Abstract
Hyponatremia is the most common electrolyte disorder and also a predictor of mild cognition impairment. However, the association between hyponatremia and dementia in long follow up periods is rarely investigated. A retrospective cohort study was performed using the claims data of all insured residents who were covered by Taiwan’s universal health insurance from 2000 to 2011. A total of 4900 hyponatremia patients and 19545 matched comparisons were recruited for the analysis. The incidences of hyponatremia and dementia were diagnosed with clinical protocol and defined using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Cox proportional hazard regression and Kaplan–Meier curves were used for the analyses. Independent of adjusting factors, hyponatremia patients had 2.36-fold higher chances of suffering dementia, including Alzheimer’s disease (AD) and non-AD dementia, than the comparisons. Severe hyponatremia patients had higher risks of suffering dementia than the non-severe hyponatremia patients (adjusted hazard ratio: 4.29 (95% CI: 3.47–5.31) versus 2.08 (95% CI: 1.83–2.37)). A dose response relationship was observed between hyponatremia and dementia. Those hyponatremia patients with baseline or incident stroke had significantly higher chances of suffering dementia compared with those patients without hyponatremia and stroke. Stroke is a significant modifier of the relationship between hyponatremia and dementia. Cerebrovascular disease after incident hyponatremia must be prevented to reduce the incidence of dementia.
Introduction
Hyponatremia is not only the most common electrolyte disorder but is also associated with higher infection rate [1], cardiovascular disease [2, 3], and mortality risk [4, 5]. Central nervous system (CNS) symptoms are the main manifestations of hyponatremia [6]. Symptoms of acute hyponatremia include confusion, coma, and seizure that is triggered through cerebral edema. In chronic hyponatremia, the degree of cerebral edema becomes mild through osmotic equilibrium, thereby reducing the severity of CNS symptoms. Several studies reported that chronic hyponatremia resulted in mild cognition impairment (MCI) [7, 8], which in turn was associated with increased risk of progression to dementia [9] or death [10]. However, the MCI among patients with hyponatremia was transient and would return to its normal state when the hyponatremia was corrected [8, 11].
Dementia is a progressive, incurable disease that greatly influences the quality of life of the affected patients. About 35.6 million individuals worldwide are suffering from this disease, and this number is expected to triple in value by 2050[12]. Dementia requires a higher medical cost than cancer and heart disease combined[13]. Therefore, many studies attempted to explore the modifiable risk factors of dementia, such as diabetes mellitus (DM), hypertension, and hyperlipidemia [14]. However, hyponatremia, as a clear cause of MCI, is not well-documented for dementia[15]. In fact, cerebrovascular diseases are currently considered as underlying pathologic hallmarks of various types of dementias[16]. Hyponatremia has also been suggested an important predictor of cerebrovascular diseases, such as stroke [2, 3]. Therefore, the association between hyponatremia and dementia must be explored further.
This study is the first to explore such association based on a retrospective cohort study from a nationwide database. This study attempts to determine the following: (1) the association between hyponatremia and dementia, including Alzheimer’s disease (AD) and non-AD dementia; (2) the relationship among hyponatremia, stroke, and dementia; and (3) the dose response relationship between hyponatremia severity and dementia.
Materials and methods
Data source
The Taiwan Department of Health consolidated 13 insurance programs to establish the Taiwan National Health Insurance program (NHI program) in March 1995. Over 99% of Taiwanese residents are covered by this program. The National Health Insurance Research Database (NHIRD) comprises NHI program registration files and original claims data for reimbursement. This database was established by the National Health Research Institute, which was authorized to manage insurance data. As a sub-dataset of NHIRD, the Longitudinal Health Insurance Database (LHID 2000) includes claims data for one million people who have been randomly selected from the total insured population between 1996 and 2011. We selected our sample from LHID 2000 to investigate the association between hyponatremia and dementia. This database also contained information on the demographic status of the insured individuals and the claims data for inpatient and outpatient care. The personal information of the participants was concealed to protect their privacy. We ensured that all data were de-identified and analyzed anonymously. This study was also exempted from full ethical review by the institutional review board of China Medical University (CMUH104-REC2-115). The diagnosis of each patient was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM).
Study population
Using the retrospective population-based cohort design, we identified 4900 new hyponatremia (ICD-9-CM code 276.1) patients, whose first-time hyponatremia diagnoses were used as index year (2000 to 2009). Given that severe symptomatic hyponatremia patients could be treated with hypertonic saline (3% saline) in clinical settings, we defined the severe hyponatremia group as those hyponatremia patients who required 3% sodium chloride treatment. By contrast, the non-severe hyponatremia group included hyponatremia patients that did not require 3% sodium chloride treatment. For comparison cohort (non-hyponatremia cohort), we randomly selected 19545 subjects without hypernatremia (ICD-9-CM: 276.0) and hyponatremia diagnosis at a 1:4 ratio. These cohorts were frequency-matched by age (per five years), gender, and index year.
