Abstract
Fossil plant stomata reveal insights into the evolution of atmospheric composition, trends in plant genome size, and the biology of the living plant prior to fossilization.
The presence of stomata is a diagnostic trait of all living and extinct land plants with the exception of liverworts. They are preserved widely in the fossil record from anatomically pristine stomatal complexes on permineralized and charcoalified stems of the earliest land plants dating back >400 million years to isolated guard cell pairs in quaternary aged palynological samples. Detailed study of fossil stomatal complexes has been used to track the evolution of genome size and to reconstruct atmospheric composition, to circumscribe new species to science, and to bring ancient landscapes to life by providing both habitat information and insights on fossil plant ecophysiological function and life form. This review explores how fossil stomata can be used to advance our understanding of plant, environment, and atmospheric evolution over the Phanerozoic. We compare the utility of qualitative (e.g. presence/absence of stomatal crypts) versus quantitative stomatal traits (e.g. amphistomaty ratio) in paleoecological reconstructions. A case study on Triassic-Jurassic Ginkgoales is provided to highlight the methodological difficulty of teasing apart the effect of genome size, ploidy, and environment on guard cell size evolution across mass extinction boundaries. We critique both empirical and mechanistic stomatal-based models for paleoCO2 reconstruction and highlight some key limitations and advantages of both approaches. Finally, we question if different stomatal developmental pathways have ecophysiological consequence for leaf gas exchange and ultimately the application of different stomatal-based CO2 proxy methods. We conclude that most studies currently only capture a fraction of the potential invaluable information that can be gleaned from fossilized stomata and highlight future approaches to their study that better integrate across the disciplinary boundaries of paleobotany, developmental biology, paleoecology, and plant physiology.
The fossil record of land plants (embryophytes) dates back unequivocally to the Middle Ordovician (∼460 million years ago [mya]). This is supported by the presence of spore tetrads contained within an enveloping sporangium (Wellman et al., 2003). Since Wellman’s discovery, the fossil spore record has revealed older and older spores of various morphologies (naked, enveloped) and configurations (singular, paired, etc.) that may eventually push back even further the accepted date of the oldest land plant (Wellman and Strother, 2015). This early phase in land plant evolution is complex to interpret, however, since no stomata have been discovered so far on the earliest fossilized land plants, suggesting perhaps that they may have been absent, as is the case for the early land plants’ algal predecessors. As soon as sheets of fossilized cuticle with true stomata started to appear in Siluran aged (443–419 mya) sediment samples, our ability to taxonomically separate charophyacean algae from land plants based on fragmentary fossil evidence improved greatly because the presence of stomata is the defining anatomical trait of all living and extinct land plant sporophytes with the exception of liverworts. Therefore, it is unsurprising that the use of fossilized stomata for taxonomic purposes and to elucidate the phylogeny of land plants as revealed by the fossil record has a long history in paleobotany.
Stomatal traits that are considered of utility for fossil plant taxonomy and systematics are numerous, including stomatal presence or absence, size, geometry and orientation, and association with subsidiary cells (Table I), whether they are sunken, raised, or flush with epidermal cells or plugged with wax, are kidney or dumbbell shaped, are overarched by papillate subsidiary/epidermal cells, or are completely encircled by a ring of fused subsidiaries (Cleal and Zodrow, 1989; Hill and Pole, 1992; Carpenter and Jordan, 1997; Denk and Velitzelos, 2002; Krings et al., 2003; Carpenter et al., 2005; Kerp et al., 2006; Cleal, 2008; Pole, 2008; Hernandez-Castillo et al., 2009; Pott and McLoughlin, 2009; Bomfleur and Kerp, 2010; Cleal and Shute, 2012). Guard cell lignification (Lacourse et al., 2016), striations (Barclay et al., 2007), and the presence of two size classes of stomata, including giant stomata (Fišer Pečnikar et al., 2012), have also been examined for taxonomic purposes. The problem of using fossil stomatal traits in taxonomy and systematics is that some traits “show some genetically uncontrolled variability” (Baranova, 1992) with specific traits showing greater variability than others (Barclay et al., 2007; Cleal and Shute, 2012; Jordan et al., 2014; Lacourse et al., 2016).
Table I. Stomatal complex types recognized from mature stomata and used in taxonomy and systematics from Cleal and Shute (2012) and Rudall et al. (2013).
See the Cuticle Database (http://cuticledb.eesi.psu.edu/) and Barclay et al. (2007) for illustrations of mature stomatal types.
| Descriptive Mature Stomatal Complex Types |
Examples of Extant and Extinct Taxa |
|---|---|
| Stomata Absent | Liverworts |
| Anomocytic | Hornworts, mosses, rhyniophytes, lycophytes |
| Lacking subsidiary cells | Trimerophytes, Psilotaleae, Ophioglossaceae, Polipodieae, progymnosperms, paleozoic pteridosperms, Medullosales, Ginkgoales, Peltaspermales, Cycadales, Cordaitales, Coniferales, Gnetales, Glossopteridales, Caytoniales, angiosperms |
| Stephanocytic | Marattiales, Corystospermales (incomplete ring) |
| An encircling ring of subsidiary cells | Ginkgoales, ?Cycadales, ?Cheirolepidaceae, ?Peltaspermales, Glossopteridales, Caytoniales, Medullosales |
| Paracytic | Equisetales, Corystospermales, Gnetales, Bennettitales, angiosperms, Medullosales |
| One or more pairs of lateral subsidiary cells orientated parallel with guard cells | |
| Anisocytic | Angiosperms (e.g. Peperoma), Marattiales |
| Three subsidiary cells surrounding guard cells, one of which is smaller than the other two |
Over the past three decades, since publication of Woodward’s (Woodward, 1987) seminal article on the inverse relationship between stomatal density and atmospheric CO2 concentration, the “uncontrolled variability” in stomatal traits such as stomatal density (SD) and stomatal index (SI) has been seized on by paleobiologists as an opportunity to extract meaningful paleo-climatic and paleoecological information from fossil plant stomata (McElwain et al., 2005; Roth-Nebelsick, 2005; Wagner et al., 2005; Kürschner et al., 2008; Lammertsma et al., 2011; Steinthorsdottir et al., 2011b; Franks et al., 2014; Maxbauer et al., 2014; Bai et al., 2015; Montañez et al., 2016; Steinthorsdottir et al., 2016a, 2016b). This has led to a subtle tension in the field of paleobotany, where at one extreme some studies have focused almost exclusively on the taxonomic and systematic utility of stomata with insufficient consideration of environmentally driven variability, while at the other extreme some reconstructions of paleoatmospheric composition have been undertaken using fossil stomatal traits without due consideration for taxonomic determination of the fossils used. The aim of this article is to briefly review both long-established and novel uses of fossil stomata, including their use, and (1) to infer the paleoecology of ancient landscapes from fossil plant assemblages, (2) to elucidate the genomic history of embryophytes, (3) to reconstruct paleoatmospheric trends in carbon dioxide concentration (pCO2) over the past ∼400 mya, and (4) to gain insights into plant developmental biology, in particular that of the leaf. We provide a case study demonstrating how genome size and ploidy can be estimated from fossil guard cell size of Triassic-Jurassic Ginkgoales. Furthermore, we highlight two paleo-pCO2 cross-calibration examples for the Late Pennsylvanian-Early Permian (311–296 mya) and Late Triassic-Early Jurassic (∼209–199 mya), where pCO2 estimates are derived by applying different stomatal models and calibration approaches to the same stomatal datasets. A secondary, but equally important, aim of this review is to highlight potential future avenues for synthesis of these myriad uses of fossil stomata in order to maximize the genotypic and phenotypic information that can be gathered from their frequency, size, geometry, distribution, developmental pathway, and association with neighboring and/or subsidiary cells.
PALEOECOLOGY AND FOSSIL STOMATA
Paleobotanists as a community use all tools available to reconstruct the climate and ecology of ancient landscapes from fossil plant assemblages. Briefly, these tools can include sedimentological information (Parrish et al., 1982); assessment of fossil taxa co-occurrence (Bowman et al., 2014); behavioral information such as leaf herbivory damage (Carvalho et al., 2014); census information on paleodiversity, relative abundance, density, and fossil plant preservation type (Cúneo et al., 2003; Wilf et al., 2005; Mander et al., 2013; Willis and McElwain, 2014; Falcon-Lang, 2015), factors that could bias fossilization potential (Mander et al., 2012); and of course organ, tissue (Royer et al., 2007; Belcher et al., 2010), and cellular level traits (Haworth and McElwain, 2008) that provide unique ecological information.
The extraction of precise paleoecological information from fossil plant stomata and their associated neighboring and subsidiary cells is not however without its pitfalls. Some of the most enduring traits of the stomatal complex considered to have paleoecological value include the presence of papillae on neighboring or subsidiary cells that overarch the stomatal aperture, sunken guard cells, and the presence of deep stomatal crypts. Traditionally, all of these modifications to the stomatal complex have been interpreted functionally as anatomical adaptations to aridity, since they reduce stomatal conductance and leaf level transpiration (Hill, 1998; Krings et al., 2003). Haworth and McElwain (2008) directly challenged this narrow interpretation, arguing that many of these morphological traits also occur in very humid and wet environments, with a function to repel liquid water from the leaf surface, as well as in self-cleaning of atmospheric particulates, spores and other irritants (Barthlott et al., 2017). They cautioned that an antitranspirant function of papillae and sunken stomata must be interpreted in conjunction with other paleoecologically significant information. A detailed stomatal trait-climate study on Proteaceae further confirmed that while deep stomatal crypts are systematically associated with climatic aridity, the presence of sunken stomata and papillae on cells of the stomatal complex are not uniquely associated with ecological aridity (Jordan et al., 2008). The living fossil, Sciadopitys (Japanese umbrella-pine), for example, possesses a deep stomatal crypt yet it occurs in modern day wet warm temperate forest.
