Abstract
IMPORTANCE
Among cranial nerve palsies, a third nerve palsy is important because a subset is caused by life-threatening aneurysms. However, there is significant disagreement regarding its incidence and the reported etiologies.
OBJECTIVE
To determine the incidence and etiologies of acquired third nerve palsy using a population-based method.
DESIGN, SETTING, AND PARTICIPANTS
All newly diagnosed cases of acquired third nerve palsy from January 1, 1978, through December 31, 2014, in Olmsted County, Minnesota, were identified using the Rochester Epidemiology Project, a record-linkage system of medical records for all patient-physician encounters among Olmsted County residents. All medical records were reviewed to confirm a diagnosis of acquired third nerve palsy and determine the etiologies, presenting signs, and symptoms. Incidence rates were adjusted to the age and sex distribution of the 2010 US white population.
MAIN OUTCOMES AND MEASURES
Incidence and etiologies of acquired third nerve palsies. The secondary outcome was incidence of pupil involvement in acquired third nerve palsies.
RESULTS
We identified 145 newly diagnosed cases of acquired third nerve palsy in Olmsted County, Minnesota, over the 37-year period. The age- and sex-adjusted annual incidence of acquired third nerve palsy was 4.0 per 100 000 (95% CI, 3.3–4.7 per 100 000). The annual incidence in patients older than 60 was greater than patients younger than 60 (12.5 vs 1.7 per 100 000; difference, 10.8 per 100 000; 95% CI, 4.7–16.9; P < .001). The most common causes of acquired third nerve palsy were presumed microvascular (42%), trauma (12%), compression from neoplasm (11%), postneurosurgery (10%), and compression from aneurysm (6%). Ten patients (17%) with microvascular third nerve palsies had pupil involvement, while pupil involvement was seen in 16 patients (64%) with compressive third nerve palsies.
CONCLUSIONS AND RELEVANCE
This population-based cohort demonstrates a higher incidence of presumed microvascular third nerve palsies and a lower incidence of aneurysmal compression than previously reported in non–population-based studies. While compressive lesions had a higher likelihood of pupil involvement, pupil involvement did not exclude microvascular third nerve palsy and lack of pupil involvement did not rule out compressive third nerve palsy.
Among all cases of ocular misalignment from cranial nerve palsies, third nerve palsies are important because a subset of these are caused by life-threatening aneurysms. The true incidence of third nerve palsies and the relative incidence of the various etiologies are largely unknown. Prior studies1–4 have indicated that aneurysms account from anywhere from 10% to 30% of cases. These studies were from academic tertiary referral centers and, therefore, were not population-based and may suffer from a referral bias toward more severe patients.
Using the Rochester Epidemiology Project (REP), a record-linkage system of medical records for all patient-clinician encounters among residents of Olmsted County, Minnesota, we were able to perform a population-based analysis of third nerve palsies. The goal of this study was to evaluate the population-based incidence of acquired third nerve palsy and determine the relative frequency of the various etiologies. In addition, this study reviewed the presenting signs and symptoms of acquired third nerve palsies. Patients with congenital third nerve palsy were excluded from the study because symptoms and recovery do not apply to this subset of patients.
Methods
All medical records of patients with newly diagnosed third nerve palsies identified using the REP5–7 were retrospectively reviewed. These records capture medical care in Olmsted County at Mayo Clinic, Olmsted Medical Center, and their affiliated hospitals, and the few private clinicians. The Mayo Clinic institutional review board waived the need for informed consent for this study because this was a retrospective medical record review with minimal risk. In addition, consent for review of medical records was not possible to obtain in individuals who were deceased or were no longer in the immediate area.