Study variables
The follow-up person–years at the end of 2011 were calculated for each subject until the diagnosis of dementia (ICD-9-CM: 290, 294.1, and 331.0) or withdrawal from the insurance system. We categorized dementia into AD (ICD-9-CM: 331.0) and non-AD (ICD-9-CM: 290 and 294.1). Those subjects with a history of dementia at the baseline were excluded from the study. All dementia-related comorbidities, including diabetes (ICD-9-CM: 250), hypertension (ICD-9-CM: 401 to 405), hyperlipidemia (ICD-9-CM: 272), ischemic heart disease (ICD-9-CM: 410 to 414), heart failure (ICD-9-CM: 428), mental illness (ICD-9-CM: 290 to 319), stroke (ICD-9-CM: 430 to 438), liver cirrhosis (ICD-9-CM: 571.2, 571.5, and 571.6), atrial fibrillation (ICD-9-CM: 427.31), chronic renal disease (ICD-9-CM: 585), parkinsonism (ICD-9-CM: 332), and cancer (ICD-9-CM: 140 to 208), were identified before the index date. Diuretics use was evaluated before the index date and was then divided into furosemide, thiazide, and other diuretics. In addition, we defined the visiting numbers as sum of both outpatient and inpatient visit for hyponatremia per year to be representative of severity of hyponatremia. To explore the interaction effect between hyponatremia and stroke on dementia risk, we defined baseline stroke as the occurrence of stroke event before the index date of hyponatremia and defined incident stroke as the occurrence of stroke event after the index date of hyponatremia.
Statistical analysis
We performed chi-square and student's t tests to examine the differences among the categorical and continuous variables, respectively. We employed analysis of variance to analyze the differences in the mean age and follow-up years of patients in the severe hyponatremia group, patients in the non-severe hyponatremia group, and comparison cohorts. The incidence rate ratio of dementia was measured via Poisson regression analysis. The multivariable Cox proportional hazards model was used to adjust dementia-related comorbidities and to estimate the risk of dementia, which was represented by adjusted hazard ratio (aHR) and 95% confidence interval (95% CI). Kaplan–Meier analysis was performed to measure the cumulative dementia incidence for the three study groups, and the log-rank test was performed to assess the differences of cumulative incidence among these groups. All statistical analyses were performed using the SAS 9.4 statistical package (SAS Institute Inc., NC, USA), with a significance level of P <0.05 in the two-tailed tests.
Results
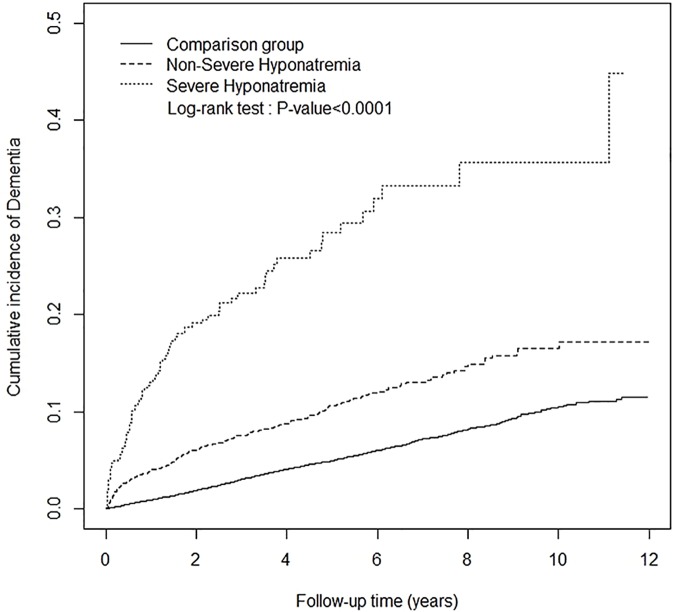
Table 1 shows the demographic characteristics, status of each comorbidity and uses of diuretics by the hyponatremia (N = 4900) and non-hyponatremia cohorts (N = 19545). The cohorts in both groups had similar age and sex distributions after frequency matching. The mean age in both groups was approximately 67 years. The hyponatremia group had a higher prevalence of comorbidity and percentage of diuretics use than the non-hyponatremia group. We further defined the severe (N = 572) and non-severe hyponatremia groups (N = 4328). The severe and non-severe hyponatremia groups (chi-square test p-value < 0.0001) had different age, sex, comorbidity, and diuretics use distributions than the non-hyponatremia cohort. The mean follow-up years for dementia were 2.16, 3.20, and 5.16 in the severe hyponatremia group, non-severe hypernatremia group, and comparison group, respectively. The severe hyponatremia group showed the highest cumulative incidence of dementia by the end of the follow-up period (Fig 1; Log-rank test p-value < 0.0001).