An additional stomatal trait that is sometimes used in paleoecological studies is stomatal distribution (Bomfleur and Kerp, 2010; Cleal and Shute, 2012). Amphistomaty, where stomata occur on both leaf surfaces, is often associated with high irradiance (Fitter and Peat, 1994), high elevation (Woodward, 1986), as well as open aquatic or open desert environments (Mott et al., 1982). It facilitates increased stomatal and mesophyll conductance and is usually associated with higher phyotosynthetic rates (Mott et al., 1982). In Proteaceae, amphistomaty is strongly associated with open vegetation (Jordan et al., 2014), leading to the suggestion that amphistomaty may be a good proxy for an open habitat. However, Muir (2015) argues that the adaptive significance of this relatively rare stomatal distribution (<10% of modern global flora are amphistomatic) relates most strongly to life history traits such as relative growth rate. Annuals, biennials, and perennials were shown to have a much greater frequency of species that were amphistomatous than shrubs and trees across 599 species and 94 families (Muir, 2015).
Therefore, it appears that simple binary data regarding the presence or absence of particular stomatal traits, including crypts, sunken stomata, papillate overarching subsidiary cells, etc., cannot be used by themselves to interpret paleoecology across all phylogenetic groups but may have great utility when applied in phylogenetically restricted studies, for example, in Proteaceae (Jordan et al., 2014). However, other aspects of stomatal development, such as amphistomaty or specifically the ratio of abaxial to adaxial stomatal density (sensu; Muir, 2015), look more promising for broader application to the fossil record to interpret paleoecology, including habit and habitat. Herbaceous taxa are believed to be very poorly represented in the fossil record yet many fossil plant assemblages over the past 400 million years are characterized by an unusually high proportion of amphistomatous taxa compared with the modern flora (e.g. Dicroidium dominated floras of the Late Triassic; Bomfleur and Kerp, 2010). Were paleo-amphistomatous taxa fast growing herbaceous plants or woody shrubs and trees from open habitats? Extant herbaceous species with amphistomatous leaves are typically dorsiventral with differentiated mesophyll tissue layers, whereas extant sclerophyllous species of arid environments with amphistomatous leaves are typically isobilateral with undifferentiated mesophyll. Permineralized or charcoalified fossil leaf preservation will therefore likely be required to examine mesophyll tissue differentiation in addition to stomatal distribution in order to assess if fossil taxa were dorsiventral or isobilateral and correctly interpret their paleoecology.
Furthermore, quantitative stomatal traits such as anatomical gmax can be used in conjunction with other anatomical data such as vein density and chemistry (e.g. carbon isotopic composition) to infer important ecophysiological characteristics such as transpiration rate (Steinthorsdottir et al., 2012), assimilation rate (Brodribb et al., 2007), and water use efficiency from fossilized leaves and cuticle fragments (Franks and Beerling, 2009a; Assouline and Or, 2013; Wilson et al., 2015; McElwain et al., 2016b; Montañez et al., 2016). This opens up the possibility of using stomata to make quantitative paleoecological comparisons between cohabiting fossil taxa (Montañez et al., 2016), which may be more fruitful than grouping all fossil taxa to broadly classify the climatic preference of an entire assemblage or collection locality. The former approach was used to provide a better mechanistic explanation underlying vegetation dynamics in response to glacial-interglacial cycles of the great Carboniferous ice age (between ∼312 and 299 mya) and across the Triassic-Jurassic mass extinction boundary (∼201 mya; Wotzlaw et al., 2014; Steinthorsdottir et al., 2012). In both cases, gmax calculations based on the density and size of stomata in different fossil taxa were used to model paleo-transpiration rates and compare water use efficiencies of the taxa that suffered local extinction (giant lycopsid in the Late Pennsylvanian and Bennettittales in the latest Triassic) to those that survived and subsequently proliferated (Pecopteris and Ginkgo in the Late Pennsylvanian and Early Jurassic, respectively; Steinthorsdottir et al., 2012; Montañez et al., 2016).
Where the phylogeny of the fossil taxon under study is well known and detailed trait-environment relationships have been established, it may also be possible to characterize ecological habit using stomatal traits. For example, guard cell length appears to be tightly correlated with habit in Proteaceae with open vegetation displaying significantly longer guard cells than forested (Jordan et al., 2014).
DETERMINING GENOME SIZE AND PLOIDY FROM FOSSIL STOMATA
In rare and exceptionally fossilized specimens, genome size has been estimated from direct morphometric analysis of intact nuclei preserved within permineralized cells (Bomfleur et al., 2014). Scaling relationships between nuclear envelope size and DNA content then allowed estimation of genome size (Bomfleur et al., 2014) from which ploidy can be interpreted. Where such remarkable preservation is not available, however, alternative proxies for genome size are required. Two such proxies that have received increasing interest include pollen/spore size (Kürschner et al., 2013) and guard cell size (Wagner et al., 2000; Beaulieu et al., 2008; Lomax et al., 2009; Brodribb et al., 2013; Lomax et al., 2014), as both cell types have relatively conserved dimensions and provide an approximation of nuclear content and hence genome size (for review, see Lomax et al., 2014). Genome size is strongly positively correlated (r2 = 0.59) with guard cell size across 101 angiosperm species (Beaulieu et al., 2008; Lomax et al., 2014; Fig. 1A), and an increase in ploidy is associated with an increase in guard cell size in the gymnosperm species Ginkgo biloba (Šmarda et al., 2016). The fossil record of stomata is long-ranging, dating back almost to the earliest embryophyte, and they are preserved in many styles (permineralized, charcoalified, and dispersed cuticle) that enable approximation of guard cell dimensions. Stomata are therefore considered to offer a unique archive with which to study genome size evolution in plants (Lomax et al., 2009; Franks et al., 2012). However, the genome size-guard cell size relationship is by no means universal, as exceptions have been identified such as in the Proteaceae (Jordan et al., 2015) where large changes in guard cell size are not accompanied by similar magnitude changes in their genome.
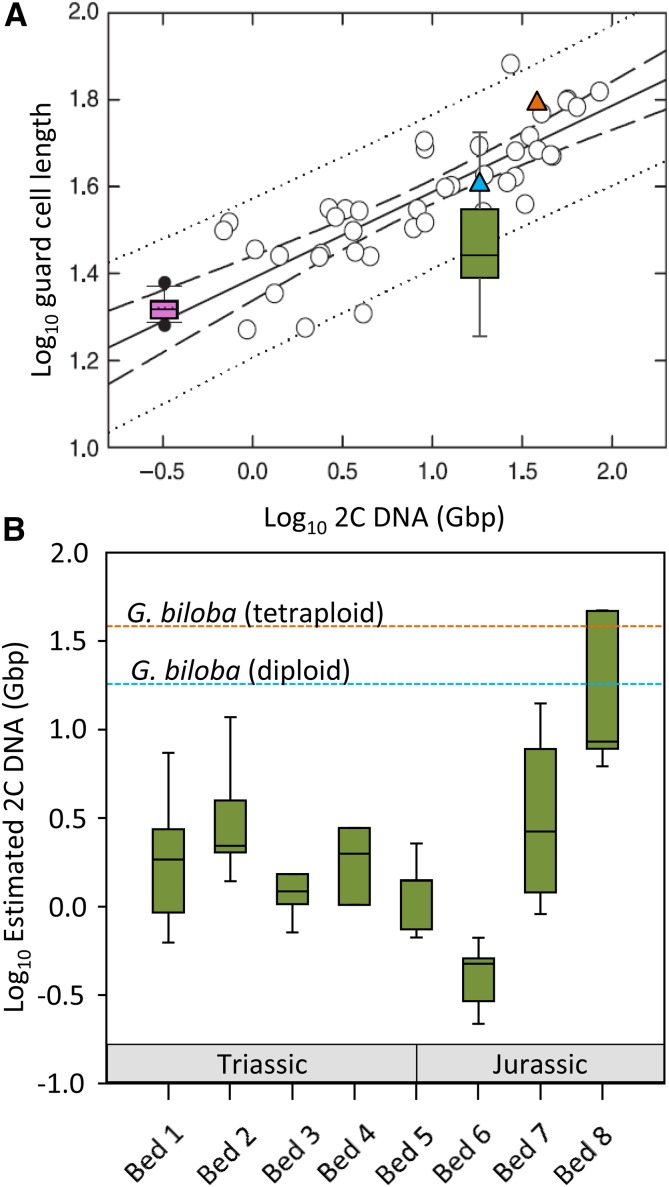
Figure 1.
Plots illustrating two possible scenarios for the relative roles of environment (A) versus genome evolution (B) on gcl of Triassic and Jurassic Ginkgoales. A, “Environment-only scenario” where the range of variability in gcl observed in Late Triassic (T) and Early Jurassic (J) fossil Ginkgoales (green box) from Steinthorsdottir et al. (2012) is assumed to be driven only by environmental change associated with the T-J interval including a CO2 exposure range of 500 to >2,500 ppmV. In this scenario, all fossil Ginkgoales samples are fixed as diploid with the same 2C DNA content as extant G. biloba. These data are then superimposed on a plot illustrating the relationship between guard cell length (log10) and the 2C DNA content (log10) of diploid angiosperms (open circles) from the modern herb data set of Beaulieu et al. (2008). Also superimposed on this data set are (1) the range of phenotypic plasticity observed in Arabidopsis gcl (pink box) when exposed to a range of environmental stressors (from Lomax et al., 2009) and (2) the relationship between gcl and 2C DNA content observed in modern diploid (blue triangle = 18.4 Gbp) and tetraploid (yellow triangle = 37.4 Gbp) G. biloba from Šmarda et al. (2016). B, “Genome-only scenario” where changes in gcl observed in Late Triassic and early Jurassic fossil Ginkgoales from Steinthorsdottir et al. (2012) are assumed to reflect genome size evolution only. The plot illustrates variability in estimated genome size of fossil Ginkgoales over an ∼8 million year time span of T-J strata from Astartekløft East Greenland (for palynostratigraphy, see Mander et al., 2013). Genome size was estimated from fossil Ginkgoales gcl (Baeira, Ginkgo, and Sphenobaiera; see Supplemental Table S1) using the linear regression in A from Beaulieu et al. (2008) (y = 0.1987x + 1.3894). Bed number refers to the sequential stratigraphic position of fossil plant assemblages in the Astartekløft section (see McElwain et al., 2007).
