Abstract
Devil Facial Tumour 1 (DFT1) is one of two transmissible neoplasms of Tasmanian devils (Sarcophilus harrisii) predominantly affecting their facial regions. DFT1’s cellular origin is that of Schwann cell lineage where lesions are evident macroscopically late in the disease. Conversely, the pre-clinical timeframe from cellular transmission to appearance of DFT1 remains uncertain demonstrating the importance of an effective pre-clinical biomarker. We show that ERBB3, a marker expressed normally by the developing neural crest and Schwann cells, is immunohistohemically expressed by DFT1, therefore the potential of ERBB3 as a biomarker was explored. Under the hypothesis that serum ERBB3 levels may increase as DFT1 invades local and distant tissues our pilot study determined serum ERBB3 levels in normal Tasmanian devils and Tasmanian devils with DFT1. Compared to the baseline serum ERBB3 levels in unaffected Tasmanian devils, Tasmanian devils with DFT1 showed significant elevation of serum ERBB3 levels. Interestingly Tasmanian devils with cutaneous lymphoma (CL) also showed elevation of serum ERBB3 levels when compared to the baseline serum levels of Tasmanian devils without DFT1. Thus, elevated serum ERBB3 levels in otherwise healthy looking devils could predict possible DFT1 or CL in captive or wild devil populations and would have implications on the management, welfare and survival of Tasmanian devils. ERBB3 is also a therapeutic target and therefore the potential exists to consider modes of administration that may eradicate DFT1 from the wild.
Introduction
The Tasmanian devil (Sarcophilus harrisii) belongs to the Dasyuridae family, it is a carnivorous marsupial that is extinct on mainland Australia and now found only on the island of Tasmania. Superficial dermal cutaneous lesions of wild Tasmanian devils can be found commonly in the form of skin sores [1] and neoplasia [2]. Spontaneous neoplasms in captive Tasmanian devils including squamous cell carcinoma of the lip and gingiva, dermal lymphosarcoma [3], trichoepithelioma, papilloma and keratoacanthoma [4] and a single devil with multiple unrelated tumours involving internal organs in combination with skin [5] have been recorded, suggestive of potential metastasis. Similar observations were made while reviewing Dasyurid archival material at the Australian Registry of Wildlife Health [6] and recently, two captive female devils with pruritus and dermatitis were diagnosed with cutaneous T-cell lymphoma [7]. None of the recorded neoplastic superficial lesions found in captive or wild Tasmanian devils appeared to mimic the firm, flattened centrally ulcerated soft tissue lesions of DFT1 affected Tasmanian devils [8].
Although the first evidence of DFT1 in wild populations occurred in 1996 when several Tasmanian devils were photographed by Christo Baars in the north east of the state with facial lesions. However, a tissue diagnosis was not obtained until 2001 [9]. Review of Tasmanian devil archival slides submitted to the Animal Health Laboratory, DPIPWE, revealed a single case in 1997 that was consistent with DFT1 [8, 10]. An emerging disease was finally recognised in 2003 [10] and subsequent investigations revealed the tumour to be a transmissible allograft being transferred from devil to devil via biting [11] with tumours tending to be located on the face, lips and oral mucosa [8].The timeframe of the pre-clinical stage of DFTD1 remains largely undetermined with observations ranging from 2–13 months [9, 12–15] but as little as 1 month has been recorded (Author unpublished observation, laboratory records, DPIPWE). Immunohistochemical examination of DFT1 suggested a possible undifferentiated neuroendocrine tumour [16, 17] although subsequent molecular testing lead to the conclusion that DFT1 is of Schwann cell origin [18]. Down-regulation mechanisms causing absence of major histocompatibility complex (MHC) class 1 cell surface antigens is a major contributing factor allowing the DFT1 allograft to evade the host devil’s immune system without rejection [19–21]. Further cytogenetic and molecular techniques have identified four karyotypic strains that are differentiated by a small number of identifiable rearrangements [22, 23]. As a consequence of this cancer, wild populations of the Tasmanian devil have been significantly reduced in Tasmania where the possibility of extinction either locally within 10–15 years [24, 25] or completely within 25–35 years [25] has been predicted. The impedance of this 2007 dire prediction includes the adaption of wild Tasmanian devils to their life history change by precocial sexual maturity [26] and through a strong collaborative scientific research and conservation management framework devised by the Save the Tasmanian Devil Program (STDP) [27]. A second transmissible tumour in Tasmanian devils, devil facial tumour 2 (DFT2), distinct from DFT1 has recently been reported [28] suggesting that the species may well be prone to transmissible cancers, increasing the urgency of biomarker identification and therapeutic intervention.
ERBB3 is expressed in early embryonal development and plays an integral role in the development of the neural crest and Schwann cells [29] regulating pathways that execute diverse cellular functions including development, cell cycle, migration, survival, proliferation and differentiation [30–34]. ERBB3 is a member of the Epidermal Growth Factor (EGF) family representing a complex group of type 1 transmembrane receptor tyrosine kinase (RTK) with differing ligands. The EGF family consists of four members and collectively the human epidermal growth factor receptor gene family members are designated EGFR/ERBB1/HER1, ERBB2/HER2, ERBB3/HER3 and ERBB4/HER4 [35]. The extracellular domain (ECD) of ERBB receptors has high structural homology although they bind selectively within a group of 11 peptide growth factor members that includes Neuregulin 1 and 2 (NRG1/NRG2) both ERBB3 ligands. [35–39]. Although the complex signalling network of ERBB receptors commonly activate the mitogen activated protein kinase (MAPK) pathway and the phosphatidylinositide 3-Kinase (PI3K) pathway [40–43], ERBB3 efficiently activates the PI3K pathway [44] due to the presence of multiple p85 binding sites in its tyrosine kinase domain.
Lateral signalling among ERBB’s is no more apparent than with receptors ERBB2 and ERBB3 that must heterodimerise with other ERBB members to signal [40] as ERBB3 has a ligand but impaired tyrosine kinase activity [45] and ERBB2 has no known ligand (orphan receptor) but a functional kinase region [46]. Although ERBB3 has long been considered impaired or termed a pseudo-kinase, it does have sufficient, although substantially reduced [47], kinase activity. How ERBB3 is able to activate other ERBB family members with its weak catalytic domain remained elusive until an allosteric mechanism termed an ‘asymmetric dimer’ enabling trans-autophosphorylation was discovered [48].
ERBB2 and ERBB3 overexpression [49–51], cooperation in neoplastic transformation [44, 52–54] and loss of ERBB3 preventing the progressive transformation of ERBB2-over expressing tumours [55] reinforces ERBB3’s pivotal role in ERBB signalling. Early studies revealed ERBB3 as a potential oncogene with overexpression due to possible increased transcription as no gene amplification was observed [56, 57] although recently oncogenic mutations have been reported [58] indicating either ERBB3 or its downstream components should represent a potential target for therapy [59].
ERBB3 is upregulated in a number of human cancers such breast, colon, gastric, ovarian and prostate [33, 60] but seldom reported in veterinary cancers [61–63] although it would appear the instrumental role that ERBB3 may play in some veterinary tumours is yet to be elucidated. DFT1’s immunohistochemical expression of ERBB3 led us to postulate that excess extracellular domain (ECD) may circulate in the host’s plasma and present itself as a possible candidate biomarker for DFT1. Literature reports five secreted alternative transcripts of ERBB3 present in serum or interstitial fluid [64, 65] which can be detected utilising ELISA methodology.
Our pilot study assessed serum ERBB3 for the for the first time in Tasmanian devils revealing that serum ERBB3 was substantially elevated in the serum of Tasmanian devils with DFT1 compared to those Tasmanian devils without DFT1. Interestingly, the inclusion of some Tasmanian devils with CL in our pilot study revealed that ERBB3 may also be a biomarker for this DFT1, although CL is clinically distinct from DFT1. We identify ERBB3 as a potential biomarker of DFT1 and highlight current literature supporting the therapeutic possibilities that can be directed towards ERBB3 overexpressing tumours that may be helpful in the elimination of DFT1 from the wild.
Materials and methods
Animal ethics statement
Serum and paraffin embedded tissue samples were collected by veterinary staff for the Save the Tasmanian Devil Program (STDP) http://www.tassiedevil.com.au/tasdevil.nsf encompassing health checks, field trapping trips, or autopsy due to animal welfare reasons. All samples were accessed from the Animal Health Laboratory archive and did not require ethics approval.
Tasmanian devil ERBB3 pilot study
A pilot study of thirty-five Tasmanian devils differing in age, sex and geographic location were selected (Table 1) to compare serum ERBB3 levels in clinically healthy Tasmanian devils (CHD), devils with DFT1 and those with CL. The Fifteen CHD’S included both adults (n = 12) and clinically healthy juvenile Tasmanian devils (CHJD, n = 3) 10 months of age. Adults included free range captive (n = 5), captive (n = 3) and wild devils (n = 4). Clinically healthy adults either had no visible disease (ND, n = 8) or had localised skin non-DFT1 dermatopathy (CHDD, n = 4) consisting of two abscesses, a skin tag and localised dermatitis. Eight Tasmanian devils with clinical DFT1 and Twelve Tasmanian devils with CL. Tasmanian devils with CL were included in the study as a severe skin condition recognised clinically but very distinct from DFT1. All dermatopathies, DFT1 and CL were confirmed histologically by the Animal Health Laboratory.
Table 1. Tasmanian devil pilot study individuals.
| Devil | Microchip Identification | Laboratory accession | Age (years) | Sex (M/F) | Geographic location | Clinical status |
|---|---|---|---|---|---|---|
| 1 | 982000190997443 | 13/3712 | 1 | F | Freycinet a | CHD |
| 2 | 982000123211124 | 13/3683 | 3 | F | Freycinet a | CHD |
| 3 | 982009104963600 | 13/3680 | 4 | M | Freycinet a | CHD |
| 4 | 982009104860765 | 13/3713 | 4 | M | Freycinet a | CHD |
| 5 | 982000123130282 | 13/3716 | 2 | M | Freycinet a | CHD |
| 6 | 982009105111670 | 09/4200 | 3 | F | West Pencil Pine b | CHD |
| 7 | 982009105849999 | 09/3957 | 2 | M | Tullah b | CHD |
| 8 | 985154000001063 | 09/1051 | 1 | M | Cressy c | CHD |
| 9 | 982009104269684 | 08/1805 | 2 | M | Narawntapu b | CHDD |
| 10 | 982009106039877 | 10/0156 | 2 | M | Dunalley b | CHDD |
| 11 | 982009104236464 | 08/0798 | 1 | F | Taroona c | CHDD |
| 12 | 982009104357109 | 09/2009 | 4 | F | Fern Tree c | CHDD |
| 13 | 985154000001151 | 09/0451 | <1 | M | Mt Pleasant d | CHJD |
| 14 | 985154000001142 | 09/0449 | <1 | F | Mt Pleasant d | CHJD |
| 15 | 985154000001130 | 09/0448 | <1 | M | Mt Pleasant d | CHJD |
| 16 | 982009104841875 | 12/2065 | 6 | F | West Pencil Pine b | DFT1 |
| 17 | 982009106034139 | 11/0767 | 2 | F | Dunalley b | DFT1 |
| 18 | 982009104719592 | 12/0820 | 4 | F | West Pencil Pine b | DFT1 |
| 19 | 982000000122095 | 12/2095 | 2 | F | Upper Natone b | DFT1 |
| 20 | 982000123128645 | 11/3917 | 2 | M | Hamilton b | DFT1 |
| 21 | 982000123216973 | 11/3918 | 1 | F | Hamilton b | DFT1 |
| 22 | 982000123209814 | 11/4493 | 2 | M | Waratah b | DFT1 |
| 23 | 000000000130406 | 13/0406 | 2 | F | Mangalore b | DFT1 |
| 24 | NC | 11/0650 | 7 | F | Mole Creek c | CL |
| 25 | 985120016024404 | 11/4290 | 8 | F | Mt Pleasant c | CL |
| 26 | 982009106314654 | 10/4001 | 8 | M | Taranna c | CL |
| 27 | 982009106585887 | 10/3765 | 5 | F | Calder b | CL |
| 28 | 982009104789818 | 14/0034 | 6 | F | Cressy c | CL |
| 29 | NC | 08/4048 | 4 | F | Circular Head b | CL |
| 30 | 982009100786171 | 09/0402 | 6 | F | Mt Pleasant c | CL |
| 31 | 982009101694833 | 10/1013 | 6 | F | Richmond c | CL |
| 32 | 982009104910854 | 13/0518 | 6 | F | Cressy c | CL |
| 33 | NC | 09/3035 | 5 | F | South Riana b | CL |
| 34 | NC | 11/1615 | 6 | F | Mole Creek c | CL |
| 35 | 982009104873582 | 13/3714 | 4 | F | Freycineta | CL* |
NC not microchipped, CHD clinically healthy devil, CHDD clinically healthy devil with dermatopathy, CHJD clinically healthy juvenile devil, DFT1 devil facial tumour 1, CL cutaneous lymphoma
a Free range enclosure
b Wild devil
c Captive devil
d captive juvenile
* no tissue diagnosis.
