Abstract
Cholinergic neurotransmission plays a key role in learning and memory. Prior research with rats indicated that a low dose of pre-training scopolamine (0.1 mg/kg), a cholinergic receptor antagonist, did not affect cued fear conditioning, but did block renewal when injected before extinguishing a conditioned tone, opening up opportunities to pharmacologically improve exposure therapy for anxiety patients. Before translating these findings to the clinic, it is important to carefully examine how scopolamine affects contextual fear memories. Here, we investigated the effects of scopolamine on encoding of contextual anxiety and its generalization in male Wistar rats. We found a profound disruption of context conditioning, suggesting that, even at a low dose, systemic scopolamine may influence contextual encoding in the hippocampus, particularly when the context is the best predictor for the presence of shocks.
Keywords: Context conditioning, contextual fear, generalization, anxiety, rats, scopolamine
Introduction
Anxiety disorders are associated with significant disability and poor quality of life, but their pathophysiological mechanisms are only beginning to be understood. Contextual and cued fear conditioning procedures are valuable tools for in-depth studies of the neurobiology of anxiety, which may open up new treatment avenues (McNally, 2007; Kindt, 2014; Mineka and Zinbarg, 2006; Fanselow, 2000; Walker and Davis, 1997). Contextual anxiety, in particular, mimics some aspects of the typical free-floating anticipatory anxiety in unpredictable situations that is seen in several anxiety disorders (Luyten et al., 2011). Another core characteristic of anxiety disorders, and a key element of what makes them so disabling, is generalization, i.e., the ready transfer of anxiety acquired for one situation to similar situations (Luyten et al., 2016). Here, we manipulated cholinergic transmission during the encoding of contextual anxiety. More specifically, we focused on the effect of mild muscarinic antagonism on the subsequent expression and generalization of contextual anxiety.
Cholinergic neurotransmission has been widely implicated in learning and memory processes, particularly in the acquisition of new information (van der Zee and Luiten, 1999). Scopolamine, a muscarinic cholinergic receptor antagonist, is a well-studied compound (Klinkenberg and Blokland, 2010) with more pronounced effects on contextual than on cued fear in adult rats, presumably mediated by a cholinergic blockade in the hippocampus (Anagnostaras et al., 1995; Anagnostaras et al., 1999; Brown et al., 2011). Accordingly, intrahippocampal infusion of scopolamine has even more manifest behavioral effects than systemic administration (Chang and Liang, 2012; Gale et al., 2001; Wallenstein and Vago, 2001). A dose-effect analysis of systemic scopolamine found that a low dose (0.1 mg/kg), in contrast to higher doses, did not affect postshock freezing during cued fear conditioning, nor subsequent freezing in the training context or to the conditioned tone (Anagnostaras et al., 1999). A recent study, however, observed that the same dose did influence contextualization of extinction. In particular, it was shown that 0.1 mg/kg systemic scopolamine renders tone fear extinction learning context-independent, and probably hippocampus-independent, thereby blocking subsequent renewal (Zelikowsky et al., 2013).
Here, we examined the effect of 0.1 mg/kg systemic scopolamine administered before context conditioning. The control group received scopolamine after conditioning, which we expected to have no effects (Zelikowsky et al., 2013; Anagnostaras et al., 1999; Kroon and Carobrez, 2009). Tests assessing the expression and generalization of contextual anxiety occurred on the second (drug-free) day. We hypothesized that low-dose systemic scopolamine would have no acute behavioral effects during acquisition nor effects on subsequent freezing in the training context (cf (Anagnostaras et al., 1999)), but that it would change the nature of context learning (cf the ‘decontextualization’ concept described by (Zelikowsky et al., 2013)), resulting in increased anxiety in a generalization context that resembles the training context. The generalization angle has clinical relevance because systemic scopolamine has been put forward as an adjunct to exposure therapy for anxiety patients (Zelikowsky et al., 2013). Such pharmacological enhancers of psychotherapy, although potentially life-changing, should be used with caution, as interfering with memory processes may have unwanted side effects (Bowers and Ressler, 2015). If cholinergic antagonism not only enhances generalization of extinction, but also increases generalization of fearful memories, this could be a disadvantage of scopolamine if a patient received it preceding an anxiety-evoking, ‘unsuccessful’ therapy session. Therefore, this study investigated the effect of the same dose of pre-training scopolamine that has been shown to prevent contextualization of extinction learning on the expression and generalization of contextual anxiety.