As we have seen in previous sections of this review, stomatal size (guard cell size) is also influenced by the environment. In the case of fossils, is it possible to tease apart the effect of paleoenvironment and paleoecology from genome size with conviction? Lomax et al. (2009) addressed this issue for the extant model plant Arabidopsis (Arabidopsis thaliana) and demonstrated a very narrow range of environmental driven variability in guard cell length following exposure to a range of abiotic stressors including high UV-B radiation, super-elevated CO2 (2,000 ppmV), pathogen attack, and water deficit. Since then, it has been suggested that a change in genome size offers a means of changing cell size in plants to optimize gas exchange (Franks et al., 2012; Brodribb et al., 2013) but that a substantial amount of environmental driven change in guard cell size can occur over evolutionary time after a genome duplication event (Jordan et al., 2015). Put simply, it appears that the size of fossil stomata provide both valuable genotypic (e.g. genome size and potentially ploidy) and phenotypic data (e.g. maximum pore area for gas exchange), the latter of which can be determined by genome size and/or environment. As argued by Jordan et al. (2015), it seems that changes in cell size such as guard cells are adaptive and that change in genome size is one of several ways in which changes in cell size can occur. However, there is not always a simple relationship between genome size and ploidy because increased genome size can be caused by amplification of repetitive DNA sequences without whole-genome duplication (Lomax et al., 2014). Careful studies assessing guard cell size variability, together with independent paleoecological and paleoclimate data, are therefore required to assess the relative contribution of each driver.
As a demonstration of the complexity of interpreting changes in guard cell size in fossils, we have reanalyzed an extensive Ginkgoales stomatal size data set from Steinthorsdottir et al. (2012) that spans the Triassic-Jurassic transition ∼201.36 ± 0.17 mya (Wotzlaw et al., 2014), marked by transient global warming and the end-Triassic mass extinction event. The data set comprises mean stomatal pore length measurements (n = ∼50 per leaf sample) from 47 discrete fossil leaf samples derived from three ginkgoalean genera: Ginkgo, Baiera, and Sphenobaiera (Supplemental Table S1). Guard cell lengths (gcl) for all samples were estimated from pore length (pl) measurements by assuming conservatively that gcl = 1.5(pl). Variability in fossil guard cell length over evolutionary time (an estimated ∼8 million years) was assessed simplistically under two extreme scenarios; the first “environment only” assumes stasis in nuclear genome size of Triassic and Jurassic fossil ginkgoalean taxa, where observed variability in guard cell length is assumed to be driven exclusively by the extreme environmental change (McElwain et al., 1999; van de Schootbrugge et al., 2007; Richoz et al., 2012; Bacon et al., 2013) associated with the Triassic-Jurassic mass extinction event (Fig. 1A). Here, the fossil genome size is assumed to be equivalent to that of extant diploid G. biloba (2C DNA = 18.4 Gbp; Šmarda et al., 2016; Fig. 1A). Under the second scenario, “genome only,” it is assumed that nuclear genome size is the predominant control on guard cell length across the Triassic-Jurassic mass extinction interval with no influence from environmental factors (Fig. 1B). It is of course more likely that both environment and genome size influenced the evolution of guard cell size and that our analysis masks likely genome size differences that occur between the different Ginkgoales genera. However, the demonstration is useful as a thought exercise as it highlights the potential impact of the two end-member scenarios. For instance if the “environment only” scenario is correct, our results suggest that, while variability in guard cell length exposed to abiotic stressors may be very constrained in some taxa and under short-term modern experimental exposure (e.g. Arabidopsis gcl data set of Lomax et al., 2009), the range of environmentally driven variability in other taxa is high given sufficient time for evolutionary adaptation (e.g. Ginkgoales gcl data set of Steinthorsdottir et al., 2012; Fig. 1A).
The “genome only” scenario is particularly interesting because the trends in estimated 2C DNA content hint at the presence of possible tetraploid Ginkgoales among individual samples of the fossil taxon Sphenobaiera spectabilis (Nathorst) florin from Early Jurassic aged fossil plant bed 8 from Astartekløft, East Greenland. Two samples of S. spectabilis are identified that have an estimated 2C DNA amount of ∼47.1 Gbp (FMNH47853) and 46.9 Gbp (FMNH 47863), respectively (Supplemental Table S1), which both exceed the known 2C DNA amounts in modern tetraploid G. biloba (37.4 Gbp; Šmarda et al., 2016). If the presence of tetraploids within the genus Sphenobaiera can be independently verified, it would add support to the suggestion by Fawcett et al. (2009) that mass extinction intervals in Earth history trigger polyploidization (whole-genome duplication) events or expose vacant niches into which existing polyploid taxa can diversify. It would also provide considerable support to long-standing, but mostly untested, hypotheses that a high prevalence of polyploidization events in land plants compared with animals is a key mechanism for generic and family level resilience and survivorship of plants at mass extinction boundaries (Willis and McElwain, 2014). High variability in the size and morphology of conifer pollen tetrads collected from Triassic-Jurassic aged sediments have been interpreted as evidence of unreduced gametes, indicating polyploidization in the extinct conifer Cheirolepidadeae (Kürschner et al., 2013). A similar but higher resolution study on size variability in fossil Ginkgoales pollen across the Triassic-Jurassic is now needed to test the hypothesis arising from fossil guard cell length data (Fig. 1B) that S. spectabilis may be represented by both diploid and tetraploid individuals within the Early Jurassic forests of East Greenland. Alternatively, increased 2C DNA amount may just reflect increased genome size in a diploid due to amplification of repetitive DNA sequences (Lomax et al., 2014).
RECONSTRUCTING PALEOATMOSPHERIC CO2 USING FOSSIL STOMATA
Assumptions and Limitations
Fossil stomata have been used extensively to reconstruct atmospheric composition through Earth history, in particular atmospheric CO2 concentration (pCO2). Paleo-CO2 reconstruction methods that use stomata as proxies include both empirical (McElwain and Chaloner, 1996; Wagner et al., 1996; Kürschner et al., 2008; Barclay et al., 2010; Maxbauer et al., 2014) and mechanistic (Wynn, 2003; Konrad et al., 2008, 2017; Franks et al., 2014) approaches. All approaches operate on the same underlying assumption—that the density and/or size of stomata are the primary control of leaf level gas exchange in vascular plants that is optimized to balance CO2 uptake for photosynthesis against water loss through transpiration (Woodward, 1987; Katul et al., 2010; Manzoni et al., 2011). All empirical proxy CO2 methods are also underpinned by the assumption of evolutionary conservatism. For example, CO2 proxies that use an inverse relationship between stomatal density (or index) and atmospheric CO2 make the implicit assumption that the slope and sign of the SD-CO2 relationship has not evolved significantly over geological time. Jordan (2011) suggested that high species variability in the magnitude and sign of stomatal density response to CO2 between different species weakens the reliability of stomatal-based CO2 proxies because it breaks the key assumption of evolutionary conservatism. We agree that this is the case for a number of families that have been investigated (Kelly and Beerling, 1995; Kürschner et al., 1997; Haworth et al., 2010); however, other families show strong conservatism in the SD-CO2 relationship with species belonging to the same genera, and even at the family or order level, clustering together with similar SD/SI values, as well as displaying similar response directions and magnitudes to changes in pCO2 (McElwain et al., 2002; Barclay et al., 2010; Haworth et al., 2011; Steinthorsdottir et al., 2011a; Steinthorsdottir et al., 2011b; Steinthorsdottir et al., 2016b). This is particularly important when operating in the pre-quaternary fossil record, which does not typically offer fossil plants that are conspecific with modern plants. The key to the selection and application of the most effective and accurate paleo-pCO2 proxy is thus better characterization of how different taxa, both extant and extinct, control their gas exchange and whether they do indeed conform to the principles of evolutionary conservatism in the SD (SI)-CO2 response required for empirical based CO2 proxies to be appropriately applied.
Part of the reason why there is such a high apparent variability in the slope and sign of SD-CO2 relationships among different plant species is due to the fact that plants control gas exchange using multiple mechanisms including developmental responses that set the maximum anatomically possible stomatal conductance (gmax) and/or through physiological control of the stomatal aperture (Haworth et al., 2013; Brodribb and McAdam, 2017). gmax is determined by both stomatal density and stomatal pore area and depth (Parlange and Waggoner, 1970; Lawson and Morison, 2004; Franks and Beerling, 2009b), and although taxa use on average 25% of this maximum potential anatomical conductance—referred to as the operational stomatal conductance (gop)—considerable species level differences in gop/gmax are apparent (Dow et al., 2014; Franks et al., 2014; McElwain et al., 2016b). Therefore, it follows that species-specific differences in the direction and magnitude of the SD/SI response to pCO2 can be compensated for by alteration to stomatal pore size and depth (Lammertsma et al., 2011) and/or by physiological control of aperture (in taxa with abscisic acid-mediated regulation of guard cell aperture; see Broddribb and McAdam, 2017), resulting in optimized control of gas exchange (Haworth et al., 2013) and a strong mechanistic underpinning for stomatal-CO2 proxies that will be discussed later in this section.
Empirical approaches use an inverse relationship between SD or SI with pCO2 to infer paleo-pCO2 from SD and SI calculations on fossil leaves (for review, see Royer, 2002; Roth-Nebelsick, 2005; Jordan, 2011; Barclay and Wing, 2016). This type of proxy is simple to apply but limited by the fact that it ideally should only be applied to fossil taxa that are known to control gas exchange by predominantly developmental control of SD/SI, termed morphological responders by Haworth et al. (2013) (e.g. G. biloba), rather than via active hormone-mediated physiological control of stomatal aperture (Brodribb and McAdam, 2011, 2017), termed physiological responders by Haworth et al. (2013). This simple dichotomy is complicated by the fact that the mode of stomatal response to CO2 may be time-dependent, with short-term CO2 fumigation experiments eliciting only physiological responses that reduce stomatal conductance with little or no significant change in SD (Roth-Nebelsick 2005), whereas long-term herbarium data sets of the same species show strong stomatal developmental changes that result in a reduction in SD in response to increasing CO2 (Barclay and Wing, 2016).
