Abstract
Objectives:
Patients experience severe pain after pectus excavatum (PE) surgery. The aim of this prospective, randomized study was to compare analgesic effects of ultrasonography-guided bilateral intercostal nerve blocks (UG-ICNBs) with those of conventional patient-controlled intravenous analgesia (PCIA) on acute pain after the Nuss procedure for PE repair in children.
Methods:
A prospective randomized study was performed in children with PE who were scheduled for the Nuss procedure. Participants were randomly assigned to receive either UG-ICNBs or PCIA for postoperative analgesia. Faces Pain Scale-Revised scores, opioid consumption, analgesia-associated side effects (respiratory depression, pruritus, nausea, vomiting) during the first 24 hours, and lengths of stay in the postanesthesia care unit (PACU) and hospital were recorded after the surgery.
Results:
Sixty-two children undergoing the Nuss procedure were enrolled in the trial. Faces Pain Scale-Revised scores were significantly decreased in the UG-ICNBs group compared with the PCIA group for up to 6 hours after surgery. The opioid doses required in the PACU and during the first 24 hours after surgery were significantly greater in the PCIA group compared with the UG-ICNBs group. Accordingly, patients in the UG-ICNBs group showed a lower incidence of analgesia-associated side effects and faster PACU discharge compared with the PCIA group.
Conclusions:
Our study suggests that UG-ICNBs might be more effective than PCIA for postoperative analgesia in children who undergo the Nuss procedure for PE.
Key Words: ultrasound guidance, intercostal nerve blocks, patient-controlled intravenous analgesia, pediatric surgery, Nuss procedure
The incidence of pectus excavatum (PE), the most common congenital chest wall deformity in children, is approximately 1 in 400.1 At our institution, severe thoracic deformities in children between 3 and 18 years old are commonly reshaped with the placement of a retrosternal steel bar that is removed several years later after reshaping has stabilized. Our surgeons use a modification of Nuss’s technique.2 Although minimally invasive, this procedure produces significant postoperative pain, which might affect the function of other organs and postoperative rehabilitation and thus increase the length of hospitalization.3,4 At present, the optimal method for postoperative analgesia remains unclear.
Epidural analgesia has been reported as the standard method for pain control in the early postoperative period. However, it is not suitable for all patients and is associated with numerous risks such as respiratory depression; dural perforation; spinal cord damage due to hematoma formation, infection, and abscess; hypotension; and urinary retention.5,6 Compared with epidural analgesia, conventional patient-controlled intravenous analgesia (PCIA) using fentanyl has been shown to be an effective technique with fewer complications in children requiring thoracoscopic PE repair.7 However, high-dose systemic opioids may increase the incidence of undesirable adverse effects, such as respiratory depression, nausea, and vomiting. In recent years, regional anesthesia techniques, including intercostal nerve blocks (ICNBs), have been implemented in children undergoing the Nuss procedure.8,9 The advantages of ICNBs include technical simplicity, good analgesia, an opioid-sparing effect, improved pulmonary mechanics, reduced central nervous system (CNS) depression, and avoidance of urinary retention.10 Ultrasound-guided blocks may improve the effectiveness and safety of intercostal blocks as has been noted for other pediatric regional techniques.11,12 The combination of ICNBs with ultrasonographic guidance could maximize their respective benefits and might be preferable to PCIA for controlling acute pain after the Nuss procedure in children.
The primary objective of this prospective, randomized controlled study was to compare analgesic effects (measured by Faces Pain Scale-Revised [FPS-R] scores) of ultrasonography-guided (UG) bilateral ICNBs (UG-ICNBs) and conventional PCIA on acute pain (within 24 h) after the Nuss procedure in children. The secondary objectives were to compare postoperative opioid consumption, lengths of stay in the postanesthesia care unit (PACU) and hospital, and analgesia-associated side effects between the 2 groups.
METHODS
Participants
This study was approved by the ethics committee of Xinhua Hospital, Shanghai Jiaotong University (Approval-No: XHEC-C-2014-079) and is registered at www.chictr.org (ChiCTR-TRC-13004410). After written informed consent was obtained, 80 children (age range, 4 to 16 y) scheduled for the Nuss procedure at Xinhua Hospital, Shanghai Jiaotong University between October 2013 and October 2014 were randomly assigned by computer-generated randomization to receive either UG-ICNBs or conventional PCIA for postoperative analgesia (Fig. 1). Assignments were enclosed in sealed opaque envelopes before the operation. The exclusion criteria were as follows: requirement for additional surgery, history of analgesic administration (eg, opioids, acetaminophen, or nonsteroidal anti-inflammatory drugs) 24 hours before premedication, history of coagulation disorders or allergy to local anesthetics, history of renal insufficiency or an American Society of Anesthesiologists (ASA) physical status that was higher than II, inability to understand PCIA device use, or parental objection to ICNBs.