Materials & Methods
Forty-eight male Wistar rats (±275 g at the time of training, obtained from Janvier Labs, France), were used for all experiments, which were approved by the KU Leuven animal ethics committee, in accordance with the Belgian Royal Decree of 29/05/2013 and European Directive 2010/63/EU. Animals were housed in pairs in cages with cage dividers and maintained on a 14h/10h light/dark cycle. All experimental sessions were scheduled using free ExpTimer software (Luyten and Van Cappellen, 2013).
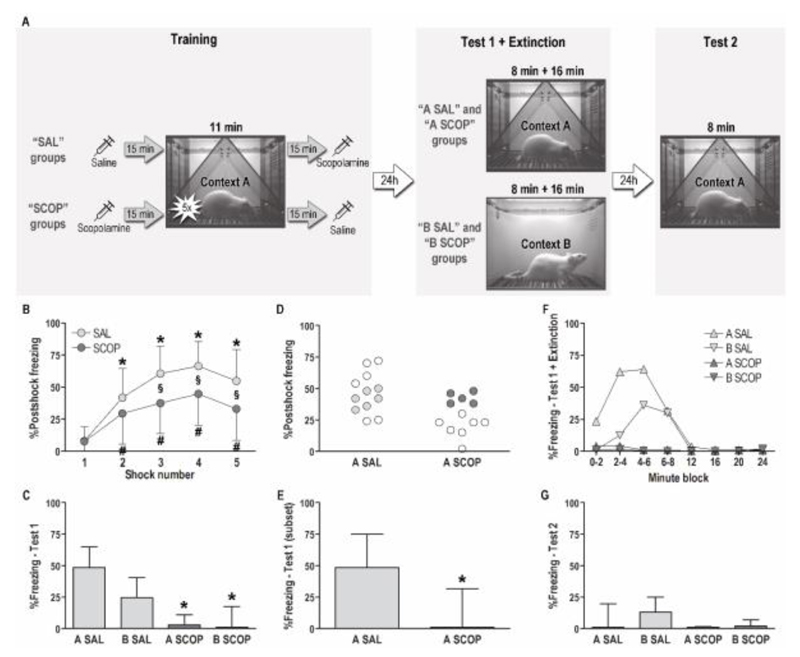
We recently developed a contextual generalization procedure for rats (Luyten et al., 2014; Luyten et al., 2016), which was extended for this study with an extinction phase and an additional spontaneous recovery/renewal test day (Fig. 1A). In brief, rats were trained in context A and afterwards tested in this context or in a perceptually similar generalization context B. Details regarding the setup, which consisted of two separate test chambers (Med Associates, USA) equipped with different grid floors, plastic chamber inserts, odors and lighting conditions, have been described previously (Luyten et al., 2014; Luyten et al., 2016). Four minutes after the start of the Training session, rats received 5 unsignaled footshocks (0.8 mA, 1 s), separated by 90 s. One minute after the last shock, animals were returned to their home cage. Twenty-four hours later, half of the rats were tested in context A and the other half in similar context B. During this test (Test 1), rats were exposed to the context for 8 minutes, without shocks (cf Luyten et al., 2016). Test 1 was immediately followed by a 16-minute extinction phase (Extinction). One day later, all animals were tested in context A for 8 minutes (Test 2). Freezing during training and the average motion index during test were measured with VideoFreeze software (Med Associates). Freezing (i.e., total absence of movement, with the exception of respiratory movements) during test was measured manually by two trained observers who were blind to the experimental condition (continuous measurement with a stopwatch from video recordings), as previous findings indicated that comparison of software-scored freezing in different contexts was not reliable (Luyten et al., 2014).