Mechanistic approaches to pCO2 reconstruction use SD plus pore length measurements to compute maximum theoretical stomatal conductance (gmax; Box 1) together with other important factors influencing gas exchange, such as boundary layer and mesophyll conductance. Total estimated conductance from fossils is then used in conjunction with carbon isotopic data (used to estimate Ci) and the Farquhar photosynthesis model to estimate paleo-pCO2 (Wynn, 2003; Konrad et al., 2008, 2017; Franks et al., 2014; McElwain et al., 2016a, 2017; Franks and Royer, 2017; Box 1). These methods take into account both developmental (from gmax measurements) and physiological control (from carbon isotopic composition) of plant gas exchange and therefore have wide application in the fossil record, particularly in relation to extinct taxa, for which the stomatal control of gas exchange is unknown. They represent significant improvements because they are not merely correlational, but provide a mechanistic basis for interpreting changes in SD and stomatal pore length. Furthermore, they do not require an assumption of evolutionary conservatism in the SD-CO2 relationship, which is a prerequisite of empirical approaches. Just like all empirical stomatal-pCO2 models, however, the newly developed mechanistic models (Konrad et al., 2008, 2017; Franks et al., 2014) are limited by a requirement to cross-calibrate with extant nearest living relative (NLR) or nearest living equivalent (NLE) taxa (McElwain et al., 2017). For example, the stomatal ratio approach of McElwain and Chaloner (1996) estimates paleo-pCO2 by comparing fossil SD or SI with that of an NLE using one of two possible calibrations, the carboniferous standardization: pCO2 (paleo) = SDNLE/SDFossil × 600, and the recent standardization, pCO2 (paleo) = SDNLE/SDFossil × pCO2NLE. The Franks et al. (2014) mechanistic model needs to be parameterized with fossil plant net assimilation rate under modern ambient pCO2 (termed A0), a task that is difficult to do without cross-comparison with modern taxa (McElwain et al., 2017). The Konrad optimization models require parameterization with multiple photosynthetic variables taken from NLRs or NLEs.
Stomatal pCO2 Proxies and the Role of Carbonic Anhydrase
A likely mechanism supporting the stomatal proxy (explaining how plants adjust their stomatal densities in response to changes in atmospheric pCO2) has been proposed: Plants use carbonic anhydrase (CA) located principally in the guard cells to detect (sense) pCO2 enveloping their leaves (Hu et al., 2010, 2015; Chater et al., 2015; Engineer et al., 2016) and control initiation of stomatal development via a signal transduction pathway that is modulated by the HIC gene (Gray et al., 2000; Brownlee, 2001). It has further been shown experimentally that transpiration rates of mature leaves correspond with stomatal densities in developing leaves, suggesting a link between the short-term control of the stomatal aperture and the long-term regulation of stomatal development (Lake and Woodward, 2008). To date, however, this has only been demonstrated for angiosperms and other reviews within this special issue (Broddribb and McAdam, 2017) highlight the complexity of evolution of stomatal control in land plants as a whole. CAs are distributed among all three domains of life, being a group of enzymes that catalyze the rapid conversion of CO2 and water to bicarbonate and protons (and back)—a fundamental reaction to all biological processes (Elleuche and Pöggeler, 2010; Cummins et al., 2014), suggesting that the process may be evolutionary highly conserved or ancestral (Frommer, 2010; Chater et al., 2015). Most of the molecular and genetic studies referred to above have involved the model plant Arabidopsis, and although CAs have also been shown to play a similar role in stomatal responses and photosynthesis in, e.g. Nicotiana (Hu et al., 2015), Flaveria (Ludwig, 2011), and even Olea (olive trees; Perez-Martin et al., 2014), much more work needs to be done to understand the relationship between the sensory and signaling mechanisms and the long-term responses of stomatal development to changing environmental conditions (Doheny-Adams et al., 2012). Future work should further illuminate the potential taxon-specific differences in stomatal response to pCO2 and other environmental factors between plant groups (Brodribb and McAdam, 2013; Merilo et al., 2014).
The way mature leaves (early shoots) control stomatal development of younger leaves through long-distance signaling (Lake et al., 2001, 2002) may in part explain early failures to replicate the inverse relationship of stomata to pCO2 in some modern CO2 enrichment experiments (Royer, 2001; Reid et al., 2003; Tricker et al., 2005; Ainsworth and Rogers, 2007) as well as indicate that plants may need to be grown in atmospheric experimental conditions for longer time intervals than has typically been the case, in order to obtain a reliable SD/SI response to elevated pCO2. Experimental studies to date have usually been short-term, limited mostly to a single growing season or less, probably in most cases not providing the proper conditions for stomatal responses to pCO2 to be morphologically expressed. Strongly supporting this assumption is a recent growth chamber experiment, exposing Betula nana to three levels of pCO2 (150, 450 and 800 ppm) over two successive growing seasons, finding that while some adjustment of stomatal parameters took place in the first growing season, amplified adjustment of stomatal properties, such as SD, occurred mostly in the experiments’ second year (Hincke et al., 2016). An additional reason why so few growth chamber enrichment studies have documented a reduction in SD in response to elevated pCO2 may be counting error (Barclay and Wing, 2016). Barclay and Wing’s reanalysis of elevated pCO2 grown G. biloba from growth chamber experiments revealed that 45% of stomata were malformed and likely lacked functionality compared with 35% of ambient chamber grown plants. The malformed stomata represent cells that have arrested at various stages in the developmental pathway to guard cell pair formation and therefore should not be included in a stomatal density or index count as they do not contribute to the leaf’s overall diffusivity.
Insights from Stomatal-pCO2 Model Cross-Comparison Studies
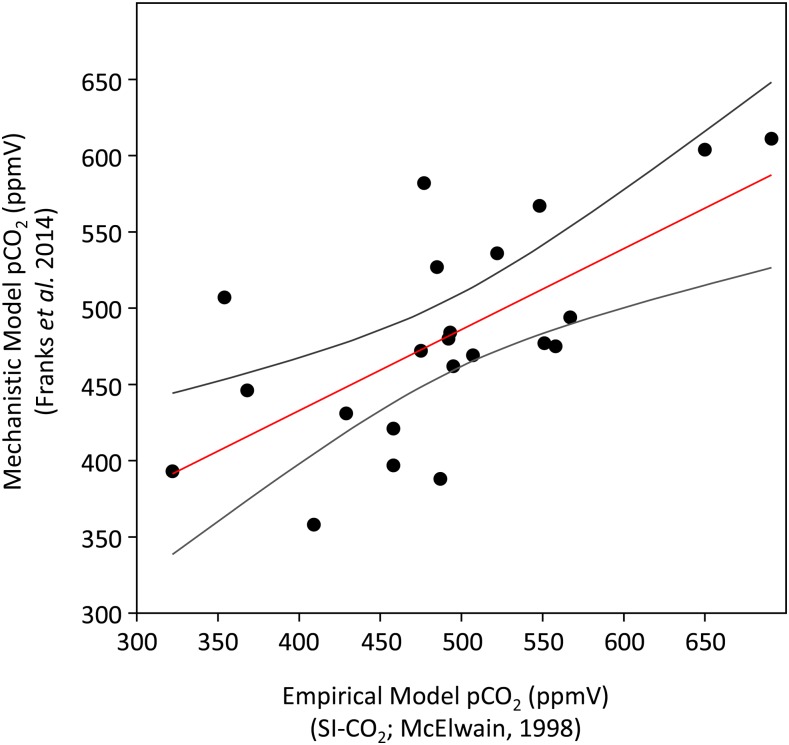
A recent high-resolution paleo-pCO2 reconstruction spanning 16 million years of the Carboniferous and Early Permian (∼312–296 mya) uses three methods: the paleosol-pCO2 proxy (Breecker, 2013; Montañez, 2013), an empirical stomatal-based proxy model (McElwain and Chaloner, 1995, 1996), and a mechanistic stomatal based model (Franks et al., 2014) to derive a consensus pCO2 record (Montañez et al., 2016). Glacial-interglacial fluctuations in atmospheric pCO2 were reconstructed that correlate well with inferred sea level and modeled polar ice volume records (Montañez et al., 2016). Interestingly, both empirical and mechanistic paleo-pCO2 models applied to the same fossil taxa (Medullosa, an extinct gymnosperm) yield very comparable pCO2 estimates throughout the Carboniferous (Fig. 2; r2= 0.4399, P = 0.00076). The generally good correlation between pCO2 estimates based on the different calibration approaches confirms that SD (and SI) is likely the major control of gas exchange in the medullosan seed ferns studied. Furthermore, the good consistency between the two stomatal based methods supports the robustness of the simpler stomatal-ratio method that only requires SD or SI measurements from fossil leaves to estimate pCO2. Unlike application of the Franks et al., (2014) model, it does not require detailed measurements of stomatal pore length and depth, which can be difficult to observe in all fossil preservation types and in fossil taxa with sunken guard cells.
Figure 2.
A cross-comparison of pCO2 estimates spanning glacial-interglacial cycles of the Late Pennsylvanian ice age (303–312 mya) derived from empirical (McElwain, 1998) and mechanistic stomatal models (Franks et al., 2014) applied to stomatal data from the same fossil plant specimens of Neuropteris and Macroneuropteris (two extinct Medullosales seed plant taxa). Stomatal density, pore size, and depth data measured from fossils were used as the primary stomatal inputs into the Franks et al. (2014) model, while SI measurements from the same fossil were compared with SI values of nearest living equivalent taxa in order to apply the McElwain (1998) SI-CO2 stomatal ratio calibration. See Supplemental Table S3 in Montañez et al. (2016) for the complete stomatal data set, pCO2 estimates, and detailed description of the methodology.