This study was approved by the Mayo Clinic and Olmsted County institutional review boards. All patient medical records were reviewed to identify patients with newly diagnosed third nerve palsy presenting from January 1, 1978, through December 31, 2014. The REP database was searched for a diagnosis of third nerve palsy, and the medical records were reviewed to confirm the diagnosis. The REP database was also searched for other diagnoses that may include third nerve palsy, including the diagnosis of strabismus, hypertropia, and miscellaneous ophthalmoplegia, and was reviewed for a clinical diagnosis of third nerve palsy. Each occurrence of acquired third nerve palsy was considered a separate incidence case; 51 of 145 of these patients (35.2%) have been included in previous reports of pediatric cranial nerve palsies and adult strabismus, but those articles did not include patients of all ages or a detailed analysis of causes and presenting signs.8,9
In cases of confirmed acquired third nerve palsy, the medical records were also reviewed to determine the cause of the third nerve palsy. In addition, the details of the acquired third nerve palsy at presentation were also documented, including age, imaging that was performed, pupil involvement, ptosis, degree of ophthalmoplegia, presence of eye pain or headache, other neurologic symptoms, recovery, and aberrant regeneration. Pupil involvement was defined by at least 0.5 mm of anisocoria, with the larger pupil being on the affected side. Neurologically isolated palsies were defined as the absence of other signs or symptoms other than headache or periorbital pain. A presumed microvascular third nerve palsy was diagnosed in patients who demonstrated spontaneous recovery or substantial improvement within 4 months without signs of aberrant regeneration, had no other neurologic symptoms or signs, and had other causes of the palsy excluded, such as trauma and compression.10 Patients with myasthenia gravis, thyroid eye disease, Miller Fisher syndrome, or other causes of strabismus that could mimic a third nerve palsy were excluded from this study. Congenital third nerve palsies were also excluded from the analyses because these are not acquired and the presence of symptoms and recovery are not relevant for congenital third nerve palsy.
Simple comparisons of categorical factors between groups were completed using χ2 tests. The overall incidence of acquired third nerve palsy was estimated using the age- and sex-specific population figures for Olmsted County US Census data for 1978 through 2014. Yearly incidence rates for age and sex group were determined by dividing the number of cases with acquired third nerve palsy within that group by the estimated total Olmsted County resident population of the group for that given year. Because 86% of the Olmsted County population is white, these incidence rates were also age- and/or sex-adjusted to the 2010 census figures for the US white population, so that the data could be compared with national estimates. The 95% CIs for the rates were calculated assuming Poisson error distribution. Overall comparisons of the incidence among age groups, between sexes, and across time were investigated using Poisson regression models.
Results
Overall Incidence
Over the 37-year period, 145 new cases of acquired third nerve palsy were diagnosed in the defined population. The number of cases per year ranged from 1 to 12. There were 58 males (40%) and 87 females (60%); 74 were right eye palsies, 68 were left eye palsies, and 3 were both. The overall age- and sex-adjusted annual incidence of acquired third nerve palsy was 4.0 in 100 000 (95% CI, 3.3–4.7). The incidence was fairly low among children and young adults. While there was a mild increase of incidence in middle-aged adults, patients older than 60 years had a larger increase in incidence of acquired third nerve palsy (P < .001) Figure 1). The incidence in patients older than 60 years was 12.5 per 100 000 (95% CI, 9.9–15.5) compared with 1.7 per 100 000 per year (95% CI, 1.3–2.2) for patients younger than 60 years (difference of 10.8 per 100 000; 95% CI, 4.7–16.9; P < .001). The incidence was not different between males and females (P =.18) and there was no trend over time (P = .49).
Figure 1. Incidence of Acquired Third Nerve Palsy per 100 000 Person-years by Age in Olmsted County, Minnesota, 1978 to 2014.