Fig. 1. The effects of 0.1 mg/kg pre-training scopolamine in a contextual generalization procedure.
(A) Study design. N = 12 per group, 48 rats in total. (B) %Freezing (mean ± SD) after each shock during the Training session in rats that received an intraperitoneal pre-training saline (SAL) or 0.1 mg/kg scopolamine (SCOP) injection, *significantly higher than after the first shock in SAL rats, #significantly higher than after the first shock in SCOP rats, §significantly different between SAL and SCOP rats (p ≤ .01). (C) %Freezing (median + interquartile range) during the 8-minute Test 1 session, *significantly lower than A SAL rats (p < .01). (D) %Freezing after shocks for A SAL and A SCOP rats. Colored circles indicate animals that were included in the subset of rats with postshock freezing scores close to the average of saline rats. (E) %Freezing (median + interquartile range) during the 8-minute Test 1 in the subset of six A SAL and five A SCOP rats with ‘average’ postshock freezing scores, *significantly lower than A SAL rats (p < .01). (F) %Freezing (median) during the 8-minute Test 1 and subsequent 16-minute Extinction phase. (G) %Freezing (median + interquartile range) during the 8-minute Test 2.
The aim of this study was to investigate the effects of pre-training scopolamine on the expression, generalization, extinction and spontaneous recovery or renewal of contextual anxiety. Fresh drug solutions were prepared daily. Half of the rats (groups A SCOP and B SCOP) received an intraperitoneal injection (0.1 mg/kg in 1 ml/kg) of scopolamine dissolved in saline (Scopolamine HBr Sterop, Brussels, Belgium) 15 minutes before the start of Training and an intraperitoneal injection of saline (1 ml/kg) 15 minutes after the end of Training. The other half of the rats (groups A SAL and B SAL) served as controls and received a pre-training injection of saline and a post-training injection of scopolamine. This design allowed for a specific examination of the effects of muscarinic antagonism on encoding, largely cancelling out potential (additional) effects on consolidation, and equating all animals for drug exposure on the Training day. Based upon previous studies (Anagnostaras et al., 1999), we expected no acute behavioral effects (on baseline or postshock freezing during Training) of the 0.1 mg/kg dose of scopolamine and no effects of scopolamine on contextual freezing on Test 1 in the rats that were re-exposed to context A (i.e., A SCOP = A SAL). Our main hypothesis was that the cholinergic antagonism during encoding, which presumably made the learning hippocampus-independent (cf (Zelikowsky et al., 2013)), would result in increased generalization in context B (i.e., B SCOP > B SAL). We had no clear-cut predictions regarding the effects on extinction and spontaneous recovery/renewal, but we made these additions to the protocol to better understand the effects of pre-training scopolamine on generalized anxiety.
Unexpectedly, we noticed that freezing on Test 1 was quite low in both A SAL and B SAL groups. To investigate this in more detail, we compared the data from this study with historical control groups. These control groups consisted of animals (N = 78 in total) that were either naïve or had received a tail vein puncture and a systemic saline injection or oral water administration within two minutes after training (Luyten et al., 2016). To evaluate freezing during Test 1 in A SAL and B SAL animals in the current study as compared with control rats in our previous experiments, we conducted a factorial ANOVA with factors Experiment (previous and current) and Context (A and B).
