Abstract
The pathway and timing of starch turnover in guard cells differs from mesophyll cells and is linked to stomatal opening in the light.
This update focuses on the starch that accumulates in the guard cells that control stomatal pore size and thus the exchange of water vapor, CO2, and O2 between the leaf and the atmosphere. Transitory starch in these cells plays a key role in determining the velocity of stomatal opening in the light. This significantly differs from the transitory starch in the mesophyll leaves, which acts primarily as a carbohydrate reserve to sustain plant metabolism during the night. We discuss how the unique function of transitory starch in guard cells is reflected in the timing of its deposition and mobilization, along with differences from mesophyll cells in the pathways and regulation of starch metabolism.
Starch is a nonstructural polysaccharide synthesized inside plastids of plants and algae. It consists of two types of α-1,4-linked glucan polymers—amylose and amylopectin—that differ in chain length and frequency of α-1,6-branches. These polymers adopt complex secondary and tertiary structures that organize into insoluble, semicrystalline granules to store energy in a dense, osmotically inert form (Pfister and Zeeman, 2016).
Starch is a vital substance for plants, both for short- and long-term storage of carbohydrates. In heterotrophic organs, such as potato tubers, cassava roots, cereal seed endosperm, and the stems of woody perennials, starch is synthesized in specialized amylopasts from imported sucrose (Suc) and stored over the seasons, or even for many years. Remobilization of this long-term storage starch takes place during seed germination, tuber sprouting, or regrowth, when photosynthesis has either not yet resumed or is insufficient to meet the demand for energy and carbon skeletons (Lloyd and Kossmann, 2015). In photosynthetic tissues, starch is synthesized in the chloroplasts of mesophyll cells during the day and remobilized at night to provide carbon and energy for maintenance and growth (Stitt and Zeeman, 2012). These short-term reserves, known as transitory starch, are formed directly from intermediates of the Calvin-Benson cycle in the light and when broken down at night provide substrates for respiration in the leaf and synthesis of Suc that can be exported to growing sink organs. Within the leaf epidermis, transitory starch is also present in the chloroplasts of the guard cells that surround the stomatal pore.
Guard cells play an essential role for plant survival and productivity. Through reversible changes in their turgor pressure, they control the opening and closing of the stomatal pore in response to internal and external environmental factors to maximize the uptake of CO2 for photosynthesis, while preventing excessive water loss through transpiration (for review, see Kollist et al., 2014; Munemasa et al., 2015; Murata et al., 2015). The presence of starch in guard cells was first observed at the beginning of the last century (Lloyd, 1908), but its function has been experimentally challenging to study, and so the significance of guard cell starch has remained controversial for many years. However, recent technical advances and discoveries have shed new light on the functions of starch metabolism in guard cells, showing that it is critical for rapid stomatal opening in the light and in response to water deficit (Prasch et al., 2015; Horrer et al., 2016). This unique function of transitory starch in guard cells is reflected in the timing, pathways, and regulation of its deposition and mobilization, which differ in important ways from those in mesophyll cells.
In this article, we focus on recent advances in our understanding of transitory starch metabolism in guard cells, highlighting differences and similarities with transitory starch metabolism in mesophyll cells. We identify gaps in our knowledge that need to be addressed in the future and discuss the prospects for modifying starch metabolism in guard cells to improve stomatal responsiveness and kinetics, and ultimately crop plant performance in the field.
PATTERNS OF TRANSITORY STARCH DEPOSITION AND MOBILIZATION
Much of our knowledge of the pathway and regulation of transitory starch turnover comes from studies of Arabidopsis (Arabidopsis thaliana). This species stores up to 50% of its photoassimilate as starch, representing a major investment by the plant. Therefore, the efficiency with which this reserve is used by the plants affects the growth potential and final biomass of the plant (Sulpice et al., 2009, 2014).
Under short-day conditions (photoperiod <12 h), Arabidopsis plants degrade their starch in a linear manner that is timed to dawn, such that by the end of the night the plant has remobilized most but not quite all of its starch (Fig. 1). By carefully controlling the rate of starch degradation, the plant not only makes maximal use of its reserves for growth, but also avoids running out of carbon before the end of the night (Gibon et al., 2004). Premature exhaustion of starch reserves before dawn could trigger carbon starvation responses such as autophagy, resulting in loss of proteins and other cellular components that are metabolically expensive to replace, thereby having a negative impact on growth (Stitt and Zeeman, 2012; Avin-Wittenberg et al., 2015). We know from light-dark shift experiments and analysis of circadian clock mutants that the circadian clock plays a major role in this regulation, enabling the plant to predict the length of the coming night (Graf et al., 2010; Graf and Smith, 2011). Current models envisage the plant sensing the total amount of starch accumulated during the day and then dividing this amount by the predicted length of the night to set an appropriate rate of starch degradation (Scialdone et al., 2013). One of the most important features of the circadian clock is its robustness under fluctuating environmental conditions, including its ability to compensate for changes in temperature. This is reflected in the ability of plants to adjust their rate of starch breakdown in response to temperature changes during the night, ensuring that the reserves are not exhausted before dawn (Pyl et al., 2012; Pilkington et al., 2015). Under long-day conditions (>12-h photoperiod), Arabidopsis plants accumulate more than enough starch to last through the shorter nights, and a moderate starch excess at dawn is often observed. This suggests that the clock plays a less important role in controlling starch remobilization under long-day conditions when the timing of starch degradation is not so critical (Stitt and Zeeman, 2012).
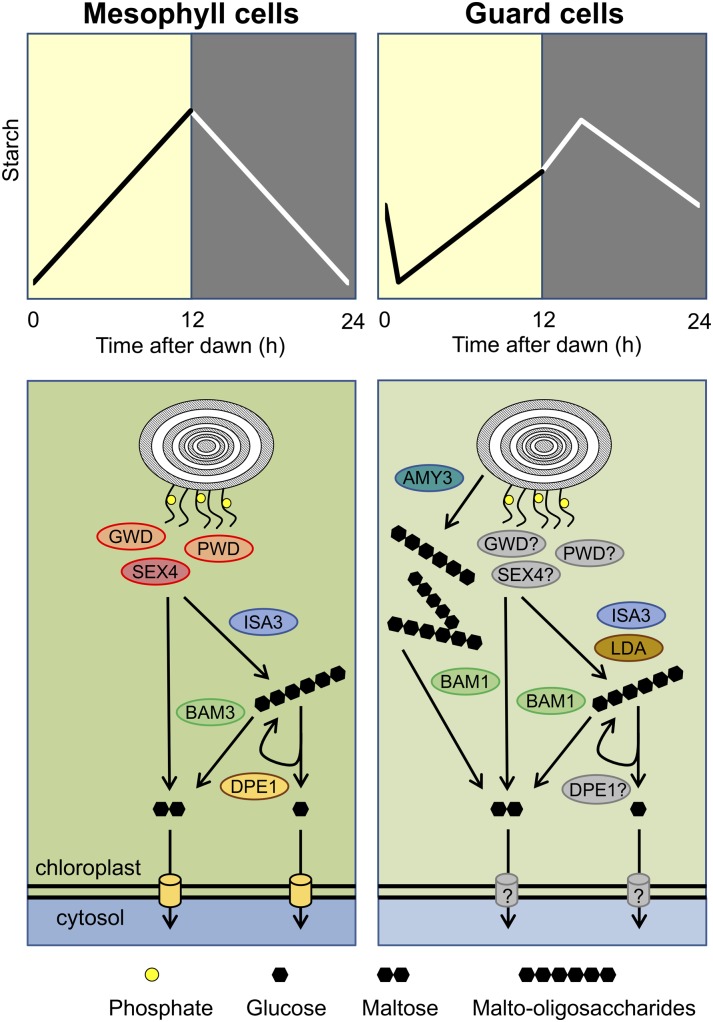
Figure 1.
