Abstract
Background
In experimental studies viral infections have been shown to induce type 2 inflammation in asthmatics, but whether this is a feature of naturally occurring virus-induced asthma exacerbations is unknown. Thymic stromal lymphopoietin (TSLP) released from the airway epithelium in response to damage, has been suggested as a link between viral infection and type 2 inflammation, but the role of TSLP in asthma exacerbations is unknown.
Objective
To assess whether type 2 inflammation, as measured by sputum eosinophils and fractional exhaled nitric oxide (FeNO), is a feature of naturally occurring virus-induced exacerbations of asthma and whether TSLP is associated with this type 2 inflammation.
Methods
Patients presenting to hospital with acute asthma were examined during the exacerbation, and after 4 weeks recovery. The assessments included spirometry, FeNO and induced sputum for differential counts and TSLP mRNA levels. Nasal swabs were collected for viral detection.
Results
Sputum eosinophils and FeNO were similar between virus-positive (n = 44) and negative patients (n = 44). In virus-positive patients, TSLP expression was lower at exacerbation than follow-up (p = 0.03). High TSLP at exacerbation was associated with lower sputum eosinophils (p = 0.01) and higher FEV1 (p = 0.03). In virus-positive patients, %-predicted FEV1 negatively correlated with both FeNO and sputum eosinophils (p = 0.02 and p = 0.05, respectively).
Conclusion
Our findings support that type 2 inflammation is present in patients during virus-induced asthma exacerbations, to the same degree as non-viral exacerbations, and correlate negatively with FEV1. However, in virus-positive patients, high TSLP expression during exacerbation was associated with low sputum eosinophils, suggesting that the effect of TSLP in vivo, in the setting of an asthma exacerbation, might be different than the type 2 inducing effects observed in experimental studies.
Keywords: Asthma, Exacerbation, Eosinophils, Viral infection, TSLP
Highlights
-
•
Sputum eosinophils and FeNO are similar in virus-induced and non-viral exacerbations.
-
•
Sputum eosinophils and FeNO correlate with FEV1 during exacerbation.
-
•
TSLP correlate negatively with sputum eosinophils during virus-induced exacerbations.
1. Introduction
With an estimated prevalence of nearly 10%, asthma is one of the more frequent chronic diseases in Western countries, and exacerbations are the major cause of morbidity and health care utilization [1]. Respiratory viruses are one of the most common triggers of asthma exacerbations [2], but the underlying mechanisms are not fully understood.
Overall, around half of asthma patients have prominent type 2 inflammation during stable disease [3], characterized by IL-5 driven eosinophilia in both sputum and blood and mucus hypersecretion, airway hyperresponsiveness and increased IgE production, driven by IL-4 and IL-13. This Th2-high phenotype has been associated with more severe disease [4], [5] and an increased risk of exacerbations [6]. Whether aggravated type 2 inflammation is a feature of both virus-induced and non-viral exacerbations, is currently unknown and is likely to have implications for the efficacy of emerging biological treatment in reducing the rate and severity of these exacerbations.
Allergen sensitization and exposure is the established cause of increased type 2 inflammation. However, in vitro studies have suggested that respiratory viruses are capable of independently inducing type 2 inflammation, by causing a release of pro-inflammatory cytokines, including thymic stromal lymphopoietin (TSLP), from the airway epithelium [7], [8]. In this innate immune pathway, TSLP causes release of type 2 cytokines IL-4, IL-5 and IL-13, from T-cells through activation of dendritic cells [9] and by direct action on innate lymphoid cells (ILC) [10]. TSLP is increased in patients with asthma, compared to controls [11] and anti-TSLP antibodies have been shown to reduce airway inflammation in response to an allergen provocation test [12]. Epithelial cells from asthmatics seem to be particular prone to TSLP release following exposure to viral infection in vitro [8] but whether TSLP is induced in asthmatics during viral infection in vivo and stimulates type 2 inflammation is unknown.
Asthma patients, particularly those with type 2 inflammation, experience more severe symptoms when experimentally infected with respiratory viruses [13], [14] and, although controversial, the mechanism is proposed to involve a reduced production of interferons [15], [16], [17], [18]. Whether patients with high type 2 inflammation during a real-life viral infection experience more severe exacerbations, is unclear.
The aim of the present study was to test the hypothesis that sputum eosinophilia and increased FeNO are features of naturally occurring virus-induced exacerbations. Secondarily, that the level of sputum eosinophilia and FeNO are associated with the degree of TSLP expression in sputum. Finally we wished to test whether any type 2 inflammation during a virus-induced exacerbation would be associated with more severe exacerbations, measured by FEV1. Exploratory outcomes included rhinovirus subtype and systemic markers of inflammation.
2. Methods
2.1. Study design
The study was a prospective observational study conducted at Bispebjerg University Hospital between July 2013 and September 2015. Patients were recruited both from the hospital Emergency Department, and from the acute asthma clinic within the hospital research unit and were seen within 24 h of contact. Exacerbations were defined as worsening of symptoms surpassing the daily variation that required a change in treatment in line with ERS/ATS recommendations [19]. After 4 weeks, participants were re-examined in the research unit. All patients had a doctor's diagnosis of asthma and had the diagnosis confirmed during the study in line with GINA recommendations [1]. Patients were between 16 and 45 years of age. Pregnant women and patients with pulmonary diseases other than asthma were excluded. Both current and past smokers were included.
The study was approved by the local scientific ethics committee (protocols nr: H-2-2013-046, H-3-2011-121, H-15003691).