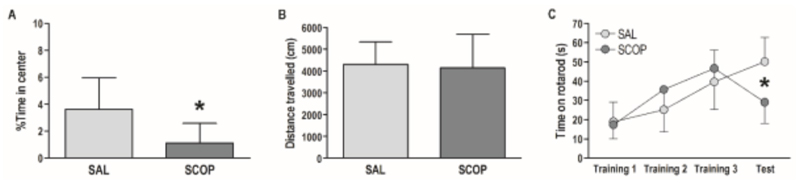
To further characterize the acute behavioral effects of 0.1 mg/kg scopolamine, we also conducted tests in the open field and on the accelerating rotarod. We hypothesized that this low dose of scopolamine would have no acute behavioral effects in either of these tests. One day after Test 2, 16 of the animals that had taken part in the main experiment received a 0.1 mg/kg scopolamine or saline injection 15 minutes before being introduced in the open field (80 cm x 80 cm) for 10 minutes. Percentage time spent in the center (25% of the central surface of the open field) and total distance travelled were calculated with in-house developed software (Luyck et al., in press). Another 16 of the animals from the main experiment were trained and tested on the accelerating rotarod (from 0 to 40 rpm in 4 minutes) (IITC Life Science, USA). The first session took place 5 days after Test 2 of the main experiment. Rats first received 3 days of rotarod training (3 consecutive trials on each day), followed by 1 day with the actual rotarod test (3 consecutive trials), 15 minutes earlier preceded by a 0.1 mg/kg scopolamine or saline injection. The time until falling off the rotarod was calculated as the average of the 3 daily trials.
For statistical analyses (Statistica 12, StatSoft), parametric tests (unpaired t-test, repeated-measures ANOVA with Tukey’s posthoc tests or factorial ANOVA) were used if all assumptions (normality, homoscedasticity, sphericity) were met. Data analyzed with parametric tests are graphically presented (Prism 7, GraphPad Software) as means with standard deviation. Grubbs’ tests were used to detect outliers. Data sets that did not meet one or more assumptions for parametric tests, were analyzed using non-parametric alternatives (Mann-Whitney U test, Kruskal-Wallis ANOVA by ranks with multiple comparisons or Wilcoxon matched-pairs test). These data are depicted as medians with interquartile range. All analyses were conducted with the significance level set at p < .05.
Results
We hypothesized that 0.1 mg/kg scopolamine would have no acute behavioral effects during the training session. However, while baseline freezing was low (mean ± standard deviation: 1 ± 3%) in all groups during the first 4 minutes of the session, scopolamine rats showed significantly less postshock freezing than control rats (Fig. 1B). A repeated-measures ANOVA analyzing freezing after shocks 1 to 5 showed a significant main effect of drug (F(1,46) = 12.01, p < .01), a main effect of shock number (F(4,184) = 67.40, p < .0001) and a significant interaction between both (F(4,184) = 4.55, p < .01). Tukey’s posthoc tests (all p’s ≤ .01) indicated that the overall lower postshock freezing in the scopolamine versus control rats seemed to be a rather consistent effect, with scopolamine rats reaching lower maximal freezing levels than control rats.
On Test 1, we expected significantly less freezing in B SAL rats compared to A SAL rats, due to generalization decrement (Luyten et al., 2016). A planned comparison (one-tailed unpaired t-test: t(22) = 1.88, p = .04) showed less freezing in B SAL versus A SAL. Accordingly, further analyses indicated that the average motion index was significantly higher in B SAL versus A SAL rats (537 ± 299 versus 247 ± 246, one-tailed unpaired t-test: t(22) = 2.59, p < .01).
To compare all 4 groups on Test 1 (Fig. 1C), we used a non-parametric Kruskal-Wallis ANOVA by ranks (the assumptions for a factorial ANOVA were not met), which showed a significant effect of group (p < .01). Multiple comparisons indicated more freezing in A SAL than in A SCOP and B SCOP rats (both p < .01). Thus, although we predicted no effect of scopolamine in A SCOP, the data indicated less freezing in this group compared with A SAL. Additionally, freezing in B SCOP was lower than in B SAL, but this difference did not survive correction for multiple testing. We have no evidence that SCOP rats discriminated between contexts A and B, with very low freezing percentages in both groups.