Tasmanian devil serum sample and collection
Blood samples from Tasmanian devils (Table 1) were collected by wildlife veterinarians through jugular venepuncture, whilst the animals were restrained by a trained field officer. Ten millilitres of blood was collected in sterile serum separation tubes, stored on ice for transport to the laboratories, centrifuged and serum removed for archival storage at -20°C. Serum samples were retrieved from the frozen archive and thawed at room temperature immediately before analysis.
Histology
Tasmanian Devil tissues were fixed in 10% Neutral Buffered Formaldehyde (Confix, ACFC, Australian Biostain, Traralgon, Victoria, Australia) for 24 hours and selected tissues were cassetted and processed overnight using a standard 15 hour overnight procedure in the TP1050 tissue processor (Leica Microsystems, Wetzlar, Germany). Tissues were orientated on the EG1160 (Leica), embedded in paraffin wax (Surgipath Paraplast, 39601006, Leica) and sectioned at 3 microns using Leica RM2245 microtome and adhered to microscope slides (Menzel Glaser, Braunschweig, Germany) for 20 minutes at 60°C. Sections were deparaffinised, rehydrated and stained using Jung autostainer XL (Leica) for Haematoxylin (Harris’ Haematoxylin, AHHNA, Australian Biostain) and Eosin, dehydrated cleared and mounted in CV Mount (Leica, 046430011).
Immunohistochemistry
Archival Tasmanian devil tissues and tumours were sectioned at 3 microns, floated onto Superfrost plus slides (Menzel Glaser) and subjected to standard deparaffinisation and rehydration techniques using an automated stainer (Leica). Antigen retrieval in tissue sections was conducted in citrate buffer at pH 6.0 (Reveal Decloaker, Biocare Medical, California, USA) at 120°C for 8 minutes using a Pascal pressure chamber (Dako, Glostrup, Denmark) then cooled to 20°C. Endogenous peroxidase activity was quenched using 3% hydrogen peroxide (Ajax Finechem, Sydney, Australia, 260) in methanol (Ajax, 723) for 30 minutes. Detection of primary antibodies was achieved using Mach1 Universal HRP-Polymer detection kit (Biocare Medical, California, USA, M1U539GL10). Protein block (Background Sniper BS966L10) was applied for 20 minutes prior to application of primary antibodies. Monoclonal rabbit anti-human ERBB3 (Abcam, clone SP71, ab93739, internal region) was diluted 1:50 with antibody diluent (Dako, S0809) and applied to both devil tumour and normal devil control tissues at room temperature for 30 minutes. Negative control was omission of primary antibody with buffer substitution. Universal HRP-polymer was applied for 30 minutes (MRH538L10) followed by 1 drop of Betazoid DAB Chromogen 3,3 Diaminobenzidine (BDB900G) in 1ml of substrate buffer (DB900) applied for 4 minutes. Tris buffered saline (Biocare Medical, TWB945) was used to rinse between all steps. Slides were rinsed, stained with Carazzi’s Haematoxylin for 5 minutes, washed for 3 minute in tap water, dehydrated, cleared and mounted in CV mount. Sections were viewed under light microscopy using Olympus BX41 (Olympus corporation, Tokyo, Japan) and selected areas were photographed using an Olympus digital camera (DP20).
ERBB3 ELISA assay
Serum ERBB3 levels were measured using the RayBio anti-human ERBB3 ELISA Kit (ELH-ERBB3, RayBiotech Inc, GA, USA) according to manufacturer’s instructions. Briefly, serum samples were diluted 1/5 in Assay Diluent A and 100 uL of standard or diluted sample were added in duplicate to wells of a 96 well assay plate and incubated for 24 hrs at 4°C. The supernatant was removed and wells were washed 4 times with 300 uL of 1X wash solution using an Immunowash 1575 (BioRad Laboratories, CA, USA). One hundred microliters of prepared biotinylated anti-ERBB3 was added to each well and the assay plate incubated for 1 hour at room temperature. The assay plate was washed as described after which 100 uL of prepared HRP-streptavadin conjugate was added to each well and the assay plate incubated for 45 minutes at RT. The assay plate was again washed as described and 100 uL of TMP substrate was added and the plate incubated for 30 minutes at room temperature in the dark, after which 50 uL of stop reagent was added to each well. The absorbance of each well was measured at 450 nm using a Tecan Infinite M200 microplate reader (Tecan, Salzburg, AUT).
Data analysis
The ELISA standard curve was plotted using Prism v5 (GraphPad, CA, USA) and results for each serum interpolated and corrected for dilution. The significance of differences in serum ERBB3 between groups was determined using a Kruskal-Wallis test with Dunn’s Multiple Comparison utilizing Prism v5 (GraphPad, CA, USA).
Results
Histology and Immunohistochemstry
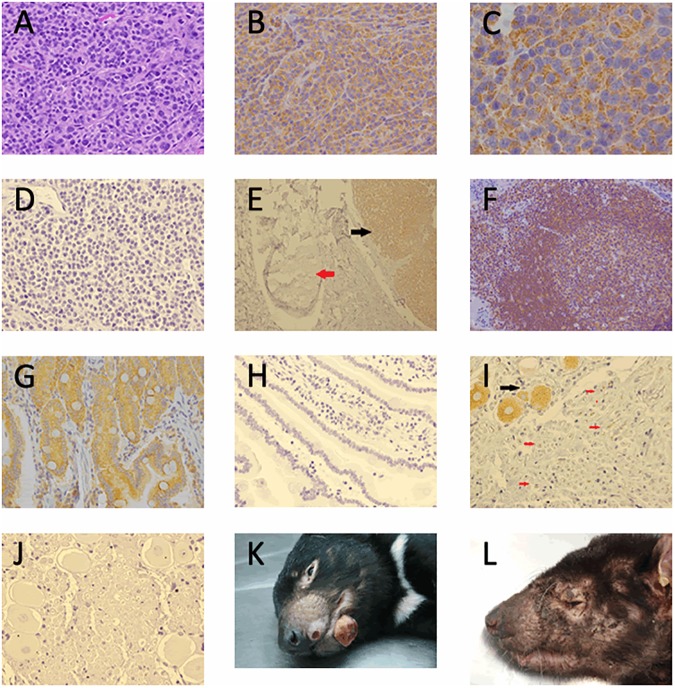
DFT1 histology (Fig 1A) and Haematoxylin and Eosin demonstrates small round cells with indistinct cell membranes arranged in cords and packets. ERBB3 IHC on average revealed moderate to strong expression in 75% of cells in both primary and secondary DFT1 tumours in cytogenetically determined strains 1 to 5 of DFT1. Typical granular cytoplasmic expression (Fig 1B) demonstrated by DFT1 strain 3 cells with small and large aggregates noted. Higher magnification (Fig 1C) shows accumulation in and around vacuolar structures within the cytoplasm. In sections of devil skin and subcutous (Fig 1E), peripheral nerve was seldom positive for ERBB3 (red arrow) in keeping with downregulation of ERBB3 in the adult in contrast to DFT1 ERBB3 expression (black arrow). ERBB3 expression was noted in Tasmanian devil lymphoid follicle (Fig 1F) where cytoplasmic expression of ERBB3 is present in both T (germinal centre) and B (mantle) cells. Devils with CL were not included in the ERBB3 immunohistochemical staining. Trigeminal nerve section (Fig 1I) showed ERBB3 expression in nerve bodies (black arrow) and occasional ERBB3 expression in the adaxonal area (red arrows) but generally small myelinated nerves were negative. Positive control included devil bowel (Fig 1G) which exhibited a similar expression pattern to human ERBB3 and negative controls DFT1 (Fig 1D), bowel (Fig 1H) and Trigeminal nerve (Fig 1J). The monoclonal rabbit anti-human ERBB3 clone SP71 is a synthetic peptide corresponding to an internal sequence of Human ERBB3. Although the exact sequence is a proprietary secret ERBB3 sequence alignment between Human and Tasmanian devil in this region has high homology (S1 Fig. ERBB3 Orthologue protein alignment).
Fig 1. DFT1 staining and skin manifestation.
(A) Haematoxylin and Eosin stained DFT1 x40, (B) ERBB3 Immunohistochemical expression in DFT1 strain 3 x40, (C) ERBB3 immunohistochemical expression in DFT1 strain 3 x100, (D) DFT1 negative control, (E) Tasmanian devil skin and subcutis section with peripheral nerve (red arrow) and DFT1 (black arrow) x10, (F) Tasmanian devil lymph node ERBB3 expression lymphoid follicle x20, (G) Tasmanian devil bowel ERBB3 positive control x40, (H) ERBB3 IHC negative control bowel, (I) trigeminal nerve shows ERBB3 positive nerve body (black arrow) and occasional adaxonal ERBB3 positivity (red arrows) x40, (J) ERBB3 IHC negative control trigeminal nerve, (K) Tasmanian Devil gross appearance of DFT1. Photo credit: DPIPWE archive, (L) Tasmanian devil gross appearance cutaneous lymphoma. Photo credit DPIPWE archive.
Serum ERBB3 in Tasmanian devils
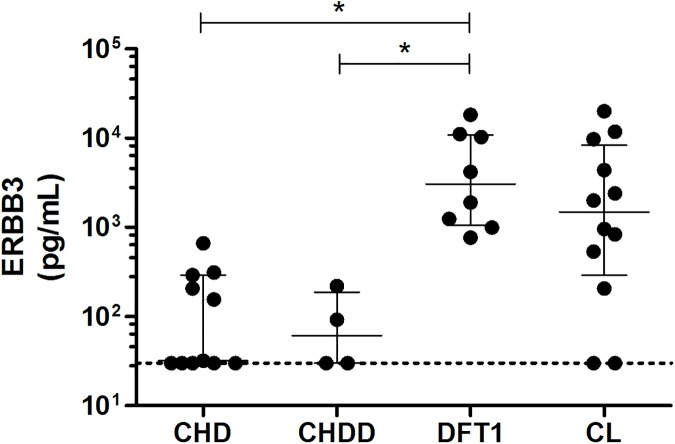
Serum ERBB3 levels are shown in Table 2 and graphically in Fig 2. Serum ERBB3 in the Fifteen Tasmanian devils without neoplasia (devils 1–15 includes CHD,CHDD and CHJD) ranged from <30–663 pg/ml with a median of 32 pg/mL (30–220; interquartile range). Serum ERBB3 levels in the eight Tasmanian devils (devils 16–23) with clinical DFT1 ranged from 766–18,254 pg/ml with median of 3051 pg/mL (1060–10879; interquartile range. In the twelve Tasmanian devils with cutaneous lymphoma (devils 24–35) serum ERBB3 levels ranged from <30–20,021 pg/ml with a median of 1485 pg/mL (289–7901; interquartile range).