2.2. Examinations
Fractional expiratory nitric oxide (FeNO) (NioxMinor, Aerocrine, Sweden) was assessed following the recommendations of the ERS and ATS [20] to use the mean of two measurements. Patients completed spirometry (EasyOne, NDD, Switzerland) according to ERS guidelines [21], using NHANES III for lung function predicted values [22]. Because patients were examined during an exacerbation, they were not asked to withhold beta-2-agonists prior to spirometry, but the time since the last dose of reliever was taken was recorded. Induced sputum was processed as described by Pavord et al. [23]: In brief, after inhalation of 1 mg terbutaline, sputum was induced by inhalation of hypertonic saline in increasing concentrations (3%, 4%, and 5%) for 5 min each (total duration 15 min). Sputum plugs were selected and processed, cytospins were prepared, and a differential cell count of 400 non-squamous cells was completed. The hospital clinical laboratory completed a full white blood cell differential count and measured high-sensitivity C-reactive protein (hsCRP), total IgE and specific IgE to common allergens (grasses, house dust mite, birch, mugwort, dog, cat, horse, Cladosporium herbarum and Alternaria tenuis). Atopy was defined as the presence of specific IgE (>0.35 IU/ml) to one or more of these allergens. Asthma control was assessed with the 5-item Asthma Control Questionnaire (ACQ) [24].
2.3. Detection of respiratory pathogens
Flocked nasal swabs (Copan, Italy) were used to sample turbinate nasal. Samples were assessed for human adenovirus species B-D; human bocavirus; coronaviruses OC43, 229E, HKU1 and NL63; influenza viruses A, B and C; parainfluenzaviruses 1–4; KI and WU polyomaviruses; respiratory syncytial virus types A and B and human metapneumovirus, using a tandem multiplex real-time PCR assay [25], [26].
A PCR directed at picornavirus sequences in the 5′UTR was used to detect RV. The products were identified to species level as RV-A, RV-B or RV-C by sequencing of this 260 bp product and analysed using ClustalX software [27], [28]. The local hospital laboratory at Bispebjerg Hospital completed bacterial cultures on spontaneous expectorates collected during the exacerbation [29].
2.4. Quantification of TSLP mRNA expression
Sputum cell pellets were stored in RNAlater (Ambion, USA) and kept at −80 °C until processing. RNeasy Mini kit (Invitrogen, USA) was used to extract mRNA following the manufacturers recommendations. After the RNA extraction, cDNA was synthesised using SSIII reverse transcriptase (Invitrogen, USA) as described by the manufacturer. TSLP was quantified using digital droplet PCR (QX200, Bio-Rad, USA) with TaqMan primers ands probes. GAPDH was used for normalization. For the analyses participants were divided into TSLP high and TSLP low by the median expression.
2.5. Statistical analyses
The data was analysed with SPSS version 22.0 (IBM SPSS, USA). Normally distributed data are reported as mean ± standard deviation and analysed using students t-test for continuous variables, and chi-squared test for categorical variables. Log-transformed variables are reported as geometric mean (GM) with 95% CI. For log-transformation of sputum eosinophil percentage, cases with 0% were set to 0.1% prior to transformation. Non-normally distributed data are reported as median (range) and comparisons are made using the Mann-Whitney U-test. Regarding TSLP, we used the median expression to classify patients as either TSLP-high (above the median) or TSLP-low (below the median) at exacerbation.
A multiple linear regression model, stratified by virus detection, was used to examine determinants of FEV1%-predicted at the time of exacerbation. Due to multicollinearity each inflammatory marker was assessed separately with adjustment for predefined covariates: gender, age, smoking, bacterial infection and ICS usage.
For all analyses a p-value of <0.05 was considered significant.
3. Results
3.1. Respiratory viruses and bacteria detected at exacerbation
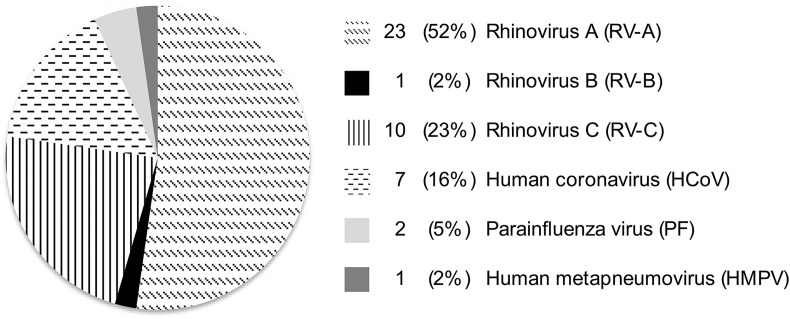
A total of 88 patients were included in the study, of which 44 (50%) had a detectable respiratory virus at exacerbation. Viruses detected were rhinovirus (77%), human coronavirus (16%), parainfluenza virus (5%) and human metapneumovirus (2%) (Fig. 1 ). Among patients with a respiratory virus, six had a bacterial co-infection (3 H. influenzae, 1 S. pneumoniae, 1 S. aureus and 1 M. catarrhalis). There were three patients with bacterial infections in the virus-negative group (2 S. pneumoniae and 1 Gemella haemolysans) (p = 0.29). In the comparisons of virus-positive and negative groups, the nine patients with bacterial infections were included. All conclusions remained unchanged when patients with bacterial infections were excluded from the analyses.
Fig. 1.
Prevalence of respiratory viruses at exacerbation (n = 44).
