Sentenac H, Thibaud JB, Dreyer I (2008) Heteromeric K+ channels in plants. Plant J 54: 1076–1082 [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim YW, Jeon BW, Park KY, Suh SJ, Seo J, Kwak JM, Martinoia E, Hwang I, Lee Y (2007) Phosphatidylinositol 4,5-bisphosphate is important for stomatal opening. Plant J 52: 803–816 [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Marten H, Hedrich R, Roelfsema MR (2007) Blue light inhibits guard cell plasma membrane anion channels in a phototropin-dependent manner. Plant J 50: 29–39 [DOI] [PubMed] [Google Scholar]
- Marten I, Deeken R, Hedrich R, Roelfsema MR (2010) Light-induced modification of plant plasma membrane ion transport. Plant Biol (Stuttg) (Suppl) 12: 64–79 [DOI] [PubMed] [Google Scholar]
- McLachlan DH, Lan J, Geilfus CM, Dodd AN, Larson T, Baker A, Hõrak H, Kollist H, He Z, Graham I, Mickelbart MV, Hetherington AM (2016) The breakdown of stored triacylglycerols is required during light-induced stomatal opening. Curr Biol 26: 707–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, Giraudat J, Leung J (2007) Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet-Parramona C, Wang Y, Hills A, Vialet-Chabrand S, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2016) An optimal frequency in Ca2+ oscillations for stomatal closure is an emergent property of ion transport in guard cells. Plant Physiol 170: 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S (2015) Diverse stomatal signaling and the signal integration mechanism. Annu Rev Plant Biol 66: 369–392 [DOI] [PubMed] [Google Scholar]
- Nagatoshi Y, Mitsuda N, Hayashi M, Inoue S, Okuma E, Kubo A, Murata Y, Seo M, Saji H, Kinoshita T, Ohme-Takagi M (2016) GOLDEN 2-LIKE transcription factors for chloroplast development affect ozone tolerance through the regulation of stomatal movement. Proc Natl Acad Sci USA 113: 4218–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126: 467–475 [DOI] [PubMed] [Google Scholar]
- Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX (2007) Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics 6: 1711–1726 [DOI] [PubMed] [Google Scholar]
- Okumura M, Inoue S, Kuwata K, Kinoshita T (2016) Photosynthesis activates plasma membrane H+-ATPase via sugar accumulation. Plant Physiol 171: 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M, Inoue S, Takahashi K, Ishizaki K, Kohchi T, Kinoshita T (2012) Characterization of the plasma membrane H+-ATPase in the liverwort Marchantia polymorpha. Plant Physiol 159: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Arinaga N, Umezawa T, Katsura S, Nagamachi K, Tanaka H, Ohiraki H, Yamada K, Seo SU, Abo M, et al. (2013) Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 25: 609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Watanabe T, Sugano SS, Ueta R, Ishihara R, Shinozaki K, Osakabe K (2016) Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci Rep 6: 26685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran L-SP (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol 202: 35–49 [DOI] [PubMed] [Google Scholar]
- Palmgren MG. (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52: 817–845 [DOI] [PubMed] [Google Scholar]
- Papanatsiou M, Scuffi D, Blatt MR, García-Mata C (2015) Hydrogen sulfide regulates inward-rectifying K+ channels in conjunction with stomatal closure. Plant Physiol 168: 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen LR, López-Marqués RL, Pedas PR, McDowell SC, Brown E, Kunze R, Harper JF, Pomorski TG, Palmgren M (2015) A phospholipid uptake system in the model plant Arabidopsis thaliana. Nat Commun 6: 7649. [DOI] [PubMed] [Google Scholar]
- Ren H, Gray WM (2015) SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol Plant 8: 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudashevskaya EL, Ye J, Jensen ON, Fuglsang AT, Palmgren MG (2012) Phosphosite mapping of P-type plasma membrane H+-ATPase in homologous and heterologous environments. J Biol Chem 287: 4904–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Lunn JE (2017) Transitory starch metabolism in guard cells: unique features for a unique function. Plant Physiol 174: 539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sato Y, Fukao Y, Fujiwara M, Umezawa T, Shinozaki K, Hibi T, Taniguchi M, Miyake H, Goto DB, Uozumi N (2009) Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem J 424: 439–448 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Hirt H, Meskiene I (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci 9: 236–243 [DOI] [PubMed] [Google Scholar]
- Scuffi D, Álvarez C, Laspina N, Gotor C, Lamattina L, García-Mata C (2014) Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166: 2065–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J, Lee HY, Choi H, Choi Y, Lee Y, Kim YW, Ryu SB, Lee Y (2008) Phospholipase A2β mediates light-induced stomatal opening in Arabidopsis. J Exp Bot 59: 3587–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Iino M, Zeiger E (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319: 324–326 [Google Scholar]