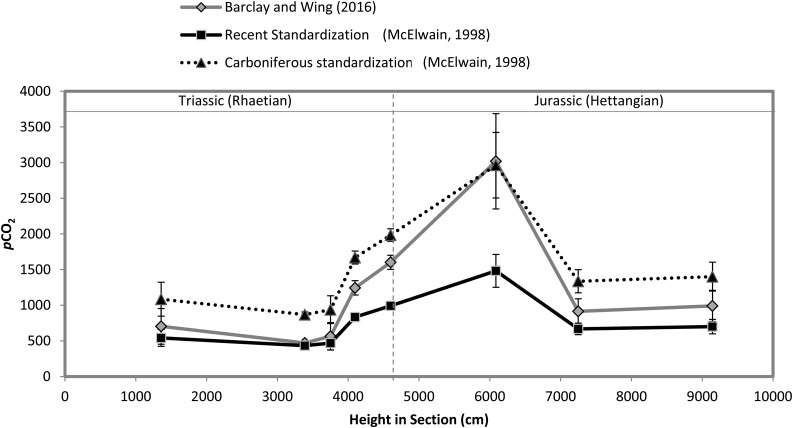
Similar congruence in pCO2 estimates are observed in a cross-comparison of different empirical based calibrations (e.g. McElwain, 1998; Barclay and Wing, 2016) applied to the same fossil Ginkgoales leaves spanning the Triassic-Jurassic mass extinction boundary (Fig. 3). McElwain’s (1998) NLE method yields almost identical pCO2 estimates to Barclay and Wing’s (2016) updated SI-CO2 transfer function based on modern G. biloba applied to extinct Ginkgo, Baiera, and Sphenobaiera (Fig. 2). Could these two case study examples for the Carboniferous (Fig. 2) and Triassic-Jurassic (Fig. 3) suggest a high degree of stasis in the evolutionary relationship between stomatal frequency and pCO2 giving confidence to the assumption of uniformitarianism that underpins the stomatal-CO2 proxy method?
Figure 3.
A comparison of two different empirical pCO2 proxy models applied to stomatal index data (from Steinthorsdottir et al., 2011b) of Triassic and Jurassic fossil Ginkgoales. The Barclay and Wing (2016) model is based on an SI-pCO2 response curve from historical herbarium specimens over a CO2 range from 290 to 429 ppm (CO2 = 9920.9 × (SIfossil)−1.484). The McElwain (1998) calibration is based on a comparison of fossil SI with that of an NLE: Carboniferous standardization: pCO2 (paleo) = SDNLE/SDFossil × 600 and the recent standardization, pCO2 (paleo) = SDNLE/SDFossil × pCO2NLE.
In other cases, however, empirical and mechanistic methods do not fully agree, even when based on the same fossil stomatal dataset. This has been the case for some Cenozoic pCO2 reconstructions, where, for example, estimates for Late Eocene and Early and Late Oligocene pCO2, although broadly comparable quantitatively, do not always agree on pCO2 trends through time (see Roth-Nebelsick et al., 2012; Grein et al., 2013; Steinthorsdottir et al., 2016a). Part of the issue here may be that the optimization model of Konrad et al. (2008, 2017) requires calibrating pCO2 using multiple contemporaneous (overlapping) species to derive a best estimate of pCO2, making it difficult to compare to single-species databases.
Much of the variability in pCO2 estimates derived using various stomatal proxy CO2 models when examined more broadly stem from differences in stratigraphic position and/or resolution, differences in fossil sample number/replication, and due to different methodological approaches to raw data collection and subsequent calibration. In addition, although SD-pCO2 calibration models are continuously being updated with improved data sets and methodological approaches (Ginkgo in Barclay and Wing, 2016), pCO2 estimates based on outdated calibration models remain part of the literature (e.g. Retallack, 2001; Royer et al., 2001). Very few true cross-calibration studies are available to test the performance of different stomatal models and calibrations using the same fossil stomatal datasets. Detailed cross-comparison studies of the different stomatal based methods are now urgently required for a range of phylogenetic groups and a range of geological time intervals to improve our understanding of the weaknesses and strengths inherent in all methods where stomatal data are used in whole or in part to propagate paleo-CO2 estimates. The challenge for the future is to develop a truly taxon independent paleo-pCO2 proxy that is not hampered by phylogenetic differences in the rate, magnitude, and sign of stomatal, isotopic, and photosynthetic responses to atmospheric pCO2. In the meantime, the robustness of paleo-pCO2 estimates based on fossil stomata can be greatly improved by taking a multiproxy approach (Jordan, 2011) that incorporates both mechanistic and empirical models, through careful cuticle preparation and counting protocols (Barclay and Wing, 2016), and by undertaking calibration experiments that span at least two rather than one growth season where empirical approaches are used.
DEVELOPMENTAL INSIGHTS FROM THE FOSSIL STOMATAL COMPLEX
Studies of fossil stomata and their associated subsidiary and neighboring cells can provide insights into the origin and evolution of developmental pathways of the stomatal complex (Barbacka and Bóka, 2000; Barclay et al., 2007; Bomfleur and Kerp, 2010; Rudall et al., 2013). This is more than just an academic exercise as a deeper understanding of the origin, evolution, and diversity of stomatal developmental pathways in both extant and extinct lineages underpins genetic engineering programs for altered stomatal conductance, assimilation rates, and leaf cooling capacities in modern crop plants. This is because traits that are physiologically important to plant carbon acquisition and water loss, such as maximum anatomical stomatal conductance (Franks and Beerling, 2009b; de Boer et al., 2016; McElwain et al., 2016b), are all ultimately controlled by stomatal development. According to Rudall et al. (2013), the perigenous stomatal complex was likely ancestral in land plants, whereby neighboring and subsidiary cells adjacent to guard cells developed from different meristemoids than those from which guard cells developed. An alternative developmental pathway called mesogenous, where guard 2017 and subsidiary cells developed from the same meristemoid, likely developed later (for review, see detail in Rudall et al., 2013). A combination pathway called mesoperigenous, where subsidiary and/or neighboring cells derive from both perigenous and mesogenous routes is also evident in some fossil lineages, for example, in the extinct gymnosperm lineage Bennettitales.
“Fossil fingerprints” may in the future help to identify the stomatal developmental pathways for the many extinct lineages that remain unknown (Rudall et al., 2013). Fingerprints or developmental markers of a mesogenous stomatal developmental pathway include (Rudall et al., 2013) (1) nonrandom stomatal orientation in relation to the leaf long axis, (2) two size classes of stomata present on the same leaf, and (3) variability in epidermal cell size, including alteration of long and short epidermal cells. Identifying these fingerprints in fossils requires excellent anatomical preservation and positional information along a fossil leaf. For example, Bennettitiales show strong morphological disparity in all of these traits moving from the basal to apical portion of the leaf (Fig. 3 in Steinthorsdottir et al., 2011a).
Of interest from the perspective of this review is the likely physiological significance of different developmental pathways, if any. Paleo-pCO2 proxies based on empirical relationships between SD and SI responses to pCO2 are limited by the fact that some taxa are CO2 sensitive in their developmental response (Haworth et al., 2013), while others are not (Haworth et al., 2011). To date, no study has investigated if the stomatal density response to CO2 is modulated in some way by the different developmental pathways of the stomatal complex. The study of Barclay and Wing (2016) illustrated that elevated CO2 increased malformation of the stomatal complex but their focus was on guard cell formation rather than on subsidiary and/or neighboring cells within the stomatal complex. Equally, the debate regarding the evolution of active versus passive stomatal control (Brodribb and McAdam, 2011; 2017) is ongoing, yet no study to our knowledge has attempted to correlate physiological responsiveness of the stomatal pore to abiotic stimuli with the developmental pathway of the stomatal complex. If the magnitude of stomatal aperture opening and closing responses to abiotic stimuli or the sensitivity of SD response to pCO2 could be meaningfully linked to perigenous versus mesogenous stomatal complex development, this would open up the possibility of using fossil developmental fingerprints to categorize the utility of fossil taxa as paleoatmospheric proxies and assess the likely stomatal control of extinct lineages. An example working hypothesis is that mesogenous taxa are highly responsive to CO2 via a stomatal density response because asymmetrical divisions may provide greater flexibility to alter stomatal spacing without altering total cell number.
Another interesting avenue would be to explore the energetic cost of different stomatal complex types in extinct and extant taxa using de Boer et al.’s (2016) proxy measure of the “cost” of stomata (termed fgc), which is calculated as the fraction of epidermal cells allocated to stomata. Perhaps the fgc function should be expanded to include all mesogenous cells within the stomatal complex or all subsidiary cells whatever their developmental pathway if they serve a supporting functional role to guard cells. The wide variability in mature stomatal complex morphology observed in fossils is also of interest (Table I). Why do some taxa have many obvious subsidiary cells, while others have neighboring cells that cannot be distinguished from normal epidermal cells? In part, the disparity may be due to the style of fossil preservation (cuticle versus permineralization etc.) and the type of microscopy used to observe fossil stomata (e.g. bright-field, epifluorescent, scanning electron microscopy). For example, while bright-field microscopy may not always be able to distinguish subtle differences in the morphology of cells adjacent to guard cells from normal epidermal cells, epifluorescence can readily distinguish these subsidiary cells from neighboring cells via differences in their autofluorescence.
Distinct functional roles have been identified in the subsidiary cells of modern plants, including sequestration and isolation of metal ions, such as nickel in the Brassicaceae species Thalaspi montanum var siskiyouense (Heath et al., 1997), providing mechanical support to guard cells (Franks and Farquhar, 2007) and providing a source of water and/or ions to guard cells. It is also hypothesized that the role of subsidiary cells is to balance differences in growth rate between normal epidermal pavement cells and guard cells (Rudall et al., 2013). Perhaps then different subsidiary cell arrangements are related to whole plant relative growth rate? Cleal and Shute (2012) observed a wide variety of different subsidiary cell arrangements in Carboniferous Medullosales (Table I) with an apparent trend in mature stomatal complex types through time from simple anomocytic to stephanocytic and paracytic. Could these differences observed within Medullosales stomatal complex type reflect changes in the relative growth rate of the fronds belonging to different species? It certainly appears as though detailed assessment of fossil stomatal complex developmental pathways (Rudall et al., 2013) together with quantitative paleoecological traits such as the ratio of amphistomaty (SD adaxial surface/SD abaxial surface; Muir, 2015) have the potential to yield important insights on plant habit in the future.