Table 1. Demographic profiles of patients with hyponatremia, divided into severe and non-severe hyponatremia.
| Hyponatremia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe | Non-Severe | All | Comparison | |||||||
| N = 572 | N = 4328 | N = 4900 | N = 19545 | |||||||
| n | % | n | % | n | % | n | % | p-value1 | p-value2 | |
| Age, year | 0.89 | < 0.0001 | ||||||||
| < 65 | 136 | 23.8 | 1661 | 38.4 | 1797 | 36.7 | 7188 | 36.8 | ||
| ≥ 65 | 436 | 76.2 | 2667 | 61.6 | 3103 | 63.3 | 12357 | 63.2 | ||
| Mean (SD) | 72.1 (13.5) | 66.7 (16.5) | 67.1 (16.2) | 67.3 (16.3) | 0.32† | < 0.0001# | ||||
| Gender | 0.90 | 0.94 | ||||||||
| Women | 260 | 45.5 | 1935 | 44.7 | 2195 | 44.8 | 8736 | 44.7 | ||
| Men | 312 | 54.5 | 2393 | 55.3 | 2705 | 55.2 | 10809 | 55.3 | ||
| Comorbidity | ||||||||||
| Diabetes | 237 | 41.4 | 1761 | 40.7 | 1998 | 40.8 | 4206 | 21.5 | < 0.0001 | < 0.0001 |
| Hypertension | 436 | 76.2 | 2918 | 67.4 | 3354 | 68.4 | 10313 | 52.8 | < 0.0001 | < 0.0001 |
| Hyperlipidemia | 179 | 31.3 | 1432 | 33.1 | 1611 | 32.9 | 5389 | 27.6 | < 0.0001 | < 0.0001 |
| Ischemic heart disease | 269 | 47.0 | 1699 | 39.3 | 1968 | 40.2 | 5854 | 30.0 | < 0.0001 | < 0.0001 |
| Heart failure | 121 | 21.2 | 716 | 16.5 | 837 | 17.1 | 1572 | 8.04 | < 0.0001 | < 0.0001 |
| Mental illness | 299 | 52.3 | 2003 | 46.3 | 2302 | 47.0 | 6797 | 34.8 | < 0.0001 | < 0.0001 |
| Stroke | 324 | 56.6 | 1621 | 37.5 | 1945 | 39.7 | 3954 | 20.2 | < 0.0001 | < 0.0001 |
| Liver cirrhosis | 50 | 8.74 | 358 | 8.27 | 408 | 8.33 | 358 | 1.83 | < 0.0001 | < 0.0001 |
| Atrial fibrillation | 58 | 10.1 | 264 | 6.10 | 322 | 6.57 | 513 | 2.62 | < 0.0001 | < 0.0001 |
| CKD | 50 | 8.74 | 353 | 8.16 | 403 | 8.22 | 594 | 3.04 | < 0.0001 | < 0.0001 |
| Parkinsonism | 47 | 8.22 | 222 | 5.13 | 269 | 5.49 | 417 | 2.13 | < 0.0001 | < 0.0001 |
| Cancer | ||||||||||
| Brain | 3 | 0.52 | 9 | 0.21 | 12 | 0.24 | 3 | 0.02 | < 0.0001 | < 0.0001 |
| Non-brain | 98 | 17.1 | 606 | 14.0 | 704 | 14.4 | 870 | 4.45 | < 0.0001 | < 0.0001 |
| Diuretics | ||||||||||
| Furosemide | 277 | 48.4 | 1701 | 39.3 | 1978 | 40.4 | 3398 | 17.4 | < 0.0001 | < 0.0001 |
| Thiazide | 57 | 10.0 | 221 | 5.11 | 278 | 5.67 | 669 | 3.42 | < 0.0001 | < 0.0001 |
| Others | 57 | 10.0 | 244 | 5.64 | 301 | 6.14 | 400 | 2.05 | < 0.0001 | < 0.0001 |
| Follow-up years | ||||||||||
| Mean (SD) | 2.16 (2.53) | 3.20 (2.91) | 3.10 (2.89) | 5.16 (2.81) | < 0.0001† | < 0.0001# | ||||
SD, standard deviation; Chi-square
†t-test
#ANOVA. p-value
1: the p-value between all hyponatremia and comparison groups. p-value
2: the p-value for different types of dysnatremia relative to that for the comparison group
Fig 1. Cumulative dementia risk in the severe hyponatremia, non-severe hyponatremia, and comparison groups.