FIGURE 1.
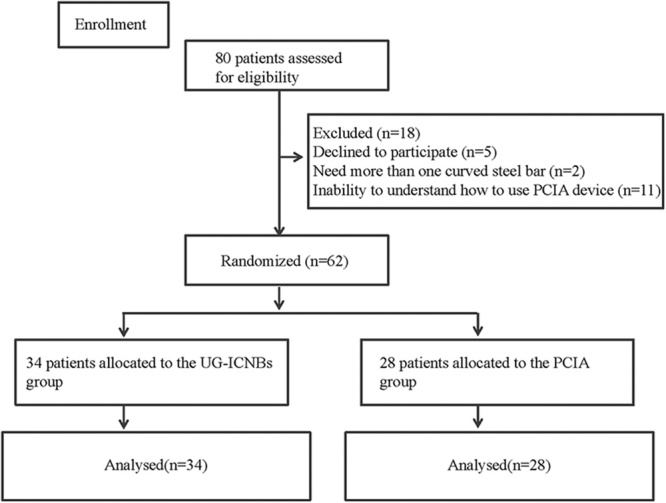
CONSORT flow diagram. PCIA indicates patient-controlled intravenous analgesia; UG-ICNBs, ultrasonography-guided bilateral intercostal nerve blocks.
Study Protocol
All children were premedicated with midazolam 0.3 mg/kg and acetaminophen 15 mg/kg (maximum 650 mg) orally. After peripheral intravenous catheterization, anesthesia was induced by midazolam 0.2 mg/kg, propofol 3 mg/kg, fentanyl 2 μg/kg, and rocuronium 0.6 mg/kg. After tracheal intubation, general anesthesia was maintained with sevoflurane (end-tidal concentration, 2%) in oxygen-nitrous oxide (fraction of inspired oxygen, 0.5). Intravenous remifentanil (0.2 μg/kg/min) was continuously injected for intraoperative analgesia. Before recovery, patients received intravenous ondansetron hydrochloride 0.1 mg/kg. Isotonic saline was used for intraoperative fluid maintenance.
After induction, an ultrasonographic probe (M-Turbo with the L25× transducer; SonoSite Inc.) was used to scan laterally from the midaxillary line to identify the required anatomic landmarks, while the patients were in a supine position. The ribs were identified as hyperechoic streaks, while the pleura appeared as hyperechoic lines between and below the ribs. In the UG-ICNBs group, 0.25% ropivacaine hydrochloride (Table 1) was introduced at the level of the incision using a 22-G short-bevel needle placed in-plane. Anesthetic agent spread was monitored by ultrasonography. The needle was advanced until the distal tip was just superficial to the pleura. A tissue plane between the internal and innermost intercostal muscles was delineated as the local anesthetic agent. Expansion of this potential space indicated appropriate spread (Fig. 2). Normal saline solution (0.9%) was injected in the PCIA group. This procedure was repeated bilaterally in one space above and one space below. Accordingly, a total of 6 injections were administered to each patient. All nerve blocks were administered by the same, experienced anesthesiologist (S.S.). Postoperatively, the PCIA group received PCIA with fentanyl, while the UG-ICNB group received normal saline solution for 48 hours. The patients and health care personnel were blinded to the groups. For the PCIA regimen, the pump was programmed with a basal infusion of 0.1 μg/kg/h, bolus dose of 0.2 μg/kg, and lockout interval of 10 minutes.7
TABLE 1.
Total Volume of Local Anesthetic Used by Patient Weight
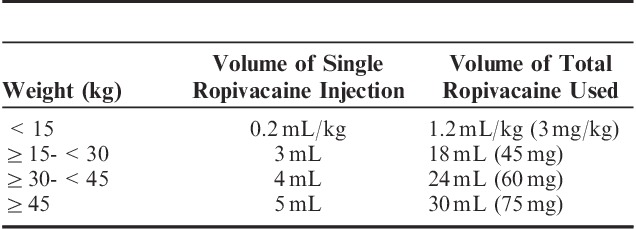
FIGURE 2.