Starch dynamics and preferential pathways of starch degradation in mesophyll cells (left) and guard cells (right). Essential enzymes in each cell type are highlighted. Maltose and glucose (Glc) produced from starch breakdown in the chloroplast are exported to the cytosol via the maltose (MEX1) and Glc (GlcT) transporters, respectively (yellow cylinders, left). Question marks refer to enzymes and transporters whose involvement in the guard cell starch degradation pathway has not been experimentally verified.
Recent technical advances in starch staining, using periodic acid and the fluorophore propidium iodide, have allowed quantitative analysis of starch granules in individual guard cells. These have revealed that the diurnal pattern of starch accumulation and breakdown in guard cells differs in several respects from that in mesophyll cells. The starch content of guard cells continues to increase for several hours after dusk (Fig. 1; Horrer et al., 2016). After peaking in the first half of the night, the starch content of the guard cells then begins to fall, but not all of the starch is degraded during the night. A substantial amount of starch remains in guard cells at dawn, but this is rapidly degraded when the plant is illuminated, coinciding with the opening of the stomata. The sugars released by rapid starch degradation will increase the osmotic potential within the guard cells and so contribute to stomatal opening, although most is likely to be used for malate synthesis (via glycolysis and phosphoenolpyruvate carboxylase (PEPC), providing a counterion for the potassium (K+) ions taken up from the apoplast (Jezk and Blatt, 2017). After falling to near zero in the first hour after dawn, starch levels then begin to rise again, with net accumulation continuing past dusk into the early hours of the night (Horrer et al., 2016). Guard cell chloroplasts contain Rubisco and seem capable of net CO2 assimilation (Lawson et al., 2002, 2003), so some of the carbon for starch synthesis in the light may come from guard cell photosynthesis but is likely to be supplemented by import of sugars from the apoplast or from sugars stored in the guard cells during the previous light phase (Daloso et al., 2016; Santelia and Lawson, 2016).
It is not known whether the clock also mediates starch turnover in guard cells, as it does in mesophyll cells. Stomatal aperture shows well-defined circadian rhythms, which persist under constant-light conditions (Webb, 2003; T. Kinoshita et al., 2011), and regulation of starch turnover in guard cells might be one of the ways by which the clock exerts influence on stomatal aperture.
MOLECULAR PATHWAYS OF TRANSITORY STARCH DEGRADATION
Using forward and reverse genetics, more than 20 genes encoding enzymes and other proteins involved in starch degradation have so far been identified in Arabidopsis (Table I). These include enzymes for debranching, hydrolysis, or phosphorolysis of starch, and some noncatalytic proteins with putative regulatory functions. Research in this field over the past 10 years has thrown up a number of surprises. Although most of these enzymes are located inside plastids and are active on native starch granules and/or the derived soluble malto-oligosaccharides, only some are absolutely required for mesophyll starch degradation at night (Table I). Other isoforms are extraplastidial (Yu et al., 2005; Lu et al., 2006; Glaring et al., 2007; Reinhold et al., 2011) and have in some cases acquired a distinct function, for example, acting as transcriptional regulators in the nucleus (Reinhold et al., 2011). The multiplicity of isoforms of starch degrading enzymes probably reflects diversification in the functions of starch metabolism during the evolution of multicellular plants, with expansion of gene families arising from genome duplications allowing paralogous genes to evolve new or more specialized functions in particular cell or tissue types (Ball and Morell, 2003; Proost et al., 2011).
Table I. Arabidopsis genes encoding enzymes and other proteins involved in starch degradation.
Only 10 out of 27 identified starch-related degrading proteins are required for nighttime leaf starch metabolism. Of the remainder, some are inactive; others have acquired specialized functions in other tissues and/or conditions, and in some cases are extraplastidial. N.A. Not applicable.
| Enzyme/Transporter | EC Number | Reaction Catalyzed | No. of Isoforms | Required for Nighttime Leaf Starch Degradation | Not Essential for Nighttime Leaf Starch Degradation |
|---|---|---|---|---|---|
| Glucan water dikinase | EC 2.7.9.4 | Transfer of β-phosphate of ATP to glucosyl residues of starch | 3 | GWD1, PWD | GWD2 |
| Phosphoglucan phosphatase | EC 2.7.9.5 | Hydrolysis of starch-bound phosphate | 3 | SEX4, LSF1 | LSF2 |
| β-Amylase | EC 3.2.1.2 | Exoamylase, hydrolytic cleavage of α-1,4-glucosidic bonds liberating β-maltose | 9 | BAM3, BAM4 | BAM1, BAM2, BAM5-BAM9 |
| α-Amylase | EC 3.2.1.1 | Endoamylase, hydrolytic cleavage of α-1,4-glucosidic bonds liberating linear and branched oligosaccharides | 3 | – | AMY1–AMY3 |
| Debranching enzyme | EC 3.2.1.68 | Hydrolytic cleavage of α-1,6-glucosidic bonds releasing linear oligosaccharides | 2 | ISA3 | LDA |
| Glucan phosphorylase | EC 2.4.1.1 | Phosphorolytic cleavage of α-1, 4-glucosidic bonds liberating Glc-1-P (Glc1P) and inorganic phosphate (Pi) | 2 | – | PHS1, PHS2 |
| Disproportionating enzyme | EC 2.4.1.25 | Glucanotransferase, transfers maltosyl units from one 1,4-α-glucan to another, liberating Glc | 2 | DPE1, DPE2 | – |
| Maltose transporter | N.A. | Maltose exchange facilitator between plastid and cytosol | 1 | MEX1 | – |
| Glc transporter | N.A. | Glc exchange facilitator between plastid and cytosol | 1 | – | GlcT |
| Putative Glc1P/Pi translocator | N.A. | Putative counter exchange of Glc1P and Pi between plastid and cytosol (both directions) | 1 | – | Glc1PT |
| Total number | 27 | 10 | 17 |
Our current model of starch degradation in Arabidopsis leaves at night (Fig. 1) indicates that the first steps in the pathway are the sequential phosphorylation of the starch granule by glucan, water dikinase (GWD1), and phosphoglucan, water dikinase (PWD; Ritte et al., 2006), increasing the accessibility of the glucan chains at the granule surface to degradative enzymes (Hejazi et al., 2008, 2009; Blennow and Engelsen, 2010). The phosphate groups introduced by GWD1 and PWD are subsequently removed by the STARCH EXCESS4 (SEX4) and LIKE SEX FOUR2 (LSF2) phosphoglucan phosphatases (Kötting et al., 2009; Santelia et al., 2011). As the surface of the starch granule is made accessible via this cycle of starch phosphorylation and dephosphorylation, the glucan chains are attacked by debranching enzymes—isoamylase3 (ISA3) and limit dextrinase (LDA)—that cleave the α-1,6-branch points, liberating long malto-oligosaccharides into the chloroplast stroma, and by β-amylases, which are exoamylases that attack the nonreducing end of the glucan chains to release maltose (Edner et al., 2007; Kötting et al., 2009). β-Amylase3 (BAM3) is the predominant isoform of β-amylase in mesophyll cell chloroplasts, although BAM2 and BAM4 are also expressed in mesophyll cells, as is BAM1 under osmotic stress conditions (Fulton et al., 2008; Valerio et al., 2011). ISA3 and BAM3 are unable to hydrolyze malto-oligosaccharides with fewer than four Glc units (i.e. maltose and maltotriose). Disproportionation of maltotriose by the plastidial disproportionating enzyme1 (DPE1) yields Glc and maltopentaose, which can then be attacked by β-amylases to release maltose (Critchley et al., 2001; O’Neill et al., 2015).