After considering about the same conditions of dementia-related comorbidities, Table 2 shows that the hyponatremia cohort has 2.36-fold higher chances of suffering dementia than the comparison cohort (95% CI: 2.09 to 2.66) using the multivariable Cox proportional hazards model. Compared with the non-hyponatremia cohort, the risk of dementia significantly increased in the severe (aHR: 4.29, 95% CI: 3.47 to 5.31) and non-severe hyponatremia groups (aHR: 2.08, 95% CI: 1.83 to 2.37). In addition, we evaluated the visiting numbers per year for hyponatremia cohorts and further explored the effect of visiting numbers on dementia risk. The risk of dementia increased from 1.54 (95% CI: 1.33 to 1.77) for those patients with two or fewer visiting numbers up to 15.7 (95% CI: 13.1 to 19.0) for those patients with more than two visiting numbers (p-value < 0.0001 for trend). Hyponatremia patients had 2.31- (95% CI: 1.35 to 3.95) and 2.36-fold (95% CI: 2.09 to 2.66) higher chances of suffering AD and non-AD dementia than the non-hyponatremia patients. Non-severe hyponatremia patients had statistically significant higher risks of suffering AD than the comparison cohort (aHR: 2.35, 95% CI: 1.34 to 4.10). However, an insignificant increased risk was observed between severe hyponatremia and AD. Compared with that of the comparison cohort, the risk of non-AD was 4.38 (95% CI: 3.53 to 5.43) and 2.07 (95% CI: 1.81 to 2.36) in the severe and non-severe hyponatremia groups, respectively.
Table 2. Incidence rate and hazard ratios of dementia in different severity and visiting numbers of hyponatremia.
| Outcome | Event | Person–Years | Rate | Crude HR (95% CI) | Adjusted HR (95% CI) # |
|---|---|---|---|---|---|
| Dementia (ICD-9-CM 290, 294.1 and 331.0) | |||||
| Comparison cohort | 1056 | 100778 | 10.5 | 1.00 | 1.00 |
| Hyponatremia cohort | 424 | 15081 | 28.1 | 2.61(2.33 to 2.93)*** | 2.36(2.09 to 2.66)*** |
| Non-severe hypernatremia | 324 | 13843 | 23.4 | 2.19(1.93 to 2.48)*** | 2.08(1.83 to 2.37)*** |
| Severe hypernatremia | 100 | 1238 | 80.8 | 7.32(5.96 to 9.00)*** | 4.29(3.47 to 5.31)*** |
| Hyponatremia visiting numbers, per year | |||||
| ≤ 2 | 248 | 14465 | 17.2 | 1.64(1.49 to 1.79)*** | 1.54(1.33 to 1.77)*** |
| > 2 | 176 | 616 | 285.7 | 27.3(24.6 to 30.3)*** | 15.7(13.1 to 19.0)*** |
| p-value for trend | < 0.0001 | < 0.0001 | |||
| Alzheimer disease (ICD-9-CM 331.0) | |||||
| Comparison cohort | 61 | 100778 | 0.61 | 1.00 | 1.00 |
| Hyponatremia cohort | 19 | 15081 | 1.26 | 2.13(1.27 to 3.57)** | 2.31(1.35 to 3.95)** |
| Non-severe hypernatremia | 17 | 13843 | 1.23 | 2.07(1.21 to 3.55)** | 2.35(1.34 to 4.10)** |
| Severe hypernatremia | 2 | 1238 | 1.62 | 2.81(0.69 to 11.5) | 2.04(0.49 to 8.53) |
| Non-Alzheimer dementia (ICD-9-CM 290, and 294.1) | |||||
| Comparison cohort | 995 | 100778 | 9.87 | 1.00 | 1.00 |
| Hyponatremia cohort | 405 | 15081 | 26.9 | 2.64(2.35 to 2.97)*** | 2.36(2.09 to 2.66)*** |
| Non-severe hypernatremia | 307 | 13843 | 22.2 | 2.19(1.93 to 2.49)*** | 2.07(1.81 to 2.36)*** |
| Severe hypernatremia | 98 | 1238 | 79.2 | 7.57(6.15 to 9.33)*** | 4.38(3.53 to 5.43)*** |
Medical visit, including outpatient and inpatient visits. PY, person–year; Rate, incidence rate (per 1,000 person–years); IRR, incidence rate ratio; #Adjusted for age, gender, comorbidity, and medicine used