To evaluate if the reduced freezing in A SCOP versus A SAL during Test 1 was merely a consequence of differences in shock sensitivity and/or unconditioned response during Training, we conducted an exploratory analysis only including rats with ‘average’ postshock freezing levels, i.e., freezing in a limited range around the mean of the SAL rats (predefined range: [mean – 1 standard deviation; mean + 0.5*standard deviation], i.e., [31%; 53%]). This approach ensured comparable postshock freezing during Training in both subgroups, excluding rats that may not have perceived the shocks as aversive, and resulted in a subset of six A SAL rats and five A SCOP rats (Fig. 1D). The subset analysis confirmed that freezing during Test 1 was significantly lower in A SCOP rats than in A SAL rats (Mann-Whitney U test: Z = 2.38, p < .01) (Fig. 1E).
To exclude rats that may not have learned anything during Training, we repeated all analyses without two rats. These animals did not qualify as outliers according to a Grubbs’ test, but nevertheless showed very low freezing during training (one A SCOP rat with 2% freezing and one B SCOP rat with 5% freezing). These low freezing values may be due to the pre-training injection of scopolamine or the rats just being ‘bad learners’ or a combination of both. All other rats showed ≥15% postshock freezing. Exclusion of these two rats did not change any of the conclusions.
We noticed that freezing on Test 1 was remarkably low in the both saline groups, which was confirmed by a comparison with historical controls (Suppl. Fig. 1). Postshock freezing was equivalent in previous experiments (45% on average) and the current study (46%), suggesting that the saline injection 15 minutes prior to training in the current experiment did not have any effects on postshock freezing during the Training session. However, during the test session 24 hours later, we found significant effects of Experiment (previous > current, F(1,98) = 11.07, p = .001) and Context (A > B, F(1,98) = 19.61, p < .0001) and no interaction. In other words, freezing on Test 1 in A SAL and B SAL was indeed lower in this study than in previous experiments.
Given the unexpected findings for Training and Test 1, it is difficult to interpret the results of the Extinction phase and Test 2. Therefore, we will only mention some observations that may be of interest for future studies. As illustrated in Fig. 1F, we find virtually complete extinction in all groups, with very low freezing levels at the end of the 24-minute session (median freezing varied between 0% and 2% in all groups during this final minute). On Test 2 in context A (Fig. 1G), we find no spontaneous recovery in A SAL or A SCOP rats, and no renewal in B SAL or B SCOP rats. Note that, in B SAL rats, median freezing did increase from 2% during the final minute of Extinction to 13% during Test 2, but this increase was not significant (Wilcoxon matched-pairs test: Z = 1.36, p = .17).
To further characterize the effects of 0.1 mg/kg scopolamine in male Wistar rats, we conducted two additional behavioral tests. Contrary to our predictions, scopolamine induced behavioral changes in the open field test and on the accelerating rotarod (Fig. 2). Scopolamine rats spent slightly less time in the center of the open field than control rats (unpaired t-test: t(14) = 2.39, p = .03), but were comparably active as indexed by the distance travelled during the 10-minute test (unpaired t-test: t(14) = 0.26, p = .79). Average latency until falling off the rotarod improved over the 3 training days, but was significantly different on the test day, when scopolamine or saline was given (t(14) = 3.58, p < .01). Scopolamine rats fell off the rotarod on average after 29 s, while control rats stayed on the rotarod for 50 s.
Fig. 2. The effects of 0.1 mg/kg scopolamine in the open field test and on the accelerating rotarod.
(A) %Time (mean + SD) spent in the center of the open field during a 10-minute test, *significantly shorter than SAL rats (p = .03). (B) Distance travelled (mean + SD) during the 10-minute open field test. (C) Average time (mean ± SD) until falling off the rotarod on three drug-free training days and a subsequent test day when rats were given saline (SAL) or scopolamine (SCOP), *significantly different between SAL and SCOP rats (p < .01). N = 8 per group.