CONCLUSION
What has emerged from this brief review is that fossil stomata are being underutilized as tools in most studies and have the potential to provide integrated insights on paleoecology, taxonomy, paleoatmospheric composition, genome size, and developmental biology (see Outstanding Questions). For best application of empirical based proxies for paleo-pCO2 reconstruction, we recommend the use of multiseason elevated pCO2 experiments and/or multiseason herbarium data sets to generate appropriate transfer functions and calibrations. Mechanistic models for paleo-CO2 reconstructions represent very significant advances in the field. Careful assessment of whether stomata have been aborted or are fully developed and functional is also a key prerequisite of obtaining accurate stomatal density and index counts from fossils and neobotanical specimens. Where possible, the application of multiple paleo-pCO2 proxies to fossil stomatal data sets is also recommended as these will improve the robustness of pCO2 estimates by reporting the consensus pCO2. We suggest that trends in guard cell length data are interpreted from two end member perspectives: an environment-only and a genome-only scenario unless independent data sets allow the relative impact of environment and genome to be assessed with confidence. We recommend moving away from attempts to characterize the entire ecological setting of a fossil assemblage using presence/absence traits associated with stomata. Instead, we advocate the use of quantitative traits that provide comparative estimates of water use efficiency, maximum gas exchange capacity, and leaf level transpiration of co-occurring fossil taxa within a fossil assemblage. Finally, of all the possible stomatal-based traits that can be observed in fossils, it appears that quantitative assessment of stomatal distribution across both leaf surfaces (Muir’s stomatal distribution ratio; Muir, 2015) together with estimated genome size (Beaulieu et al., 2008) will yield meaningful inferences on relative growth rate and plant habit and help to assess hypotheses on the likely growth form of extinct taxa.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Fossil Ginkgoales stomatal data used to estimate genome size (2C DNA amount).
Supplementary Material
Acknowledgments
We thank C. Evans-Fitzgerald and A. Porter (University College Dublin) for providing feedback on the manuscript. We also thank G. Jordan and two anonymous reviewers for feedback and comments that greatly improved the manuscript.
Glossary
- mya
million years ago
- gcl
guard cell length
- SD
stomatal density
- SI
stomatal index
- NLE
nearest living equivalent
- NLR
nearest living relative
- CA
carbonic anhydrase
Footnotes
This work was supported by a Science Foundation Ireland Principal Investigator Award 11/PI/1103, by European Research Council Award ERC-2011-StG 279962 to J.C.M., and by Swedish Research Council Starting Grant (VR NT-7 2016 04905) and the Bolin Centre for Climate Research, Stockholm University, to M.S.
J.C.M. and M.S. cowrote the manuscript.
Articles can be viewed without a subscription.
References
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Assouline S, Or D (2013) Plant water use efficiency over geological time--evolution of leaf stomata configurations affecting plant gas exchange. PLoS One 8: e67757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon KL, Belcher CM, Haworth M, McElwain JC (2013) Increased Atmospheric SO2 Detected from Changes in Leaf Physiognomy across the Triassic-Jurassic Boundary Interval of East Greenland. PLoS One 8: e60614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YJ, Chen LQ, Ranhotra PS, Wang Q, Wang YF, Li CS (2015) Reconstructing atmospheric CO2 during the Plio-Pleistocene transition by fossil Typha. Glob Change Biol 21: 874–881 [DOI] [PubMed] [Google Scholar]
- Baranova M. (1992) Principles of comparative stomatographic studies of flowering plants. Bot Rev 58: 49–99 [Google Scholar]
- Barbacka M, Bóka K (2000) The stomatal ontogeny and structure of the Liassic pteridosperm Sagenopteris (Caytoniales) from Hungary. Int J Plant Sci 161: 149–157 [DOI] [PubMed] [Google Scholar]
- Barclay R, McElwain J, Dilcher D, Sageman B (2007) The cuticle database: developing an interactive tool for taxonomic and paleoenvironmental study of the fossil cuticle record. Cour Forsch-Inst Senckenberg 258: 39–55 [Google Scholar]
- Barclay RS, McElwain JC, Sageman BB (2010) Carbon sequestration activated by a volcanic CO2 pulse during Ocean Anoxic Event 2. Nat Geosci 3: 205–208 [Google Scholar]
- Barclay RS, Wing SL (2016) Improving the Ginkgo CO2 barometer: implications for the early Cenozoic atmosphere. Earth Planet Sci Lett 439: 158–171 [Google Scholar]
- Barthlott W, Mail M, Bhushan B, Koch K (2017) Plant surfaces: structures and functions for biomimetic innovations. Nano-Micro Lett 9: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179: 975–986 [DOI] [PubMed] [Google Scholar]
- Belcher CM, Mander L, Rein G, Jervis FX, Haworth M, Hesselbo SP, Glasspool IJ, McElwain JC (2010) Increased fire activity at the Triassic/Jurassic boundary in Greenland due to climate-driven floral change. Nat Geosci 3: 426–429 [Google Scholar]
- Bomfleur B, Kerp H (2010) Dicroidium diversity in the Upper Triassic of north Victoria Land, East Antarctica. Rev Palaeobot Palynol 160: 67–101 [Google Scholar]
- Bomfleur B, McLoughlin S, Vajda V (2014) Fossilized nuclei and chromosomes reveal 180 million years of genomic stasis in royal ferns. Science 343: 1376–1377 [DOI] [PubMed] [Google Scholar]
- Bowman VC, Francis JE, Askin RA, Riding JB, Swindles GT (2014) Latest Cretaceous–earliest Paleogene vegetation and climate change at the high southern latitudes: palynological evidence from Seymour Island, Antarctic Peninsula. Palaeogeogr Palaeoclimatol Palaeoecol 408: 26–47 [Google Scholar]
- Breecker DO. (2013) Quantifying and understanding the uncertainty of atmospheric CO2 concentrations determined from calcic paleosols. Geochem Geophys Geosyst 14: 3210–3220 [Google Scholar]
- Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144: 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Jordan GJ, Carpenter RJ (2013) Unified changes in cell size permit coordinated leaf evolution. New Phytol 199: 559–570 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2013) Unique responsiveness of angiosperm stomata to elevated CO2 explained by calcium signalling. PLoS One 8: e82057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2017) Evolution of the stomatal regulation of plant water content. Plant Physiol 174: 639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee C. (2001) The long and the short of stomatal density signals. Trends Plant Sci 6: 441–442 [DOI] [PubMed] [Google Scholar]
- Carpenter RJ, Jordan GJ (1997) Early Tertiary macrofossils of Proteaceae from Tasmania. Aust Syst Bot 10: 533–563 [Google Scholar]
- Carpenter RJ, Hill RS, Jordan GJ (2005) Leaf cuticular morphology links Platanaceae and Protaceae. Int J Plant Sci 166: 843–855 [Google Scholar]
- Carvalho MR, Wilf P, Barrios H, Windsor DM, Currano ED, Labandeira CC, Jaramillo CA (2014) Insect leaf-chewing damage tracks herbivore richness in modern and ancient forests. PLoS One 9: e94950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater C, Peng K, Movahedi M, Dunn JA, Walker HJ, Liang Y-K, McLachlan DH, Casson S, Isner JC, Wilson I, et al. (2015) Elevated CO2-induced responses in stomata require ABA and ABA signalling. Curr Biol 25: 2709–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal CJ. (2008) Palaeofloristics of Middle Pennsylvanian medullosaleans in variscan Euramerica. Palaeogeogr Palaeoclimatol Palaeoecol 268: 164–180 [Google Scholar]
- Cleal CJ, Shute CH (2012) The systematic and palaeoecological value of foliage anatomy in Late Palaeozoic medullosalean seed-plants. J Syst Palaeontology 10: 765–800 [Google Scholar]
- Cleal CJ, Zodrow EL (1989) Epidermal structure of some medullosan Neuropteris foliage from the Middle and Upper Carboniferous of Canada and Germany. Palaeontology 32: 837–882 [Google Scholar]
- Cummins EP, Selfridge AC, Sporn PH, Sznajder JI, Taylor CT (2014) Carbon dioxide-sensing in organisms and its implications for human disease. Cell Mol Life Sci 71: 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cúneo NR, Taylor EL, Taylor TN, Krings M (2003) In situ fossil forest from the upper Fremouw Formation (Triassic) of Antarctica: paleoenvironmental setting and paleoclimate analysis. Palaeogeogr Palaeoclimatol Palaeoecol 197: 239–261 [Google Scholar]
- de Boer HJ, Price CA, Wagner-Cremer F, Dekker SC, Franks PJ, Veneklaas EJ (2016) Optimal allocation of leaf epidermal area for gas exchange. New Phytol 210: 1219–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk T, Velitzelos D (2002) First evidence of epidermal structures of Ginkgo from the Mediterranean Tertiary. Rev Palaeobot Palynol 120: 1–15 [Google Scholar]
- Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE (2012) Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos Trans R Soc Lond B Biol Sci 367: 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow GJ, Bergmann DC, Berry JA (2014) An integrated model of stomatal development and leaf physiology. New Phytol 201: 1218–1226 [DOI] [PubMed] [Google Scholar]
- Elleuche S, Pöggeler S (2010) Carbonic anhydrases in fungi. Microbiology 156: 23–29 [DOI] [PubMed] [Google Scholar]
- Engineer CB, Hashimoto-Sugimoto M, Negi J, Israelsson-Nordström M, Azoulay-Shemer T, Rappel W-J, Iba K, Schroeder JI (2016) CO2 sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci 21: 16–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon-Lang HJ. (2015) A calamitalean forest preserved in growth position in the Pennsylvanian coal measures of South Wales: implications for palaeoecology, ontogeny and taphonomy. Rev Palaeobot Palynol 214: 51–67 [Google Scholar]