** p < 0.01
*** p < 0.001.
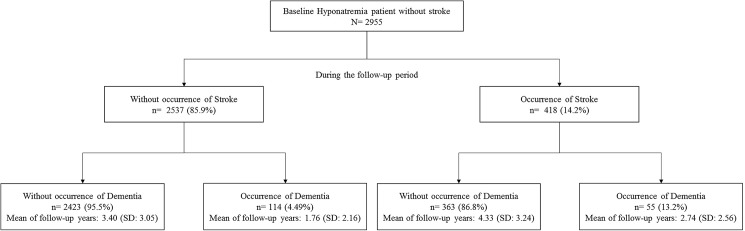
Table 3 illustrates the joint effect of baseline or incident stroke and hyponatremia on dementia outcomes. Hyponatremia patients with baseline stroke had significantly higher risks of suffering dementia (aHR: 4.63, 95% CI: 4.00 to 5.37) than non-hyponatremia patients without stroke. The association between incident stroke and dementia risk for hyponatremia patients were elucidated during the study period (Fig 2). A total of 2955 hyponatremia patients had no baseline stroke. A total of 418 hyponatremia patients had incident stroke (14.2%) during the follow-up period, among which 55 developed signs of dementia by the end of the follow-up period. A total of 114 patients without incident stroke developed dementia. During the study period, the risk of dementia increased from 1.95 (95% CI: 1.63 to 2.33) for hyponatremia patients without incident stroke to 4.04 (95% CI: 3.05 to 5.34) for hyponatremia patients with incident stroke (Table 3).
Table 3. The joint effect between baseline or incident stroke as well as hyponatremia on dementia risk.
| Variable | N | Event | PY | Rate | IRR (95% CI) | Adjusted HR# | p-value | |
|---|---|---|---|---|---|---|---|---|
| (95% CI) | ||||||||
| Hyponatremia | Baseline Stroke | 0.7310 | ||||||
| No | No | 15591 | 636 | 83488 | 7.62 | 1.00 | 1.00 | |
| No | Yes | 3954 | 420 | 17289 | 24.3 | 3.19(2.94 to 3.46)*** | 1.82(1.60 to 2.06)*** | |
| Yes | No | 2955 | 169 | 10164 | 16.6 | 2.18(1.95 to 2.44)*** | 2.65(2.24 to 3.15)*** | |
| Yes | Yes | 1945 | 255 | 4917 | 51.9 | 6.81(6.19 to 7.49)*** | 4.63(4.00 to 5.37)*** | |
| Hyponatremia | Incident Stroke | 0.1233 | ||||||
| No | No | 14193 | 469 | 74939 | 6.26 | 1.00 | 1.00 | |
| No | Yes | 1398 | 167 | 8549 | 19.5 | 3.12(2.82 to 3.46)*** | 1.95(1.63to 2.33)*** | |
| Yes | No | 2537 | 114 | 8441 | 13.5 | 2.16(1.92 to 2.43)*** | 2.77(2.25 to 3.40)*** | |
| Yes | Yes | 418 | 55 | 1722 | 31.9 | 5.10(4.34 to 6.01)*** | 4.04(3.05 to 5.34)*** | |
PY, person–year; Rate, incidence rate (per 1,000 person–years); IRR, incidence rate ratio
#Adjusted for age and gender; p-value for interaction
*** p < 0.001.
Fig 2. Follow-up occurrence of dementia and incident stroke among hyponatremia patients without baseline stroke.
Discussion
To the best of our knowledge, this study is the first nationwide database study to reveal the significant influence of hyponatremia on the occurrence of dementia. Hyponatremia patients had 2.36-fold higher chances of suffering dementia after adjusting other confounding factors. Our findings were consolidated by the effects of the significant dose response relationship between severe and non-severe hyponatremia on dementia occurrence.
Dementia can be divided into different subtypes according to their cause [17]. As the most common subtype, AD accounts for about 50% of all dementia cases. The pathological hallmarks of AD include amyloid plaque and neurofibrillary tangles that damage the structure and function of neurons and synapses. Vascular dementia, which is triggered by a cerebrovascular disease that hinders blood from flowing to the brain, accounts for the majority of the non-AD dementia causes. However, mixed dementia, or the coexistence of AD and non-AD dementia, has been recently identified as the leading cause of dementia [18–20]. The overlap of AD neuropathology (amyloid plaques and neurofibrillary tangles) with cerebrovascular lesions is observed in up to 50% of dementia cases[21]. Therefore, AD and non-AD dementia cannot be easily distinguished in clinical practice. In our study, those patients with non-AD dementia outnumbered those with AD in both normal- and hypo-natremia groups, which indicated that our neurologists tended to classify those patients with signs of mixed dementia to the non-AD group. Regardless of dementia subtype, hyponatremia was significantly associated with higher risks of suffering either AD or non-AD dementia. However, the number of severe hyponatremia patients in our study is too small to show significant results. Dementia has many risk factors[22], including age, sex, DM, hypertension, hyperlipidemia, atrial fibrillation, coronary artery diseases, mental illness, and Parkinson’s disease, which we considered as confounding factors of the disease. Hyponatremia remained an independent strong factor of dementia after these confounding factors were adjusted.