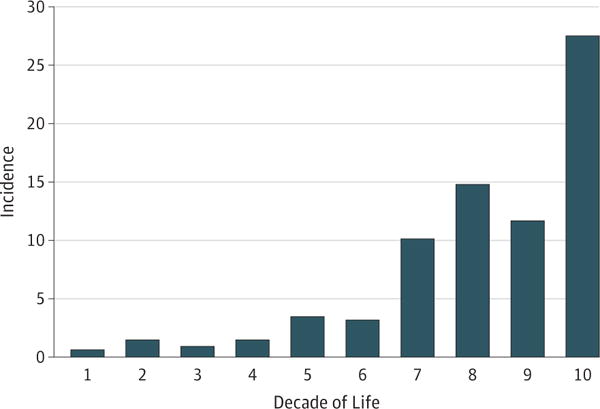
The overall age- and sex-adjusted annual incidence of acquired third nerve palsy was 4.0 in 100 000 (95% CI, 3.3–4.7).
Causes of the Acquired Third Nerve Palsy
The most common causes were presumed microvascular (42%), trauma (12%), compression from neoplasm (11%), post-neurosurgery (10%), compression from aneurysm (6%), other cause (5%), stroke (4%), undetermined (4%), pituitary apoplexy (2%), Tolosa-Hunt syndrome (2%), and giant cell arteritis (1%). The “other” category (5%) included single cases of carotid cavernous sinus fistula, zoster, carcinomatous meningitis, ophthalmoplegic migraine, cavernous sinus thrombosis, and postviral palsy.
Six of the 14 patients with postneurosurgical third nerve palsies were from clipping of an aneurysm. Therefore, aneurysms directly and indirectly accounted for 15 cases (10%) when both compression and postsurgical cases were combined.
Among the 6 cases of undetermined third nerve palsy, 3 patients had chronic third nerve palsies without recovery and did not have magnetic resonance imaging (MRI) due to their older age, one of whom had a simultaneous ipsilateral fourth nerve palsy. One patient developed third and sixth nerve palsies that resolved spontaneously; results from computed tomography and MRI were unremarkable. Two patients did not have imaging because the third nerve palsy resolved spontaneously within 24 hours.
Causes of Acquired Third Nerve Palsy by Age
In childhood and young adulthood, trauma was the common cause of acquired third nerve palsy (Figure 2). An increasing number of microvascular third nerve palsies were seen beginning in the fifth decade of life and were largely responsible for the increase in incidence of acquired third nerve palsy in the sixth decade of life (Figure 1 and Figure 2). The mean (SD) age of a patient with a microvascular third nerve palsy was 68 (13) years (range, 45–96 years). There was a fairly equal number of third nerve palsies from compression in all age groups (Figure 2). Compression from aneurysm was only seen later in life.
Figure 2. Frequency and Causes of Acquired Third Nerve Palsy by Decade of Life.
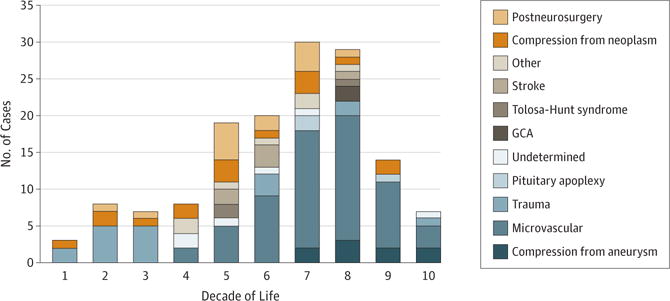
GCA indicates giant cell arteritis.
Characteristics of Acquired Third Nerve Palsy
The clinical characteristics of the various acquired third nerve palsy etiologies are illustrated in the Table. Overall, 62 of 145 patients (43%) with acquired third nerve palsy had pupil involvement at the time of presentation. Pupil involvement was seen in 16% of microvascular third nerve palsies, 71% of traumatic third nerve palsies, 71% of postneurosurgical third nerve palsies, and 64% of compressive third nerve palsies. Among the compressive third nerve palsies, 33% of aneurysms had pupil involvement at presentation, while 81% of nonaneurysmal compressive third nerve palsies had pupil involvement. All 3 patients with posterior communicating artery aneurysm presented with pupil involvement (≥2 mm of anisocoria), while all of the 5 patients with intracavernous sinus aneurysm initially presented with pupil-sparing third nerve palsies but then developed pupil involvement over time. There was a single case of a patient with a posterior cerebral artery aneurysm that did not have anisocoria, but the patient also had bilateral Adie tonic pupil that complicated evaluation of the pupils.