3.2. Clinical characteristics of virus-positive vs. virus-negative patients
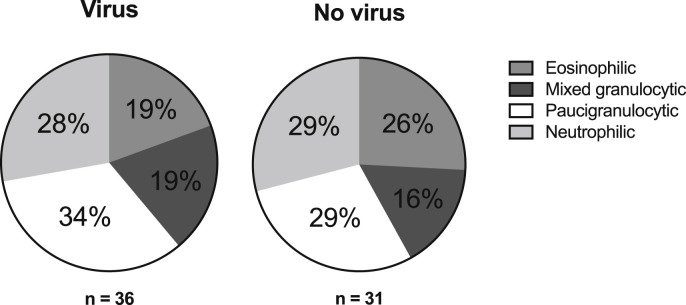
Demographics of patients with, or without, viral infection were similar, as were the level of treatment required at exacerbation (Table 1 ). Likewise, lung function and inflammatory markers were largely similar, with the exception of a higher CRP in the virus-positive group (GM 8.4, 95% CI 3.5–11.5, p = 0.02) compared to the virus-negative group (GM 4.9, 95% CI 3.5–7.0). Sputum induction was successful in 67 patients (76%, 31 without virus and 36 with virus). The absolute number of both eosinophils and neutrophils were higher in virus-positive patients, compared to virus-negative patients, but the percentages of each cell type were not different between virus-positive and negative (Table 1). Consequently, the distribution of patients into established sputum phenotypes [30] was similar between viral and non-viral exacerbations (Fig. 2 ).
Table 1.
Clinical characteristics of patients at presentation with exacerbation with, and without, detectable viral infection.
| No virus detected (n = 44) |
Virus detected (n = 44) |
p-value | |
|---|---|---|---|
| Gender (% females) | 61 | 68 | 0.50 |
| Age | 34 (10) | 32 (12) | 0.35 |
| Smokers (% never; former; current) | 59; 14; 27 | 75; 11; 14 | 0.23 |
| Packyears (among former and current smokers) | 11.7 (11.2) | 6.9 (5.0) | 0.19 |
| Atopic (%) | 68 | 57 | 0.27 |
| ICS dose (daily budesonide eq.) | 0 (0–1600) | 400 (0–2400) | 0.13 |
| Required burst of OCS (%) | 80 | 71 | 0.33 |
| Were admitted (%) | 68 | 59 | 0.38 |
| ACQ | 3.1 (1.2) | 3.1 (1.5) | 0.94 |
| FEV1%pred. | 77.9 (21.9) | 80.3 (16.7) | 0.57 |
| FVC %pred. | 84.0 (20.3) | 89.0 (14.1) | 0.20 |
| Beta-2-agonist within 8 h? (%) | 52 | 42 | 0.32 |
| FeNO (ppb)a | 35 (26–46) | 28 (22–34) | 0.21 |
| Blood eosinophils (× 109 cells/L) | 0.07 (0–1.16) | 0.08 (0–1.32) | 0.79 |
| Blood neutrophils (× 109 cells/L) | 6.0 (1.7–16.4) | 5.5 (2.1–14.1) | 0.99 |
| Total IgE (× 103 IU/L)a | 150 (78–286) | 148 (84–259) | 0.98 |
| CRP (mg/L)a |
4.9 (3.5–7.0) |
8.4 (3.5–11.5) |
0.02 |
| n = 31 |
n = 36 |
||
| Sputum total cell count (× 106/ml)a | 0.5 (0.3–0.8) | 1.3 (0.8–1.9) | 0.003 |
| Sputum eosinophils (× 106/ml)a | 0.03 (0.01–0.05) | 0.06 (0.04–0.11) | 0.04 |
| Sputum %-eosinophilsa | 1.4 (0.6–3.4) | 1.1 (0.5–2.3) | 0.61 |
| Sputum neutrophils (× 106/ml)a | 0.2 (0.1–3.2) | 0.6 (0.3–1.1) | 0.003 |
| Sputum %-neutrophilsa | 34.0 (23.1–50.1) | 47.2 (35.1–63.5) | 0.17 |
Log-transformed and presented as geometric mean (95% CI).
Fig. 2.
Distribution of sputum inflammatory phenotypes in patients with, or without, detectable virus at exacerbation (n.s.). Paucigranulocytic (<3% eosinophils & <61% neutrophils), eosinophilic (≥3% eosinophils & <61% neutrophils), neutrophilic (<3% eosinophils & ≥61% neutrophils), mixed granulocytic (≥3% eosinophils & ≥61% neutrophils).
In the virus-induced group FeNO was higher at exacerbation (GM 28, 95% CI 22–35, p = 0.001) compared to follow-up (GM 18, 95% CI 14–24), which was also the case in the non-viral group (GM 35, 95% CI 26–49 vs. 23, 95% CI 18–30, respectively, p = 0.001). Sputum eosinophils were not significantly different at follow-up compared to the percentages during exacerbation in either group.
In the period between the initial and the follow-up visit patients were treated following clinical guidelines (GINA). Following admission 75% received an oral course of prednisolone (37.5 mg once daily for 10 days), while the rest were managed by increasing their inhaled steroid. No other treatment was initiated by the study team, but existing treatment with LABA, leukotrienes or any other asthma medication was continued following exacerbation. There were no significant differences in clinical management between the virus-positive and virus-negative group.