Although we lacked data on the level of serum sodium, we partially compensated for this limitation by investigating the dose response relationship between severe and non-severe hyponatremia. The severity of hyponatremia was measured based on symptoms instead of serum sodium level. All patients with severe symptomatic hyponatremia also received 3% saline in practice[6]. Therefore, our definition of severe hyponatremia was logical. Previous studies suggested that the cognition impairments triggered by hyponatremia could still be treated[8, 9]. However, we found that hyponatremia in long follow-up periods carried a much higher risk of dementia. Therefore, hyponatremia must be prevented to reduce the burden of dementia.
The association between hyponatremia and dementia could be explained in several ways. Hyponatremia could lead to brain damage through multiple mechanisms, predisposing to dementia. Hyponatremia leads to cerebral edema in the acute phase. Cerebral edema was closely linked to neuronal death by the Na/K ATPase dysfunction and the formation of reactive oxygen species[23]. Moreover, hypoxia is a major factor in hyponatremia that contributes to brain damage. This condition is usually observed among patients that face hyponatremia encephalopathy through two mechanisms, namely, neurogenic pulmonary edema and hypercapnia respiratory failure.[24] Hypoxia not only deteriorates cerebral edema[25] but also contributes to increased resting blood pressure and cerebral vascular resistance[26]; in addition, hypoxia induces the expression of proinflammatory transcription factors that can lead to endothelial dysfunction, atherosclerosis, and stroke[27].
In chronic hyponatremia, hyponatremia is associated with dementia through multiple mechanisms. First, renin–angiotensin–system (RAS) activation may have a role in the formation of dementia. Hyponatremia serves not only as a marker but also as a contributory factor to RAS activation[28]. Such activation may result in atherosclerosis and lead to plaque rupture in the atherosclerotic lesions of patients with stroke and brain damage[29–31]. Second, hyponatremia is considered a marker of inflammation[32]. Elevated levels of inflammatory cytokines have been observed among hyponatremia patients. Chronic inflammation is also associated with dementia[33]. These cytokines increase the concentration of amyloidogenic peptides, which are central pathogeneses of AD. Third, previous studies showed that hyponatremia could induce mitochondria dysfunction[34] and oxidative stress[35], which also served key pathogenic roles in AD[16]. The abovementioned evidence also suggested the convergence of pathogenic factors on cerebral blood vessels, which could lead to stroke and dementia[20]. Fourth, hippocampus has a major role in triggering dementia. Chronic hyponatremia induces memory loss by decreasing the ATP production and energy of hippocampus cells and by decreasing the long-term potentiation of hippocampal synapses[34]. Fifth, in the animal model, hyponatremia increases the sensitivity of beta amyloid, thereby leading to cognitive impairment[36].
Stroke is notorious for doubling the risk of suffering dementia (post-stroke dementia), including both AD and non-AD dementia[37]. Recurrent or multiple strokes carry much higher risks of dementia[38]. In our study, both baseline and incident stroke increased the risk of dementia, and their interactions with hyponatremia could increase such risk further. These findings indicated the cumulative damage of stroke and hyponatremia. Several risk factors of post-stroke dementia were also identified[38]. Based on our findings, hyponatremia should also be considered when investigating the causes of dementia.
Hyponatremia has a mutual interaction with stroke. Hyponatremia was associated with increased incidence of cerebrovascular diseases [2, 3] and a common complication after cerebrovascular diseases [39]. Fig 2 shows that among those patients without baseline stroke, about 30% (55/169) have developed dementia after suffering a stroke. In conclusion, stroke is an important, yet not essential, modifier on dementia because hyponatremia is an independent risk factor for dementia. Therefore, the abovementioned mechanisms, including vascular or non-vascular factors, must be considered to explain the association between hyponatremia and dementia.