Discussion
In this study, we examined the effect of pre-training scopolamine on the expression and generalization of contextual anxiety, using a dose that has previously been shown to prevent the contextualization of extinction of cued fear. Our main hypothesis was not confirmed, as pre-training scopolamine almost completely abolished contextual freezing in rats that were re-exposed to the training context one day after training, and had comparable effects in rats that were tested in a perceptually similar generalization context. Several additional hypotheses were not confirmed either, with acute effects of scopolamine on post-shock freezing, behavior in the open field test (time in center only, distance travelled was unaffected) and on the accelerating rotarod. Overall, 0.1 mg/kg scopolamine had surprisingly profound behavioral effects in our male Wistar rats.
Dose
The choice for a 0.1 mg/kg dose was based upon findings in male and female Long-Evans rats (Anagnostaras et al., 1999; Zelikowsky et al., 2013), but inter-strain differences in drug sensitivity may exist (Entlerova et al., 2013). Still, the effects of such a low dose of scopolamine on postshock freezing and on the subsequent expression of contextual anxiety were unexpected given the existing literature on systemic scopolamine injections in male Wistar rats. In a comprehensive review, Klinkenberg and Blokland enumerated the behavioral effects of scopolamine in various strains and species (Klinkenberg and Blokland, 2010). In Wistar rats, 0.1 mg/kg scopolamine generally did not affect discrimination tasks, but it did reduce performance in presumably more demanding delayed conditional discrimination tasks and object recognition tasks. Working memory and behavior in passive avoidance tasks were usually not impaired. Thus, although 0.1 mg/kg scopolamine appears to influence behavior on some tasks, this dose is considered (much) too low to produce behavioral effects in many other tests.
Although our findings were unexpected, and do not give a decisive answer about the effects of pre-training scopolamine on contextual generalization, it is worthwhile to take a closer look at the results.
Acute effects of 0.1 mg/kg scopolamine
First of all, we found acute effects during context conditioning, with scopolamine-treated rats reaching lower maximal postshock freezing levels. This might be interpreted as an anxiolytic effect of scopolamine, and is in line with the findings of Anagnostaras and colleagues, who found lower postshock freezing, albeit only with doses that were 10-1000 times higher than ours (Anagnostaras et al., 1999). It is unlikely that the observed effect on postshock freezing is a pure locomotion effect, as 0.1 mg/kg scopolamine did not induce hyperactivity (distance travelled) in the open field test. Then again, scopolamine rats did fall off the rotarod more quickly than saline rats, but it is difficult to conclude what exactly explains this effect (e.g., motor or vision impairment, attention deficits, drowsiness, dizziness). In any case, these effects were surprising, given the low dose of scopolamine. In contrast with the observed lower postshock freezing, the slightly shorter time spent in the center of the open field by scopolamine versus saline rats rather suggests an anxiogenic effect. Higher doses of scopolamine have repeatedly been described to elicit anxiogenic effects (Klinkenberg and Blokland, 2010). Note that our combination of different behavioral tests has proven to be a useful approach to avoid premature conclusions regarding the acute effect of this drug on anxiety.
Effects of 0.1 mg/kg scopolamine on encoding and consolidation of contextual anxiety
To examine the effects of pre-training scopolamine on freezing during test in the conditioned context, without the possibly confounding differences in postshock freezing, we conducted a subset analysis of groups A SAL and A SCOP, including only rats that showed ‘average’ freezing during training and found that, in this subset, A SAL rats still froze significantly more than A SCOP rats during Test 1. This supports the idea that the difference in freezing between A SAL and A SCOP rats on Test 1 cannot be fully attributed to differences in unconditioned responding or shock sensitivity during Training. In particular, this indeed suggests that the group differences arise from an effect of scopolamine on the encoding quality of the memory (i.e., effects on contextual processing during Training, some kind of state-dependent learning (Bouton, 2002) and/or on very early consolidation). Note that differences between A SAL and A SCOP on Test 1 cannot be attributed to consolidation processes taking place starting 15 min after Training, because, at that point, the A SAL group was under influence of scopolamine as well.