3.3. TSLP mRNA expression
There was no difference in sputum TSLP mRNA expression between patients with, or without, a viral infection at exacerbation (GM 3.2, 95% CI 1.4–7.1 vs. 3.5, 95% CI 2.0–6.0, respectively, p = 0.84) (Fig. 3 ). In all, there were 36 paired measurements of TSLP (exacerbation and follow-up; 19 from virus-positive patients, 17 from virus-negative patients). In virus-positive patients TSLP expression was lower at exacerbation (GM 2.8, 95% CI 1.2–6.8, p = 0.03) compared to follow-up (GM 6.1, 95% CI 2.8–13.4), whereas there was no difference between exacerbation (GM 2.8, 95% CI 1.1–7.0, p = 0.74) and follow-up (GM 2.4, 95% CI 0.9–6.4) in the virus-negative group (Fig. 3).
Fig. 3.
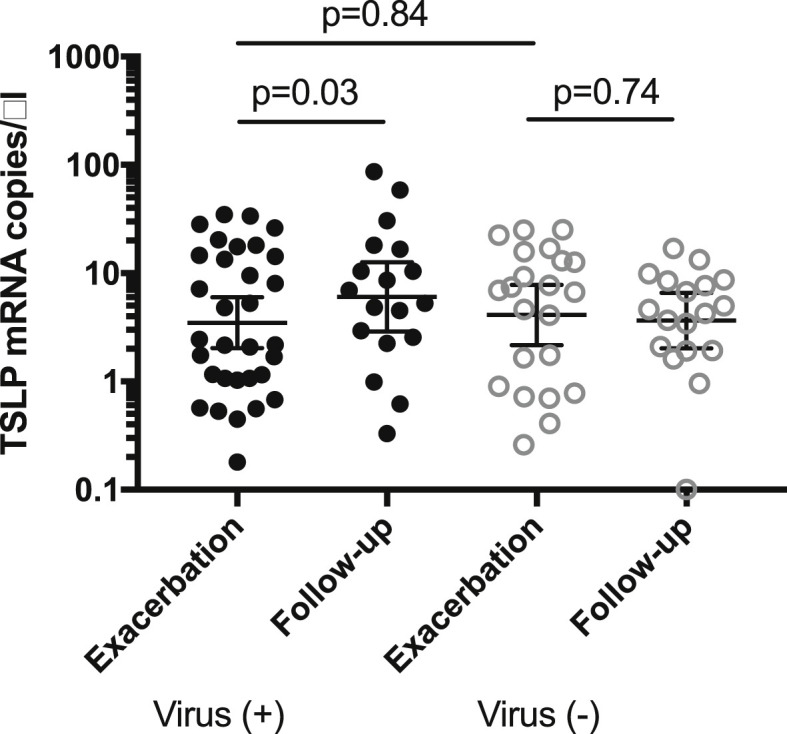
TSLP mRNA expression in sputum at exacerbation in patients with, or without, viral infection. Normalized to GAPDH = 5000 copies/μl.
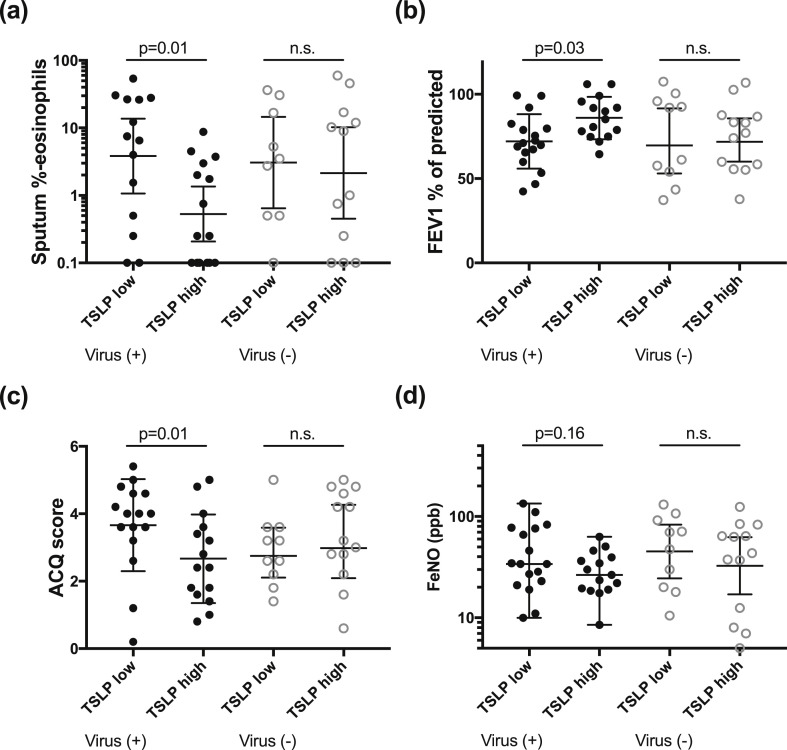
In patients without a virus infection, there was no difference in inflammatory phenotype, FEV1 or ACQ between those with high or low TSLP expression (n = 12 and n = 9, respectively) (Fig. 4 ). However, in virus-positive patients, those with high TSLP expression levels (n = 15) had significantly lower sputum eosinophil percentage (GM 0.5, 95% CI 0.2–1.4, p = 0.01 vs. 3.8, 95% CI 1.1–13.8), higher FEV1 (86.2 ± 12.4, p = 0.03 vs. 75.1 ± 14.9), lower ACQ score (2.5 ± 1.2, p = 0.01 vs. 3.7 ± 1.4), higher blood neutrophils (median 4.3 × 109 cells/L (range 2.3–10.2), p = 0.04 vs. 6.0 × 109 cells/L (2.8–13.5)) and a trend for lower FeNO (GM 27 ppb, 95% CI 20–36, p = 0.16 vs. 38 ppb 95% CI 26–56) compared to those with low TSLP expression levels (n = 15) (Fig. 4). There was no difference in ICS usage between the TSLP high and low groups.