This study has several limitations. First, we had no detailed information about the level of serum sodium, but tried to compensate for this limitation by dividing the sample into severe and non-severe hyponatremia patients. Second, we could not consider all possible confounders of dementia, such as smoking habits, alcohol use, other prescription drugs, vitamin deficiency, education, physical activity, and genetic factors. Third, we could not differentiate the severity and location of stroke, which could also influence the occurrence of dementia.
Conclusions
In sum, hyponatremia increases the risk of dementia, including both AD and non-AD dementia. Severe hyponatremia carries a much higher risk of dementia. Baseline or incident stroke can modify the relationship between hyponatremia and dementia. To prevent the occurrence of dementia after hyponatremia, clinical physicians must prevent secondary insults, such as cerebrovascular diseases.
Data Availability
The authors confirm that some access restrictions apply to the present data based on the law restriction of the release of individualized data from the National Health Insurance database. Requests for data can be sent to the data owners, Collaboration Center of Health Information Application, Ministry of Health and Welfare, Taiwan (stcarolwu@mohw.gov.tw).
Funding Statement
This study is supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019), the China Medical University Hospital (DMR-105-081), the Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), the NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039 -005), the Tseng-Lien Lin Foundation in Taichung, Taiwan, the Taiwan Brain Disease Foundation in Taipei, Taiwan, and the Katsuzo and Kiyo Aoshima Memorial Funds in Japan. These funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mandai S, Kuwahara M, Kasagi Y, Kusaka K, Tanaka T, Shikuma S, et al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol. 2013;14:276 doi: 10.1186/1471-2369-14-276 ; PubMed Central PMCID: PMC3878351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wannamethee SG, Shaper AG, Lennon L, Papacosta O, Whincup P. Mild hyponatremia, hypernatremia and incident cardiovascular disease and mortality in older men: A population-based cohort study. Nutr Metab Cardiovasc Dis. 2016;26(1):12–9. doi: 10.1016/j.numecd.2015.07.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HW, Ryu GW, Park CH, Kang EW, Park JT, Han SH, et al. Hyponatremia Predicts New-Onset Cardiovascular Events in Peritoneal Dialysis Patients. PLoS One. 2015;10(6):e0129480 doi: 10.1371/journal.pone.0129480 ; PubMed Central PMCID: PMC4460085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Combs S, Berl T. Dysnatremias in patients with kidney disease. Am J Kidney Dis. 2014;63(2):294–303. doi: 10.1053/j.ajkd.2013.09.017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gankam-Kengne F, Ayers C, Khera A, de Lemos J, Maalouf NM. Mild hyponatremia is associated with an increased risk of death in an ambulatory setting. Kidney Int. 2013;83(4):700–6. doi: 10.1038/ki.2012.459 . [DOI] [PubMed] [Google Scholar]
- 6.Fraser CL, Arieff AI. Epidemiology, pathophysiology, and management of hyponatremic encephalopathy. Am J Med. 1997;102(1):67–77. . [DOI] [PubMed] [Google Scholar]
- 7.Xu R, Pi HC, Xiong ZY, Liao JL, Hao L, Liu GL, et al. Hyponatremia and Cognitive Impairment in Patients Treated with Peritoneal Dialysis. Clin J Am Soc Nephrol. 2015;10(10):1806–13. doi: 10.2215/CJN.02240215 ; PubMed Central PMCID: PMC4594065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71 e1–8. doi: 10.1016/j.amjmed.2005.09.026 . [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323–34. doi: 10.1176/appi.ajp.2014.14070878 . [DOI] [PubMed] [Google Scholar]
- 10.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis. 2010;56(4):693–703. doi: 10.1053/j.ajkd.2010.07.003 . [DOI] [PubMed] [Google Scholar]
- 11.Ahluwalia V, Heuman DM, Feldman G, Wade JB, Thacker LR, Gavis E, et al. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J Hepatol. 2015;62(1):75–82. doi: 10.1016/j.jhep.2014.07.033 ; PubMed Central PMCID: PMC4272614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wortmann M. Dementia: a global health priority—highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4(5):40 doi: 10.1186/alzrt143 ; PubMed Central PMCID: PMC3580397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Lin PJ, Levey A. Monetary costs of dementia in the United States. N Engl J Med. 2013;369(5):489 doi: 10.1056/NEJMc1305541#SA1 . [DOI] [PubMed] [Google Scholar]
- 14.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94. doi: 10.1016/S1474-4422(14)70136-X . [DOI] [PubMed] [Google Scholar]