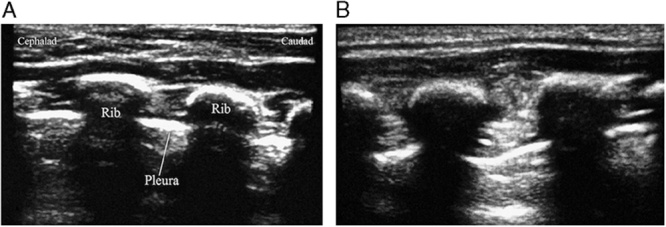
Ultrasound image of the landmarks and local anesthetic injection for ultrasonography-guided bilateral intercostal nerve blocks. A, The hyperechoic ribs and the pleura. B, After the administration of the local anesthetic, the bolus increased, and expansion of the potential space is visible.
Postoperative Follow-up
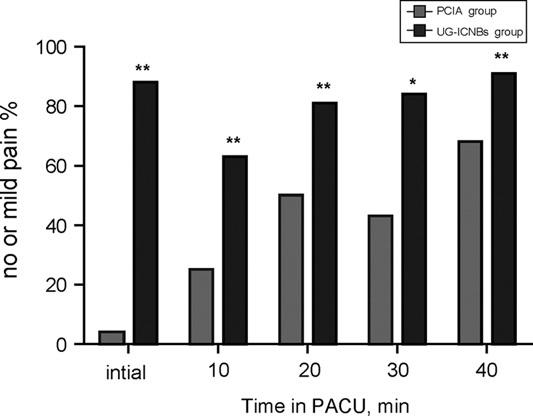
After surgery, the patients were transferred to the PACU. When the children regained consciousness, a blinded research team member (Y.S.) assessed their postoperative pain levels using the FPS-R13 at 10 minutes intervals in both groups (10, 20, 30, and 40 or more minutes). Fentanyl 0.2 μg/kg was administered as a rescue analgesic according to the patient-reported FPS-R score to decrease the pain score <4. FPS-R scores, opioid dose, and analgesia-associated side effects, including respiratory depression14 (defined as respiratory rate <8 bpm, requirement of naloxone, and/or peripheral oxygen saturation <90% as evaluated by one of the authors [L.N.]), pruritus, nausea, and vomiting were recorded during the first 24 hours after surgery.
Blinding
The pediatric anesthesiologists (M.L., X.L., L.N., S.S., Y.S) and other operating room staff members (nurses) were all blinded. Only one author (Y.C.) took care of randomization and prepared all local anesthetic solutions, drugs, and PCIA pumps. Patients, other authors, medical staff members, and the statistician were unaware of the treatment groups.
Statistics
All statistical analyses were performed using the standard software packages SPSS version 18.0 and GraphPad Prism5. The average FPS-R scores during the first hour in the PACU were used to calculate the sample size in a pilot study. The power (β) and confidence level (α) for the study were set at 0.8 and 0.05, respectively. A total of 25 participants in each treatment group would provide an 80% power to detect a difference in FPS-R scores between the UG-ICNBs and PCIA groups (2-sided significance level of 0.05). Accordingly, we enrolled 62 children to account for any loss in follow-up. The Mann-Whitney test was used for the comparison of FPS-R scores between groups. Age, weight, and the duration of surgery were compared using 2-tailed t tests, while the incidence of analgesia-associated side effects and the proportion of patients with scores representing mild pain (0 to 3) were compared using 2-tailed Fisher exact tests. A 2-sided P-value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the Study Participants