Fig. 4.
Inflammatory and clinical characteristics of virus positive patients with TSLP expression at exacerbation below the median (TSLP low) and above the median (TSLP high).
3.4. Type 2 inflammation and FEV1 at exacerbation
To examine the impact of type 2 inflammation on the clinical severity of exacerbation, the association with sputum eosinophils, FeNO and FEV1 at exacerbation was examined in both the virus-positive and negative group.
The percentage of sputum eosinophils correlated negatively with FEV1 in patients with a virus detected at exacerbation (rho −0.42, p = 0.01) and in patients with non-viral exacerbation (rho −0.60, p < 0.001). When adjusted for gender, age, smoking status, bacterial infection and ICS usage in a multiple linear regression, the associations remained significant (p = 0.05 and p = 0.02, respectively) (Table 4). In univariate analyses FeNO was negatively correlated with FEV1 in the virus-positive group (rho −0.37, p = 0.01) but not significantly associated with FEV1 in virus-negative patients (rho −0.23, p = 0.13). After adjustment for the same potential confounders, the association remained significant in the virus-positive group (p = 0.02), yet not in the virus-negative group (p = 0.98) (Table 4).
Table 4.
Multivariate linear regression of sputum eosinophils (a) and FeNO (b) to predict FEV1%-predicted at the time of an asthma exacerbation, corrected for gender, age, smoking status, bacterial infection and ICS usage. Sputum differential counts were available from 67 patients (31 without virus, 36 with virus).
| (a) | All patients (n = 67) |
No virus detected (n = 31) |
Virus detected (n = 36) |
|||||
|---|---|---|---|---|---|---|---|---|
| SEB | p-value | SEB | p-value | SEB | p-value | |||
| Constant | <0.001 | <0.001 | <0.001 | |||||
| Sputum eosinophils (%)* | −0.42 | 0.001 | −0.45 | 0.02 | −0.36 | 0.05 | ||
| Gender | 0.15 | 0.21 | 0.19 | 0.28 | 0.09 | 0.63 | ||
| Age (years) | −0.08 | 0.48 | −0.20 | 0.25 | 0.01 | 0.94 | ||
| ICS (budesonide eq.) | 0.11 | 0.35 | 0.02 | 0.93 | 0.05 | 0.76 | ||
| Bacteria detected | 0.02 | 0.84 | 0.04 | 0.84 | −0.05 | 0.78 | ||
| Smoking status** |
−0.01 |
0.92 |
0.11 |
0.58 |
−0.21 |
0.23 |
||
| (b) | All patients (n = 88) | No virus detected (n = 44) | Virus detected (n = 44) | |||||
| SEB |
p-value |
SEB |
p-value |
SEB |
p-value |
|||
| Constant | <0.001 | 0.003 | <0.001 | |||||
| FeNO (ppb)* | −0.17 | 0.12 | −0.01 | 0.98 | −0.37 | 0.02 | ||
| Gender | 0.31 | 0.01 | 0.47 | 0.003 | 0.06 | 0.68 | ||
| Age (years) | −0.07 | 0.48 | −0.23 | 0.10 | −0.04 | 0.78 | ||
| ICS (budesonide eq.) | 0.04 | 0.71 | −0.08 | 0.56 | 0.06 | 0.72 | ||
| Bacteria detected | −0.04 | 0.69 | −0.07 | 0.60 | −0.06 | 0.68 | ||
| Smoking status** | −0.05 | 0.65 | 0.11 | 0.44 | −0.29 | 0.06 | ||
*Log-transformed. **Never smoker vs. former + current smoker.
As exploratory predictors of FEV1, we also measured blood eosinophils, total IgE and sputum neutrophils. Using the same analysis, both blood eosinophils and total IgE were associated with FEV1 with no difference between virus-positive and negative patients, while sputum neutrophils were not associated with FEV1 in either group (data not shown).
3.5. Impact of the type of virus detected
Patients infected with a rhinovirus had lower %-predicted FEV1 at exacerbation (77.2 ± 16.0, p = 0.02) compared to patients with other respiratory viruses (90.8 ± 15.5), and a higher ACQ score (3.4 ± 1.4, p = 0.03 vs. 2.3 ± 1.4, respectively) (Table 2 ). There were no significant differences in demographics, spirometry or inflammatory markers between patients infected with RV-C (n = 10) compared to patients infected with RV-A (n = 23) (Table 3 ).
Table 2.
Characteristics of patients at presentation with exacerbation with rhinovirus or other viruses at exacerbation.