- 15.Pentimone F, Del Corso L. [Hyponatremia, cause of reversible dementia in the elderly]. Minerva Psichiatr. 1992;33(3):165–7. . [PubMed] [Google Scholar]
- 16.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2015. Epub 2015/07/16. doi: 10.1038/jcbfm.2015.164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JH, Lin KP, Chen YC. Risk factors for dementia. J Formos Med Assoc. 2009;108(10):754–64. Epub 2009/10/30. doi: 10.1016/S0929-6646(09)60402-2 . [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. Epub 2007/06/15. doi: 10.1212/01.wnl.0000271090.28148.24 . [DOI] [PubMed] [Google Scholar]
- 19.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29(10):1587–90. Epub 2007/05/01. doi: 10.1016/j.neurobiolaging.2007.03.008 ; PubMed Central PMCID: PMCPMC3437222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–66. Epub 2013/11/26. doi: 10.1016/j.neuron.2013.10.008 ; PubMed Central PMCID: PMCPMC3842016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17 Epub 2013/04/19. doi: 10.3389/fnagi.2013.00017 ; PubMed Central PMCID: PMCPMC3622231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–51. Epub 2005/08/24. doi: 10.1212/01.wnl.0000172914.08967.dc ; PubMed Central PMCID: PMCPMC1619350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasantes-Morales H, Tuz K. Volume changes in neurons: hyperexcitability and neuronal death. Contrib Nephrol. 2006;152:221–40. Epub 2006/10/27. doi: 10.1159/000096326 . [DOI] [PubMed] [Google Scholar]
- 24.Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy. Noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest. 1995;107(2):517–21. . [DOI] [PubMed] [Google Scholar]
- 25.Kokko JP. Symptomatic hyponatremia with hypoxia is a medical emergency. Kidney international. 2006;69(8):1291–3. doi: 10.1038/sj.ki.5000252 . [DOI] [PubMed] [Google Scholar]
- 26.Foster GE, Brugniaux JV, Pialoux V, Duggan CT, Hanly PJ, Ahmed SB, et al. Cardiovascular and cerebrovascular responses to acute hypoxia following exposure to intermittent hypoxia in healthy humans. The Journal of physiology. 2009;587(Pt 13):3287–99. doi: 10.1113/jphysiol.2009.171553 ; PubMed Central PMCID: PMC2727037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64(7):631–6. doi: 10.1136/thx.2008.105577 . [DOI] [PubMed] [Google Scholar]
- 28.Graudal NA, Galloe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. Jama. 1998;279(17):1383–91. . [DOI] [PubMed] [Google Scholar]
- 29.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide population-based study. Diabetes care. 2013;36(4):894–900. doi: 10.2337/dc12-0916 ; PubMed Central PMCID: PMC3609481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37(4):1047–52. . [DOI] [PubMed] [Google Scholar]
- 31.Grote K, Drexler H, Schieffer B. Renin-angiotensin system and atherosclerosis. Nephrol Dial Transplant. 2004;19(4):770–3. Epub 2004/03/20. doi: 10.1093/ndt/gfh030 . [DOI] [PubMed] [Google Scholar]
- 32.Park SJ, Shin JI. Inflammation and hyponatremia: an underrecognized condition? Korean J Pediatr. 2013;56(12):519–22. doi: 10.3345/kjp.2013.56.12.519 ; PubMed Central PMCID: PMC3885786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer's disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3(4):169–76. doi: 10.1111/j.1474-9728.2004.00101.x [DOI] [PubMed] [Google Scholar]
- 34.Fujisawa H, Sugimura Y, Takagi H, Mizoguchi H, Takeuchi H, Izumida H, et al. Chronic Hyponatremia Causes Neurologic and Psychologic Impairments. J Am Soc Nephrol. 2015. doi: 10.1681/ASN.2014121196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286(12):10864–75. doi: 10.1074/jbc.M110.155002 ; PubMed Central PMCID: PMC3060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibbs ME, Gibbs CL. Deleterious effects of soluble beta amyloid on cognition, antagonism by saline and noradrenaline, a role for microglia. Neuroscience. 2013;230:62–71. doi: 10.1016/j.neuroscience.2012.10.070 . [DOI] [PubMed] [Google Scholar]
- 37.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4(11):752–9. Epub 2005/10/22. doi: 10.1016/S1474-4422(05)70221-0 . [DOI] [PubMed] [Google Scholar]
- 38.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–18. Epub 2009/09/29. doi: 10.1016/S1474-4422(09)70236-4 . [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues B, Staff I, Fortunato G, McCullough LD. Hyponatremia in the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(5):850–4. Epub 2013/08/21. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.011 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that some access restrictions apply to the present data based on the law restriction of the release of individualized data from the National Health Insurance database. Requests for data can be sent to the data owners, Collaboration Center of Health Information Application, Ministry of Health and Welfare, Taiwan (stcarolwu@mohw.gov.tw).