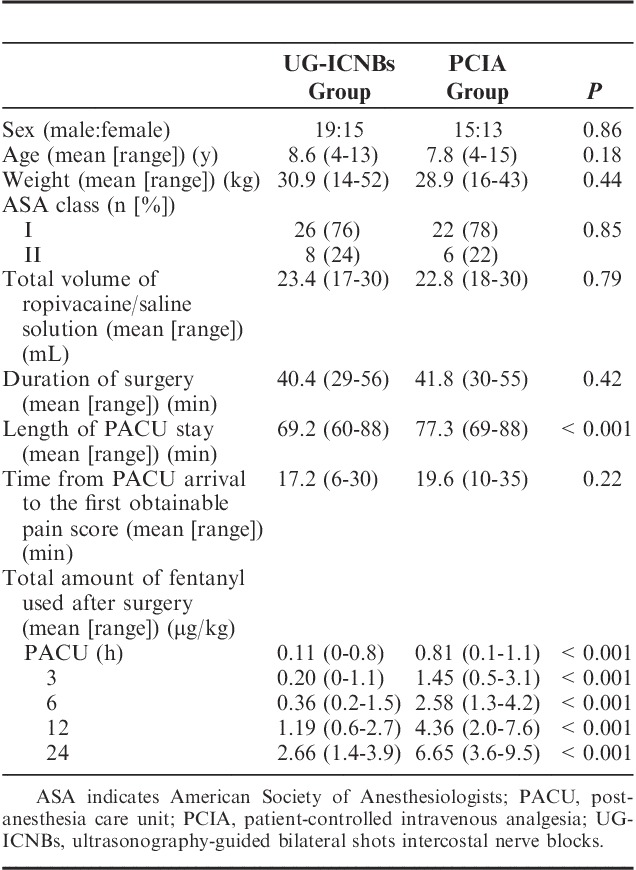
Of the 80 patients, 18 were excluded because of the requirement for additional surgery (more than 1 curved steel bar, n=2), refusal to participate (n=5), or the inability to understand how to use the PCA device (n=11). Eventually, 62 patients were successfully randomized to receive UG-ICNBs (n=34) or PCIA (n=28; Fig. 1). There were no significant differences in age, sex, weight, ASA classification, total amount of ropivacaine or saline solution used, and time from PACU arrival to the first obtainable pain score between the 2 groups (Table 2). No complications associated with UG-ICNBs use such as pneumothorax, pleural puncture, penetration of the peritoneum and abdominal viscera, nerve injury, and intravascular injection were observed.
TABLE 2.
Demographic and Clinical Characteristics of Patients Who Underwent the Nuss Procedure
FPS-R Scores
FPS-R scores were significantly lower in the UG-ICNBs group than in the PCIA group after PACU arrival (first obtainable, P<0.001; 10 min, P<0.05; 20 min, P<0.001; 30 min, P=0.002; and 40 min or longer, P<0.001) (Table 3). The patients were stratified by FPS-R scores into mild pain (FPS-R score ≤3), moderate pain (4 ≤FPS-R score ≤6), and severe pain (7 ≤FPS-R score ≤10). Rescue analgesics were administered if moderate or severe pain was verified. FPS-R scores in the initial 6 postoperative hours were significantly lower in the UG-ICNBs group compared with the PCIA group (Fig. 3, P<0.05).
TABLE 3.
Comparison of Faces Pain Scale-Revised (FPS-R) Scores (Mean, Range) in the Postanesthesia Care Unit (PACU) Between the Treatment Groups
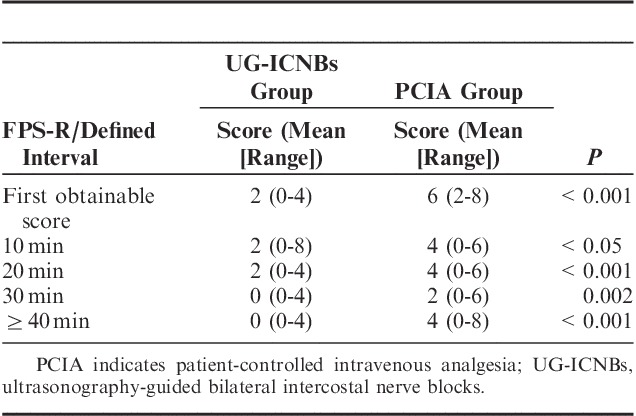
FIGURE 3.
Faces Pain Scale-Revised (FPS-R) scores after surgery. PCIA indicates patient-controlled intravenous analgesia; UG-ICNBs, ultrasonography-guided bilateral intercostal nerve blocks. **P<0.01 (mean±SD).