Table 2. Tasmanian devil serum ERBB3 and clinical history.
| Devil | Serum Erbb3 (pg/ml) | Weight (Kg) | Serum transit (days) | clinical history | BCS (0–5) | DFT1 strain | DFT1 1o No (range cm) | Mets No |
|---|---|---|---|---|---|---|---|---|
| 1 | 155 | N/A | 1 | CHD, NAD | ||||
| 2 | 663 | N/A | 1 | CHD, Localised alopecia | ||||
| 3 | 207 | OW | 1 | CHD, Multiple punctures | ||||
| 4 | 313 | N/A | 1 | CHD, Multiple punctures | ||||
| 5 | 291 | N/A | 1 | CHD, Multiple minor wounds | ||||
| 6 | <30 | N/A | 1 | CHD, Few wounds, lactating | ||||
| 7 | <30 | N/A | 2 | CHD, N/A | ||||
| 8 | <30 | 6 | 1 | CHD, Great condition | ||||
| 9 | 220 | 10.5 | 1 | CHDD, Abscess/scab on face | ||||
| 10 | 92 | N/A | 2 | CHDD, Abscess left neck. | ||||
| 11 | <30 | 4.7 | 1 | CHDD, Skin tag on left ear | ||||
| 12 | <30 | N/A | 1 | CHDD, Dermatitis upper flank | ||||
| 13 | <30 | 4.2 | 1 | CHJD, Health check | ||||
| 14 | 32 | 3.4 | 1 | CHJD, Health check | ||||
| 15 | <30 | 4.6 | 1 | CHJD, Health check. | ||||
| 16 | 18,254 | N/A | 1 | DFT1, weak | 2 | 2 | 2 (1.0–2.5) | 3 |
| 17 | 999 | 6.1 | 3 | DFT1, Reared 4 young | 2 | 3 | 4 (1.0–1.5) | 5 |
| 18 | 11,090 | 4.8 | 1 | DFT1, Poor body condition | 1–2 | 1 | 4 (2.0–3.0) | 1 |
| 19 | 1903 | 3.7 | 1 | DFT1, Emaciated disorientated | 0 | 1 | 2 (1.6–5.2) | 10 |
| 20 | 10,247 | 10 | 3 | DFT1, Multiple lesions | 3 | 2 | 4 (1.0–2.0) | 2 |
| 21 | 1241 | 5 | 3 | DFT1, Poor body condition | 2.5 | 2 | 3 (1.0–1.5) | 1 |
| 22 | 4198 | 9.3 | 4 | DFT1, Advanced DFT1 | 2 | 4 | 7 (1.0–2.5) | 0 |
| 23 | 766 | N/A | 1 | DFT1, Emaciated | 2 | 1 | 7 (1.0–4.7) | 1 |
| 24 | 4383 | 6.7 | 1 | CL, Generalised alopecia | N/A | |||
| 25 | <30 | 8.2 | 1 | CL, cutaneous plaques chest | N/A | |||
| 26 | <30 | 8.0 | 1 | CL, percutaneous plaque | N/A | |||
| 27 | 2008 | 5.9 | 1 | CL, Skin lesions | N/A | |||
| 28 | 837 | 5.9 | 1 | CL, Alopecia | Poor | |||
| 29 | 9703 | 5.3 | 1 | CL, Generalised alopecia | Poor | |||
| 30 | 2403 | 8.2 | 1 | CL, Alopecia ventrally | N/A | |||
| 31 | 536 | 7.4 | 1 | CL, Alopecia left neck, pouch | N/A | |||
| 32 | 962 | 6.7 | 1 | CL, Alopecia ventrally | N/A | |||
| 33 | 11,837 | 5.4 | 1 | CL, Widespread alopecia | 1–2 | |||
| 34 | 207 | 5.7 | 1 | CL, Multifocal dermatitis, cutaneous lump (acanthoma) | Poor | |||
| 35 | 20,021 | N/A | 1 | CL, Multifocal alopecia | N/A |
N/A not available, NAD no abnormality detected, OW over weight, BCS—body condition score, DFT1 strain–cytogenetically determined strain, DFT1 1o No–number and size of primary tumours recorded, Mets No—number of metastasis recorded, CHD clinically healthy devil, CHDD clinically healthy devil with dermatopathy, CHJD clinically healthy juvenile devil, DFT1 devil facial tumour 1, CL cutaneous lymphoma
Fig 2. Serum ERBB3 levels in Tasmanian devils.
Serum ERBB3 levels were measured by ELISA in clinically healthy Tasmanian devils CHD (n = 11), clinically healthy Tasmanian devils with dermatopathy CHDD (n = 4), clinically diagnosed DFT1 (n = 8) and those with cutaneous lymphoma CL (n = 12). Horizontal dashed line indicates the limit of detection of the ELISA assay at 30 pg/mL. Results of individual devils are shown with the median and interquartile range identified by the whiskers. Significance testing using a Kruskal-Wallis test with Dunn’s Multiple Comparison Testing shown with * representing p < 0.05.
Discussion
ERBB3 in devils without DFT1
Fifteen Tasmanian devils without neoplasia (twelve adults either wild caught, free range or captive enclosures and three captive juveniles encompassing CHD, CHDD and CHJD) were studied with an average serum ERBB3 of 32 pg/ml. Collectively, CHD Tasmanian devils serum ERBB3 levels ranged from <30–663 pg/ml which could be considered representative of the reference range for Tasmanian devils. Wild caught devils 6 and 7 were unremarkable and had serum ERBB3 levels <30 pg/ml however devil 9 (220 pg/ml) and devil 10 (92 pg/ml) both recorded skin abscesses. The ERBB3 levels in the CHDD group (devils 9, 10, 11 and 12) ranged from <30–220 pg/ml all had a small isolated dermatopathy such as abscess (devil 9), pyogranuloma (devil 10), skin tag with associated inflammation (devil 11) and small focus of dermatitis (devil 12) all recorded a low serum ERBB3 levels of <92 pg/ml. The CHJD (devils 13, 14 and 15) approximately 10 months old had an unremarkable clinical history that indicated serum was collected for a health check only, reflected in the low serum ERBB3 level of <30 pg/ml.
Further assessment of data and clinical history (Table 2) revealed that four out of five Tasmanian devils from the Freycinet free range enclosure (devils 1–5) had higher serum ERBB3 ranging from 155–663 pg/ml compared to most other clinically healthy devils having serum ERBB3 levels <30 pg/ml. The Freycinet free range enclosure (FRE) consists of a 22 Hectare natural reserve that creates living conditions that are more similar to the wild than traditional captive conditions. The structure is fenced completely enclosing an insurance population of healthy devils with density caped to approximately one devil per hectare. This type of enclosure allows devils the opportunity to compete at feeding and breeding times and bite wounds are therefore common (David Schaap, personal communication). In contrast, captive devils are housed in small enclosures that measure approximately 100 m2 containing capped at one devil per 100 m2.
We noted that skin injuries were commonly recorded although no abnormality was noted for devil 1, alopecia bilaterally around the hind limbs and flank was present on one mother due to her 3 pouch young (devil 2) and multiple puncture wounds were present on the remainder (devils 3, 4 and 5). Given that these devils were otherwise clinically healthy it would suggest that skin wounds caused by biting may contribute to some elevation in the serum ERBB3 of Tasmanian devils. There is also the possibility that simply being a Tasmanian devil living in a free range enclosure as opposed to wild populations may in itself be contributory to elevation in serum ERBB3 due to more frequent devil-devil engagement. Our results indicate that Tasmanian devils without injuries or an isolated skin lesion have serum ERBB3 levels <30 pg/ml whereas Tasmanian devils with multiple injuries or large abscesses have serum ERBB3 levels ranging from 92–663 pg/ml. Together, these results suggest that cancer-free Tasmanian devils have a serum ERBB3 range of <30–663 pg/ml.
ERBB3 in devils with DFT1
All devils with DFT1 were wild caught and all subjected to field autopsy with most serum samples reaching the laboratory within one to three days. We assessed the available clinical history (Table 2) including animal weight, body condition score (BCS 1–5) where 1 = emaciated, 2 = moderately thin, 3 = average, 4 = good and 5 = obese (Sarah Peck, personal communication), number of primary and metastatic DFT1’s and cytogenetic strain ensuring the consideration of any factors that may contribute to the ERBB3 range in DFT1 affected Tasmanian devils. No correlation was established between levels of ERBB3 and extent of DFT1 when comparing the number and size of primary DFT1 lesions and any metastatic disease (see Table 2). For example, the devil with the highest serum ERBB3 of 18,254 pg/ml (devil 16), had 2 primary lesions with 3 metastases whereas the lowest serum ERBB3 of 766 pg/ml (devil 23) had seven primary DFT1 lesions and one metastasis. No correlation was established between serum ERBB3 levels and the BCS as most were low (BCS 1–2) with only one devil (devil 20) having a BCS of three out of five, indicating average body condition. Cytogenetic strain did not appear to correlate to serum ERBB3 levels and reflects the immunohistochemical findings that ERBB3 expression was present in all cytogenetic strains of DFT1. Our results indicate that Tasmanian devils with DFT1 have elevated serum ERBB3 levels compared to clinically healthy Tasmanian devils ranging from 766–18,254 pg/ml and that the extent of DFT1 does not readily correlate directly with the serum ERBB3 levels. Further investigations beyond the pilot study encompassing a larger study group of Tasmanian devils with advanced DFT1 and metastases would be necessary to establish any relationship with serum ERBB3 and the extent of DFT1.
ERBB3 in devils with cutaneous lymphoma
We included Tasmanian devils with cutaneous lymphoma (CL) in the study for two reasons. Firstly, they were non-DFT1 devils with a severe skin condition that can affect the facial regions and secondly, the disease presentation of alopecia, excoriation and thickened plaques is distinct from DFT1 (Fig 1E and 1F). Our results revealed that some Tasmanian devils with CL had elevated serum ERBB3 levels, a result that was most unexpected. Although ERBB3 immunohistochemistry on Tasmanian devils with CL was beyond the scope of this research, ERBB3 Immunohistochemical staining of Tasmanian devil lymph node (Fig 1D) did reveal ERBB3 expression in the lymphoid follicle where cytoplasmic expression of ERBB3 is present in both T (germinal centre) and B (mantle) cells. CL devils were in the older age bracket ranging from 4–8 years where the maximum age of a wild devil would be considered 5–6 years (Sarah Peck, personal communication). Bodyweights ranging from 5.4–8.2 Kg compared to the mean weight of 6.6Kg for female and 8.3Kg for male [66] shows possible female underweight wild devils and overweight captive devils. Age or weight did not appear to correlate to the broad range of serum ERBB3 of 30–20,021 pg/ml. Interestingly, 11 of the 12 devils with CL were female. We noted that devils with widespread alopecia (devils 24, 29, 33 and 35), did exhibit increased serum ERBB3 levels ranging from 4383–20,021 pg/ml, suggesting that the severity of CL manifesting clinically as widespread alopecia may contribute to increased serum ERBB3 levels. Together, the elevated serum ERBB3 results in devils with CL is unlikely to cause confusion with DFT1 as CL tends to affects devils in the older age group and the clinical signs of CL are also distinct from DFT1 in established disease. Additionally, if elevated serum ERBB3 levels in Tasmanian devils indicative of CL could be established (pre-clinical) this would improve the healthy captive breeding populations of Tasmanian devils to ensure survival of the species by excluding these devils from this program.
Potential source of serum ERBB3
The capture and detection of antibody in our ELISA assay is selective for the extracellular domain (ECD) of transmembrane ERBB3 in serum or plasma, thus ERBB3’s ECD is cleaved and shed from the plasma membrane would be a natural assumption. In contrast the ERBB3 receptor is internalised, although very slowly, for negative regulation and inactivation [67–71] utilising pathways such as caveolin or micropinocytosis and clathrin-and caveolin independent pathways [72, 73]. ERBB3 has also been shown to be endocytosed independent of phosphorylation and without ligand in clathrin-dependent manner [74]. ERBB3 is degraded by proteasomes catalysed by two E3 ubiquitin ligases; NRDP1 (Neuregulin Receptor Degradation Protein -1) [75], now known as RNF41 (Ring Finger 41, E3 Ubiquitin Protein Ligase) [76–78], and NEDD4 (Neural Cell Precursor Expressed, Developmentally Down-regulated 4, E3 Ubiquitin Protein Ligase) [79] that regulate steady-state ERBB3 levels influencing NRG1 signalling.
Defective internalisation, recycling and degradation of cell surface proteins and ligands is an emerging feature of cancer [80]. It is therefore conceivable that DFT1 is subjected to the same dysregulation and inefficient degradation and recycling resulting in over expression of ERBB3 receptor at the plasma membrane and subsequent detectable levels of serum ERBB3. While dysregulated endocytosis, deregulation and recycling may theoretically account for excess ERBB3 ECD detectable in serum, secreted isoforms of ERBB3 must also be considered as an alternative explanation for the presence of excess ERBB3.
As well as functional transmembrane forms, secreted soluble forms of Epidermal Growth Factor Receptors have been well documented for ERBB1 [81–84], ERBB2 [85–88] and ERBB4 [89–91]. Alternative transcripts for ERBB3 resulting in naturally occurring soluble truncated isoforms including a 1.4 kb transcript of ERBB3 in gastric cancer cell lines [64] and an additional four novel transcripts (1.6, 1.7, 2.1, and 2.3kb) from ovarian cancer cell lines [65] encouraged researchers to identify these secreted isoforms of ERBB3 in Prostate [92–95], liver [96], breast [97, 98] and squamous cell carcinoma [99]. ERBB3 isoforms have also been expressed intracellularly in breast cancer cell lines [97] as well as in the nucleus of Schwann cells [100, 101], prostate [102–104] and breast [105, 106]. Secreted ERBB3 isoform p85 has been shown to inhibit the action of its ligand Neuregulin [98, 107], nuclear translocations act as co-transcriptional activators [108], possible post-translation modification and the tumour microenvironment are instructive to serum ERBB3 secretion from the cell [96] and functions yet to be determined.