| Rhinovirus (n = 34) |
Other viruses (n = 10) |
p-value | |
|---|---|---|---|
| Gender (% females) | 68 | 70 | 0.89 |
| Age | 29 (9) | 41 (17) | 0.06 |
| Smokers (% never; former; current) | 65; 15; 20 | 60; 30; 10 | 0.47 |
| Packyears (among former and current smokers) | 6.0 (5.4) | 1.7 (1.6) | 0.15 |
| Atopic (%) | 62 | 40 | 0.22 |
| ICS dose (daily budesonide eq.) | 200 (0–2400) | 800 (0–800) | 0.34 |
| ACQ | 3.4 (1.4) | 2.3 (1.4) | 0.03 |
| FEV1%pred. | 77.2 (16.0) | 90.8 (15.5) | 0.02 |
| FVC %pred. | 87.1 (14.4) | 94.9 (11.8) | 0.13 |
| Beta-2-agonist within 8 h? (%) | 46 | 25 | 0.29 |
| FeNO (ppb)a | 29 (23–37) | 24 (15–38) | 0.41 |
| Blood eosinophils (× 109 cells/L) | 0.07 (0–1.32) | 0.13 (0–0.41) | 0.84 |
| Blood neutrophils (× 109 cells/L) | 6.2 (2.1–14.1) | 3.6 (2.9–10.2) | 0.13 |
| Total IgE (× 103 IU/L)a | 167 (85–328) | 96 (31–298) | 0.41 |
| CRP (mg/L)a |
8.5 (6.0–12.0) |
8.2 (3.5–19.1) |
0.93 |
| n = 27 |
n = 9 |
||
| Sputum total cell count (× 106/ml)a | 1.0 (0.6–1.7) | 2.2 (0.9–5.4) | 0.11 |
| Sputum eosinophils (× 106/ml)a | 0.07 (0.04–0.13) | 0.06 (0.02–0.16) | 0.80 |
| Sputum %-eosinophilsa | 1.2 (0.5–3.0) | 0.9 (0.3–3.2) | 0.75 |
| Sputum neutrophils (× 106/ml)a | 0.5 (0.2–0.9) | 1.1 (0.3–3.8) | 0.20 |
| Sputum %-neutrophilsa | 45.9 (32.0–66.0) | 51.3 (28.3–93.0) | 0.75 |
Log-transformed and presented as geometric mean (95% CI).
Table 3.
Characteristics of patients infected with rhinovirus A (RV-A) compared with patients infected with rhinovirus C (RV-C).
| RV-A (n = 23) |
RV-C (n = 10) |
p-value | |
|---|---|---|---|
| Gender (% females) | 74 | 60 | 0.42 |
| Age | 28 (9) | 30 (9) | 0.83 |
| Smokers (% never; former; current) | 65; 22; 13 | 80; 0; 20 | 0.27 |
| Packyears (among former and current smokers) | 7 (0.1–17) | 1 (0.1–6) | 0.29 |
| Atopic (%) | 65 | 60 | 0.78 |
| ICS dose (daily budesonide eq.) | 400 (0–1600) | 0 (0–2400) | 0.83 |
| FEV1%pred. | 77.7 (15.9) | 76.2 (17.7) | 0.81 |
| FVC %pred. | 86.0 (15.0) | 87.4 (12.3) | 0.80 |
| FeNO (ppb)a | 26 (19–36) | 38 (25–56) | 0.18 |
| ACQ | 3.5 (1.3) | 3.4 (1.5) | 0.98 |
| Blood eosinophils (× 109 cells/L) | 0.06 (0–0.52) | 0.18 (0.02–1.32) | 0.04 |
| Blood neutrophils (× 109 cells/L) | 6.3 (2.1–14.1) | 6.3 (2.8–11.2) | 0.95 |
| Total IgE (× 103 IU/L)a | 214 (98–470) | 142 (35–574) | 0.70 |
| CRP (mg/L)a |
9.1 (6.2–13.4) |
7.8 (3.3–18.8) |
0.56 |
| n = 16 |
n = 10 |
||
| Sputum total cell count (× 106/ml)a | 0.8 (0.4–1.5) | 1.9 (1.0–3.6) | 0.06 |
| Sputum eosinophils (× 106/ml)a | 0.04 (0.02–0.10) | 0.19 (0.09–0.40) | 0.01 |
| Sputum %-eosinophilsa | 1.1 (0.3–3.4) | 1.6 (0.2–11.4) | 0.66 |
| Sputum neutrophils (× 106/ml)a | 0.3 (0.1–0.8) | 1.0 (0.3–3.1) | 0.12 |
| Sputum %-neutrophilsa | 42.4 (24.7–72.9) | 52.5 (29.1–94.4) | 0.58 |
Log-transformed and presented as geometric mean (95% CI).
4. Discussion
In this study, type 2 inflammation and TSLP mRNA expression were present in virus-induced exacerbations to a similar extent as in non-viral exacerbations. Furthermore, both sputum eosinophils and FeNO were negatively correlated with FEV1 at exacerbation.
To our knowledge, this is the first report on TSLP mRNA expression during naturally occurring asthma exacerbations. In contrast to our hypothesis, we found that participants with high sputum TSLP had lower sputum eosinophils, lower FeNO and higher %FEV1 at exacerbation than participants with low sputum TSLP suggesting that TSLP does not promote type 2 inflammation in the airways during virus-induced exacerbations of asthma. It is possible that the timing of sample collection and changes in post-translational modification of the TSLP mRNA could have impacted on the final amount of TSLP protein being produced, although, previous studies have found good concordance between TSLP gene expression and protein concentration [11], [31]. An inhibitory effect of corticosteroids cannot be ruled out, but it would not explain the difference between the virus-positive and virus-negative group.
Although ex vivo studies have proposed that TSLP acts as a mediator of eosinophilia in response to viral infection [32], this has not been documented in vivo. Our findings suggests that this effect might be more complex in the setting of asthma exacerbations and suggests that other pathways (e.g. IL-33 or other modulators of ILC2s) in the innate immune system are contributing to initiating type 2 inflammation in response to viral infection.
We attempted to measure TSLP protein using a highly sensitive ELISA (Bio-Plex Pro, Bio-Rad on a Luminex platform, lower limit of detection 0.8 pg/ml) but did not detect TSLP in any of the samples. This may have been due to the addition of dithiothreitol (DTT) to the sputum samples as part of the standard method for sputum sample processing, since DTT is known to alter the tertiary structure of a number of proteins, and thereby could have interfered with the ELISA [33]. Unfortunately this could not be determined as no DTT-free samples were available for re-analysis.