- Fawcett JA, Maere S, Van de Peer Y (2009) Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event. Proc Natl Acad Sci USA 106: 5737–5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fišer Pečnikar Ž, Kulju KK, Sierra SE, Baas P, Van Welzen PC (2012) Leaf anatomy of Mallotus and the related genera Blumeodendron and Hancea (Euphorbiaceae sensu stricto). Bot J Linn Soc 169: 645–676 [Google Scholar]
- Fitter AH, Peat HJ (1994) The ecological flora database. J Ecol 82: 415–425 [Google Scholar]
- Franks PJ, Beerling DJ (2009a) CO(2)-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7: 227–236 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Beerling DJ (2009b) Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc Natl Acad Sci USA 106: 10343–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD (2007) The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol 143: 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Freckleton RP, Beaulieu JM, Leitch IJ, Beerling DJ (2012) Megacycles of atmospheric carbon dioxide concentration correlate with fossil plant genome size. Philos Trans R Soc Lond B Biol Sci 367: 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Royer DL (2017) Comment on “Was atmospheric CO2 capped at 1000ppm over the past 300 million years?” by McElwain JC et al. Palaeogeogr Palaeoclimatol Palaeoecol 472: 256–259 [Google Scholar]
- Franks PJ, Royer DL, Beerling DJ, Van de Water PK, Cantrill DJ, Barbour MM, Berry JA (2014) New constraints on atmospheric CO2 concentration for the Phanerozoic. Geophys Res Lett 41: 4685–4694 [Google Scholar]
- Frommer WB. (2010) Biochemistry. CO2mmon sense. Science 327: 275–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JE, Holroyd GH, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM (2000) The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716 [DOI] [PubMed] [Google Scholar]
- Grein M, Oehm C, Konrad W, Utescher T, Kunzmann L, Roth-Nebelsick A (2013) Atmospheric CO2 from the Late Oligocene to early Miocene based on photosynthesis data and fossil leaf characteristics. Palaeogeogr Palaeoclimatol Palaeoecol 374: 41–51 [Google Scholar]
- Haworth M, Elliott-Kingston C, McElwain JC (2013) Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171: 71–82 [DOI] [PubMed] [Google Scholar]
- Haworth M, Fitzgerald A, McElwain JC (2011) Cycads show no stomatal-density and index response to elevated carbon dioxide and subambient oxygen. Aust J Bot 59: 629–638 [Google Scholar]
- Haworth M, Heath J, McElwain JC (2010) Differences in the response sensitivity of stomatal index to atmospheric CO2 among four genera of Cupressaceae conifers. Ann Bot (Lond) 105: 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, McElwain J (2008) Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Palaeogeogr Palaeoclimatol Palaeoecol 262: 79–90 [Google Scholar]
- Heath SM, Southworth D, D’Allura JA (1997) Localization of nickel in epidermal subsidiary cells of leaves of Thlaspi montanum var. siskiyouense (Brassicaceae) using energy-dispersive X-ray microanalysis. Int J Plant Sci 158: 184–188 [Google Scholar]
- Hernandez-Castillo GR, Stockey RA, Rothwell GW, Mapes G (2009) Reconstruction of the Pennsylvanian-age walchian conifer Emporia cryptica sp nov (Emporiaceae: Voltziales). Rev Palaeobot Palynol 157: 218–237 [Google Scholar]
- Hill RS. (1998) Fossil evidence for the onset of xeromorphy and scleromorphy in Australian Proteaceae. Aust Syst Bot 11: 391–400 [Google Scholar]
- Hill RS, Pole MS (1992) Leaf and shoot morphology of extant Afrocarpus, Nageia and Retrophyllum (Podocarpaceae) species, and species with similar leaf arrangement, from Tertiary sediments in Australia. Aust Syst Bot 5: 337–358 [Google Scholar]
- Hincke AJ, Broere T, Kürschner WM, Donders TH, Wagner-Cremer F (2016) Multi-year leaf-level response to sub-ambient and elevated experimental CO2 in Betula nana. PLoS One 11: e0157400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rappel WJ, Occhipinti R, Ries A, Böhmer M, You L, Xiao C, Engineer CB, Boron WF, Schroeder JI (2015) Distinct cellular locations of Carbonic Anhydrases mediate Carbon Dioxide control of stomatal movements. Plant Physiol 169: 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan GJ. (2011) A critical framework for the assessment of biological palaeoproxies: predicting past climate and levels of atmospheric CO(2) from fossil leaves. New Phytol 192: 29–44 [DOI] [PubMed] [Google Scholar]
- Jordan GJ, Carpenter RJ, Brodribb TJ (2014) Using fossil leaves as evidence for open vegetation. Palaeogeogr Palaeoclimatol Palaeoecol 395: 168–175 [Google Scholar]
- Jordan GJ, Carpenter RJ, Koutoulis A, Price A, Brodribb TJ (2015) Environmental adaptation in stomatal size independent of the effects of genome size. New Phytol 205: 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan GJ, Weston PH, Carpenter RJ, Dillon RA, Brodribb TJ (2008) The evolutionary relations of sunken, covered, and encrypted stomata to dry habitats in Proteaceae. Am J Bot 95: 521–530 [DOI] [PubMed] [Google Scholar]
- Katul G, Manzoni S, Palmroth S, Oren R (2010) A stomatal optimization theory to describe the effects of atmospheric CO2 on leaf photosynthesis and transpiration. Ann Bot (Lond) 105: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CK, Beerling DJ (1995) Plant life form, stomatal density and taxonomic relatedness - a reanalysis of Salisbury (1927). Funct Ecol 9: 422–431 [Google Scholar]
- Kerp H, Abu Hamad A, Vording B, Bandel K (2006) Typical Triassic Gondwanan floral elements in the Upper Permian of the paleotropics. Geology 34: 265–268 [Google Scholar]
- Konrad W, Roth-Nebelsick A, Grein M (2008) Modelling of stomatal density response to atmospheric CO2. J Theor Biol 253: 638–658 [DOI] [PubMed] [Google Scholar]
- Konrad W, Katul G, Roth-Nebelsick A, Grein M (2017) A reduced order model to analytically infer atmospheric CO2 concentration from stomatal and climate data. Adv Water Resour 10.1016/j.advwatres.2017.03.018 [Google Scholar]
- Krings M, Kerp H, Taylor TN, Taylor EL (2003) How Paleozoic vines and lianas got off the ground: On scrambling and climbing Carboniferous-early Permian pteridosperms. Bot Rev 69: 204–224 [Google Scholar]
- Kürschner WM, Batenburg SJ, Mander L (2013) Aberrant Classopollis pollen reveals evidence for unreduced (2n) pollen in the conifer family Cheirolepidiaceae during the Triassic-Jurassic transition. Proc Biol Sci 280: 20131708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürschner WM, Kvacek Z, Dilcher DL (2008) The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proc Natl Acad Sci USA 105: 449–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kürschner WM, Wagner F, Visscher EH, Visscher H (1997) Predicting the response of leaf stomatal frequency to a future CO2-enriched atmosphere: constraints from historical observations. Geol Rundsch 86: 512–517 [Google Scholar]
- Lacourse T, Beer KW, Hoffman EH (2016) Identification of conifer stomata in pollen samples from western North America. Rev Palaeobot Palynol 232: 140–150 [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI (2001) Plant development. Signals from mature to new leaves. Nature 411: 154. [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI (2008) Response of stomatal numbers to CO2 and humidity: control by transpiration rate and abscisic acid. New Phytol 179: 397–404 [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI, Quick WP (2002) Long-distance CO(2) signalling in plants. J Exp Bot 53: 183–193 [DOI] [PubMed] [Google Scholar]
- Lammertsma EI, de Boer HJ, Dekker SC, Dilcher DL, Lotter AF, Wagner-Cremer F (2011) Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc Natl Acad Sci USA 108: 4035–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Morison JI (2004) Stomatal Function and Physiology. The Evolution of Plant Physiology; from Whole Plants to Ecosystems. Elsevier Academic, Cambridge, UK [Google Scholar]
- Lomax BH, Hilton J, Bateman RM, Upchurch GR, Lake JA, Leitch IJ, Cromwell A, Knight CA (2014) Reconstructing relative genome size of vascular plants through geological time. New Phytol 201: 636–644 [DOI] [PubMed] [Google Scholar]
- Lomax BH, Woodward FI, Leitch IJ, Knight CA, Lake JA (2009) Genome size as a predictor of guard cell length in Arabidopsis thaliana is independent of environmental conditions. New Phytol 181: 311–314 [DOI] [PubMed] [Google Scholar]
- Ludwig M. (2011) The molecular evolution of β-carbonic anhydrase in Flaveria. J Exp Bot 62: 3071–3081 [DOI] [PubMed] [Google Scholar]
- Mander L, Kürschner WM, McElwain JC (2013) Palynostratigraphy and vegetation history of the Triassic-Jurassic transition in East Greenland. J Geol Soc London 170: 37–46 [Google Scholar]
- Mander L, Wesseln CJ, McElwain JC, Punyasena SW (2012) Tracking taphonomic regimes using chemical and mechanical damage of pollen and spores: an example from the Triassic-Jurassic mass extinction. PLoS One 7: e49153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni S, Vico G, Katul G, Fay PA, Polley W, Palmroth S, Porporato A (2011) Optimizing stomatal conductance for maximum carbon gain under water stress: a meta‐analysis across plant functional types and climates. Funct Ecol 25: 456–467 [Google Scholar]
- Maxbauer DP, Royer DL, LePage BA (2014) High Arctic forests during the middle Eocene supported by moderate levels of atmospheric CO2. Geology 42: 1027–1030 [Google Scholar]
- McElwain JC, Beerling DJ, Woodward FI (1999) Fossil plants and global warming at the Triassic–Jurassic boundary. Science 285: 1386–1390 [DOI] [PubMed] [Google Scholar]
- McElwain JC, Chaloner WG (1995) Stomatal density and index of fossil plants track atmospheric carbon-dioxide in the Paleozoic. Ann Bot (Lond) 76: 389–395 [Google Scholar]
- McElwain JC, Chaloner WG (1996) The fossil cuticle as a skeletal record of environmental change. Palaios 11: 376–388 [Google Scholar]