Table.
Characteristics of Acquired Third Nerve Palsya
| Cause | Cases, No. | No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pupil Involvement | Ptosis | Complete External Third Nerve Dysfunction | Recovery | Neurologically Isolated (Not Including Eye Pain or Headache) | Eye Pain and/or Headache | Aberrant Regeneration | ||
| Microvascular | 61 | 10 (16) | 56 (92) | 20 (33) | 58 (95) | 58 (95) | 37 (61) | 0 |
| Stroke | 6 | 4 (67) | 4 (67) | 1 (17) | 4 (67) | 1 (17) | 3 (50) | 0 |
| Compression | 25 | 16 (64) | 20 (80) | 6 (24) | 6 (24) | 9 (36) | 15 (60) | 4 (16) |
| Aneurysm | 9 | 3 (33) | 8 (89) | 2 (22) | 3 (33) | 6 (67) | 7 (78) | 1 (11) |
| Meningioma | 5 | 4 (80) | 3 (60) | 2 (40) | 2 (40) | 0 | 1 (20) | 0 |
| Metastasis | 5 | 4 (80) | 4 (80) | 1 (20) | 0 | 1 (20) | 4 (80) | 0 |
| Pituitary adenoma | 2 | 2 (100) | 2 (100) | 1 (50) | 0 | 1 (50) | 0 | 0 |
| Other | 4 | 3 (75) | 3 (75) | 0 | 1 (25) | 1 (25) | 3 (75) | 3 (75) |
| Trauma | 18 | 14 (78) | 16 (89) | 3 (17) | 4 (22) | 4 (22) | 17 (95) | 4 (22) |
| MVC | 12 | 12 (100) | 10 (83) | 3 (25) | 2 (17) | 0 | 12 (100) | 4 (33) |
| Other | 6 | 2 (33) | 6 (100) | 0 | 2 (33) | 4(67) | 5 (83) | 0 |
| Postneurosurgery | 14 | 10 (71) | 12 (86) | 4 (29) | 5 (36) | 2 (14) | 11 (79) | 2 (14) |
| Clipping of aneurysm | 6 | 4 (67) | 4 (67) | 0 | 2 (33) | 0 | 6 (100) | 0 |
| Meningioma | 2 | 1 (50) | 2 (100) | 1 (50) | 0 | 1 (50) | 0 | 0 |
| Other | 6 | 5 (83) | 6 (100) | 3 (50) | 3 (50) | 1 (17) | 5 (83) | 2 (33) |
| Undetermined | 6 | 1 (17) | 4 (67) | 1 (17) | 3 (50) | 4 (67) | 3 (50) | 1 (17) |
| Pituitary apoplexy | 3 | 2 (67) | 3 (100) | 2 (67) | 2 (67) | 0 | 3 (100) | 0 |
| Tolosa-Hunt syndrome | 3 | 0 | 3 (67) | 0 | 3 (100) | 0 | 2 (67) | 0 |
| Giant cell arteritis | 2 | 0 | 2 (100) | 0 | 2 (100) | 1 (50) | 2 (100) | 0 |
Abbreviation: MVC, motor vehicle crash.
Percentages do not equal 100% owing to rounding. There was a single case of carotid-cavernous sinus fistula, hemorrhage, zoster, carcinomatous meningitis, ophthalmoplegic migraine, postviral palsy, and cavernous sinus thrombosis causing third nerve palsy.
One hundred twenty-five of 145 patients (86%) with acquired third nerve palsy had ptosis at time of presentation, which was not different among the various causes of acquired third nerve palsy (Table). Complete external third nerve dysfunction was seen in 38 patients (26%) at time of presentation, which was also not different among the various causes of acquired third nerve palsy (Table).