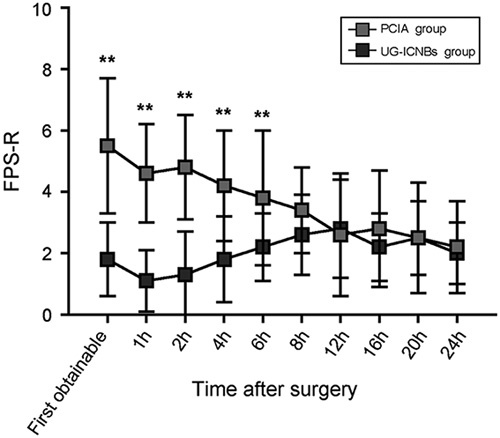
The proportion of children with absent or mild pain that did not require rescue analgesia with fentanyl in the PACU was significantly higher in the UG-ICNBs group than in the PCIA group (Fig. 4). Opioid consumption levels in the PACU and within 24 hours after surgery were significantly lower in the UG-ICNBs group compared with the PCIA group (Table 2). The length of PACU stay was statistically shorter in the UG-ICNBs group than in the PCIA group (Table 2), while the duration of surgery (40.4 vs. 41.8 min; P=0.42) and length of hospital stay (4.7 vs. 5.1 d; P=0.25) were not significantly different between the 2 groups (Table 2). The incidence of respiratory depression was markedly lower in the UG-ICNBs group in the PACU (0% vs. 21.4%, P=0.006) and in the first 24 hours (2.9% vs. 28.6%, P=0.008), with respiratory events observed more frequently in the PACU than post-PACU after surgery (Table 4). There were no significant differences in the incidences of nausea and vomiting (2.9% vs. 7.1%, P=0.585, PACU; 5.9% vs. 17.8%, P=0.228, 24 h) or pruritus (0% vs. 0%, P=1.0, PACU; 2.9% vs. 0%, P=1.0, 24 h) between the 2 groups (Table 4).
FIGURE 4.
Proportion of patients with absent or mild pain (Faces Pain Scale-Revised score≤3) in the postanesthesia care unit (PACU). PCIA indicates patient-controlled intravenous analgesia; UG-ICNBs: ultrasonography-guided bilateral intercostal nerve blocks. *P<0.05, **P<0.001, 2-tailed Fisher exact test.
TABLE 4.
Analgesia-associated Side Effects in the PACU and 24 Hours Postoperatively
DISCUSSION
In the present study, UG-ICNBs provided superior postoperative analgesia compared with the PCIA regimen for pediatric patients undergoing the Nuss procedure. Postoperative FPS-R scores were reduced for the first 6 hours and opioid consumption decreased during the first 24 hours in the UG-ICNBs group. The patients in the UG-ICNBs group also showed a decrease in postoperative opioid-related adverse effects and length of PACU stay.
Besides conventional PCIA regimens, epidural analgesia is also effective in controlling pain after the Nuss procedure. However, for many practitioners, the risk of potential permanent neurological injury may outweigh these benefits.15,16 The addition of thoracic epidural analgesia is considered an independent risk factor for permanent neurological injury.17 Of the 5 reported cases of permanent neurological injury associated with thoracic epidural analgesia in pediatric patients, 3 were undergoing Nuss repair.18,19 In addition, epidural analgesia is associated with side effects such as pulmonary complications, urinary retention, nausea and vomiting, hypotension, and technique failure.6,7 These observations have raised concerns about the safety of thoracic epidural analgesia in combination with the Nuss procedure, particularly in children.
Numerous studies have been published since 1987, when Shelly and Park20 first reported ICNBs techniques in children. However, minimal data are available for UG-ICNBs; no randomized controlled trials have been published describing UG-ICNBs success rates, analgesic effects, or complications. To the best of our knowledge, this is the first study to use UG-ICNB techniques for pediatric patients undergoing the Nuss procedure. Preoperatively administered ICNBs may block noxious afferent inputs from the surgical site to the CNS and decrease CNS sensitization. Meanwhile, local anesthetic infiltration of the dominant peripheral nerve area at the surgical site has been found to decrease the inflammatory reaction and subsequent pain.21 However, ICNBs may also cause some side effects. Pneumothorax is a common concern with ICNBs, but the reported incidence is low at 0.073%.22 Other complications of ICNBs may include penetration of the peritoneum and abdominal viscera, and Horner syndrome.23 For these reasons, ICNBs require greater expertise to avoid potential complications. Fortunately, rapid advances in ultrasonography techniques allow anesthesiologists to deliver the local anesthetic or other drugs to the desired location. In the present study, all blocks were performed by an experienced anesthesiologist (S.S.) who had administered more than 200 UG-ICNBs. This might explain why there were no associated complications.