The antigenic peptide used for this assay is located within the N-terminal domain of the full length ERBB3 protein. Full length ERBB3 translates into a 180 kDa protein whereas ERBB3 transcripts, created by intron read through and alternative polyadenylation signals result in serum ERBB3 isoforms translating into various proteins ranging in size from 22–75 kDa [109]. Secreted isoforms such as ERBB3-S (1.4kb, 140aa homologous to the N terminus and a 43aa unique carboxy terminal sequence) equates to approximately half of domain I, p50 (1.6kb, 351aa homologous to the N terminus and a 30aa unique carboxy terminal sequence) equates to domain I, II and some of domain III, p45 (1.7kb, 310aa homologous to the N terminus and a 2aa unique carboxy terminal sequence) equates to domain I, II and some of domain III, p85 (2.1kb, 519aa homologous to the N terminus and a 24aa unique carboxy terminal sequence) equates to domain I, II,III and some of domain IV, p75 (2.3kb, 474aa homologous to the N terminus and a 41aa unique carboxy terminal sequence) equates to domain I, II and III [64, 65, 109] ERBB3 isoforms have been detected by a number of methods such as immunoprecipitation [65, 97, 107], immunohistochemistry [92] and ELISA [94–96]. Isoforms that have been detected using ELISA assays include p45 sERBB3 utilising a capture antibody of sequence aa20-643 (detection antibody sequence was not recorded) [94, 95] and 40-50kDa secreted isoforms (possible p45/p50) utilising both capture and detection antibodies with a sequence aa20-643 [96]. The Raybio ELISA kit utilised in our research uses a capture and detection antibody of sequence aa20-643 (personal communication Raybio) which accounts for most of the extracellular domain of ERBB3 and therefore would be able to capture and detect both truncated isoforms as well as the transmembrane ERBB3.
The correlation of serum levels with disease severity and progression would be the foundation of a good biomarker [96] as well; the expected biomarker should be in excess when compared to clinically healthy individuals [81] or possess additional qualities such as theranostic and tertiary prevention [84]. The use of serum ERBB’s as an indicator of human cancer appears useful however, its prognostic and theranostic value remains controversial and continued investigations will be required [81–96, 99]. The development of a diagnostic test for preclinical DFT1 would assist in the field operations if individuals could be identified before they become infectious[110], therefore application of serum ERBB3 as a diagnostic biomarker of DFT1 has great potential. The simplicity of the ELISA Serum ERBB3 methodology is easily incorporated into routine batch testing or rapid turnaround of results for urgent cases if required. Our research suggests that serum ERBB3 can be used as a biomarker for DFT1 and CL irrespective of transmembrane or truncated forms being detected in the serum of affected animals and therefore the potential of serum ERBB3 as a biomarker of early DFT1 detection should be explored.
Schwann cell neoplasms
ERBB3 is crucial to the sequential transition from precursor to immature and finally mature Schwann cells where ERBB3 is down-regulated as myelination proceeds [111]. The adult peripheral nervous system requires maintenance when injured and the NRG1/ERBB system is crucial to Schwann cell dedifferentiation, proliferation, and subsequent regeneration and remyelination where ERBB3 and NRG1 is upregulated and only switched off after axon regeneration illustrating the plasticity of the Schwann cell [112–114]. Peripheral nerve sheath tumours [neurofibroma, malignant peripheral nerve sheath tumours (MPNST)] and schwannoma arise from the Schwann cell lineage and can be genetically characterised as Neurofibromas (either dermal or plexiform) and MPNST’s [Neurofibromatosis 1 (NF1)], or Schwannomas [Neurofibromatosis 2 (NF2)], Schwannomatosis and Carney complex type 1. Although distinct characterisation of these complex diseases is possible, frequent overlapping features make diagnosis difficult and must also include other tumours with a Schwannian component such as Neuroblastic and Granular Cell Tumours [reviewed in [115–119]]. Veterinary Schwann cell neoplasms have been recorded [120–124] although ERBB3 expression in Schwann cell neoplasia has not previously been reported in veterinary literature. ERBB3 receptor has been expressed in human Schwann cell neoplasms including neurofibroma, MPNST, Schwannoma, neuroblastic [125, 126] and ganglioneuroma (GN) tumours [127]. Interestingly, the down regulation of MHC class 1 and 2 molecules in a MPNST cell line [128] contrasting normal expression [129, 130] may indeed be similar to the MHC class 1 downregulation of DFT1 [19–21] resulting in defective antigen processing and presentation of the malignant Schwann cell neoplasm.
ERBB3 as a therapeutic target
Despite evidence for multiple resistance mechanisms for existing therapeutic targeting of ERBB1/2 [131–141] numerous researchers have over the last decade explored the potential of ERBB3 as a therapeutic target [reviewed in [33, 60, 142–150]] using monoclonal antibodies [57, 151–176], histone inhibitors [177], TKI [178], surrobodies [179], locked nucleic acid (LNA)-based ERBB3 antisense oligonucleotide (ASO) [180], peptide mimics and vaccine [181], anti-anginal drug [182] and disulphide disrupting agent [183].
However, managing wildlife disease is considerably more difficult than human disease because of limited data, the effect of the disease on the host and the transmission of disease within a dynamic population makes it difficult to model [184]. Previous efforts to eradicate DFT1 from wild populations by selective culling has proven unsuccessful because of the frequency-dependent transmission of DFT1 and the latency period [110, 184, 185]. TKI’s as a therapeutic approach may be limited due primarlily to the early observation that kinase region of ERBB3 had substantialy reduced activity, however cancer immunotherapy broadly categorised as passive (including monoclonal antibodies, Cytokines, adoptive cell transfer) or active (including therapeutic cancer vaccine, immune checkpoint inhibitors) remains optimistic [186–191]. Many of these successful human immunotherapeutics do hold similar promise in veterinary medicine [192–194] however, drug administration to wild Tasmanian devils is very different from the clinical setting of human and companion animals and therefore treatments such as adoptive cell transfer would be difficult to implement. The fact that DFT1 expresses tumour associated antigens (TAA’s) such as ERBB3 invites the application of monoclonal antibodies and therapeutic cancer vaccines as prospective treatments. The passive administration of monoclonal antibodies to ERBB3 primarily focused on blocking receptor epitopes are still experimental [57, 151–176] and any humanised anti-ERBB3 would certainly have to be become species specific (devil anti-ERBB3) to prevent adverse immunologic reactions [195]. Very few monoclonal antibodies have been developed in veterinary oncology although two caninised antibodies anti-ERBB1 [196] and anti-CD20 [197] show promise. Therapeutic cancer vaccination modalities applicable to wildlife include antigen delivery vaccines that utilise inactivated cancer cells (autologous or allogenic) or peptide vaccines that mimic antigen sequences. Results using an inactivated cancer cell vaccine trial (allogenic DFT1 cell line) are eagerly awaited (http://www.utas.edu.au/news/2015/10/16/19-world-first-trial-of-tasmanian-devil-vaccine-begins-in-the-wild/). Confidence that immunisation can be successful stems from research showing that Tasmanian devils have a competent immune system [21, 198–200] and can produce cytotoxic antibodies [14, 201]. An alternative antigen presentation modality to cancer cell vaccine is a peptide vaccine, where single or multiple amino acid sequences (long or short) representing a defined antigen is combined with adjuvant to elicit an immune response [202]. Development of just a single ERBB3 peptide vaccine can be found in the literature [181] however, peptide vaccines targeting ERBB1 [203, 204], ERBB2 [205–207] or both ERBB1/2 [208] including monoclonal antibody against tyrosine related protein 1 (TRP-1) and altered peptide sequence to gp100 for mouse melanoma [209] all show promise. Overcoming self-tolerance is a major hurdle, one such strategy is the use of Xenoantigens, that is the exact same antigen but from a different species that has considerable sequence homology, differing only by several amino acids which appear to the host as altered epitopes or as “altered self” and therefore tolerance can be broken causing a T-cell response against the endogenous self-antigen [210]. Veterinary xenogeneic vaccinations include a DNA plasmid vaccine encoding human Tyrosinase (TYR) [211] the only veterinary therapeutic tumour vaccine licensed by the United States department of Agriculture (USDA) for the use of oral and digital melanoma, now marketed as OnceptTM.
Recent investigations reveal that the tumour microenvironment of metastatic DFT1 expressed B7-H1 and DFT1 cell lines could upregulate B7-H1[212]. Immune-suppressive tumour microenvironment created by tumour cells that escape ‘immunoediting’ allowing tumour growth and proliferation [213] where certain checkpoint pathways will be used advantageously by tumour cells to confer immune resistance [214]. Hence, checkpoint blockades (monoclonal antibodies) targeting Programmed Cell Death 1 (PD1 or PDCD1) and its ligand PD-L1 (B7-H1) and Cytotoxic T Lymphocyte Antigen 4 (CTLA-4) are now attractive therapeutical targets [215]. Recent views consider cancer immunotherapy invaluable although a single treatment mode may be suitable for some cases, more combinatorial approach will be needed for others [216, 217].
Our research has highlighted ERBB3 as a potential therapeutic target however treatment of Tasmanian devils with DFT1 with therapeutic regimes such as chemotherapy and radiotherapy are impractical. However, a combinatorial approach using therapeutic cancer vaccines including inactivated allogenic DFT1 cancer vaccine, ERBB3 monoclonal antibody, ERBB3 Peptide or xenogeneic vaccine in combination with anti-immune checkpoint blockade therapy would be easier to implement in the field as well as providing a sustained immunological response against DFT1.
Conclusion
ERBB3 had previously avoided scrutiny due to its kinase inactivity; however, ERBB3 has now been the subject of intense investigation over the past decade and is now recognised as a potent partner of the epidermal growth receptor family. ERBB3 upregulation during developmental, dedifferentiation and regenerative processes encapsulates the Schwann cell’s inherent plasticity and imparts certain characteristics of malignant transformation advantageous to transmission of DFT1. Our pilot study has shown for the first time that ERBB3 is consistently expressed immunohistochemically and that ERBB3 is also elevated in the serum of Tasmanian devils with advanced DFT1 and cutaneous lymphoma. Therefore, our research indicates that serum ERBB3 has the potential to be employed as a biomarker of DFT1 or CL in Tasmanian devils to assist conservationists in the management and welfare of Tasmanian devils and species survival. The simplicity of the ELISA Serum ERBB3 methodology is easily incorporated into routine laboratory batch testing and equally applied to include rapid turnaround of results for urgent cases. Extension of this research is necessary to include greater numbers of healthy Tasmanian devils both with and without visible injuries, devils with large and small DFT1 lesions as well as pre-clinical DFT1. This will firmly establish the normal reference range for serum ERBB3 from which potential pre-clinical DFT1 may be identified. In addition, ERBB3 is now recognised as a therapeutic target and therefore the potential exists to consider modes of administration in addition to existing whole cell vaccination such as ERBB3 monoclonal antibody, peptide or xenogeneic vaccines including checkpoint inhibitors. A combinatorial immunotherapeutic approach will enhance cytotoxic destruction, provide long term immunity from DFT1 and therefore eradicate this transmissible tumour from the wild.
Supporting information
(DOCX)
Acknowledgments
This research was funded by the Dr Eric Guiler Save the Tasmanian Devil Research Grant and we are extremely grateful to Save the Tasmanian Devil Program especially the program manager David Pemberton, University of Tasmania, Department of Primary Industries, Water and Environment and the University of Adelaide for their continued support. We are grateful to all STDP field staff including Colette Harmsen, DPIPWE staff including veterinary officer Sarah Peck and senior keeper David Schaap. Pathologists Jim Taylor, Graeme Knowles, Andrew Davis. Librarians Margaret Quill and Toni Venettacci. Molecular biologists Richard Morrison and Teresa Wilson. Histological Support from Alistair Townsend, Tony Van Galen and Dhirendra Prasad—Royal Hobart Hospital, Mike Burley—Hobart Pathology, Dr Terry Brain and Karen Wolfswinkel—Launceston General Hospital, Jann Brauer—Launceston Pathology and Catherine Marshall—Mt Pleasant laboratories.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was funded by the Dr Eric Guiler Tasmanian Devil Research grant through the University of Tasmanian and the Save the Tasmanian Devil Appeal (STDP) to DH and SS http://www.tassiedevil.com.au/tasdevil.nsf. The Department of Primary Industries water and Environment (DPIPWE) and the University of Adelaide provided financial and in kind support.
References
- 1.Guiler ER. Tasmanian devils in agriculture. Tasmanian Journal of Agriculture. 1970;41(2):134-&. [Google Scholar]
- 2.Munday BL. Marsupial disease. Proceedings No36 of Course for Veterinarians—Fauna. 1978:335–85.