A total of 81 of 145 cases (56%) were neurologically isolated (excluding headache or eye pain) (Table). Other neurologic signs included optic neuropathy, simultaneous cranial nerve palsies, hemiplegia, sensory loss, ataxia, Horner syndrome, and mental status changes. Fifty-eight of 61 cases (95%) of microvascular third nerve palsies were neurologically isolated, while the remaining 3 cases were found incidentally during admission for unrelated altered mental status and/or dementia. Other causes of acquired third nerve palsy having a lower chance of being neurologically isolated included compression from aneurysm in 6 of 9 patients (67%), traumatic third nerve palsy in 4 of 18 (22%), and compression from neoplasm in 3 of 16 (19%).
Aberrant regeneration was seen in 11 of 145 cases (8%): 4 cases of traumatic third nerve palsy, 4 cases of compression (2 cases cavernous sinus mass, 1 intracavernous aneurysm, 1 teratoma), 2 cases of postneurosurgery, and 1 case of undetermined third nerve palsy (further imaging was not pursued owing to the patient’s advanced age).
Overall, 91 of 145 patients (63%) with acquired third nerve palsy had complete recovery. Fifty-eight of 61 patients (95%) with a microvascular third nerve palsy had complete recovery; the 3 remaining patients had a substantial but incomplete recovery. The other causes of acquired third nerve palsy were associated with a much lower chance of complete recovery, which included 5 of 14 patients (36%) with postneurosurgery-induced third nerve palsy, 3 of 9 (33%) with compression from aneurysm, 4 of 18 (22%) with traumatic third nerve palsy, and 3 of 16 (19%) with compression from neoplasm (Table).
Eye pain and/or headache were common, occurring in 100 of 145 cases of acquired third nerve palsy (69%). Thirty-seven of 61 patients with microvascular third nerve palsies (61%) had eye pain or headache at time of presentation. Seven of 9 patients with aneurysm (78%) had eye pain or headaches at time of presentation, compared with 61% from microvascular third nerve palsies (P = .32).The lack of pain in the remaining 2 cases of aneurysm led to a delay in imaging by 1 to 2 weeks, which ultimately revealed intracavernous sinus aneurysms.
Discussion
In this population-based study, we describe the incidence and etiologies of acquired third nerve palsy in a geographically de-fined population. We found the overall age- and sex-adjusted incidence of acquired third nerve palsy to be 4.0 per 100 000 (95% CI, 3.3–4.7). To our knowledge, this study is the first to describe the incidence of acquired third nerve palsy in the United States using a population-based approach. We demonstrate that the incidence was fairly low among children and young adults in whom trauma was the most common cause. There was an increase in incidence among patients older than 60 years, predominantly due to a large increase in microvascular third nerve palsies.
In our series, 42% of acquired third nerve palsies were from a presumed microvascular etiology, while only 6% were from aneurysmal compression. Other studies in Western countries have found a lower incidence of microvascular third nerve palsies, ranging from 11% to 21%, and a higher incidence of aneurysmal third nerve palsies, ranging from 10% to 30%.1–4 The present study is likely to be more representative of the actual etiologic incidences of acquired third nerve palsy because it is a population-based study that does not suffer from inherent referral bias.11
Sixteen percent of patients with microvascular third nerve palsies had pupil involvement at time of presentation compared with 64% for compressive third nerve palsies. This is in line with findings in prior studies that have reported pupil involvement in 14% to 38% of microvascular third nerve palsies, which is typically mild (usually ≤1 mm).1,12–14 In addition, it has also been reported that compressive third nerve palsies, even from aneurysm, can sometimes present without pupil involvement.15–18 Therefore, anisocoria alone cannot definitively differentiate microvascular from compressive third nerve palsies, which supports many findings of prior studies.