Previous studies have examined the analgesic effects of conventional8–9,24–26 and ultrasound-guided27,28 ICNBs in patients undergoing percutaneous nephrolithotomy,24,27 open cholecystectomy,25 breast surgery,26,28 and the Nuss procedure.8,9 They demonstrated that ICNBs could decrease pain intensity and improve the health-related quality of life in the immediate postoperative period. Lukosiene et al9 evaluated the effects of conventional techniques for ICNBs on acute pain after the Nuss procedure and showed that ICNBs decreased visual analog scale scores for up to 3 hours and morphine dose for up to 6 hours after surgery, which provided evidence for using the ultrasound device in the present study. Furthermore, the success rates for conventional approaches are highly dependent on operator skills and are associated with potential serious complications.29 Therefore, these blocks are often underutilized despite potential benefits with regard to patient outcomes. Qi et al30 evaluated the effectiveness of an ultrasound-guided bilateral thoracic paravertebral block for postoperative pain control in children undergoing the Nuss procedure, and showed that the ultrasound-guided bilateral thoracic paravertebral block improved postoperative analgesia compared with PCIA and decreased the incidence of postoperative behavioral disturbance. The use of ultrasound guidance provides real-time information about the needle tip location and local anesthetic delivery to the desired location,31 which has also been demonstrated to improve success rates for ilioinguinal and transversus abdominis plane blocks, decrease the volume of local anesthetic solution, and avoid inadvertent needle trauma to surrounding structures.12,32 In summary, ultrasound guidance could increase the accuracy and efficacy of nerve blocks and decrease the incidence of complications.
We compared FPS-R scores obtained at defined intervals. This scale shows a close linear relationship with the visual analog pain scales used and has been validated for children aged 4 to 16 years.13,33 Accordingly, we used this age range as an inclusion criterion and compared the efficacy of 2 different analgesia regimens. Furthermore, pain after the Nuss procedure is determined by several factors, such as deformity severity1 and patient age.1,34 A single injection may not cover all nerve segments and may be inadequate for inhibiting the noxious stimuli of the Nuss procedure. We administered bilateral multiple ICNBs, which can inhibit noxious stimuli more effectively. Moreover, we administered local anesthesia before initiation of the Nuss procedure. The patient may benefit from pre-emptive analgesia and be spared from the noxious stimuli of surgery.
Ropivacaine, a relatively long-acting local anesthetic with superior postoperative analgesic effects compared with those of lidocaine, has been used for pediatric ilioinguinal nerve blocks,35 and is a well-tolerated regional anesthetic with an efficacy that is broadly similar to that of bupivacaine. However, it may be a preferred option for the management of postoperative acute pain because of a lower propensity for motor blockade, and less neurotoxicity and cardiotoxicity.36 Compared with bupivacaine and lidocaine, ropivacaine is likely to provide a longer duration of anesthesia in digital nerve blocks.37 Further studies are required to clarify whether similar findings could be obtained for ICNBs. In the present study, UG-ICNBs decreased FPS-R scores for up to 6 hours and opioid consumption for up to 24 hours after surgery as compared with PCIA. To provide more satisfactory postoperative analgesia, the feasibility of applying longer-acting local anesthetics such as neosaxitoxin or adding epinephrine to ropivacaine for UG-ICNBs should be further investigated.
This study has some limitations. First, pain after the Nuss procedure was highly variable. This was determined by several factors such as deformity severity1 and patient age.1,34 We did not make further comparisons of analgesia effectiveness or side effects in young children or older adolescents. Second, we did not directly compare bilateral UG-ICNB techniques with conventional (unguided) ICNBs. Third, we focused on analgesic management with opioids and did not use benzodiazepines or antiepileptics to counter muscle spasms or neuropathic components, respectively. Lastly, we were interested in acute pain control; investigating long-term analgesic effects and life assessments such as rehabilitation time might provide more information. Further studies are necessary to improve the duration of ICNBs and determine the effect of ICNBs on chronic pain after the Nuss procedure in children.
In conclusion, the present study suggests that UG-ICNBs are more effective than PCIA for postoperative analgesia in children who undergo the Nuss procedure for PE during the acute postoperative period.
Footnotes
M.L. and X.L. contributed equally.
The authors declare no conflict of interest.