Little has been published on the association between inflammatory phenotype and severity of airway obstruction specifically during virus-induced asthma exacerbations. In the present study, both sputum eosinophils and FeNO negatively correlated with FEV1 in virus-positive patients in line with a recent experimental study [14]. To eliminate any seasonal impact on inflammatory markers, patients were recruited during all four seasons, however no seasonal differences in inflammatory markers at exacerbation were observed. Sensitization and exposure to allergens in conjunction with viral infection has been shown to increase the risk of admission [34] and biological treatment options targeting type 2 inflammation, have reported reductions in exacerbation rates of 50–80% [35], [36], [37], [38]. Yet, whether predominantly virus-induced exacerbations were being prevented, or non-viral exacerbations, is unknown.
It is possible that the inflammatory phenotype expressed during stable disease is representative of the response developed at exacerbation, regardless of trigger. A prospective study of asthma patients phenotyped during stable disease and followed until exacerbation is required to investigate this.
The mechanisms by which increased type 2 inflammation might negatively affect the course of a virus infection in asthmatics are not clear, but recent mechanistic studies have suggested type 2 inflammatory pathways may lead to reduced production of interferon [15], [16], [17], [18]. The role of type 2 inflammation in relation to naturally occurring viral infections in asthma patients, has been much less examined. Denlinger and colleagues sampled asthma patients with symptoms of a cold, and found that patients who went on to develop a clinical exacerbation were characterized by higher sputum neutrophils, whereas an association with eosinophils or FeNO was not observed. However, it should be noted that the study was not aimed at describing the severity of the exacerbations, and the potential association with sputum inflammatory phenotype [39]. Wark and colleagues have found that degranulation of both neutrophils and eosinophils was associated with a longer time in hospital for patients admitted with virus-induced acute asthma [40]. In the present study, sputum neutrophil count, though elevated in virus-positive exacerbations compared to non-viral exacerbations, did not correlate with FEV1.
More severe wheezing illness have been reported in children infected with RV-C, compared to RV-A [41], however, this has not been established in adults [42]. In the present study, RV-C comprised 30% of all rhinovirus infections – a proportion similar to that reported by Wark and colleagues [43]. We found no difference in clinical measurements or sputum inflammatory phenotype between patients with RV-C and RV-A. We did note that patients infected with RV-C had higher blood eosinophils at exacerbation that were not mediated by a difference in ICS dose; however, this should be interpreted with caution as no other type 2 markers were increased.
In conclusion, we have found that type 2 inflammation was present during virus-induced exacerbations to the same extent as non-viral exacerbations, and was associated with a lower FEV1 at exacerbation. Unexpectedly, TSLP expression correlated negatively with sputum eosinophils in virus-induced exacerbations, suggesting that other pathways (including epithelial cytokines such as IL-33) might be responsible for driving innate pathways leading to type 2 inflammation in acute virus induced asthma.
Conflicts of interest
None.
Acknowledgements
The study was in part funded by unrestricted grants from The Lundbeck Foundation (Grant/Award Number: R140-2013-13394) and Norpharma A/S.
References
- 1.Global Initiative for Asthma (GINA) Program. GINA Guideline; 2015. [Google Scholar]
- 2.Nicholson K.G., Kent J., Ireland D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993 Oct 16;307(6910):982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douwes J., Gibson P., Pekkanen J., Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002 Jul;57(7):643–648. doi: 10.1136/thorax.57.7.643. BMJ Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis R., Lau L.C., Bron A.O., Roldaan A.C., Radermecker M., Djukanović R. The relationship between airways inflammation and asthma severity. Am. J. Respir. Crit. Care Med. 2000 Jan;161(1):9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 5.Shaw D.E., Berry M.A., Hargadon B., McKenna S., Shelley M.J., Green R.H. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007 Dec;132(6):1871–1875. doi: 10.1378/chest.07-1047. American College of Chest Physicians. [DOI] [PubMed] [Google Scholar]
- 6.Kupczyk M., Brinke ten A., Sterk P.J., Bel E.H., Papi A., Chanez P. Frequent exacerbators–a distinct phenotype of severe asthma. Clin. Exp. Allergy. 2014 Feb;44(2):212–221. doi: 10.1111/cea.12179. [DOI] [PubMed] [Google Scholar]
- 7.Kouzaki H., O'Grady S.M., Lawrence C.B., Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J. Immunol. 2009 Jul 15;183(2):1427–1434. doi: 10.4049/jimmunol.0900904. American Association of Immunologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uller L., Leino M., Bedke N., Sammut D., Green B., Lau L. Double-stranded RNA induces disproportionate expression of thymic stromal lymphopoietin versus interferon-beta in bronchial epithelial cells from donors with asthma. Thorax. 2010 Jul;65(7):626–632. doi: 10.1136/thx.2009.125930. [DOI] [PubMed] [Google Scholar]