Pain was present in 61% of patients with microvascular third nerve palsies, a rate that did not differ from that of patients with aneurysm (78%). Others have also found pain to be a common finding in microvascular ocular motor cranial nerve palsies, ranging from 54% to 88%,1,10,12,19 which was similar to the reported prevalence of pain in aneurysmal third nerve palsies ranging from 31% to 92%.1,20–23 The quality of the pain has been previously studied and found to be similarly unsupportive.24 Therefore, pain is similarly unreliable to differentiate a microvascular etiology from aneurysmal compression.25
Intracranial aneurysms are the most feared cause of third nerve palsy and can represent a true neurologic emergency because of the risk of subarachnoid hemorrhage, which carries substantial morbidity and mortality.22,26,27 One-third of our aneurysmal third nerve palsies were from posterior communicating artery aneurysm, which presented acutely with painful pupil involving third nerve palsies, including 1 patient who died from rupture and subarachnoid hemorrhage, while another had subarachnoid hemorrhage requiring emergent craniotomy and clipping of the aneurysm. Others have reported posterior communicating artery aneurysms as the most common cause of an isolated third nerve palsy,21,25,28 which may be a referral bias because posterior communicating artery aneurysms tend to present more acutely as neurologic emergencies. Among the 9 cases of aneurysmal third nerve palsies in our study, 5 were from cavernous sinus aneurysms. Cavernous sinus aneurysms seldom rupture, and if they do rupture, they rarely cause life-threatening subarachnoid hemorrhage but rather cause carotid-cavernous fistula.29–32 In conjunction with this, the patients in our study with cavernous sinus aneurysms presented insidiously, often without pupil involvement at initial presentation. Cavernous sinus aneurysms tend to produce other ocular motor cranial neuropathies or Horner syndrome because of the close proximity of the third nerve palsy to the other nerves within the cavernous sinus.25 However, 3 of the 5 patients with cavernous sinus aneurysms presented with an isolated third nerve palsy.
As can be seen in our series, the presentation of aneurysmal third nerve palsy can be quite variable with regard to pupil involvement and pain. Clinical signs and symptoms cannot distinguish microvascular from compression; therefore, neuroimaging is recommended for all acquired third nerve palsies without obvious known cause, such as an expected complication from a neurosurgical procedure.
Limitations
A limitation of the study is that Olmsted County has primarily a white population and, therefore, the results are best extrapolated to other white US populations. There is also the evolution of neuroimaging over the course of the study. Without MRI or gadolinium in the early years of the study, some diagnoses may have been missed. However, the diagnoses were evenly distributed throughout the years, and, therefore, imaging yield bias likely played an insignificant role in the etiologies found in this study. Despite these limitations, a population-based approach provides valuable information most applicable to practitioners outside of large referral centers.
Conclusions
We provide population-based incidence and etiologies of acquired third nerve palsy, which have demonstrated a higher incidence of microvascular and a lower incidence of aneurysmal third nerve palsies than previously reported. The incidence upsurges in the sixth decade of life associated with an increase in microvascular third nerve palsies. While pupil involvement was more common in compressive lesions, pupil involvement was seen in some cases of microvascular third nerve palsies, and pupil sparing was seen in some cases of compressive lesions, including aneurysm.
Key Points.
Question
What is the incidence and etiologies of acquired third nerve palsy?
Findings
In this case series in Olmsted County, Minnesota, the age- and sex-adjusted annual incidence of acquired third nerve palsy was 4.2 per 100 000. There was a higher incidence of microvascular and lower incidence of aneurysm than previously reported.
Meaning
Because of its population-based method, this study provides evidence regarding etiologies of acquired third nerve palsies that can be considered by clinicians when evaluating patients with such palsies.
Acknowledgments
Funding/Support: This work was supported, in part, by an unrestricted grant to the Department of Ophthalmology by Research to Prevent Blindness Inc, New York, New York. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under grant R01AG034676.