Notably, effects of cholinergic manipulations on encoding (Hasselmo, 2006) and consolidation (Power et al., 2003) in several behavioral tasks have been described previously, often with local infusions in the brain. Nonetheless, we expected that systemic scopolamine administered áfter training would not influence freezing in the conditioned context A on Test 1, given the reported absence of any effects on freezing to the context or tone in a cued fear conditioning procedure, even with doses that were 500 times higher (Anagnostaras et al., 1995; Anagnostaras et al., 1999). We found, however, that freezing on Test 1 was unusually low in both A SAL and B SAL groups, suggesting that 0.1 mg/kg post-training scopolamine had an unexpected detrimental effect on consolidation. Note that this conclusion should be drawn with caution, as we did not directly compare both conditions in one experiment. Finally, despite these potential effects of scopolamine on consolidation, the significant difference in freezing between the A SCOP and A SAL groups (and subsets) indicates that scopolamine primarily affected the earliest stages of learning (encoding and early consolidation within 15 minutes after the end of Training).
Recent hypotheses regarding the involvement of acetylcholine in learning and memory suggest a role in hippocampal modulation (Hasselmo, 2006; Hasselmo and McGaughy, 2004; Klinkenberg and Blokland, 2010). High acetylcholine would enable acquisition of new information, whereas low acetylcholine would facilitate memory consolidation and recall. Our data show that even mild cholinergic antagonism disrupts encoding of contextual anxiety. In addition, our findings suggest no facilitation, but rather an impairment, of memory consolidation, which appears to be in contrast with other reports (Hasselmo and McGaughy, 2004).
Pharmacological manipulation of associative fear memories
In search of better treatment options for anxiety patients, several approaches have been proposed that combine psycho- and pharmacotherapy. Some have focused on boosting extinction, e.g. with d-cycloserine or cortisol, without necessarily changing the context-dependent nature of extinction (Walker et al., 2002; Woods and Bouton, 2006; de Quervain et al., 2011). Others have attempted to fully erase the initial fear memory, e.g. with propranolol administration after reactivation, which has been suggested to block reconsolidation (Debiec and Ledoux, 2004; Beckers and Kindt, 2017). Here, we looked into the possibility of pharmacologically decontextualizing a fear or extinction memory. To our knowledge, this approach is quite novel and has only been implemented twice: by Zelikowsky and colleagues (using scopolamine before extinction training) and Haaker and colleagues (using L-DOPA after extinction training) (Haaker et al., 2013; Zelikowsky et al., 2013).
Our study could not provide conclusive insights in the effects of scopolamine on contextual generalization, given the unanticipated behavioral effects of 0.1 mg/kg scopolamine during acquisition of contextual anxiety and on the subsequent recall during re-exposure to the same context one day later. Nevertheless, our data do provide novel information regarding the effects of systemic scopolamine on context conditioning. In line with the effects described by Zelikowsky and colleagues, we find effects on contextual encoding, even with this relatively low dose. Whereas this dose does not disrupt cued fear (Anagnostaras et al., 1999), it may influence the hippocampus just enough to have effects on contextual encoding, especially when the context is the best or only predictor for the absence (cf (Zelikowsky et al., 2013)) or presence (cf our findings) of shocks.
Supplement
Acknowledgments
We would like to thank Tim Tambuyzer for technical assistance with the video analysis of the open field test.
Financial disclosures
This work was supported by the Research Foundation – Flanders (FWO) Postdoctoral Fellowship 1295613N and Research Grant 1504614N (to L. Luyten) and KU Leuven Center for Excellence on Generalization Research Grant PF/10/005 (to T. Beckers). Preparation of this manuscript was further supported by ERC Consolidator Grant 648176 (to T. Beckers).