Maltose is exported from the plastids by the maltose exchange facilitator MEX1 (Niittylä et al., 2004) and further metabolized by the cytosolic α-glucanotransferase disproportionating enzyme2 (DPE2), with one Glc moiety being transferred to a soluble heteroglycan and then released as Glc1-phosphate (Glc1P) by cytosolic glucan phosphorylase2 (PHS2), while the other Glc moiety is released as free Glc (Chia et al., 2004; Malinova et al., 2013). This Glc, along with Glc produced by DPE1 and exported to the cytosol by the plastidial Glc transporter GlcT (Cho et al., 2011), is phosphorylated by hexokinase, producing Glc6-phosphate (Glc6P). The cytosolic pools of Glc1P and Glc6P provide substrates for maintenance respiration in the leaves and synthesis of Suc for export to growing sink organs at night.
Starch breakdown is generally compromised in mutants that lack one or more of these enzyme activities, so not all of their starch is degraded during the night and the mutants retain more starch at dawn than wild-type plants, resulting in a so-called starch excess (sex) phenotype (Streb and Zeeman, 2012). The severity of the sex phenotype depends on which enzyme is missing, with gwd1 (sex1) mutants having the most severe sex phenotype (Yu et al., 2001). The starch content and diurnal profiles in lsf2 and lda mutants are essentially the same as in wild-type plants due to functional redundancy with SEX4 and ISA3, respectively, as shown by the additive effects in sex4lsf2 and isa3lda double mutants (Santelia et al., 2011; Streb et al., 2012). The plastidial starch phosphorylase (PHS1) is also not essential for starch breakdown in mesophyll cells (Zeeman et al., 2004).
Although expressed in mesophyll cells and present in the chloroplasts, the BAM4 protein has no measurable β-amylase activity, but bam4 mutant plants show a sex phenotype (Fulton et al., 2008). Likewise, Arabidopsis plants lacking the inactive phosphoglucan phosphatase LSF1 also have altered starch levels at the end of the night (Comparot-Moss et al., 2010). It has been speculated that BAM4 and LSF1 have regulatory rather than catalytic functions (Fulton et al., 2008; Comparot-Moss et al., 2010). These observations suggest that the pathway of starch degradation in leaves is more complicated than initially thought, and it is likely that other, so-far-unidentified components are involved in the pathway and regulation of starch degradation.
Starch degradation in guard cells is far less well understood than in mesophyll cells, although it is clear it follows a distinct pathway (Fig. 1). It has been recently shown that the major starch-degrading enzyme in guard cells is BAM1 (Valerio et al., 2011; Prasch et al., 2015; Horrer et al., 2016). Upon illumination, BAM1 rapidly mobilizes starch in conjunction with the chloroplastic α-amylase3 (AMY3), an endoamylase that hydrolyzes α-1,4 bonds within glucan chains at random (Horrer et al., 2016). Interestingly, loss of BAM1 and AMY3 alone or in combination has no impact on starch metabolism in mesophyll cells under normal conditions, indicating that neither of these enzymes is required for starch degradation in this cell type. Similar to mesophyll cells, ISA3 is the major starch debranching activity in guard cells, as the isa3 mutant has elevated guard cell starch levels, while those of the lda mutant are similar to wild type (Horrer et al., 2016). However, the even higher starch content of guard cells in the isa3lda double mutant shows that LDA does contribute to starch debranching in guard cells in the absence of ISA3 (Horrer et al., 2016), as it does in mesophyll cells (Delatte et al., 2006).
The phosphorylation and dephosphorylation of starch in guard cells has not yet been investigated. However, it was demonstrated that BAM1 activity in vitro is greatly stimulated by the presence of GWD1 and SEX4 (Edner et al., 2007; Kötting et al., 2009). This suggests that β-amylolysis of starch in guard cells by BAM1 could be at least partly dependent on reversible starch phosphorylation. In vitro, α-amylases, such as AMY3, are able to attack intact starch granules (Seung et al., 2013), but it is not known whether AMY3 can degrade guard cell starch granules in vivo without prior phosphorylation of the starch by GWD1 and PWD. Thus, some enzymes, such as ISA3, play a similar role in starch degradation in both mesophyll and guard cells, while others, such as BAM1 and AMY3, are specifically or predominantly involved in degrading starch in guard cells. Further studies are needed to define the role of other starch degrading enzymes (e.g. PHS1) and the chloroplast maltose (MEX1) and Glc (GlcT) transporters in guard cells. For enzymes that are important or essential for starch degradation in mesophyll cells, e.g. GWD1, constitutive null mutants may not be informative because of pleiotropic effects from the severe defect in mesophyll cell starch degradation. In such cases, silencing of expression specifically in guard cells may be required.
FACTORS CONTROLLING TRANSITORY STARCH DEGRADATION
As outlined above, transitory starch produced in leaves and guard cells serves different functions. It is therefore likely that specific mechanisms operate in mesophyll and guard cells to regulate starch turnover according to the respective functions of starch in these cell types. Various studies over the last decade, mainly on Arabidopsis mutants, have revealed the complexity of mechanisms involved in the regulation of starch in mesophyll cells. There are still many gaps in our knowledge of individual mechanisms and how these are integrated with each other, and with other cellular processes such as growth. Nevertheless, our fragmentary knowledge of the regulation of starch degradation in mesophyll cells is far in advance of research on the mechanisms controlling starch degradation in guard cells, which is still in its infancy.
Transcriptional versus Posttranslational Control
It has long been known that the expression of starch-related genes is diurnally regulated (Harmer et al., 2000; Smith et al., 2004; Bläsing et al., 2005; Lu et al., 2005). In particular, a group of genes encoding mostly enzymes of leaf starch degradation show coordinated expression, with a peak at the end of the day, just prior to the onset of starch breakdown (Smith et al., 2004). This expression pattern is retained and continues to oscillate with a 24-h periodicity when plants are grown in continuous light, consistent with being under the control of the circadian clock (Lu et al., 2005; Harmer, 2009). Very recently, diurnal changes in a novel histone H3 modification signature (H3K9ac|H3K27ac|H3S28p) were found to be correlated with the diurnal expression of several starch-degrading genes (Baerenfaller et al., 2016). Of these, genes encoding essential components of nighttime starch degradation, such as GWD1, PWD, SEX4, and DPE2 (Table I), had higher histone modification levels at the end of day than at the end of the night, potentially explaining the observed oscillations in transcript abundance. BAM1 was the only gene to have higher levels of all three chromatin modifications at the end of the night (Baerenfaller et al., 2016). This is interesting as, unlike most starch-degrading enzyme genes, BAM1 expression peaks at the end of the night and falls in the first few hours of the day (Smith et al., 2004). This expression pattern matches the specific function of BAM1 in guard cells, where starch degradation is triggered by the transition from dark to light at dawn, in contrast to the light to dark transition at dusk that triggers starch degradation in mesophyll cells.