REFERENCES
- 1.Grosen K, Pfeiffer-Jensen M, Pilegaard HK. Postoperative consumption of opioid analgesics following correction of pectus excavatum is influenced by pectus severity: a single-centre study of 236 patients undergoing minimally invasive correction of pectus excavatum. Eur J Cardiothorac Surg. 2010;37:833–839. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Jiang Z, Xiao H, et al. A novel modified Nuss procedure for pectus excavatum: a new steel bar. Ann Thorac Surg. 2015;99:1788–1792. [DOI] [PubMed] [Google Scholar]
- 3.Coln D, Gunning T, Ramsay M, et al. Early experience with the Nuss minimally invasive correction of pectus excavatum in adults. World J Surg. 2002;26:1217–1221. [DOI] [PubMed] [Google Scholar]
- 4.St Peter SD, Weesner KA, Sharp RJ, et al. Is epidural anesthesia truly the best pain management strategy after minimally invasive pectus excavatum repair? J Pediatr Surg. 2008;43:79–82. [DOI] [PubMed] [Google Scholar]
- 5.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–223. [DOI] [PubMed] [Google Scholar]
- 6.Brull R, McCartney CJ, Chan VW, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg. 2007;104:965–974. [DOI] [PubMed] [Google Scholar]
- 7.Butkovic D, Kralik S, Matolic M, et al. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectus excavatum repair in children. Br J Anaesth. 2007;98:677–681. [DOI] [PubMed] [Google Scholar]
- 8.Lukosiene L, Rugyte DC, Macas A, et al. Postoperative pain management in pediatric patients undergoing minimally invasive repair of pectus excavatum: the role of intercostal block. J Pediatr Surg. 2013;48:2425–2430. [DOI] [PubMed] [Google Scholar]
- 9.Lukosiene L, Macas A, Trepenaitis D, et al. Single shot intercostal block for pain management in pediatric patients undergoing the Nuss procedure: a double-blind, randomized, controlled study. J Pediatr Surg. 2014;49:1753–1757. [DOI] [PubMed] [Google Scholar]
- 10.Kopacz DJ, Thompson GE. Intercostal blocks for thoracic and abdominal surgery. Tech Reg Anesth Pain Manag. 1998;2:25–29. [Google Scholar]
- 11.Willschke H, Marhofer P, Bösenberg A, et al. Ultrasonography for ilioinguinal/iliohypogastric nerve blocks in children. Br J Anaesth. 2005;95:226–230. [DOI] [PubMed] [Google Scholar]
- 12.Willschke H, Bösenberg A, Marhofer P, et al. Ultrasonographic-guided ilioinguinal/iliohypogastric nerve block in pediatric anesthesia: what is the optimal volume? Anesth Analg. 2006;102:1680–1684. [DOI] [PubMed] [Google Scholar]
- 13.Hicks CL, von Baeyer CL, Spafford P, et al. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar JL, Benhamou D, Bonnet F, et al. ESRA guidelines for the use of epidural opioids. Int Monit Reg Anesth. 1997;9:3–8. [Google Scholar]
- 15.Krasopoulos G, Dusmet M, Ladas G, et al. Nuss procedure improves the quality of life in young male adults with pectus excavatum deformity. Eur J Cardiothorac Surg. 2006;29:1–5. [DOI] [PubMed] [Google Scholar]
- 16.Krasopoulos G, Goldstraw P. Minimally invasive repair of pectus excavatum deformity. Eur J Cardiothorac Surg. 2011;39:149–159. [DOI] [PubMed] [Google Scholar]
- 17.Attar S, Hankins JR, Turney SZ, et al. Paraplegia after thoracotomy: report of five cases and review of the literature. Ann Thorac Surg. 1995;59:1410–1416. [DOI] [PubMed] [Google Scholar]
- 18.Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. 2010;252:1072–1081. [DOI] [PubMed] [Google Scholar]
- 19.Meyer MJ, Krane EJ, Goldschneider KR, et al. Neurological complications associated with epidural analgesia in children: a report of 4 cases of ambiguous etiologies. Anesth Analg. 2012;115:1365–1370. [DOI] [PubMed] [Google Scholar]
- 20.Shelly MP, Park GR. Intercostal nerve blockade for children. Anesthesia. 1987;42:541–544. [DOI] [PubMed] [Google Scholar]