- 3.Griner LA. Neoplasms in Tasmanian devils (Sarcophilus harrisii) J Natl Cancer Inst. 1979;62(3):589–95. [DOI] [PubMed] [Google Scholar]
- 4.Canfield PJ, Hartley WJ, Reddacliff GL. Spontanious proliferations in Australian marsupials—A survey and review .2.Dasyurids and Bandicoots. J Comp Pathol. 1990;103(2):147–58. [DOI] [PubMed] [Google Scholar]
- 5.Canfield PJ, Cunningham AA. Disease and mortality in Australasian marsupials held at London zoo, 1872–1972 J Zoo Wildl Med. 1993;24(2):158–67. [Google Scholar]
- 6.Ladds P. Pathology of Australian native wildlife. Ladds P, editor. Collingwood, Australia: CSIRO Publishing; 2009. 640 p. [Google Scholar]
- 7.Scheelings TF, Dobson EC, Hooper C. Cutaneous T-cell lymphoma in two captive Tasmanian devils (Sarcophilus harrisii) J Zoo Wildl Med. 2014;45(2):367–71. doi: 10.1638/2013-0217R.1 [DOI] [PubMed] [Google Scholar]
- 8.Loh R, Bergfeld J, Hayes D, O'Hara A, Pyecroft S, Raidal S, et al. The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). Vet Pathol. 2006;43(6):890–5. doi: 10.1354/vp.43-6-890 [DOI] [PubMed] [Google Scholar]
- 9.Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, Lazenby B, et al. Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biol Conserv. 2006;131(2):307–24. [Google Scholar]
- 10.Chadwick B. Outbreak of facial tumours in Tasmanian devils. Journal of Wildlife Diseases. 2003;39(4Supplement):7–8. [Google Scholar]
- 11.Pearse AM, Swift K. Transmission of devil facial-tumour disease—An uncanny similarity in the karyotype of these malignant tumours means that they could be infective. Nature. 2006;439(7076):549-. doi: 10.1038/439549a [DOI] [PubMed] [Google Scholar]
- 12.McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, et al. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology. 2009;90(12):3379–92. [DOI] [PubMed] [Google Scholar]
- 13.Hamede RK, McCallum H, Jones M. Biting injuries and transmission of Tasmanian devil facial tumour disease. J Anim Ecol. 2013;82(1):182–90. doi: 10.1111/j.1365-2656.2012.02025.x [DOI] [PubMed] [Google Scholar]
- 14.Pye R, Hamede R, Siddle HV, Caldwell A, Knowles GW, Swift K, et al. Demonstration of immune responses against devil facial tumour disease in wild Tasmanian devils. Biology Letters. 2016;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karu N, Wilson R, Hamede R, Jones M, Woods GM, Hilder EF, et al. Discovery of Biomarkers for Tasmanian Devil Cancer (DFTD) by Metabolic Profiling of Serum. J Proteome Res. 2016;15(10):3827–40. doi: 10.1021/acs.jproteome.6b00629 [DOI] [PubMed] [Google Scholar]
- 16.Loh R, Hayes D, Mahjoor A, O'Hara A, Pyecroft S, Raidal S. The immunohistochemical characterization of devil facial tumor disease (DFTD) in the Tasmanian Devil (Sarcophilus harrisii). Vet Pathol. 2006;43(6):896–903. doi: 10.1354/vp.43-6-896 [DOI] [PubMed] [Google Scholar]
- 17.Pyecroft SB, Pearse AM, Loh R, Swift K, Belov K, Fox N, et al. Towards a case definition for devil facial tumour disease: What is it? EcoHealth. 2007;4(3):346–51. [Google Scholar]
- 18.Murchison EP, Tovar C, Hsu A, Bender HS, Kheradpour P, Rebbeck CA, et al. The Tasmanian Devil Transcriptome Reveals Schwann Cell Origins of a Clonally Transmissible Cancer. Science. 2010;327(5961):84–7. doi: 10.1126/science.1180616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddle HV, Kreiss A, Tovar C, Yuen CK, Cheng YY, Belov K, et al. Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proc Natl Acad Sci U S A. 2013;110(13):5103–8. doi: 10.1073/pnas.1219920110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddle HV, Kaufman J. Immunology of naturally transmissible tumours. Immunology. 2015;144(1):11–20. doi: 10.1111/imm.12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods GM, Howson LJ, Brown GK, Tovar C, Kreiss A, Corcoran LM, et al. Immunology of a Transmissible Cancer Spreading among Tasmanian Devils. J Immunol. 2015;195(1):23–9. doi: 10.4049/jimmunol.1500131 [DOI] [PubMed] [Google Scholar]
- 22.Pearse AM, Swift K, Hodson P, Hua B, McCallum H, Pyecroft S, et al. Evolution in a transmissible cancer: a study of the chromosomal changes in devil facial tumor (DFT) as it spreads through the wild Tasmanian devil population. Cancer Genet. 2012;205(3):101–12. doi: 10.1016/j.cancergen.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Deakin JE, Bender HS, Pearse AM, Rens W, O'Brien PCM, Ferguson-Smith MA, et al. Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour. PLoS genetics. 2012;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachish S, Jones M, McCallum H. The impact of disease on the survival and population growth rate of the Tasmanian devil. J Anim Ecol. 2007;76(5):926–36. doi: 10.1111/j.1365-2656.2007.01272.x [DOI] [PubMed] [Google Scholar]
- 25.McCallum H, Tompkins DM, Jones M, Lachish S, Marvanek S, Lazenby B, et al. Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth. 2007;4(3):318–25. [Google Scholar]
- 26.Jones ME, Cockburn A, Hamede R, Hawkins C, Hesterman H, Lachish S, et al. Life-history change in disease-ravaged Tasmanian devil populations. Proc Natl Acad Sci U S A. 2008;105(29):10023–7. doi: 10.1073/pnas.0711236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg CJ, Grueber CE, Pemberton D, Fox S, Lee AV, Ivy JA, et al. "Devil Tools & Tech": A Synergy of Conservation Research and Management Practice. Conserv Lett. 2017;10(1):133–8. [Google Scholar]
- 28.Pye RJ, Pemberton D, Tovar C, Tubio JMC, Dun KA, Fox S, et al. A second transmissible cancer in Tasmanian devils. Proc Natl Acad Sci U S A. 2016;113(2):374–9. doi: 10.1073/pnas.1519691113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Advances in anatomy, embryology, and cell biology. 2007;190:1–65. [PubMed] [Google Scholar]
- 30.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–25. [DOI] [PubMed] [Google Scholar]
- 31.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–65. doi: 10.1038/35077225 [DOI] [PubMed] [Google Scholar]
- 32.Lemmon MA, Schlessinger J. Cell Signaling by Receptor Tyrosine Kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15(7):413–48. doi: 10.1038/cgt.2008.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh AC, Moasser MM. Targeting HER proteins in cancer therapy and the role of the non-target HER3. Br J Cancer. 2007;97(4):453–7. doi: 10.1038/sj.bjc.6603910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 36.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ. Functional selectivity of EGF family peptide growth factors: Implications for cancer. Pharmacol Ther. 2009;122(1):1–8. doi: 10.1016/j.pharmthera.2008.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. [DOI] [PubMed] [Google Scholar]
- 38.Montero JC, Rodriguez-Barrueco R, Ocana A, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Neuregulins and cancer. Clin Cancer Res. 2008;14(11):3237–41. doi: 10.1158/1078-0432.CCR-07-5133 [DOI] [PubMed] [Google Scholar]
- 39.Mei L, Nave KA. Neuregulin-ERBB Signaling in the Nervous System and Neuropsychiatric Diseases. Neuron. 2014;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 41.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi: 10.1038/nrm1962 [DOI] [PubMed] [Google Scholar]
- 42.Warren CM, Landgraf R. Signaling through ERBB receptors: Multiple layers of diversity and control. Cell Signal. 2006;18(7):923–33. doi: 10.1016/j.cellsig.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 43.Li YW, Tennekoon GI, Birnbaum M, Marchionni MA, Rutkowski JL. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci. 2001;17(4):761–7. doi: 10.1006/mcne.2000.0967 [DOI] [PubMed] [Google Scholar]
- 44.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. Embo J. 2000;19(13):3159–67. doi: 10.1093/emboj/19.13.3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL. Insect cell-expressed P180(ERBB3) possesses an impaired tyrosine kinase-activity Proc Natl Acad Sci U S A. 1994;91(17):8132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999;96(9):4995–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi FM, Telesco SE, Liu YT, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107(17):7692–7. doi: 10.1073/pnas.1002753107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang XW, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125(6):1137–49. doi: 10.1016/j.cell.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 49.Kraus MH, Issing W, Miki T, Popescu NC, Aaronson SA. Isolation and characterization of ERBB3, a 3rd member of the ERBB/epidemal growth factor receptor family: Evidence for overexpression in a subset of human mammary tumours. Proc Natl Acad Sci U S A. 1989;86(23):9193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yarden Y, Peles E. Biochemical analysis of the ligand for the Neu oncogene receptor Biochemistry. 1991;30(14):3543–50. [DOI] [PubMed] [Google Scholar]
- 51.Lemoine NR, Barnes DM, Hollywood DP, Hughes CM, Smith P, Dublin E, et al. Expression of the ERBB3 gene product in breast cancer Br J Cancer. 1992;66(6):1116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, et al. Cooperative signalling of ERBB3 and ERBB2 in neoplastic transformation and human mammary carcinomas Oncogene. 1995;10(9):1813–21. [PubMed] [Google Scholar]
- 53.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo J. 1999;18(8):2149–64. doi: 10.1093/emboj/18.8.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284(1):99–110. [DOI] [PubMed] [Google Scholar]
- 55.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 Is Required for HER2-Induced Preneoplastic Changes to the Breast Epithelium and Tumor Formation. Cancer Res. 2012;72(10):2672–82. doi: 10.1158/0008-5472.CAN-11-3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkumar T, Gullick WJ. The type 1 growth factor receptors in human breast cancer Breast Cancer Res Treat. 1994;29(1):3–9. [DOI] [PubMed] [Google Scholar]
- 57.Reschke M, Mihic-Probst D, van der Horst EH, Knyazev P, Wild PJ, Hutterer M, et al. HER3 is a determinant for poor prognosis in melanoma. Clin Cancer Res. 2008;14(16):5188–97. doi: 10.1158/1078-0432.CCR-08-0186 [DOI] [PubMed] [Google Scholar]
- 58.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 Mutations in Human Cancers. Cancer Cell. 2013;23(5):603–17. doi: 10.1016/j.ccr.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 59.Gullick WJ. The c-erb/HER3 receptor in human cancer. Cancer Surv. 1996;27:339–49. [PubMed] [Google Scholar]