While the diurnal fluctuations in chromatin modification patterns might explain the coordinated transcriptional changes in starch-degrading enzyme genes, their contribution to regulation of starch degradation seems doubtful because the maximal activities or the protein amount of most starch enzymes appears to remain constant throughout the light-dark cycle (Smith et al., 2004; Lu et al., 2005). Similar discrepancies between transcript and protein abundance or activities have been observed for many other enzymes (Gibon et al., 2004). It has been speculated that the dynamic behavior of enzyme transcripts provides robustness to metabolic networks, enabling them to be adjusted in response to a change in environmental conditions, but only if the change is sustained over several days. Whatever the significance of the diurnal fluctuations in transcripts, it seems clear that posttranscriptional mechanisms are very important for regulation of starch-degrading enzyme activities. These could include translational as well as posttranslational mechanisms, with evidence beginning to emerge that reversible protein phosphorylation and redox modulation could play significant roles (for review, see Kötting et al., 2010).
Phosphopeptides of several starch-degrading proteins have been identified in Arabidopsis by large-scale phosphoproteomic approaches (de la Fuente van Bentem et al., 2008; Sugiyama et al., 2008; Lohrig et al., 2009; Reiland et al., 2009). However, in many cases, the phosphorylated residues are located within the chloroplast transit peptide and so unlikely to be involved in regulation of activity in the chloroplasts, as they will be absent from the mature proteins. Several starch-degrading enzymes, such as GWD1, SEX4, BAM1, and AMY3, contain cysteine residues that render the enzyme susceptible to redox modulation, with the reduced (dithiol) forms of the enzymes having higher activity in vitro than the oxidized (disulfide) forms (Mikkelsen et al., 2005; Sparla et al., 2006; Seung et al., 2013; Silver et al., 2013). Given that the chloroplast stroma is more reducing in the light than in the dark, the redox sensitivity of GWD1 and SEX4 should make them more active in the light than in the dark, which appears to be counterintuitive. However, expression of a mutagenized, redox-insensitive form of GWD1 was able to fully complement the gwd1 mutant, restoring wild-type-like patterns of starch turnover, thus showing that redox modulation of GWD1 is not essential for regulation of starch breakdown in vivo (Skeffington et al., 2014). Furthermore, it is now evident that phosphorylation and dephosphorylation of starch granules occur during the synthesis of starch, as well as during degradation (Ritte et al., 2004; Santelia et al., 2011; Hejazi et al., 2014). It has been speculated that phosphorylation of starch during synthesis might determine the accessibility of the glucan chains to the various starch synthases and (de)branching enzymes, preventing premature crystallization of the amylopectin glucan chains until the correct chain length and branching structure has been formed. It is plausible that the redox sensitivity of GWD1 and SEX4 allows the two activities to be coregulated to prevent excessive phosphorylation of starch during its synthesis, which might otherwise interfere with starch degradation in the dark.
Although no role for GWD1 and SEX4 in guard cell starch degradation has so far been established, light-driven changes in the reducing potential of the chloroplast stroma would potentially activate these enzymes at the beginning of the day, when rapid starch degradation occurs to drive stomatal opening. Likewise, the redox-sensitive BAM1 and AMY3, which are known to play a central role in guard cell starch degradation, should become activated in the light. However, experimental evidence that this type of posttranslational regulation is required for their action in guard cells has not yet been reported. Future research should be directed toward guard cell-specific mutant complementation studies with redox-insensitive forms of the BAM1 and AMY3 proteins.
Metabolite Control
Maltose, and ultimately Suc, are the major products of transitory starch degradation in mesophyll cells, and there is evidence that both can exert feedback inhibition on starch breakdown. The Arabidopsis mex1 and dpe2 mutants accumulate high levels of maltose due to defects in its export from the chloroplasts or catabolism in the cytosol, respectively (Chia et al., 2004; Niittylä et al., 2004). Both mutants also have a sex phenotype, suggesting that the accumulation of maltose inhibits starch degradation in some way, although the target and mechanism are unknown. Maltose does not inhibit BAM3 directly, but the enzyme was recently shown to be sensitive to inhibition by maltotriose, potentially linking BAM3 activity to stromal and cytosolic Glc levels via DPE1 and GlcT (Li et al., 2017). The sweet11;12 mutant, which is defective in Suc loading into the phloem, accumulates Suc in the leaves and also has a sex phenotype (Chen et al., 2012b). Suc could affect the breakdown of transitory starch in two ways: via effects on the circadian clock or via the Suc-signaling metabolite trehalose 6-phosphate (T6P).
Light is usually the dominant stimulus for entrainment of the clock in natural as well as controlled environment conditions, with entrainment being affected by both the level and spectral quality of the light (Millar, 2016; Sanchez and Kay, 2016). Light perception and signaling via phytochromes is a major input, although other direct light signaling systems (e.g. cryptochromes and phototropins) might also be involved. Light can also act indirectly via effects on the rates of photosynthesis and the production of Suc, which might be a direct factor in clock entrainment or modify the effects of other entraining factors (Haydon et al., 2013). Suc appears to have relatively little impact on the expression of core clock genes themselves under standard growth conditions (Flis et al., 2016) but does under conditions where the plants are carbon-limited (Haydon et al., 2013), and it is also thought to affect the outputs from the core clock and thus clock-regulated processes such as starch degradation (Kato et al., 2007; Graf et al., 2010).
T6P is the intermediate of trehalose biosynthesis, being synthesized by T6P synthase (TPS) and then dephosphorylated to trehalose by T6P phosphatase (TPP). The Suc-T6P nexus model postulates that T6P is both a signal and negative feedback regulator of Suc levels (Lunn et al., 2006; Yadav et al., 2014), regulating the production of Suc in source leaves (Martins et al., 2013; Figueroa et al., 2016) and the utilization of Suc for growth of sink organs (Schluepmann et al., 2003; Zhang et al., 2009). In Arabidopsis and other species, tps and tpp mutants show a diverse range of phenotypic defects, demonstrating that the pathway of trehalose biosynthesis is essential for normal growth and development (for review, see Figueroa and Lunn, 2016). Induced, short-term increases in T6P lead to inhibition of transitory starch breakdown in leaves at night (Martins et al., 2013). T6P appears to act, directly or indirectly, on one of the early steps in starch degradation in the chloroplasts, but the precise molecular mechanisms are unknown. However, as T6P levels change in parallel with Suc, their inhibitory effect potentially links starch degradation to Suc levels in the leaf, which in turn are determined by the relative rates of Suc production (from starch breakdown), its export via the phloem, and its consumption by growing sink tissues (Fig. 2). Martins et al. (2013) proposed a model that envisages the maximum permissible rate of starch degradation being set by the circadian clock, as described above, but the actual rate of degradation also being determined by the levels of Suc and T6P (Fig. 2). If sink demand for Suc is low (e.g. due to low nighttime temperatures), Suc would be expected to accumulate in the leaves, triggering a rise in T6P and inhibition of starch breakdown. Conversely, if sink demand exceeds supply, leaf Suc levels will fall, as will T6P, lifting the inhibition of starch degradation and allowing the rate to accelerate up to the maximum value set by the clock. Via this feedback circuit, the plant would make best use of its starch reserves when conditions are favorable for growth but avoid wasting its starch reserves under less favorable conditions.