- 9.Soumelis V., Reche P.A., Kanzler H., Yuan W., Edward G., Homey B. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002 Jul;3(7):673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 10.Mjösberg J., Bernink J., Golebski K., Karrich J.J., Peters C.P., Blom B. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012 Oct 19;37(4):649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Ying S., O'Connor B., Ratoff J., Meng Q., Fang C., Cousins D. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008 Aug 15;181(4):2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 12.Gauvreau G.M., O'Byrne P.M., Boulet L.-P., Wang Y., Cockcroft D., Bigler J. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014 May 29;370(22):2102–2110. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 13.Message S.D., Laza-Stanca V., Mallia P., Parker H.L., Zhu J., Kebadze T. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. U. S. A. 2008 Sep 9;105(36):13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson D.J., Makrinioti H., Rana B.M.J., Shamji B.W.H., Trujillo-Torralbo M.-B., Footitt J. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014 Dec 15;190(12):1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contoli M., Message S.D., Laza-Stanca V., Edwards M.R., Wark P.A.B., Bartlett N.W. Role of deficient type III interferon-λ production in asthma exacerbations. Nat. Med. 2006 Aug 13;12(9):1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 16.Gill M.A., Bajwa G., George T.A., Dong C.C., Dougherty I.I., Jiang N. Counterregulation between the Fc RI Pathway and antiviral responses in human plasmacytoid dendritic cells. J. Immunol. 2010 May 18;184(11):5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durrani S.R., Montville D.J., Pratt A.S., Sahu S., DeVries M.K., Rajamanickam V. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J. Allergy Clin. Immunol. 2012 Aug;130(2):489–495. doi: 10.1016/j.jaci.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contoli M., Ito K., Padovani A., Poletti D., Marku B., Edwards M.R. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015 Aug;70(8):910–920. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 19.Reddel H.K., Taylor D.R., Bateman E.D., Boulet L.-P., Boushey H.A., Busse W.W. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society, European Respiratory Society ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 2005 Apr 15;171(8):912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 21.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am. J. Respir. Crit. Care Med. 1999 Jan;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Pavord I.D., Pizzichini M.M., Pizzichini E., Hargreave F.E. The use of induced sputum to investigate airway inflammation. Thorax. 1997 Jun;52(6):498–501. doi: 10.1136/thx.52.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juniper E.F., O'Byrne P.M., Guyatt G.H., Ferrie P.J., King D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999 Oct;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 25.Chidlow G.R., Harnett G.B., Shellam G.R., Smith D.W. An economical tandem multiplex real-time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses. 2009 Jun;1(1):42–56. doi: 10.3390/v1010042. Molecular Diversity Preservation International. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chidlow G.R., Laing I.A., Harnett G.B., Greenhill A.R., Phuanukoonnon S., Siba P.M. Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J. Clin. Virol. 2012 Jul;54(3):235–239. doi: 10.1016/j.jcv.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochkov Y.A., Grindle K., Vang F., Evans M.D., Gern J.E. Improved molecular typing assay for rhinovirus species A, B, and C. J. Clin. Microbiol. 2014 Jul;52(7):2461–2471. doi: 10.1128/JCM.00075-14. American Society for Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W.-M., Kiesner C., Pappas T., Lee I., Grindle K., Jartti T. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgensen J.H., Pfaller M.A. American Society for Microbiology; 2015. Manual of Clinical Microbiology, Chapter 18; p. 1. [Google Scholar]
- 30.Simpson J.L., Scott R., Boyle M.J., Gibson P.G. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006 Jan;11(1):54–61. doi: 10.1111/j.1440-1843.2006.00784.x. Blackwell Science Pty. [DOI] [PubMed] [Google Scholar]
- 31.Shikotra A., Choy D.F., Ohri C.M., Doran E., Butler C., Hargadon B. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012 Jan;129(1):104–111. doi: 10.1016/j.jaci.2011.08.031. e1–9. [DOI] [PubMed] [Google Scholar]
- 32.Lee H.-C., Headley M.B., Loo Y.-M., Berlin A., Gale M., Debley J.S. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012 Nov;130(5):1187–1196. doi: 10.1016/j.jaci.2012.07.031. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolhouse I.S., Bayley D.L., Stockley R.A. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax. 2002 Aug;57(8):667–671. doi: 10.1136/thorax.57.8.667. BMJ Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green R.M., Custovic A., Sanderson G., Hunter J., Johnston S.L., Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. BMJ. 2002 Mar 30;324(7340):763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanania N.A., Noonan M., Corren J., Korenblat P., Zheng Y., Fischer S.K. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015 May 22;70(8) doi: 10.1136/thoraxjnl-2014-206719. BMJ Publishing Group Ltd and British Thoracic Society, thoraxjnl–2014–206719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel S., Ford L., Pearlman D., Spector S., Sher L., Skobieranda F. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013 Jun 27;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 37.Ortega H.G., Liu M.C., Pavord I.D., Brusselle G.G., FitzGerald J.M., Chetta A. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014 Sep 25;371(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 38.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012 Aug 18;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 39.Denlinger L.C., Sorkness R.L., Lee W.-M., Evans M.D., Wolff M.J., Mathur S.K. Lower airway Rhinovirus burden and the seasonal risk of asthma exacerbation. Am. J. Respir. Crit. Care Med. 2011 Nov;184(9):1007–1014. doi: 10.1164/rccm.201103-0585OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wark P.A.B., Johnston S.L., Moric I., Simpson J.L., Hensley M.J., Gibson P.G. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur. Respir. J. 2002 Jan;19(1):68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 41.Bizzintino J., Lee W.M., Laing I.A., Vang F., Pappas T., Zhang G. Association between human rhinovirus C and severity of acute asthma in children. Eur. Respir. J. 2011 Apr 30;37(5):1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCulloch D.J., Sears M.H., Jacob J.T., Lyon G.M., Burd E.M., Caliendo A.M. Severity of rhinovirus infection in hospitalized adults is unrelated to genotype. Am. J. Clin. Pathol. 2014 Aug;142(2):165–172. doi: 10.1309/AJCPHIKRJC67AAZJ. The Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wark P.A.B., Tooze M., Powell H., Parsons K. Viral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmission. Respirology. 2013 Aug;18(6):996–1002. doi: 10.1111/resp.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]