- 60.Jiang N, Saba NF, Chen ZG. Advances in Targeting HER3 as an Anticancer Therapy. Chemotherapy research and practice. 2012;2012:817304 doi: 10.1155/2012/817304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JH, Im KS, Kim NH, Yhee JY, Nho WG, Sur JH. Expression of HER-2 and nuclear localization of HER-3 protein in canine mammary tumors: Histopathological and immunohistochemical study. Vet J. 2011;189(3):318–22. doi: 10.1016/j.tvjl.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 62.Doster AR, Yhee JY, Kim JH, Im KS, Sur JH. CDX-2 and HER-3 Expression in Canine Gastric and Colorectal Adenocarcinomas. J Comp Pathol. 2011;145(1):12–9. doi: 10.1016/j.jcpa.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Matsuyama S, Nakamura M, Yonezawa K, Shimada T, Ohashi F, Takamori Y, et al. Expression patterns of the erbB subfamily mRNA in canine benign and malignant mammary tumors. J Vet Med Sci. 2001;63(9):949–54. [DOI] [PubMed] [Google Scholar]
- 64.Katoh M, Yazaki Y, Sugimura T, Terada M. c-erbb3 gene encodes secreted as well as transmembrane receptor tyrosine kinase Biochem Biophys Res Commun. 1993;192(3):1189–97. doi: 10.1006/bbrc.1993.1542 [DOI] [PubMed] [Google Scholar]
- 65.Lee H, Maihle NJ. Isolation and characterization of four alternate c-erbB3 transcripts expressed in ovarian carcinoma-derived cell lines and normal human tissues. Oncogene. 1998;16(25):3243–52. doi: 10.1038/sj.onc.1201866 [DOI] [PubMed] [Google Scholar]
- 66.Peck S, Corkrey R, Hamede R, Jones M, Canfield P. Hematologic and serum biochemical reference intervals for wild Tasmanian devils (Sarcophilus harrisii). Vet Clin Pathol. 2015;44(4):519–29. doi: 10.1111/vcp.12304 [DOI] [PubMed] [Google Scholar]
- 67.Roepstorff K, Grovdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129(5):563–78. doi: 10.1007/s00418-008-0401-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315(4):683–96. [DOI] [PubMed] [Google Scholar]
- 69.Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273(22):13819–27. [DOI] [PubMed] [Google Scholar]
- 70.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490(3):142–52. [DOI] [PubMed] [Google Scholar]
- 71.Baulida J, Kraus MH, Alimandi M, DiFiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271(9):5251–7. [DOI] [PubMed] [Google Scholar]
- 72.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–12. doi: 10.1038/nrm2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6(2):112–26. doi: 10.1038/nrm1571 [DOI] [PubMed] [Google Scholar]
- 74.Sak MM, Breen K, Ronning SB, Pedersen NM, Bertelsen V, Stang E, et al. The oncoprotein ErbB3 is endocytosed in the absence of added ligand in a clathrin-dependent manner. Carcinogenesis. 2012;33(5):1031–9. doi: 10.1093/carcin/bgs128 [DOI] [PubMed] [Google Scholar]
- 75.Qiu XB, Goldberg AL. Nrdpl/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci U S A. 2002;99(23):14843–8. doi: 10.1073/pnas.232580999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diamonti AJ, Guy PM, Ivanof C, Wong K, Sweeney C, Carraway KL. An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc Natl Acad Sci U S A. 2002;99(5):2866–71. doi: 10.1073/pnas.052709799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouyain S, Leahy DJ. Structure-based mutagenesis of the substrate-recognition domain of Nrdp1/FLRF identifies the binding site for the receptor tyrosine kinase ErbB3. Protein Sci. 2007;16(4):654–61. doi: 10.1110/ps.062700307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao ZW, Wu XL, Yen L, Sweeney C, Carraway KL. Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol. 2007;27(6):2180–8. doi: 10.1128/MCB.01245-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Z, Choi BK, Mujoo K, Fan X, Fa M, Mukherjee S, et al. The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling. Oncogene. 2015;34(9):1105–15. doi: 10.1038/onc.2014.56 [DOI] [PubMed] [Google Scholar]
- 80.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8(11):835–50. doi: 10.1038/nrc2521 [DOI] [PubMed] [Google Scholar]
- 81.Maramotti S, Paci M, Manzotti G, Rapicetta C, Gugnoni M, Galeone C, et al. Soluble Epidermal Growth Factor Receptors (sEGFRs) in Cancer: Biological Aspects and Clinical Relevance. Int J Mol Sci. 2016;17(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baron AT, Cora EM, Lafky JM, Boardman CH, Buenafe MC, Rademaker A, et al. Soluble epidermal growth factor receptor (sEGFR/sErbB1) as a potential risk, screening, and diagnostic serum biomarker of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(2):103–13. [PubMed] [Google Scholar]
- 83.Pitteri SJ, Amon LM, Buson TB, Zhang YZ, Johnson MM, Chin A, et al. Detection of Elevated Plasma Levels of Epidermal Growth Factor Receptor Before Breast Cancer Diagnosis among Hormone Therapy Users. Cancer Res. 2010;70(21):8598–606. doi: 10.1158/0008-5472.CAN-10-1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baron AT, Wilken JA, Haggstrom DE, Goodrich ST, Maihle NJ. Clinical implementation of soluble EGFR (sEGFR) as a theragnostic serum biomarker of breast, lung and ovarian cancer. IDrugs. 2009;12(5):302–8. [PubMed] [Google Scholar]
- 85.Baric M, Kulic A, Sirotkovic-Skerlev M, Plavetic ND, Vidovic M, Horvatic-Herceg G, et al. Circulating Her-2/Neu Extracellular Domain in Breast Cancer Patients-Correlation with Prognosis and Clinicopathological Parameters Including Steroid Receptor, Her-2/Neu Receptor Coexpression. Pathol Oncol Res. 2015;21(3):589–95. doi: 10.1007/s12253-014-9859-6 [DOI] [PubMed] [Google Scholar]
- 86.Lam L, McAndrew N, Yee M, Fu T, Tchou JC, Zhang HT. Challenges in the clinical utility of the serum test for HER2 ECD. Biochim Biophys Acta-Rev Cancer. 2012;1826(1):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siampanopoulou M, Galaktidou G, Dimasis N, Gotzamani-Psarrakou A. Profiling serum HER-2/NEU in prostate cancer. Hippokratia. 2013;17(2):108–12. [PMC free article] [PubMed] [Google Scholar]
- 88.Carney WP. The emerging role of monitoring serum HER-2/neu oncoprotein levels in women with metastatic breast cancer. Lab Med. 2003;34(1):58–64. [Google Scholar]
- 89.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275(14):10379–87. [DOI] [PubMed] [Google Scholar]
- 90.Feng SM, Sartor CI, Hunter D, Zhou H, Yang XH, Caskey LS, et al. The HER4 cytoplasmic domain, but not its c terminus, inhibits mammary cell proliferation. Mol Endocrinol. 2007;21(8):1861–76. doi: 10.1210/me.2006-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feng SM, Muraoka-Cook RS, Hunter D, Sandahl MA, Caskey LS, Miyazawa K, et al. The E3 Ubiquitin Ligase WWP1 Selectively Targets HER4 and Its Proteolytically Derived Signaling Isoforms for Degradation. Mol Cell Biol. 2009;29(3):892–906. doi: 10.1128/MCB.00595-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vakar-Lopez F, Cheng CJ, Kim J, Shi GG, Troncoso P, Tu SM, et al. Up-regulation of MDA-BF-1, a secreted isoform of ErbB3, in metastatic prostate cancer cells and activated osteoblasts in bone marrow. J Pathol. 2004;203(2):688–95. doi: 10.1002/path.1568 [DOI] [PubMed] [Google Scholar]
- 93.Chen NY, Ye XC, Chu K, Navone NM, Sage EH, Yu-Lee LY, et al. A secreted isoform of ErbB3 promotes osteonectin expression in bone and enhances the invasiveness of prostate cancer cells. Cancer Res. 2007;67(14):6544–8. doi: 10.1158/0008-5472.CAN-07-1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin SH, Cheng CJ, Lee YC, Ye X, Tsai WW, Kim J, et al. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27(39):5195–203. doi: 10.1038/onc.2008.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin SH, Lee YC, Choueiri MB, Wen SJ, Mathew P, Ye XC, et al. Soluble ErbB3 levels in bone marrow and plasma of men with prostate cancer. Clin Cancer Res. 2008;14(12):3729–36. doi: 10.1158/1078-0432.CCR-08-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hsieh SY, He JR, Yu MC, Lee WC, Chen TC, Lo SJ, et al. Secreted ERBB3 Isoforms Are Serum Markers for Early Hepatoma in Patients with Chronic Hepatitis and Cirrhosis. J Proteome Res. 2011;10(10):4715–24. doi: 10.1021/pr200519q [DOI] [PubMed] [Google Scholar]
- 97.Srinivasan R, Leverton KE, Sheldon H, Hurst HC, Sarraf C, Gullick WJ. Intracellular expression of the truncated extracellular domain of c-erbB-3/HER3. Cell Signal. 2001;13(5):321–30. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi M, Hasegawa Y, Ikeda Y, Wada Y, Tajiri M, Ariki S, et al. Suppression of Heregulin beta Signaling by the Single N-Glycan Deletion Mutant of Soluble ErbB3 Protein. J Biol Chem. 2013;288(46):32910–21. doi: 10.1074/jbc.M113.491902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Funayama T, Nakanishi T, Takahashi K, Taniguchi S, Takigawa M, Matsumura T. Overexpression of c-erbB-3 in various stages of human squamous cell carcinomas. Oncology. 1998;55(2):161–7. [DOI] [PubMed] [Google Scholar]
- 100.Raabe TD, Deadwyler G, Varga JW, Devries GH. Localization of neuregulin Isoforms and erbB receptors in myelinating glial cells. Glia. 2004;45(2):197–207. doi: 10.1002/glia.10311 [DOI] [PubMed] [Google Scholar]
- 101.Adilakshmi T, Ness-Myers J, Madrid-Aliste C, Fiser A, Tapinos N. A Nuclear Variant of ErbB3 Receptor Tyrosine Kinase Regulates Ezrin Distribution and Schwann Cell Myelination. J Neurosci. 2011;31(13):5106–19. doi: 10.1523/JNEUROSCI.5635-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koumakpayi IH, Le Page C, Delvoye N, Saad F, Mes-Masson AM. Macropinocytosis Inhibitors and Arf6 Regulate ErbB3 Nuclear Localization in Prostate Cancer Cells. Mol Carcinog. 2011;50(11):901–12. doi: 10.1002/mc.20766 [DOI] [PubMed] [Google Scholar]
- 103.Koumakpayi IH, Diallo JS, Le Page C, Lessard L, Gleave M, Begin LR, et al. Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res. 2006;12(9):2730–7. doi: 10.1158/1078-0432.CCR-05-2242 [DOI] [PubMed] [Google Scholar]
- 104.Cheng CJ, Ye XC, Vakar-Lopez F, Kim J, Tu SM, Chen DT, et al. Bone microenvironment and androgen status modulate subcellular localization of ErbB3 in prostate cancer cells. Mol Cancer Res. 2007;5(7):675–84. doi: 10.1158/1541-7786.MCR-06-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reif R, Adawy A, Vartak N, Schroder J, Gunther G, Ghallab A, et al. Activated ErbB3 Translocates to the Nucleus via Clathrin-independent Endocytosis, Which Is Associated with Proliferating Cells. J Biol Chem. 2016;291(8):3837–47. doi: 10.1074/jbc.M115.686782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157(6):929–39. doi: 10.1083/jcb.200109033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee H, Akita RW, Sliwkowski MX, Maihle NJ. A naturally occurring secreted human ErbB3 receptor isoform inhibits heregulin-stimulated activation of ErbB2, ErbB3, and ErbB4. Cancer Res. 2001;61(11):4467–73. [PubMed] [Google Scholar]
- 108.Brand TM, Iida M, Luthar N, Wleklinski MJ, Starr MM, Wheeler DL. Mapping C-Terminal Transactivation Domains of the Nuclear HER Family Receptor Tyrosine Kinase HER3. PLoS One. 2013;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Maihle NJ, Lee H. Soluble ErbB3 methods of detection and antibodies United States patent and Trade Mark Office. 2010;Patent number US 7,744,882(June 29, 2010):1–39 [cited 14th July 2016) available from: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=7,744,882.PN.&OS=PN/7,744,882&RS=PN/7,744,882.