- 21.Bagry H, de la Cuadra Fontaine JC, Asenjo JF, et al. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med. 2008;33:17–23. [DOI] [PubMed] [Google Scholar]
- 22.Moore DC. Intercostal nerve block for postoperative somatic pain following surgery of thorax and upper abdomen. Br J Anaesth. 1975;47:284–286. [PubMed] [Google Scholar]
- 23.Blechman KM, Zervos M. Post-thoracotomy Horner syndrome associated with extrapleural infusion of local anesthetic. Interact Cardiovasc Thorac Surg. 2009;9:309–310. [DOI] [PubMed] [Google Scholar]
- 24.Honey RJ, Ghiculete D, Ray AA, et al. A randomized, double-blinded, placebo-controlled trial of intercostal nerve block after percutaneous nephrolithotomy. J Endourol. 2013;27:415–419. [DOI] [PubMed] [Google Scholar]
- 25.Vizcarra-Román MA, Bahena-Aponte JA, Cruz-Jarquín A, et al. Effectiveness of intercostal nerve block with ropivacaine in analgesia of patients undergoing emergency open cholecystectomy under general anesthesia. Rev Gastroenterol Mex. 2012;77:9–14. [PubMed] [Google Scholar]
- 26.Vemula R, Kutzin M, Greco G, et al. The use of intercostal nerve blocks for implant-based breast surgery. Plast Reconstr Surg. 2013;132:178–180. [DOI] [PubMed] [Google Scholar]
- 27.Ozkan D, Akkaya T, Karakoyunlu N, et al. Effect of ultrasound-guided intercostal nerve block on postoperative pain after percutaneous nephrolithotomy: prospective randomized controlled study. Anaesthesist. 2013;62:9889–9894. [DOI] [PubMed] [Google Scholar]
- 28.Diéguez García P, Fajardo Pérez M, López Álvarez S, et al. Ultrasound-assisted approach to blocking the intercostal nerves in the mid-axillary line for non-reconstructive breast and axilla surgery. Rev Esp Anestesiol Reanim. 2013;60:365–370. [DOI] [PubMed] [Google Scholar]
- 29.Holzer A, Kapral S, Hellwagner K, et al. Severe pneumothorax after intercostal nerve blockade. A case report. Acta Anaesthesiol Scand. 1998;42:1124–1126. [DOI] [PubMed] [Google Scholar]
- 30.Qi J, Du B, Gurnaney H, et al. A prospective randomized observer-blinded study to assess postoperative analgesia provided by an ultrasound-guided bilateral thoracic paravertebral block for children undergoing the Nuss procedure. Reg Anesth Pain Med. 2014;39:208–213. [DOI] [PubMed] [Google Scholar]
- 31.Dolan J, Lucie P, Geary T, et al. The rectus sheath block: accuracy of local anesthetic placement by trainee anesthesiologists using loss of resistance or ultrasound guidance. Reg Anesth Pain Med. 2009;34:247–250. [DOI] [PubMed] [Google Scholar]
- 32.Dingeman RS, Barus LM, Chung HK, et al. Ultrasonography-guided bilateral rectus sheath block vs. local anesthetic infiltration after pediatric umbilical hernia repair, a prospective randomized clinical trial. JAMA Surg. 2013;148:707–713. [DOI] [PubMed] [Google Scholar]
- 33.Gurnaney HG, Maxwell LG, Kraemer FW, et al. Prospective randomized observer-blinded study comparing the analgesic efficacy of ultrasound-guided rectus sheath block and local anaesthetic infiltration for umbilical hernia repair. Br J Anaesth. 2011;107:790–795. [DOI] [PubMed] [Google Scholar]
- 34.Nagasao T, Miyamoto J, Tamaki T, et al. Stress distribution on the thorax after the Nuss procedure for pectus excavatum results in different patterns between adult and child patients. J Thorac Cardiovasc Surg. 2007;134:1502–1507. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya N, Ichizawa M, Yoshikawa Y, et al. Comparison of ropivacaine with bupivacaine and lidocaine for ilioinguinal block after ambulatory inguinal hernia repair in children. Paediatr Anaesth. 2004;14:468–470. [DOI] [PubMed] [Google Scholar]
- 36.McClellan KJ, Faulds D. Ropivacaine: an update of its use in regional anaesthesia. Drugs. 2000;60:1065–1093. [DOI] [PubMed] [Google Scholar]
- 37.Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744–751. [DOI] [PubMed] [Google Scholar]