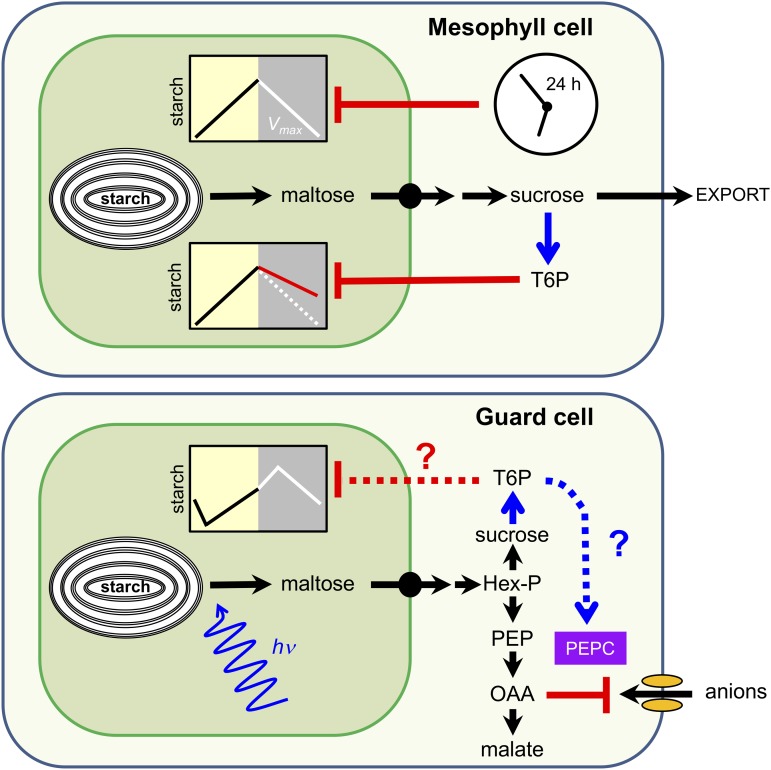
Figure 2.
Regulation of starch breakdown in mesophyll cells (top) and guard cells (bottom). Degradation of transitory starch in mesophyll cells is regulated by the circadian clock and by the Suc-signaling metabolite trehalose 6-phosphate (T6P). In a model proposed by Martins et al. (2013), the maximum allowable rate of starch degradation (Vmax) is set by the circadian clock to ensure that starch reserves are not exhausted before dawn, while the actual rate of starch degradation is linked to demand for Suc via inhibition by T6P. In guard cells, blue light (hv) is a trigger for rapid starch degradation at the beginning of the day. It is not known if starch breakdown in guard cells is regulated by the circadian clock or if it is sensitive to inhibition by T6P. However, T6P might stimulate the synthesis of malate from starch via posttranslational activation of PEPC. Known and hypothetical regulatory mechanisms are represented by solid and dashed lines, respectively; blue, activation; red, inhibition. Hex-P, Hexose phosphate; PEP, phosphoenolpyruvate; OAA, oxaloacetate. (Figure modified from Martins et al. [2013].)
The influence of T6P on starch metabolism in guard cells has so far not been studied. By extrapolation, we might speculate that T6P also inhibits the rapid starch degradation that occurs in guard cells upon illumination, perhaps preventing unnecessary starch turnover if the guard cells have sufficient Suc to drive stomatal opening (Fig. 2; Lawson et al., 2014; Daloso et al., 2016). However, given the different functions, diurnal patterns of turnover and degradative pathways in guard cells compared to mesophyll cells (Valerio et al., 2011; Prasch et al., 2015; Horrer et al., 2016), differences in the sensitivity of starch degradation to T6P are also possible. For example, the alternative pathway of starch degradation in guard cells, via BAM1 and AMY3, might render the process insensitive to T6P. There are other ways in which T6P might influence guard cell metabolism and stomatal function. For example, T6P might stimulate the synthesis of malate from starch via posttranslational activation of PEPC (Figueroa et al., 2016). In Arabidopsis, the sensitivity of stomata to abscisic acid (ABA) is also dependent on AtTPS1, AtTPPG, and AtTREHALASE1, which are particularly highly expressed in guard cells (Gómez et al., 2010; Vandesteene et al., 2010; Van Houtte et al., 2013). Constitutive changes in TPS or TPP activity lead to highly pleiotropic phenotypes that are difficult to interpret (Schluepmann et al., 2003; Yadav et al., 2014), so guard cell-specific manipulations of these enzymes, and thus the levels of T6P, will be required to investigate the contribution of T6P to regulation of starch degradation and stomatal function more generally.
IMPLICATIONS OF GUARD CELL STARCH METABOLISM FOR STOMATAL MOVEMENTS
Impaired guard cell starch metabolism has several consequences for stomatal function. In Arabidopsis, absence of BAM1 leads to reduced stomatal aperture and slower increase in stomatal conductance upon transition to light (Horrer et al., 2016). This response is exacerbated in the double mutant amy3bam1, which shows almost complete suppression of stomatal responses and reduced photosynthetic assimilation and growth, especially under higher light intensities (Horrer et al., 2016). The fact that the intercellular CO2 concentration in the stomatal cavity of the amy3bam1 mutant is also significantly lower than in wild type leads to the conclusion that defective stomatal opening in this mutant most likely limits CO2 availability for photosynthesis in the mesophyll cells. In a separate study, it was shown that soil-grown bam1 mutants subjected to a mild water deficit have improved stress tolerance and higher biomass production than wild-type plants (Prasch et al., 2015). In response to drought stress, wild-type plants partially closed their stomata, as expected, and appeared to simultaneously accumulate higher levels of starch in guard cells. In contrast, the starch content of bam1 guard cells remained high and the stomata stayed closed (Prasch et al., 2015). By conducting guard cell-specific microarray analysis, the same authors also showed significant down-regulation of genes encoding cell wall-modifying enzymes, aquaporins, and auxin response factors in bam1 mutant plants under drought stress, suggesting reduced water uptake and limited cell wall expansion (Prasch et al., 2015). These alterations are likely to affect guard cell osmotic/turgor pressure, driving the guard cell hydraulics toward stomatal closure. Altogether, these studies demonstrate that regulation of guard cell starch degradation is important for adapting stomatal opening to environmental stimuli (e.g. light intensity and abiotic stress), and its manipulation may result in crop plants with better water use efficiency.
Guard cell starch metabolism has also been implicated in high-CO2-induced stomatal closure. In a recent study, stomatal responses to shifts in [CO2] were compared in Arabidopsis mutants that are either (1) starch deficient in all plant tissues (aps1 mutants lacking the small subunit of the ADPglucose pyrophosphorylase; AGPase); or (2) accumulate starch in guard cells but not in mesophyll cells (pgi lacking the plastidial phosphoglucose isomerase that converts Fru 6-phosphate to Glc6P; Azoulay-Shemer et al., 2016). The authors demonstrated that stomatal closure upon high CO2 treatment was impaired in aps1, but not in pgi, suggesting that starch biosynthesis in guard cells, but not in mesophyll cells, is crucial for this response.