Role of the Funder/Sponsor: The funding sources did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Fang and Chen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fang, Leavitt, Holmes, Mohney, Chen.
Acquisition of data: Fang, Hodge, Holmes, Chen.
Analysis and Interpretation of data: Fang, Hodge, Holmes, Chen.
Drafting of the manuscript: Fang, Chen. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Fang, Hodge, Chen.
Obtained funding: Chen.
Administrative, technical, or material support: Fang, Mohney, Chen.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Green WR, Hackett ER, Schlezinger NS. Neuro-ophthalmologic evaluation of oculomotor nerve paralysis. Arch Ophthalmol. 1964;72:154–167. doi: 10.1001/archopht.1964.00970020154005. [DOI] [PubMed] [Google Scholar]
- 2.Keane JR. Third nerve palsy: analysis of 1400 personally examined inpatients. Can J Neurol Sci. 2010;37(5):662–670. doi: 10.1017/s0317167100010866. [DOI] [PubMed] [Google Scholar]
- 3.Rucker CW. The causes of paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1966;61(5, pt 2):1293–1298. doi: 10.1016/0002-9394(66)90258-3. [DOI] [PubMed] [Google Scholar]
- 4.Rush JA, Younge BR. Paralysis of cranial nerves III, IV, and VI: cause and prognosis in 1,000 cases. Arch Ophthalmol. 1981;99(1):76–79. doi: 10.1001/archopht.1981.03930010078006. [DOI] [PubMed] [Google Scholar]
- 5.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., III History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes JM, Mutyala S, Maus TL, Grill R, Hodge DO, Gray DT. Pediatric third, fourth, and sixth nerve palsies: a population-based study. Am J Ophthalmol. 1999;127(4):388–392. doi: 10.1016/s0002-9394(98)00424-3. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Thompson JM, Diehl NN, Holmes JM, Mohney BG. Incidence, types, and lifetime risk of adult-onset strabismus. Ophthalmology. 2014;121(4):877–882. doi: 10.1016/j.ophtha.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson DM, McCanna TD, Layde PM. Risk factors for ischemic ocular motor nerve palsies. Arch Ophthalmol. 1994;112(7):961–966. doi: 10.1001/archopht.1994.01090190109029. [DOI] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson DM. Pupil involvement in patients with diabetes-associated oculomotor nerve palsy. Arch Ophthalmol. 1998;116(6):723–72. doi: 10.1001/archopht.116.6.723. [DOI] [PubMed] [Google Scholar]
- 13.Rucker CW. Paralysis of the third, fourth and sixth cranial nerves. Am J Ophthalmol. 1958;46(6):787–794. doi: 10.1016/0002-9394(58)90989-9. [DOI] [PubMed] [Google Scholar]
- 14.Teuscher AU, Meienberg O. Ischaemic oculomotor nerve palsy. Clinical features and vascular risk factors in 23 patients. J Neurol. 1985;232(3):144–149. doi: 10.1007/BF00313889. [DOI] [PubMed] [Google Scholar]
- 15.Lustbader JM, Miller NR. Painless, pupil-sparing but otherwise complete oculomotor nerve paresis caused by basilar artery aneurysm: case report. Arch Ophthalmol. 1988;106(5):583–584. doi: 10.1001/archopht.1988.01060130633009. [DOI] [PubMed] [Google Scholar]
- 16.Tummala RP, Harrison A, Madison MT, Nussbaum ES. Pseudomyasthenia resulting from a posterior carotid artery wall aneurysm: a novel presentation: case report. Neurosurgery. 2001;49(6):1466–1468. doi: 10.1097/00006123-200112000-00034. [DOI] [PubMed] [Google Scholar]
- 17.Saito R, Sugawara T, Mikawa S, Fukuda T, Kohama M, Seki H. Pupil-sparing oculomotor nerve paresis as an early symptom of unruptured internal carotid-posterior communicating artery aneurysms: three case reports. Neurol Med Chir (Tokyo) 2008;48(7):304–306. doi: 10.2176/nmc.48.304. [DOI] [PubMed] [Google Scholar]