- McElwain JC. (1998) Do fossil plants signal palaeoatmospheric carbon dioxide concentration in the geological past? Philos Trans R Soc Lond B Biol Sci 353: 83–96 [Google Scholar]
- McElwain JC, Mayle FE, Beerling DJ (2002) Stomatal evidence for a decline in atmospheric CO2 concentration during the Younger Dryas stadial: a comparison with Antarctic ice core records. J Quaternary Sci 17: 21–29 [Google Scholar]
- McElwain JC, Montañez I, White JD, Wilson JP, Yiotis C (2016a) Was atmospheric CO2 capped at 1000ppm over the past 300 million years? Palaeogeogr Palaeoclimatol Palaeoecol 441: 653–658 [Google Scholar]
- McElwain J, Montañez I, White J, Wilson J, Yiotis C (2017) Reply to Comment on “Was atmospheric CO2 capped at 1000ppm over the past 300 million years?”. Palaeogeogr Palaeoclimatol Palaeoecol (in press) http://doi.org/10.1016/j.palaeo.2017.01.020 [Google Scholar]
- McElwain JC, Popa ME, Hesselbo SP, Haworth M, Surlyk F (2007) Macroecological responses of terrestrial vegetation to climatic and atmospheric change across the Triassic/Jurassic boundary in East Greenland. Paleobiology 33: 547–573 [Google Scholar]
- McElwain JC, Wade-Murphy J, Hesselbo SP (2005) Changes in carbon dioxide during an oceanic anoxic event linked to intrusion into Gondwana coals. Nature 435: 479–482 [DOI] [PubMed] [Google Scholar]
- McElwain JC, Yiotis C, Lawson T (2016b) Using modern plant trait relationships between observed and theoretical maximum stomatal conductance and vein density to examine patterns of plant macroevolution. New Phytol 209: 94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Jõesaar I, Brosché M, Kollist H (2014) To open or to close: species-specific stomatal responses to simultaneously applied opposing environmental factors. New Phytol 202: 499–508 [DOI] [PubMed] [Google Scholar]
- Montañez IP. (2013) Modern soil system constraints on reconstructing deep-time atmospheric CO2. Geochim Cosmochim Acta 101: 57–75 [Google Scholar]
- Montañez IP, McElwain JC, Poulsen CJ, White JD, DiMichele WA, Wilson JP, Griggs G, Hren MT (2016) Climate, pCO2 and terrestrial carbon cycle linkages during late Palaeozoic glacial-interglacial cycles. Nat Geosci 9: 824–828 [Google Scholar]
- Mott KA, Gibson AC, O’Leary JW (1982) The adaptive significance of amphistomatic leaves. Plant Cell Environ 5: 455–460 [Google Scholar]
- Muir CD. (2015) Making pore choices: repeated regime shifts in stomatal ratio. Proc R Soc B 282: 20151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlange J-Y, Waggoner PE (1970) Stomatal dimensions and resistance to diffusion. Plant Physiol 46: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JT, Ziegler A, Scotese CR (1982) Rainfall patterns and the distribution of coals and evaporites in the Mesozoic and Cenozoic. Palaeogeogr Palaeoclimatol Palaeoecol 40: 67–101 [Google Scholar]
- Perez-Martin A, Michelazzo C, Torres-Ruiz JM, Flexas J, Fernández JE, Sebastiani L, Diaz-Espejo A (2014) Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: correlation with gene expression of carbonic anhydrase and aquaporins. J Exp Bot 65: 3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole MS. (2008) Dispersed leaf cuticle from the Early Miocene of southern New Zealand. Palaeontologica Electronica 11.3:15A: 117p [Google Scholar]
- Pott C, McLoughlin S (2009) Bennettitalean foliage in the Rhaetian-Bajocian (latest Triassic-Middle Jurassic) floras of Scania, southern Sweden. Rev Palaeobot Palynol 158: 117–166 [Google Scholar]
- Reid CD, Maherali H, Johnson HB, Smith SD, Wullschleger SD, Jackson RB (2003) On the relationship between stomatal characters and atmospheric CO2. Geophys Res Lett 30: 1–3 [Google Scholar]
- Retallack GJ. (2001) A 300-million-year record of atmospheric carbon dioxide from fossil plant cuticles. Nature 411: 287–290 [DOI] [PubMed] [Google Scholar]
- Richoz S, Van De Schootbrugge B, Pross J, Püttmann W, Quan TM, Lindström S, Heunisch C, Fiebig J, Maquil R, Schouten S (2012) Hydrogen sulphide poisoning of shallow seas following the end-Triassic extinction. Nat Geosci 5: 662–667 [Google Scholar]
- Roth-Nebelsick A. (2005) Reconstructing atmospheric carbon dioxide with stomata: possibilities and limitations of a botanical pCO(2)-sensor. Trees (Berl) 19: 251–265 [Google Scholar]
- Roth-Nebelsick A, Grein M, Utescher T, Konrad W (2012) Stomatal pore length change in leaves of Eotrigonobalanus furcinervis (Fagaceae) from the Late Eocene to the Latest Oligocene and its impact on gas exchange. Review of Palaeobotany and Palynology 174: 106–112 [Google Scholar]
- Royer DL. (2001) Stomatal density and stomatal index as indicators of paleoatmospheric CO(2) concentration. Rev Palaeobot Palynol 114: 1–28 [DOI] [PubMed] [Google Scholar]
- Royer DL, Wing SL, Beerling DJ, Jolley DW, Koch PL, Hickey LJ, Berner RA (2001) Paleobotanical evidence for near present-day levels of atmospheric Co2 during part of the tertiary. Science 292: 2310–2313 [DOI] [PubMed] [Google Scholar]
- Royer DL. (2002) A critical review of CO2 proxies and models. Geochim Cosmochim Acta 66: A653 [Google Scholar]
- Royer DL, Sack L, Wilf P, Lusk CH, Jordan GJ, Niinemets U, Wright IJ, Westoby M, Cariglino B, Coley PD, et al. (2007) Fossil leaf economics quantified: calibration, Eocene case study, and implications. Paleobiology 33: 574–589 [Google Scholar]
- Rudall PJ, Hilton J, Bateman RM (2013) Several developmental and morphogenetic factors govern the evolution of stomatal patterning in land plants. New Phytol 200: 598–614 [DOI] [PubMed] [Google Scholar]
- Šmarda P, Veselý P, Šmerda J, Bureš P, Knápek O, Chytrá M (2016) Polyploidy in a ‘living fossil’ Ginkgo biloba. New Phytol 212: 11–14 [DOI] [PubMed] [Google Scholar]
- Steinthorsdottir M, Bacon KL, Popa ME, Bochner L, McElwain JC (2011a) Bennettitalean leaf cuticle fragments (here Anomozamites and Pterophyllum) can be used interchangeably in stomatal frequency-based palaeo-CO(2) reconstructions. Palaeontology 54: 867–882 [Google Scholar]
- Steinthorsdottir M, Jeram AJ, McElwain JC (2011b) Extremely elevated CO(2) concentrations at the Triassic/Jurassic boundary. Palaeogeogr Palaeoclimatol Palaeoecol 308: 418–432 [Google Scholar]
- Steinthorsdottir M, Porter A, Holohan A, Kunzmann L, Collinson M, McElwain J (2016a) Fossil plant stomata indicate decreasing atmospheric CO2 prior to the Eocene-Oligocene boundary. Clim Past 12: 439–454 [Google Scholar]
- Steinthorsdottir M, Vajda V, Pole M (2016b) Global trends of pCO2 across the Cretaceous-Paleogene boundary supported by the first Southern Hemisphere stomatal proxy-based pCO2 reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 464: 143–152 [Google Scholar]
- Steinthorsdottir M, Woodward FI, Surlyk F, McElwain JC (2012) Deep-time evidence of a link between elevated CO2 concentrations and perturbations in the hydrological cycle via drop in plant transpiration. Geology 40: 815–818 [Google Scholar]
- Tricker PJ, Trewin H, Kull O, Clarkson GJ, Eensalu E, Tallis MJ, Colella A, Doncaster CP, Sabatti M, Taylor G (2005) Stomatal conductance and not stomatal density determines the long-term reduction in leaf transpiration of poplar in elevated CO2. Oecologia 143: 652–660 [DOI] [PubMed] [Google Scholar]
- van de Schootbrugge B, Tremolada F, Rosenthal Y, Bailey TR, Feist-Burkhardt S, Brinkhuis H, Pross J, Kent DV, Falkowski PG (2007) End-Triassic calcification crisis and blooms of organic-walled ‘disaster species’. Palaeogeogr Palaeoclimatol Palaeoecol 244: 126–141 [Google Scholar]
- Wagner F, Below R, Klerk PD, Dilcher DL, Joosten H, Kürschner WM, Visscher H (1996) A natural experiment on plant acclimation: lifetime stomatal frequency response of an individual tree to annual atmospheric CO2 increase. Proc Natl Acad Sci USA 93: 11705–11708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, Dilcher DL, Visscher H (2005) Stomatal frequency responses in hardwood-swamp vegetation from Florida during a 60-year continuous CO2 increase. Am J Bot 92: 690–695 [DOI] [PubMed] [Google Scholar]
- Wagner F, Neuvonen S, Kürschner WM, Visscher H (2000) The influence of hybridization on epidermal properties of birch species and the consequences for palaeoclimatic interpretations. Plant Ecol 148: 61–69 [Google Scholar]
- Wellman CH, Osterloff PL, Mohiuddin U (2003) Fragments of the earliest land plants. Nature 425: 282–285 [DOI] [PubMed] [Google Scholar]
- Wellman CH, Strother PK (2015) The terrestrial biota prior to the origin of land plants (embryophytes): a review of the evidence. Palaeontology 58: 601–627 [Google Scholar]
- Wilf P, Johnson KR, Cúneo NR, Smith ME, Singer BS, Gandolfo MA (2005) Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. Am Nat 165: 634–650 [DOI] [PubMed] [Google Scholar]
- Willis KJ, McElwain JC (2014) The Evolution of Plants. Oxford University Press, Oxford, UK [Google Scholar]
- Wilson JP, White JD, DiMichele WA, Hren MT, Poulsen CJ, McElwain JC, Montanez IP (2015) Reconstructing extinct plant water use for understanding vegetation-climate feedbacks: Methods, synthesis and a case study using the Paleozoic era medullosan seed ferns. Palaeontol Soc Papers 21: 167–195 [Google Scholar]
- Woodward FI. (1986) Ecophysiological studies on the shrub Vaccinium myrtillus L. taken from a wide altitudinal range. Oecologia 70: 580–586 [DOI] [PubMed] [Google Scholar]
- Woodward FI. (1987) Stomatal numbers are sensetive to increase in CO2 from pre-industrial levels. Nature 327: 617–618 [Google Scholar]
- Wotzlaw J-F, Guex J, Bartolini A, Gallet Y, Krystyn L, McRoberts CA, Taylor D, Schoene B, Schaltegger U (2014) Towards accurate numerical calibration of the Late Triassic: High-precision U-Pb geochronology constraints on the duration of the Rhaetian. Geology 42: 571–574 [Google Scholar]
- Wynn JG. (2003) Towards a physically based model of CO2-induced stomatal frequency response. New Phytol 157: 394–398 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.