Footnotes
Author contributions
L. Luyten contributed to the conception and design, acquisition, analysis and interpretation of data and drafted the article. S. Nuyts contributed to the acquisition and analysis of data. T. Beckers contributed to the conception and design, and interpretation of data. S. Nuyts and T. Beckers critically revised the article. All authors approved the final manuscript.
References
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiology of learning and memory. 1995;64(3):191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Sage JR, et al. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;21(6):731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Beckers T, Kindt M. Memory reconsolidation interference as an emerging treatment for emotional disorders: strengths, limitations, challenges, and opportunities. Annual Review of Clinical Psychology. 2017;13 doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological psychiatry. 2002;52(10):976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An overview of translationally informed treatments for posttraumatic stress disorder: animal models of Pavlovian fear conditioning to human clinical trials. Biological psychiatry. 2015;78(5):E15–27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Kennard JA, Sherer DJ, et al. The context preexposure facilitation effect in mice: a dose-response analysis of pretraining scopolamine administration. Behavioural brain research. 2011;225(1):290–296. doi: 10.1016/j.bbr.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Liang KC. Roles of hippocampal GABA(A) and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: a double dissociation study. Neurobiology of learning and memory. 2012;98(1):17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Bentz D, Michael T, et al. Glucocorticoids enhance extinction-based psychotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Entlerova M, Lobellova V, Hatalova H, et al. Comparison of Long-Evans and Wistar rats in sensitivity to central cholinergic blockade with scopolamine in two spatial tasks: an active place avoidance and the Morris water maze. Physiology & behavior. 2013;120:11–18. doi: 10.1016/j.physbeh.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research. 2000;110(1–2):73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11(4):371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Haaker J, Gaburro S, Sah A, et al. Single dose of L-dopa makes extinction memories context-independent and prevents the return of fear. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):E2428–2436. doi: 10.1073/pnas.1303061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Current opinion in neurobiology. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progress in brain research. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Kindt M. A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behaviour research and therapy. 2014;62:24–36. doi: 10.1016/j.brat.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neuroscience and biobehavioral reviews. 2010;34(8):1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kroon JA, Carobrez AP. Olfactory fear conditioning paradigm in rats: effects of midazolam, propranolol or scopolamine. Neurobiology of learning and memory. 2009;91(1):32–40. doi: 10.1016/j.nlm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Luyck K, Tambuyzer T, Deprez M, et al. Electrical stimulation of the bed nucleus of the stria terminalis reduces contextual anxiety in a rat model. Translational Psychiatry. doi: 10.1038/tp.2017.2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Schroyens N, Hermans D, et al. Parameter optimization for automated behavior assessment: plug-and-play or trial-and-error? Frontiers in behavioral neuroscience. 2014;8:28. doi: 10.3389/fnbeh.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Schroyens N, Luyck K, et al. No effect of glucose administration in a novel contextual fear generalization protocol in rats. Translational Psychiatry. 2016 doi: 10.1038/tp.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, Van Cappellen F. ExpTimer: timer software to facilitate complex, multi-step procedures. Journal of Open Research Software. 2013;1(1) art. nr. e2. [Google Scholar]
- Luyten L, Vansteenwegen D, van Kuyck K, et al. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cognitive, affective & behavioral neuroscience. 2011;11(2):228–244. doi: 10.3758/s13415-011-0021-6. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical psychology review. 2007;27(6):750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it's not what you thought it was. The American psychologist. 2006;61(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiology of learning and memory. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- van der Zee EA, Luiten PG. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: a review of immunocytochemical localization in relation to learning and memory. Progress in neurobiology. 1999;58(5):409–471. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, et al. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(6):2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiology of learning and memory. 2001;75(3):245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral neuroscience. 2006;120(5):1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, et al. Cholinergic blockade frees fear extinction from its contextual dependency. Biological psychiatry. 2013;73(4):345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.