How starch is synthesized in guard cells is poorly understood (Santelia and Lawson, 2016). It is plausible that sugars and/or organic acids that have accumulated early in the day are converted back to starch via gluconeogenesis. During stomatal closure, starch may therefore act as a sink for osmolytes, promoting the necessary changes in guard cell turgor for water efflux. In support of this, early experiments with radiolabeled malate in isolated Commelina communis epidermis showed that starch formation from malic acid occurs during stomatal closure (Dittrich and Raschke, 1977). However, when stomatal closure was accelerated by ABA, guard cells lost most of the labeled malate to the medium within the first 5 min. These experiments suggest that gluconeogenesis may not be fast enough to remove malate when the loss of turgor occurs quickly, such as in response to ABA or high [CO2]. More complex explanations can be postulated. For example, some ion transport process needed for altering guard cell turgor might be specifically perturbed in the aps1 mutant, or there might be an overall imbalance in osmolyte content due to the lack of starch. Future research will be needed to examine these intriguing hypotheses and further validate the role of guard cell starch during stomatal closure.
INTEGRATION OF GUARD CELL STARCH METABOLISM WITH MEMBRANE ION TRANSPORT AND LIGHT SIGNALING
One of the most interesting aspects of guard cell starch metabolism relates to its previously unsuspected connection with guard cell signaling and ion transport. We now know that blue light is a potent activator of starch degradation in guard cells. This effect is restricted to this cell-type and depends on the PHOTOTROPIN1 (PHOT1) and PHOT2 blue light photoreceptors and their downstream signaling components (Horrer et al., 2016). Particularly relevant here is the observation that guard cell starch breakdown is severely inhibited in plants lacking the plasma membrane H+-ATPase1 (Horrer et al., 2016), which is likely the ultimate target of the blue-light signaling cascade (Inoue and Kinoshita, 2017). Experiments with fusicoccin (Fc), a fungal toxin that constitutively activates the proton pump, showed that stomatal opening and guard cell starch mobilization were restored in the phot1phot2 mutant, but not in the amy3bam1 (Horrer et al., 2016). These results clearly place the H+-ATPase upstream of the starch-degrading enzymes in the blue-light-signaling cascade in guard cells. However, the significance of this coordination is not clear. In our current model, starch is postulated to be converted to malate during stomatal opening to allow balancing of positive charges associated with proton pumping and K+ uptake, while helping to increase cell turgor for the swelling of the guard cells (Jezek and Blatt 2017; Eisenach and de Angeli, 2017; Vavasseur and Raghavendra, 2005; Lawson, 2009; Hills et al., 2012; Chen et al., 2012a). This model is based on early publications in the field, mostly correlative in nature and would need further biochemical validation. Nonetheless, it is conceivable that H+-ATPase exerts feedback inhibition on starch metabolism to avoid excessive H+ production associated with the accumulation of malate, which would lead to acidification of the cytosol. Given that proton pumping and starch degradation are physically separated by the transport barrier of the chloroplast envelope, it is difficult to imagine how this process might take place. It was recently shown that oxaloacetate (OAA) is a strong inhibitor of guard cell anion channels (Fig. 2; Wang and Blatt, 2011). During stomatal opening, OAA is formed by carboxylation of PEP, via PEPC, in the cytosol and then reduced to malate by NAD+-dependent malate dehydrogenase (Scheibe et al., 1990). OAA can also be transported across the chloroplast envelope by the 2-oxoglutarate/malate transporter (H. Kinoshita et al., 2011) or into mitochondria via the mitochondrial dicarboxylate transporter. As both the intermediate of malate synthesis and a potent inhibitor of guard cell anion channels, OAA appears to be a strong candidate for integrating anion fluxes with metabolism in guard cells through a feedback circuit (Fig. 2), and experiments to test this hypothesis should be a priority for the future.
CONCLUSION
In Arabidopsis guard cells, transitory starch follows a distinct pattern of synthesis and degradation that differs from mesophyll cells and reflects its specialized function in driving rapid stomatal opening in the light. Despite the great wealth of information that became available in the last few years, several questions still remain open, and many others are emerging (see Outstanding Questions). Development of novel methods that would allow “absolute” quantification of guard cell metabolism, including starch turnover, would be a major advance. To accelerate progress, it will be necessary to take an integrated approach, combining experimental data from guard cell-specific manipulation of enzyme and transporter activities with systems-level modeling of guard cell metabolism and transport activities. To meet such a challenge, scientists with different, complementary areas of expertise will have to work together in fruitful collaborations. It has been said of stomatal research that when one works on a hole, there is a danger of falling in. Our aim in this review has been to highlight the exciting recent advances in our understanding of guard cell metabolism, with the hope of persuading young researchers to fall in love with stomata, and take up the challenge to explore their fascinating biology.
Footnotes
This work was supported by the Swiss National Science Foundation (grant no. SNSF-Grant 31003A_166539 to D.S.) and the Max Planck Society (J.E.L.).
Articles can be viewed without a subscription.
References
- Avin-Wittenberg T, Bajdzienko K, Wittenberg G, Alseekh S, Tohge T, Bock R, Giavalisco P, Fernie AR (2015) Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell 27: 306–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay-Shemer T, Bagheri A, Wang C, Palomares A, Stephan AB, Kunz HH, Schroeder JI (2016) Starch biosynthesis in guard cells but not in mesophyll cells is involved in CO2-induced stomatal closing. Plant Physiol 171: 788–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerenfaller K, Shu H, Hirsch-Hoffmann M, Fütterer J, Opitz L, Rehrauer H, Hennig L, Gruissem W (2016) Diurnal changes in the histone H3 signature H3K9ac|H3K27ac|H3S28p are associated with diurnal gene expression in Arabidopsis. Plant Cell Environ 39: 2557–2569 [DOI] [PubMed] [Google Scholar]
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible W-R, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow A, Engelsen SB (2010) Helix-breaking news: Fighting crystalline starch energy deposits in the cell. Trends Plant Sci 15: 236–240 [DOI] [PubMed] [Google Scholar]
- Chen Z-H, Hills A, Bätz U, Amtmann A, Lew VL, Blatt MR (2012a) Systems dynamic modeling of the stomatal guard cell predicts emergent behaviors in transport, signaling, and volume control. Plant Physiol 159: 1235–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-Q, Qu X-Q, Hou B-H, Sosso D, Osorio S, Fernie AR, Frommer WB (2012b) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211 [DOI] [PubMed] [Google Scholar]
- Chia T, Thorneycroft D, Chapple A, Messerli G, Chen J, Zeeman SC, Smith SM, Smith AM (2004) A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J 37: 853–863 [DOI] [PubMed] [Google Scholar]
- Cho MH, Lim H, Shin DH, Jeon JS, Bhoo SH, Park YI, Hahn TR (2011) Role of the plastidic glucose translocator in the export of starch degradation products from the chloroplasts in Arabidopsis thaliana. New Phytol 190: 101–112 [DOI] [PubMed] [Google Scholar]
- Comparot-Moss S, Kötting O, Stettler M, Edner C, Graf A, Weise SE, Streb S, Lue W-L, MacLean D, Mahlow S, et al. (2010) A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol 152: 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26: 89–100 [DOI] [PubMed] [Google Scholar]
- Daloso DM, Williams TCR, Antunes WC, Pinheiro DP, Müller C, Loureiro ME, Fernie AR (2016) Guard cell-specific upregulation of sucrose synthase 3 reveals that the role of sucrose in stomatal function is primarily energetic. New Phytol 209: 1470–1483 [DOI] [PubMed] [Google Scholar]