- Spartz AK, Lor VS, Ren H, Olszewski NE, Miller ND, Wu G, Spalding EP, Gray WM (2017) Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in Tomato confers auxin-independent hypocotyl elongation. Plant Physiol 173: 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spartz AK, Ren H, Park MY, Grandt KN, Lee SH, Murphy AS, Sussman MR, Overvoorde PJ, Gray WM (2014) SAUR inhibition of PP2C-D phosphatases activates plasma membrane H+-ATPases to promote cell expansion in Arabidopsis. Plant Cell 26: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu N, Takami T, Ebisu Y, Watanabe H, Iiboshi C, Doi M, Shimazaki K (2014) Guard cell chloroplasts are essential for blue light-dependent stomatal opening in Arabidopsis. PLoS One 9: e108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Wang J, Gao Z, Dong J, He H, Terzaghi W, Wei N, Deng XW, Chen H (2016) Arabidopsis SAURs are critical for differential light regulation of the development of various organs. Proc Natl Acad Sci USA 113: 6071–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J-U, Sieben C, Hartel A, Eisenach C, Thiel G, Blatt MR (2007) Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr Biol 17: 1396–1402 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hayashi K, Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159: 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kinoshita T (2016) The regulation of plant cell expansion: auxin-induced turgor-driven cell elongation, In Rose R.J., ed, Molecular Cell Biology of the Growth and Differentiation of Plant Cells. CRC Press, Boca Raton, FL, pp 156–173 [Google Scholar]
- Takahashi Y, Ebisu Y, Kinoshita T, Doi M, Okuma E, Murata Y, Shimazaki K (2013) bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci Signal 6: ra48. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kinoshita T, Matsumoto M, Shimazaki K (2016) Inhibition of the Arabidopsis bHLH transcription factor by monomerization through abscisic acid-induced phosphorylation. Plant J 87: 559–567 [DOI] [PubMed] [Google Scholar]
- Takemiya A, Kinoshita T, Asanuma M, Shimazaki K (2006) Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba. Proc Natl Acad Sci USA 103: 13549–13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Shimazaki K (2016) Arabidopsis phot1 and phot2 phosphorylate BLUS1 kinase with different efficiencies in stomatal opening. J Plant Res 129: 167–174 [DOI] [PubMed] [Google Scholar]
- Takemiya A, Shimazaki K (2010) Phosphatidic acid inhibits blue light-induced stomatal opening via inhibition of protein phosphatase 1 [corrected]. Plant Physiol 153: 1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya A, Sugiyama N, Fujimoto H, Tsutsumi T, Yamauchi S, Hiyama A, Tada Y, Christie JM, Shimazaki K (2013a) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat Commun 4: 2094. [DOI] [PubMed] [Google Scholar]
- Takemiya A, Yamauchi S, Yano T, Ariyoshi C, Shimazaki K (2013b) Identification of a regulatory subunit of protein phosphatase 1 which mediates blue light signaling for stomatal opening. Plant Cell Physiol 54: 24–35 [DOI] [PubMed] [Google Scholar]
- Toda Y, Wang Y, Takahashi A, Kawai Y, Tada Y, Yamaji N, Feng Ma J, Ashikari M, Kinoshita T (2016) Oryza sativa H+-ATPase (OSA) is involved in the regulation of dumbbell-shaped guard cells of rice. Plant Cell Physiol 57: 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, Schroeder JI, Kangasjärvi J (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Hitomi K, Nishimura N, Hitomi C, Adams SR, Getzoff ED, Schroeder JI (2014) FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3: e01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SW, Li Y, Zhang XL, Yang HQ, Han XF, Liu ZH, Shang ZL, Asano T, Yoshioka Y, Zhang CG, Chen YL (2014) Lacking chloroplasts in guard cells of crumpled leaf attenuates stomatal opening: both guard cell chloroplasts and mesophyll contribute to guard cell ATP levels. Plant Cell Environ 37: 2201–2210 [DOI] [PubMed] [Google Scholar]
- Wang Y, Noguchi K, Ono N, Inoue S, Terashima I, Kinoshita T (2014a) Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc Natl Acad Sci USA 111: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shimazaki K, Kinoshita T (2014b) Multiple roles of the plasma membrane H+-ATPase and its regulation. Enzymes 35: 191–211 [DOI] [PubMed] [Google Scholar]
- Yamauchi S, Takemiya A, Sakamoto T, Kurata T, Tsutsumi T, Kinoshita T, Shimazaki K (2016) The plasma membrane H+-ATPase AHA1 plays a major role in stomatal opening in response to blue light. Plant Physiol 171: 2731–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW, Guo Y (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 22: 1313–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Ren HM, Tan YQ, Qi GN, Yao FY, Wu GL, Yang LW, Hussain J, Sun SJ, Wang YF (2016) S-type anion channels SLAC1 and SLAH3 function as essential negative regulators of inward K+ channels and stomatal opening in Arabidopsis. Plant Cell 28: 949–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Takemiya A, Kinoshita T, Shimazaki K (2007) Nitric oxide inhibits blue light-specific stomatal opening via abscisic acid signaling pathways in Vicia guard cells. Plant Cell Physiol 48: 715–723 [DOI] [PubMed] [Google Scholar]