- 18.Kissel JT, Burde RM, Klingele TG, Zeiger HE. Pupil-sparing oculomotor palsies with internal carotid-posterior communicating artery aneurysms. Ann Neurol. 1983;13(2):149–154. doi: 10.1002/ana.410130207. [DOI] [PubMed] [Google Scholar]
- 19.Wilker SC, Rucker JC, Newman NJ, Biousse V, Tomsak RL. Pain in ischaemic ocular motor cranial nerve palsies. Br J Ophthalmol. 2009;93(12):1657–1659. doi: 10.1136/bjo.2008.155150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzino G, Andreoli A, Tognetti F, et al. Orbital pain and unruptured carotid-posterior communicating artery aneurysms: the role of sensory fibers of the third cranial nerve. Acta Neurochir (Wien) 1993;120(1–2):7–11. doi: 10.1007/BF02001462. [DOI] [PubMed] [Google Scholar]
- 21.Leivo S, Hernesniemi J, Luukkonen M, Vapalahti M. Early surgery improves the cure of aneurysm-induced oculomotor palsy. Surg Neurol. 1996;45(5):430–434. doi: 10.1016/0090-3019(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 22.Okawara SH. Warning signs prior to rupture of an intracranial aneurysm. J Neurosurg. 1973;38(5):575–580. doi: 10.3171/jns.1973.38.5.0575. [DOI] [PubMed] [Google Scholar]
- 23.Soni SR. Aneurysms of the posterior communicating artery and oculomotor paresis. J Neurol Neurosurg Psychiatry. 1974;37(4):475–484. doi: 10.1136/jnnp.37.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demierre B, Safran AB. Paralysis of the IIIrd cranial nerve: pain and its value in the differential diagnosis [in French] J Fr Ophtalmol. 1981;4(2):133–141. [PubMed] [Google Scholar]
- 25.Lee AG, Hayman LA, Brazis PW. The evaluation of isolated third nerve palsy revisited: an update on the evolving role of magnetic resonance, computed tomography, and catheter angiography. Surv Ophthalmol. 2002;47(2):137–15. doi: 10.1016/s0039-6257(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 26.Bederson JB, Connolly ES, Jr, Batjer HH, et al. American Heart Association Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 27.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28(3):660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- 28.Hamer J. Prognosis of oculomotor palsy in patients with aneurysms of the posterior communicating artery. Acta Neurochir (Wien) 1982;66(3–4):173–185. doi: 10.1007/BF02074504. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg-Cohen N, Curry C, Miller NR, Tamargo RJ, Murphy KP. Long term visual and neurological prognosis in patients with treated and untreated cavernous sinus aneurysms. J Neurol Neurosurg Psychiatry. 2004;75(6):863–867. doi: 10.1136/jnnp.2003.020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiebel-Kalish H, Kalish Y, Bar-On RH, et al. Presentation, natural history, and management of carotid cavernous aneurysms. Neurosurgery. 2005;57(5):850–857. doi: 10.1227/01.neu.0000179922.48165.42. [DOI] [PubMed] [Google Scholar]
- 31.Kupersmith MJ, Stiebel-Kalish H, Huna-Baron R, et al. Cavernous carotid aneurysms rarely cause subarachnoid hemorrhage or major neurologic morbidity. J Stroke Cerebrovasc Dis. 2002;11(1):9–14. doi: 10.1053/jscd.2002.123969. [DOI] [PubMed] [Google Scholar]
- 32.Ko JH, Kim YJ. Oculomotor nerve palsy caused by posterior communicating artery aneurysm: evaluation of symptoms after endovascular treatment. Interv Neuroradiol. 2011;17(4):415–419. doi: 10.1177/159101991101700403. [DOI] [PMC free article] [PubMed] [Google Scholar]