- de la Fuente van Bentem S, Anrather D, Dohnal I, Roitinger E, Csaszar E, Joore J, Buijnink J, Carreri A, Forzani C, Lorkovic ZJ, et al. (2008) Site-specific phosphorylation profiling of Arabidopsis proteins by mass spectrometry and peptide chip analysis. J Proteome Res 7: 2458–2470 [DOI] [PubMed] [Google Scholar]
- Delatte T, Umhang M, Trevisan M, Eicke S, Thorneycroft D, Smith SM, Zeeman SC (2006) Evidence for distinct mechanisms of starch granule breakdown in plants. J Biol Chem 281: 12050–12059 [DOI] [PubMed] [Google Scholar]
- Dittrich P, Raschke K (1977) Malate metabolism in isolated epidermis of Commelina communis L. in relation to stomatal functioning. Planta 134: 77–81 [DOI] [PubMed] [Google Scholar]
- Edner C, Li J, Albrecht T, Mahlow S, Hejazi M, Hussain H, Kaplan F, Guy C, Smith SM, Steup M, et al. (2007) Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases. Plant Physiol 145: 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach C, de Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol 174: 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Feil R, Ishihara H, Watanabe M, Kölling K, Krause U, Höhne M, Encke B, Plaxton WC, Zeeman SC, et al. (2016) Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J 85: 410–423 [DOI] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE (2016) A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol 172: 7–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flis A, Sulpice R, Seaton DD, Ivakov AA, Liput M, Abel C, Millar AJ, Stitt M (2016) Photoperiod-dependent changes in the phase of core clock transcripts and global transcriptional outputs at dawn and dusk in Arabidopsis. Plant Cell Environ 39: 1955–1981 [DOI] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, Vaughan CK, Li J, Francisco P, Gil M, Reinhold H, Eicke S, Messerli G, et al. (2008) Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell 20: 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bläsing OE, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M (2004) Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Glaring MA, Zygadlo A, Thorneycroft D, Schulz A, Smith SM, Blennow A, Baunsgaard L (2007) An extra-plastidial α-glucan, water dikinase from Arabidopsis phosphorylates amylopectin in vitro and is not necessary for transient starch degradation. J Exp Bot 58: 3949–3960 [DOI] [PubMed] [Google Scholar]
- Gómez LD, Gilday A, Feil R, Lunn JE, Graham IA (2010) AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J 64: 1–13 [DOI] [PubMed] [Google Scholar]
- Graf A, Schlereth A, Stitt M, Smith AM (2010) Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A, Smith AM (2011) Starch and the clock: The dark side of plant productivity. Trends Plant Sci 16: 169–175 [DOI] [PubMed] [Google Scholar]
- Harmer SL. (2009) The circadian system in higher plants. Annu Rev Plant Biol 60: 357–377 [DOI] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290: 2110–2113 [DOI] [PubMed] [Google Scholar]
- Haydon MJ, Mielczarek O, Robertson FC, Hubbard KE, Webb AAR (2013) Photosynthetic entrainment of the Arabidopsis thaliana circadian clock. Nature 502: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Haebel S, Edner C, Paris O, Frohberg C, Steup M, Ritte G (2008) Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J 55: 323–334 [DOI] [PubMed] [Google Scholar]
- Hejazi M, Fettke J, Paris O, Steup M (2009) The two plastidial starch-related dikinases sequentially phosphorylate glucosyl residues at the surface of both the A- and B-type allomorphs of crystallized maltodextrins but the mode of action differs. Plant Physiol 150: 962–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi M, Mahlow S, Fettke J (2014) The glucan phosphorylation mediated by α-glucan, water dikinase (GWD) is also essential in the light phase for a functional transitory starch turnover. Plant Signal Behav 9: e28892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills A, Chen Z-H, Amtmann A, Blatt MR, Lew VL (2012) OnGuard, a computational platform for quantitative kinetic modeling of guard cell physiology. Plant Physiol 159: 1026–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrer D, Flütsch S, Pazmino D, Matthews JSA, Thalmann M, Nigro A, Leonhardt N, Lawson T, Santelia D (2016) Blue light induces a distinct starch degradation pathway in guard cells for stomatal opening. Curr Biol 26: 362–370 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Murakami M, Nakamura Y, Ito S, Nakamichi N, Yamashino T, Mizuno T (2007) Mutants of circadian-associated PRR genes display a novel and visible phenotype as to light responses during de-etiolation of Arabidopsis thaliana seedlings. Biosci Biotechnol Biochem 71: 834–839 [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Nagasaki J, Yoshikawa N, Yamamoto A, Takito S, Kawasaki M, Sugiyama T, Miyake H, Weber APM, Taniguchi M (2011) The chloroplastic 2-oxoglutarate/malate transporter has dual function as the malate valve and in carbon/nitrogen metabolism. Plant J 65: 15–26 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y, Ohnishi M, Nakano T, Inoue S, et al. (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: Linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Kötting O, Kossmann J, Zeeman SC, Lloyd JR (2010) Regulation of starch metabolism: The age of enlightenment? Curr Opin Plant Biol 13: 321–329 [DOI] [PubMed] [Google Scholar]
- Kötting O, Santelia D, Edner C, Eicke S, Marthaler T, Gentry MS, Comparot-Moss S, Chen J, Smith AM, Steup M, et al. (2009) STARCH-EXCESS4 is a laforin-like Phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 21: 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. (2009) Guard cell photosynthesis and stomatal function. New Phytol 181: 13–34 [DOI] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL Baker NR (2002) Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiol 128: 52–62 [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL Baker NR (2003) The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J Exp Bot 54: 1743–1752 [DOI] [PubMed] [Google Scholar]
- Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203: 1064–1081 [DOI] [PubMed] [Google Scholar]
- Li J, Zhou W, Francisco P, Wong R, Zhang D, Smith SM (2017) Inhibition of Arabidopsis chloroplast β-amylase BAM3 by maltotriose suggests a mechanism for the control of transitory leaf starch mobilisation. PLoS One 12: e0172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd F. (1908) The behaviour of stomata. In Carnegie Institute of Wasington Publication, No. 82. Carnegie Institute of Washington, Washington, DC [Google Scholar]
- Lloyd JR, Kossmann J (2015) Transitory and storage starch metabolism: Two sides of the same coin? Curr Opin Biotechnol 32: 143–148 [DOI] [PubMed] [Google Scholar]
- Lohrig K, Müller B, Davydova J, Leister D, Wolters DA (2009) Phosphorylation site mapping of soluble proteins: Bioinformatical filtering reveals potential plastidic phosphoproteins in Arabidopsis thaliana. Planta 229: 1123–1134 [DOI] [PubMed] [Google Scholar]
- Lu Y, Gehan JP, Sharkey TD (2005) Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol 138: 2280–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Steichen JM, Yao J, Sharkey TD (2006) The role of cytosolic α-glucan phosphorylase in maltose metabolism and the comparison of amylomaltase in Arabidopsis and Escherichia coli. Plant Physiol 142: 878–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible W-R, Carillo P, Hajirezaei M-R, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinova I, Steup M, Fettke J (2013) Carbon transitions from either Calvin cycle or transitory starch to heteroglycans as revealed by (14) C-labeling experiments using protoplasts from Arabidopsis. Physiol Plant 149: 25–44 [DOI] [PubMed] [Google Scholar]