- 110.Beeton N, McCallum H. Models predict that culling is not a feasible strategy to prevent extinction of Tasmanian devils from facial tumour disease. J Appl Ecol. 2011;48(6):1315–23. [Google Scholar]
- 111.Mirsky R, Jessen KR. The neurobiology of Schwann cells. Brain Pathol. 1999;9(2):293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fricker FR, Antunes-Martins A, Galino J, Paramsothy R, La Russa F, Perkins J, et al. Axonal neuregulin 1 is a rate limiting but not essential factor for nerve remyelination. Brain. 2013;136:2279–97. doi: 10.1093/brain/awt148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, et al. Axonally Derived Neuregulin-1 Is Required for Remyelination and Regeneration after Nerve Injury in Adulthood. J Neurosci. 2011;31(9):3225–33. doi: 10.1523/JNEUROSCI.2568-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ronchi G, Haastert-Talini K, Fornasari BE, Perroteau I, Geuna S, Gambarotta G. The Neuregulin1/ErbB system is selectively regulated during peripheral nerve degeneration and regeneration. Eur J Neurosci. 2016;43(3):351–64. doi: 10.1111/ejn.12974 [DOI] [PubMed] [Google Scholar]
- 115.Carroll SL. Molecular mechanisms promoting the pathogenesis of Schwann cell neoplasms. Acta Neuropathol. 2012;123(3):321–48. doi: 10.1007/s00401-011-0928-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rodriguez FJ, Stratakis CA, Evans DG. Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol. 2012;123(3):349–67. doi: 10.1007/s00401-011-0935-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123(3):295–319. doi: 10.1007/s00401-012-0954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akahane K, Kato K, Ogiso S, Sakaguchi K, Hashimoto M, Ishikawa A, et al. Malignant granular cell tumor of the breast: case report and literature review. Beast Cancer. 2015;22(3):317–23. [DOI] [PubMed] [Google Scholar]
- 119.Bourdeaut F, Ribeiro A, Paris R, Pierron G, Couturier J, Peuchmaur M, et al. In neuroblastic tumours, Schwann cells do not harbour the genetic alterations of neuroblasts but may nevertheless share the same clonal origin. Oncogene. 2008;27(21):3066–71. doi: 10.1038/sj.onc.1210965 [DOI] [PubMed] [Google Scholar]
- 120.Omi K, Kitano Y, Agawa H, Kadota K. An immunohistochemical study of peripheral neuroblastoma, ganglioneuroblastoma, anaplastic ganglioglioma, schwannoma and neurofibroma in cattle J Comp Pathol. 1994;111(1):1–14. [DOI] [PubMed] [Google Scholar]
- 121.Kagawa Y, Hirayama K, Tagami M, Tsunoda N, Yoshino T, Matsui T, et al. Immunohistochemical analysis of equine pulmonary granular cell tumours. J Comp Pathol. 2001;124(2–3):122–7. doi: 10.1053/jcpa.2000.0439 [DOI] [PubMed] [Google Scholar]
- 122.Chijiwa K, Uchida K, Tateyama S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet Pathol. 2004;41(4):307–18. doi: 10.1354/vp.41-4-307 [DOI] [PubMed] [Google Scholar]
- 123.Schoniger S, Valentine BA, Fernandez CJ, Summers BA. Cutaneous Schwannomas in 22 Horses. Vet Pathol. 2011;48(2):433–42. doi: 10.1177/0300985810377072 [DOI] [PubMed] [Google Scholar]
- 124.Duke FD, Teixeira LBC, Galle LE, Green N, Dubielzig RR. Malignant Uveal Schwannoma With Peripheral Nerve Extension in a 12-Week-Old Color-Dilute Labrador Retriever. Vet Pathol. 2015;52(1):181–5. doi: 10.1177/0300985814522811 [DOI] [PubMed] [Google Scholar]
- 125.Stonecypher MS, Byer SJ, Grizzle WE, Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24(36):5589–605. doi: 10.1038/sj.onc.1208730 [DOI] [PubMed] [Google Scholar]
- 126.Izycka-Swieszewska E, Wozniak A, Drozynska E, Kot J, Grajkowska W, Klepacka T, et al. Expression and significance of HER family receptors in neuroblastic tumors. Clin Exp Metastasis. 2011;28(3):271–82. doi: 10.1007/s10585-010-9369-1 [DOI] [PubMed] [Google Scholar]
- 127.Wilzen A, Krona C, Sveinbjornsson B, Kristiansson E, Dalevi D, Ora I, et al. ERBB3 is a marker of a ganglioneuroblastoma/ganglioneuroma-like expression profile in neuroblastic tumours. Mol Cancer. 2013;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee PR, Cohen JE, Fields RD. Immune system evasion by peripheral nerve sheath tumor. Neurosci Lett. 2006;397(1–2):126–9. doi: 10.1016/j.neulet.2005.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horste GMZ, Hu W, Hartung HP, Lehmann HC, Kieseier BC. The immunocompetence of Schwann cells. Muscle & Nerve. 2008;37(1):3–13. [DOI] [PubMed] [Google Scholar]
- 130.Tzekova N, Heinen A, Kury P. Molecules Involved in the Crosstalk Between Immune- and Peripheral Nerve Schwann Cells. J Clin Immunol. 2014;34:S86–S104. doi: 10.1007/s10875-014-0015-6 [DOI] [PubMed] [Google Scholar]
- 131.Stern DF. ERBB3/HER3 and ERBB2/HER2 duet in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):215–23. doi: 10.1007/s10911-008-9083-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nahta R. Deciphering the role of insulin-like growth factor-I receptor in trastuzumab resistance. Chemotherapy research and practice. 2012;2012:648965 doi: 10.1155/2012/648965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huang XP, Gao LZ, Wang SL, McManaman JL, Thor AD, Yang XH, et al. Heterotrimerization of the Growth Factor Receptors erbB2, erbB3, and Insulin-like Growth Factor-I Receptor in Breast Cancer Cells Resistant to Herceptin. Cancer Res. 2010;70(3):1204–14. doi: 10.1158/0008-5472.CAN-09-3321 [DOI] [PubMed] [Google Scholar]
- 134.Engelman JA, Zejnullahu K, Mitsudomi T, Song YC, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 135.Yun C, Gang L, Gu RM, Xu W, Ming XZ, Chen HQ. Essential role of Her3 in two signaling transduction patterns: Her2/Her3 and MET/Her3 in proliferation of human gastric cancer. Mol Carcinog. 2015;54(12):1700–9. doi: 10.1002/mc.22241 [DOI] [PubMed] [Google Scholar]
- 136.Wang XC, Batty KM, Crowe PJ, Goldstein D, Yang JL. The potential of panHER inhibition in cancer. Front Oncol. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7(4):733–50. [PMC free article] [PubMed] [Google Scholar]
- 138.Sergina NV, Rausch M, Wang DH, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–41. doi: 10.1038/nature05474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu BL, Ordonez-Ercan D, Fan ZY, Edgerton SM, Yang XH, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. International Journal of Cancer. 2007;120(9):1874–82. doi: 10.1002/ijc.22423 [DOI] [PubMed] [Google Scholar]
- 140.Sithanandam G, Fornwald LW, Fields J, Anderson LM. Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549. Oncogene. 2005;24(11):1847–59. doi: 10.1038/sj.onc.1208381 [DOI] [PubMed] [Google Scholar]
- 141.Amin DN, Sergina N, Lim L, Goga A, Moasser MM. HER3 signalling is regulated through a multitude of redundant mechanisms in HER2-driven tumour cells. Biochem J. 2012;447:417–25. doi: 10.1042/BJ20120724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hamburger AW. The role of ErbB3 and its binding partners in breast cancer progression and resistance to hormone and tyrosine kinase directed therapies. J Mammary Gland Biol Neoplasia. 2008;13(2):225–33. doi: 10.1007/s10911-008-9077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tinoco G, Warsch S, Gluck S, Avancha K, Montero AJ. Treating Breast Cancer in the 21st Century: Emerging Biological Therapies. J Cancer. 2013;4(2):117–32. doi: 10.7150/jca.4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–75. doi: 10.1038/nrc2656 [DOI] [PubMed] [Google Scholar]
- 145.Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res. 2010;316(7):1083–100. doi: 10.1016/j.yexcr.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 146.Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-ErbB3 monoclonals as new cancer therapeutics. Oncotarget. 2012;3(8):744–58. doi: 10.18632/oncotarget.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kol A, van Scheltinga A, Timmer-Bosscha H, Lamberts LE, Bensch F, de Vries EGE, et al. HER3, serious partner in crime Therapeutic approaches and potential biomarkers for effect of HER3-targeting. Pharmacol Ther. 2014;143(1):1–11. doi: 10.1016/j.pharmthera.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 148.Ma J, Lyu H, Huang JC, Liu BL. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer. 2014;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee Y, Ma J, Lyu H, Huang JC, Kim A, Liu BL. Role of erbB3 receptors in cancer therapeutic resistance. Acta Biochim Biophys Sin. 2014;46(3):190–8. doi: 10.1093/abbs/gmt150 [DOI] [PubMed] [Google Scholar]
- 150.Zhang N, Chang Y, Rios A, An Z. HER3/ErbB3, an emerging cancer therapeutic target. Acta Biochim Biophys Sin. 2016;48(1):39–48. doi: 10.1093/abbs/gmv103 [DOI] [PubMed] [Google Scholar]
- 151.van der Horst EH, Murgia M, Treder M, Ullrich A. Anti-HER-3 MAbs inhibit HER-3-mediated signaling in breast cancer cell lines resistant to anti-HER-2 antibodies. International Journal of Cancer. 2005;115(4):519–27. doi: 10.1002/ijc.20867 [DOI] [PubMed] [Google Scholar]
- 152.Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99(9):1415–25. doi: 10.1038/sj.bjc.6604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Treder M, Ogbagabriel S, Moor R, Schulze-Horsel U, Hettmann T, Rothe M, et al. Fully human anti-HER3 mAb U3-1287 (AMG 888) demonstrates unique in vitro and in vivo activities versus other HER family inhibitors in NSCLC models. EJC Suppl. 2008;6(12):99-. [Google Scholar]
- 154.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu LH, Nie L, et al. Therapeutically Targeting ErbB3: A Key Node in Ligand-Induced Activation of the ErbB Receptor-PI3K Axis. Sci Signal. 2009;2(77). [DOI] [PubMed] [Google Scholar]
- 155.Schoeberl B, Faber AC, Li DN, Liang MC, Crosby K, Onsum M, et al. An ErbB3 Antibody, MM-121, Is Active in Cancers with Ligand-Dependent Activation. Cancer Res. 2010;70(6):2485–94. doi: 10.1158/0008-5472.CAN-09-3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang JC, Wang SL, Lyu H, Cai B, Yang XH, Wang JX, et al. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer. 2013;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, et al. A Two-in-One Antibody against HER3 and EGFR Has Superior Inhibitory Activity Compared with Monospecific Antibodies. Cancer Cell. 2011;20(4):472–86. doi: 10.1016/j.ccr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 158.Huang S, Li CR, Armstrong EA, Peet CR, Saker J, Amler LC, et al. Dual Targeting of EGFR and HER3 with MEHD7945A Overcomes Acquired Resistance to EGFR Inhibitors and Radiation. Cancer Res. 2013;73(2):824–33. doi: 10.1158/0008-5472.CAN-12-1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Belleudi F, Marra E, Mazzetta F, Fattore L, Giovagnoli MR, Mancini R, et al. Monoclonal antibody-induced ErbB3 receptor internalization and degradation inhibits growth and migration of human melanoma cells. Cell Cycle. 2012;11(7):1455–67. doi: 10.4161/cc.19861 [DOI] [PubMed] [Google Scholar]
- 160.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, et al. Antitumor Activity of a Novel Bispecific Antibody That Targets the ErbB2/ErbB3 Oncogenic Unit and Inhibits Heregulin-Induced Activation of ErbB3. Mol Cancer Ther. 2012;11(3):582–93. doi: 10.1158/1535-7163.MCT-11-0820 [DOI] [PubMed] [Google Scholar]
- 161.Rajkumar T, Gullick WJ. A monoclonal antibody to the human c-erbb3 protein stimulates the anchorage independent growth of breast cancer cell lines Br J Cancer. 1994;70(3):459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Blackburn E, Zona S, Murphy ML, Brown IR, Chan SKW, Gullick WJ. A monoclonal antibody to the human HER3 receptor inhibits Neuregulin 1-beta binding and co-operates with Herceptin in inhibiting the growth of breast cancer derived cell lines. Breast Cancer Res Treat. 2012;134(1):53–9. doi: 10.1007/s10549-011-1908-1 [DOI] [PubMed] [Google Scholar]
- 163.Lazrek Y, Dubreuil O, Garambois V, Gaborit N, Larbouret C, Le Clorennec C, et al. Anti-HER3 Domain 1 and 3 Antibodies Reduce Tumor Growth by Hindering HER2/HER3 Dimerization and AKT-Induced MDM2, XIAP, and Fox01 Phosphorylation. Neoplasia. 2013;15(3):335-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li SR, et al. An Antibody That Locks HER3 in the Inactive Conformation Inhibits Tumor Growth Driven by HER2 or Neuregulin. Cancer Res. 2013;73(19):6024–35. doi: 10.1158/0008-5472.CAN-13-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Garrett JT, Sutton CR, Kurupi R, Bialucha CU, Ettenberg SA, Collins SD, et al. Combination of Antibody That Inhibits Ligand-Independent HER3 Dimerization and a p110 alpha Inhibitor Potently Blocks PI3K Signaling and Growth of HER2+ Breast Cancers. Cancer Res. 2013;73(19):6013–23. doi: 10.1158/0008-5472.CAN-13-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sala G, Rapposelli IG, Ghasemi R, Piccolo E, Traini S, Capone E, et al. EV20, a Novel Anti-ErbB-3 Humanized Antibody, Promotes ErbB-3 Down-Regulation and Inhibits Tumor Growth In Vivo. Transl Oncol. 2013;6(6):676–U293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mirschberger C, Schiller CB, Schraml M, Dimoudis N, Friess T, Gerdes CA, et al. RG7116, a Therapeutic Antibody That Binds the Inactive HER3 Receptor and Is Optimized for Immune Effector Activation. Cancer Res. 2013;73(16):5183–94. doi: 10.1158/0008-5472.CAN-13-0099 [DOI] [PubMed] [Google Scholar]