- Martins MCM, Hejazi M, Fettke J, Steup M, Feil R, Krause U, Arrivault S, Vosloh D, Figueroa CM, Ivakov A, et al. (2013) Feedback inhibition of starch degradation in Arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiol 163: 1142–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R, Mutenda KE, Mant A, Schürmann P, Blennow A (2005) α-Glucan, water dikinase (GWD): A plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc Natl Acad Sci USA 102: 1785–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AJ. (2016) The intracellular dynamics of circadian clocks reach for the light of ecology and evolution. Annu Rev Plant Biol 67: 595–618 [DOI] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S (2015) Diverse stomatal signaling and the signal integration mechanism. Annu Rev Plant Biol 66: 369–392 [DOI] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- O’Neill EC, Stevenson CEM, Tantanarat K, Latousakis D, Donaldson MI, Rejzek M, Nepogodiev SA, Limpaseni T, Field RA, Lawson DM (2015) Structural dissection of the maltodextrin disproportionation cycle of the Arabidopsis plastidial disproportionating enzyme 1 (DPE1). J Biol Chem 290: 29834–29853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister B, Zeeman SC (2016) Formation of starch in plant cells. Cell Mol Life Sci 73: 2781–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington SM, Encke B, Krohn N, Höhne M, Stitt M, Pyl ET (2015) Relationship between starch degradation and carbon demand for maintenance and growth in Arabidopsis thaliana in different irradiance and temperature regimes. Plant Cell Environ 38: 157–171 [DOI] [PubMed] [Google Scholar]
- Prasch CM, Ott KV, Bauer H, Ache P, Hedrich R, Sonnewald U (2015) β-amylase1 mutant Arabidopsis plants show improved drought tolerance due to reduced starch breakdown in guard cells. J Exp Bot 66: 6059–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost S, Pattyn P, Gerats T, Van de Peer Y (2011) Journey through the past: 150 million years of plant genome evolution. Plant J 66: 58–65 [DOI] [PubMed] [Google Scholar]
- Pyl E-T, Piques M, Ivakov A, Schulze W, Ishihara H, Stitt M, Sulpice R (2012) Metabolism and growth in Arabidopsis depend on the daytime temperature but are temperature-compensated against cool nights. Plant Cell 24: 2443–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiland S, Messerli G, Baerenfaller K, Gerrits B, Endler A, Grossmann J, Gruissem W, Baginsky S (2009) Large-scale Arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks. Plant Physiol 150: 889–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H, Soyk S, Simková K, Hostettler C, Marafino J, Mainiero S, Vaughan CK, Monroe JD, Zeeman SC (2011) β-Amylase-like proteins function as transcription factors in Arabidopsis, controlling shoot growth and development. Plant Cell 23: 1391–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Heydenreich M, Mahlow S, Haebel S, Kötting O, Steup M (2006) Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett 580: 4872–4876 [DOI] [PubMed] [Google Scholar]
- Ritte G, Scharf A, Eckermann N, Haebel S, Steup M (2004) Phosphorylation of transitory starch is increased during degradation. Plant Physiol 135: 2068–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez SE, Kay SA (2016) The plant circadian clock: from a simple timekeeper to a complex developmental manager. Cold Spring Harb Perspect Biol 8: 027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Kötting O, Seung D, Schubert M, Thalmann M, Bischof S, Meekins DA, Lutz A, Patron N, Gentry MS, et al. (2011) The phosphoglucan phosphatase like sex Four2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell 23: 4096–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Lawson T (2016) Rethinking guard cell metabolism. Plant Physiol 172: 1371–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Reckmann U, Hedrich R, Raschke K (1990) Malate dehydrogenases in guard cells of Pisum sativum. Plant Physiol 93: 1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M (2003) Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialdone A, Mugford ST, Feike D, Skeffington A, Borrill P, Graf A, Smith AM, Howard M (2013) Arabidopsis plants perform arithmetic division to prevent starvation at night. eLife 2: e00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seung D, Thalmann M, Sparla F, Abou Hachem M, Lee SK, Issakidis-Bourguet E, Svensson B, Zeeman SC, Santelia D (2013) Arabidopsis thaliana AMY3 is a unique redox-regulated chloroplastic α-amylase. J Biol Chem 288: 33620–33633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DM, Silva LP, Issakidis-Bourguet E, Glaring MA, Schriemer DC, Moorhead GBG (2013) Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J 280: 538–548 [DOI] [PubMed] [Google Scholar]
- Skeffington AW, Graf A, Duxbury Z, Gruissem W, Smith AM (2014) Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Plant Physiol 165: 866–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fulton DC, Chia T, Thorneycroft D, Chapple A, Dunstan H, Hylton C, Zeeman SC, Smith AM (2004) Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol 136: 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparla F, Costa A, Lo Schiavo F, Pupillo P, Trost P (2006) Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis. Plant Physiol 141: 840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Zeeman SC (2012) Starch turnover: Pathways, regulation and role in growth. Curr Opin Plant Biol 15: 282–292 [DOI] [PubMed] [Google Scholar]
- Streb S, Eicke S, Zeeman SC (2012) The simultaneous abolition of three starch hydrolases blocks transient starch breakdown in Arabidopsis. J Biol Chem 287: 41745–41756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb S, Zeeman SC (2012) Starch metabolism in Arabidopsis. Arabidopsis Book 10: e0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Nakagami H, Mochida K, Daudi A, Tomita M, Shirasu K, Ishihama Y (2008) Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol Syst Biol 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Flis A, Ivakov AA, Apelt F, Krohn N, Encke B, Abel C, Feil R, Lunn JE, Stitt M (2014) Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol Plant 7: 137–155 [DOI] [PubMed] [Google Scholar]
- Sulpice R, Pyl ET, Ishihara H, Trenkamp S, Steinfath M, Witucka-Wall H, Gibon Y, Usadel B, Poree F, Piques MC, et al. (2009) Starch as a major integrator in the regulation of plant growth. Proc Natl Acad Sci USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio C, Costa A, Marri L, Issakidis-Bourguet E, Pupillo P, Trost P, Sparla F (2011) Thioredoxin-regulated beta-amylase (BAM1) triggers diurnal starch degradation in guard cells, and in mesophyll cells under osmotic stress. J Exp Bot 62: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houtte H, Vandesteene L, López-Galvis L, Lemmens L, Kissel E, Carpentier S, Feil R, Avonce N, Beeckman T, Lunn JE, et al. (2013) Overexpression of the trehalase gene AtTRE1 leads to increased drought stress tolerance in Arabidopsis and is involved in abscisic acid-induced stomatal closure. Plant Physiol 161: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesteene L, Ramon M, Le Roy K, Van Dijck P, Rolland F (2010) A single active trehalose-6-P synthase (TPS) and a family of putative regulatory TPS-like proteins in Arabidopsis. Mol Plant 3: 406–419 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2005) Guard cell metabolism and CO2 sensing. New Phytol 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Wang Y, Blatt MR (2011) Anion channel sensitivity to cytosolic organic acids implicates a central role for oxaloacetate in integrating ion flux with metabolism in stomatal guard cells. Biochem J 439: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR. (2003) The physiology of circadian rhythms in plants. New Phytol 160: 281–303 [DOI] [PubMed] [Google Scholar]
- Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, et al. (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65: 1051–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Kofler H, Häusler RE, Hille D, Flügge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, et al. (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13: 1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T-S, Zeeman SC, Thorneycroft D, Fulton DC, Dunstan H, Lue W-L, Hegemann B, Tung S-Y, Umemoto T, Chapple A, et al. (2005) α-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J Biol Chem 280: 9773–9779 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dunstan H, Haldimann P, Bechtold N, Smith AM, Smith SM (2004) Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol 135: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]