- 168.Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, et al. MM-141, an IGF-IR- and ErbB3-Directed Bispecific Antibody, Overcomes Network Adaptations That Limit Activity of IGF-IR Inhibitors. Mol Cancer Ther. 2014;13(2):410–25. doi: 10.1158/1535-7163.MCT-13-0255 [DOI] [PubMed] [Google Scholar]
- 169.Francis D, Huang S, Werner L, Lantto J, Horak ID, Kragh M, et al. Sym013, novel pan-HER monoclonal antibody mixture, augments radiation response in human lung and head and neck tumors. Cancer Res. 2014;74(19). [Google Scholar]
- 170.Zhang L, Castanaro C, Luan B, Yang K, Fan LF, Fairhurst JL, et al. ERBB3/HER2 Signaling Promotes Resistance to EGFR Blockade in Head and Neck and Colorectal Cancer Models. Mol Cancer Ther. 2014;13(5):1345–55. doi: 10.1158/1535-7163.MCT-13-1033 [DOI] [PubMed] [Google Scholar]
- 171.Clarke N, Hopson C, Hahn A, Sully K, Germaschewski F, Yates J, et al. Preclinical pharmacologic characterization of GSK2849330, a monoclonal AccretaMab (R) antibody with optimized ADCC and CDC activity directed against HER3. Eur J Cancer. 2014;50:98–9. [Google Scholar]
- 172.D'Souza JW, Reddy S, Goldsmith LE, Shchaveleva I, Marks JD, Litwin S, et al. Combining Anti-ERBB3 Antibodies Specific for Domain I and Domain III Enhances the Anti-Tumor Activity over the Individual Monoclonal Antibodies. PLoS One. 2014;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee S, Greenlee EB, Amick JR, Ligon GF, Lillquist JS, Natoli EJ, et al. Inhibition of ErbB3 by a monoclonal antibody that locks the extracellular domain in an inactive configuration. Proc Natl Acad Sci U S A. 2015;112(43):13225–30. doi: 10.1073/pnas.1518361112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meetze K, Vincent S, Tyler S, Mazsa EK, Delpero AR, Bottega S, et al. Neuregulin 1 Expression Is a Predictive Biomarker for Response to AV-203, an ERBB3 Inhibitory Antibody, in Human Tumor Models. Clin Cancer Res. 2015;21(5):1106–14. doi: 10.1158/1078-0432.CCR-14-2407 [DOI] [PubMed] [Google Scholar]
- 175.Gu JM, Yang JS, Chang Q, Liu ZH, Ghayur T, Gu JJ. Identification of Anti-EGFR and Anti-ErbB3 Dual Variable Domains Immunoglobulin (DVD-Ig) Proteins with Unique Activities. PLoS One. 2015;10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gaborit N, Abdul-Hai A, Mancini M, Lindzen M, Lavi S, Leitner O, et al. Examination of HER3 targeting in cancer using monoclonal antibodies. Proc Natl Acad Sci U S A. 2015;112(3):839–44. doi: 10.1073/pnas.1423645112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Huang XP, Gao LZ, Wang SL, Lee CK, Ordentlich P, Liu BL. HDAC Inhibitor SNDX-275 Induces Apoptosis in erbB2-Overexpressing Breast Cancer Cells via Down-regulation of erbB3 Expression. Cancer Res. 2009;69(21):8403–11. doi: 10.1158/0008-5472.CAN-09-2146 [DOI] [PubMed] [Google Scholar]
- 178.Hickinson DM, Klinowska T, Speake G, Vincent J, Trigwell C, Anderton J, et al. AZD8931, an Equipotent, Reversible Inhibitor of Signaling by Epidermal Growth Factor Receptor, ERBB2 (HER2), and ERBB3: A Unique Agent for Simultaneous ERBB Receptor Blockade in Cancer. Clin Cancer Res. 2010;16(4):1159–69. doi: 10.1158/1078-0432.CCR-09-2353 [DOI] [PubMed] [Google Scholar]
- 179.Foreman PK, Gore M, Kobel PA, Xu L, Yee H, Hannum C, et al. ErbB3 Inhibitory Surrobodies Inhibit Tumor Cell Proliferation In Vitro and In Vivo. Mol Cancer Ther. 2012;11(7):1411–20. doi: 10.1158/1535-7163.MCT-12-0068 [DOI] [PubMed] [Google Scholar]
- 180.Wu YM, Zhang YX, Wang ML, Li Q, Qu ZX, Shi V, et al. Downregulation of HER3 by a Novel Antisense Oligonucleotide, EZN-3920, Improves the Antitumor Activity of EGFR and HER2 Tyrosine Kinase Inhibitors in Animal Models. Mol Cancer Ther. 2013;12(4):427–37. doi: 10.1158/1535-7163.MCT-12-0838 [DOI] [PubMed] [Google Scholar]
- 181.Miller MJ, Foy KC, Overholser JP, Nahta R, Kaumaya PTP. HER-3 peptide vaccines/mimics: Combined therapy with IGF-1R, HER-2, and HER-1 peptides induces synergistic antitumor effects against breast and pancreatic cancer cells. OncoImmunology. 2014;3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ren X-R, Wang J, Osada T, Mook RA Jr., Morse MA, Barak LS et al. Perhexiline promotes HER3 ablation through receptor internalization and inhibits tumor growth. Breast cancer research: BCR. 2015;17(1):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ferreira RB, Law ME, Jahn SC, Davis BJ, Heldermon CD, Reinhard M, et al. Novel agents that downregulate EGFR, HER2, and HER3 in parallel. Oncotarget. 2015;6(12):10445–59. doi: 10.18632/oncotarget.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McCallum H. Models for managing wildlife disease. Parasitology. 2016;143(7):805–20. doi: 10.1017/S0031182015000980 [DOI] [PubMed] [Google Scholar]
- 185.Lachish S, McCallum H, Mann D, Pukk CE, Jones ME. Evaluation of Selective Culling of Infected Individuals to Control Tasmanian Devil Facial Tumor Disease. Conserv Biol. 2010;24(3):841–51. doi: 10.1111/j.1523-1739.2009.01429.x [DOI] [PubMed] [Google Scholar]
- 186.Aurisicchio L, Ciliberto G. Genetic cancer vaccines: current status and perspectives. Expert Opin Biol Ther. 2012;12(8):1043–58. doi: 10.1517/14712598.2012.689279 [DOI] [PubMed] [Google Scholar]
- 187.Guo CQ, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic Cancer Vaccines: Past, Present, and Future In: Tew KD, Fisher PB, editors. Advances in Cancer Research, Vol 119 Advances in Cancer Research. 119. San Diego: Elsevier Academic Press Inc; 2013. p. 421–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Melero I, Gaudemack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–24. doi: 10.1038/nrclinonc.2014.111 [DOI] [PubMed] [Google Scholar]
- 189.Melief CJM, van Hall T, Arens R, Ossendorp F, van der Burg SH. Therapeutic cancer vaccines. J Clin Invest. 2015;125(9):3401–12. doi: 10.1172/JCI80009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Papaioannou NE, Beniata OV, Vitsos P, Tsitsilonis O, Samara P. Harnessing the immune system to improve cancer therapy. Ann Transl Med. 2016;4(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. Immunotherapeutic approaches for cancer therapy: An updated review. Artif Cell Nanomed Biotechnol. 2016;44(3):769–79. [DOI] [PubMed] [Google Scholar]
- 192.Anderson K, Modiano J. Progress in Adaptive Immunotherapy for Cancer in Companion Animals: Success on the Path to a Cure. Veterinary Sciences. 2015;2(4):363 doi: 10.3390/vetsci2040363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bergman PJ. Immunotherapy in Veterinary Oncology. Vet Clin N Am-Small Anim Pract. 2014;44(5):925-+. [DOI] [PubMed] [Google Scholar]
- 194.Regan D, Guth A, Coy J, Dow S. Cancer immunotherapy in veterinary medicine: Current options and new developments. Vet J. 2016;207:20–8. doi: 10.1016/j.tvjl.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 195.Fazekas J, Furdos I, Singer J, Jensen-Jarolim E. Why man's best friend, the dog, could also benefit from an anti-HER-2 vaccine. Oncol Lett. 2016;12(4):2271–6. doi: 10.3892/ol.2016.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singer J, Fazekas J, Wang W, Weichselbaumer M, Matz M, Mader A, et al. Generation of a Canine Anti-EGFR (ErbB-1) Antibody for Passive Immunotherapy in Dog Cancer Patients. Mol Cancer Ther. 2014;13(7):1777–90. doi: 10.1158/1535-7163.MCT-13-0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ito D, Brewer S, Modiano JF, Beall MJ. Development of a novel anti-canine CD20 monoclonal antibody with diagnostic and therapeutic potential. Leuk Lymphoma. 2015;56(1):219–25. doi: 10.3109/10428194.2014.914193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brown GK, Kreiss A, Lyons AB, Woods GM. Natural Killer Cell Mediated Cytotoxic Responses in the Tasmanian Devil. PLoS One. 2011;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pinfold TL, Brown GK, Bettiol SS, Woods GM. Mouse Model of Devil Facial Tumour Disease Establishes That an Effective Immune Response Can be Generated Against the Cancer Cells. Frontiers in immunology. 2014;5:251 doi: 10.3389/fimmu.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Howson LJ, Morris KM, Kobayashi T, Tovar C, Kreiss A, Papenfuss AT, et al. Identification of Dendritic Cells, B Cell and T Cell Subsets in Tasmanian Devil Lymphoid Tissue; Evidence for Poor Immune Cell Infiltration into Devil Facial Tumors. Anat Rec. 2014;297(5):925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kreiss A, Brown GK, Tovar C, Lyons AB, Woods GM. Evidence for induction of humoral and cytotoxic immune responses against devil facial tumor disease cells in Tasmanian devils (Sarcophilus harrisii) immunized with killed cell preparations. Vaccine. 2015;33(26):3016–25. doi: 10.1016/j.vaccine.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 202.Slingluff CL. The Present and Future of Peptide Vaccines for Cancer Single or Multiple, Long or Short, Alone or in Combination? Cancer J. 2011;17(5):343–50. doi: 10.1097/PPO.0b013e318233e5b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Foy KC, Wygle RM, Miller MJ, Overholser JP, Bekaii-Saab T, Kaumaya PTP. Peptide Vaccines and Peptidomimetics of EGFR (HER-1) Ligand Binding Domain Inhibit Cancer Cell Growth In Vitro and In Vivo. J Immunol. 2013;191(1):217–27. doi: 10.4049/jimmunol.1300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Overholser J, Ambegaokar KH, Eze SM, Sanabria-Figueroa E, Nahta R, Bekaii-Saab T, et al. Anti-Tumor Effects of Peptide Therapeutic and Peptide Vaccine Antibody Co-targeting HER-1 and HER-2 in Esophageal Cancer (EC) and HER-1 and IGF-1R in Triple-Negative Breast Cancer (TNBC). Vaccines. 2015;3(3):519–43. doi: 10.3390/vaccines3030519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Allen SD, Garrett JT, Rawale SV, Jones AL, Phillips G, Forni G, et al. Peptide vaccines of the HER-2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179(1):472–82. [DOI] [PubMed] [Google Scholar]
- 206.Gil EY, Jo UH, Lee HJ, Kang J, Seo JH, Lee ES, et al. Vaccination with ErbB-2 peptides prevents cancer stem cell expansion and suppresses the development of spontaneous tumors in MMTV-PyMT transgenic mice. Breast Cancer Res Treat. 2014;147(1):69–80. doi: 10.1007/s10549-014-3086-4 [DOI] [PubMed] [Google Scholar]
- 207.Clifton GT, Peoples GE, Mittendorf EA. The development and use of the E75 (HER2 369–377) peptide vaccine. Future Oncol. 2016;12(11):1321–9. doi: 10.2217/fon-2015-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kaumaya PTP. A paradigm shift: Cancer therapy with peptide-based B-cell epitopes and peptide immunotherapeutics targeting multiple solid tumor types: Emerging concepts and validation of combination immunotherapy. Human Vaccines Immunother. 2015;11(6):1368–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ly LV, Sluijter M, van der Burg SH, Jager MJ, van Hall T. Effective Cooperation of Monoclonal Antibody and Peptide Vaccine for the Treatment of Mouse Melanoma. J Immunol. 2013;190(1):489–96. doi: 10.4049/jimmunol.1200135 [DOI] [PubMed] [Google Scholar]
- 210.Cavallo F, Aurisicchio L, Mancini R, Ciliberto G. Xenogene vaccination in the therapy of cancer. Expert Opin Biol Ther. 2014;14(10):1427–42. doi: 10.1517/14712598.2014.927433 [DOI] [PubMed] [Google Scholar]
- 211.Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24(21):4582–5. doi: 10.1016/j.vaccine.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 212.Flies A, Woods G, Lyons AB, Hayball J. B7-H1 (PD-L1) is expressed in the Tasmanian devil facial tumor microenvironment and is strongly upregulated in response to IFN-gamma. Eur J Immunol. 2016;46:1095-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Muenst S, Laubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279(6):541–62. doi: 10.1111/joim.12470 [DOI] [PubMed] [Google Scholar]
- 214.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Topalian SL, Drake CG, Pardoll DM. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 217.van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief AJM. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–33. doi: 10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper.