Abstract
Background
The complexity of spermatogenesis makes development of appropriate in vitro testis models challenging. A novel in vitro mouse testis culture system has been reported but not yet evaluated as an alternative model for male reproductive toxicity testing. We assessed the effects of media composition on sperm differentiation and testis morphology of cultured mouse testis fragments.
Methods
Testes from postnatal day 5 B6:CBA-Tg(Acrv1-EGFP)2727Redd/J male mice were cultured in knockout serum replacement (KSR) or Albumax I (Albumax) medium. Enhanced green fluorescent protein (EGFP) expression was examined on days 35, 42, 45, and 49 of culture. Histology and flow cytometry were performed for testis morphology and spermatid differentiation.
Results
EGFP signals were first observed in round spermatids on day 22 of culture (corresponding to postnatal day 27) and were observed until the end of culture, indicating testis-specific protein expression. A-kinase anchor protein 4 expression, a marker of elongated spermatid (step 15–16) occurred earlier in explants cultured in KSR than Albumax medium (typically day 35 and after day 42 of culture, respectively). The percentage of seminiferous tubules with elongated spermatid was higher in Albumax than KSR medium from days 45 to 49 of culture.
Conclusion
Albumax medium may facilitate or support better morphology and spermatid production than KSR medium. Further studies need to improve spermatid production and refinement of this in vitro testis culture system that may be useful as a supplement to current male reproductive toxicity testing or an alternative model in cases where in vivo testing may be unfeasible.
Keywords: in vitro organ culture, mice, testes, spermatogenesis, Albumax I, knockout serum replacement
Introduction
In drug development, the assessment of toxicity as an adverse effect on the male reproductive system is crucial and typically relies on in vivo animal experiments (Sasaki et al., 2011). Recently, new experimental models, including stem cells and lower vertebrate species, such as zebrafish, have been proposed as alternatives to mammals in reproductive toxicological studies to reduce the number of animals used in testing (Stokes et al., 2002). In vitro models are being evaluated as potential research tools to supplement or streamline existing in vivo reproductive testing protocols used in the testing of chemical and drug toxicities. However, a remaining challenge is to replicate the complexity of spermatogenesis in an in vitro system. To this end, several in vitro organ culture systems have been developed (Steinberger and Steinberger, 1970); however, none allow the progression of spermatogenesis beyond the pachytene spermatocyte stage. There are also methods for culturing isolated cells obtained from the testis by trypsin treatment; these methods can include testicular cells, such as Sertoli and Leydig cells, in addition to germ cells (Hadley et al., 1985; Yu et al., 2009). However, these approaches have not been able to produce mature sperm that are capable of fertilization from spermatogonia. Additionally, testis morphology cannot be evaluated in these systems as they lack tubule organization. Testis histology remains a common and important endpoint for evaluation of male reproductive toxicity in animal models to determine how drugs and chemicals affect germ cells and other cells in the testis such as Sertoli and Leydig cells (ICH guideline S5(R2), 2005; Sasaki et al., 2011).
Sato et al. (2011a) have described a novel in vitro system that allows for complete spermatogenic development, culminating in fertile spermatozoa. Because this system involves culturing pieces of whole testis from neonatal mice, the model contains all the cell types (not only germ cells but also Leydig and Sertoli cells) found in in vivo testis, in contrast to approaches where cells are isolated by trypsin treatment. Thus, this organ culture method will be able to evaluate testis morphology and may be a potential in vitro model for male reproductive toxicity testing. This organ culture method has been used to examine potential treatments for spermatogenic defects (Sato et al., 2012); however, variability among cultures in the production of mature sperm has been reported (Sato et al., 2011a). Therefore, the aim of the present study was to evaluate culture media, time in culture, and physical factors, such as rotation of cultures, to produce elongated spermatids consistently. As B6:CBA-Tg(Acrv1-EGFP)2727Redd/J transgenic mice (Acrv1tg mice) (Reddi et al., 1999) express EGFP in postmeiotic round spermatids beginning at postnatal day (PND) 21 (Reddi et al., 1999), we used these animals to monitor spermatid differentiation. Testis fragments from Acrv1tg mice were collected at 35, 42, 45, and 49 days of culture; we examined spermatid differentiation by histology and flow cytometry.
Materials and Methods
MATERIALS
All reagents were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise indicated.
ANIMAL BREEDING
We used testis fragments from B6:CBA-Tg(Acrv1-EGF-P)2727Redd/J transgenic mice (Acrv1tg mice; 5–6 weeks old) (Reddi et al., 1999). One male and two female Acrv1tg mice were obtained from the Jackson Laboratory (Reddi et al., 1999) along with wild-type mice as mating partners. Animals were housed in pairs and maintained under a 12:12hr light/dark cycle with controlled room temperature (23°C ± 3°C) and humidity (50% ± 20%). Water and food was provided ad libitum. All animal procedures were approved by the National Center for Toxicological Research Institutional Animal Care and Use Committee and followed the guidelines set forth by the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
IN VITRO TESTIS CULTURE
Testes were collected for culture on PND 5 (day of birth = PND 0). α-Minimal Essential Medium containing knockout serum (KSR) (Life Technologies, Carlsbad, CA) or Albumax I (Albumax) (Life Technologies) were used as culture media according to Sato et al. (2011b) and Yokonishi et al. (2013). Briefly, PND 5 male pups were killed by administering carbon dioxide for less than 5 min followed by decapitation; the abdominal area was opened, and the testes removed. To assess the effects of media composition on sperm differentiation, one testis from each pup was placed into KSR medium, with the other testis cultured in Albumax medium for comparison. The tunica albuginea was removed, and each testis was cut into four pieces. Two to three testis fragments from the same testis were then placed onto small pieces of 1.5% agarose gel (Dojindo Molecular Technologies, Rockville, MD) in 6- or 12-well plates and cultured. To determine the effects of a moving culture plate on spermatid differentiation (Klöckner and Büchs, 2012), some cultures were placed on a rotary shaker (model NA-M301; Nissin Rika, Tokyo, Japan) with constant rotation at approximately 15 rpm. All cultures were incubated at 34°C in an atmosphere of 5% CO2. EGFP signals were observed at 3, 14, 19, 23, 30, 35, 42, and 49 days of culture with a Zeiss microscope and AxioVision software (Carl Zeiss Microscopy, Thornwood, NY). Samples were collected after 35, 42, 45, or 49 days of culture for histological and flow cytometric analyses.
HEMATOXYLIN AND EOSIN STAINING
Paraffin blocks of testis cultured tissue were prepared using standard procedures; 5-μm serial sections were cut and mounted on slides. After deparaffinization, sections were stained with hematoxylin QS (Vector Laboratories, Burlingame, CA) for 30 s, washed under running water, and stained with a 1% eosin/alcohol solution (Sigma-Aldrich, St. Louis, MO) for 2 min. The sections were dehydrated and mounted with Poly-Mount (Polysciences, Warrington, PA). Images were acquired with a Zeiss microscope and AxioVision software using the same exposure time for all images.
IMMUNOHISTOCHEMISTRY
To confirm the stage of spermatid differentiation (round, condensed, and elongated spermatids), acrosome and elongated spermatid differentiation was detected by immunohistochemistry using antibodies against the acrosome marker acrosin (ACR) (cat. no. NBP2-14260; Novus Biological, Littleton, CO) (de Vries et al., 1985) and A-kinase anchor protein 4 (AKAP4), a marker of step 15–16 spermatids (gift from Dr. E.M. Eddy, 2006) (Fulcher et al., 1995; Miki et al., 2002). As these proteins are expressed in only spermatogenic cells stage-specifically (Eddy et al., 2003), the use of immunohistochemistry using specific markers of germ cells differentiation will increase confidences in identification of germ cell stages. Testis sections were deparaffinized and incubated in 0.01 M citrate buffer solution (pH 6.0) at 95°C–100°C for 10–20 min and then cooled for 10 min at room temperature. The sections were blocked in Tris-buffered saline containing 0.02% Triton X-100 (TBST) along with 1% bovine serum albumin and 5% goat serum (Vector Laboratories) for 20 min at room temperature, followed by overnight incubation at 4°C with anti-ACR or anti-AKAP4 (1:2500 and 1:5000 in TBST with 1% bovine serum albumin, respectively). After three washes with TBST, sections were incubated with peroxidase-labeled rabbit IgG using the Elite ABC kit (Vector Laboratories) according to the manufacturer’s protocol. The secondary antibody was detected using 3,3′-diaminobenzidine tetrahy-drochloride (Acros Organics, Morris Plains, NJ) or ImmPACT NovaRED Peroxidase substrate kit (Vector Laboratories) as the chromogen. Testis morphology was examined, and the number of seminiferous tubules (STs) with ACR- or AKAP4-positive cells was counted by light microscopy.
QUANTIFICATION OF ST DIFFERENTIATION
To evaluate spermatid differentiation, we counted the number of STs with round or elongated spermatids and ACR- or AKAP4-positive cells, as detected by immunohistochemistry or hematoxylin and eosin (H&E) staining followed by light microscopy. For quantification, this number was then divided by the number of STs with germ cell differentiation present in whole sections. When the slide contained an entire cross section of the testis fragments, all STs that contained germ cells differentiated to spermatocyte and spermatid stages were counted as well as STs that did not contain these differentiated germ cells. In this case, this number of STs with differentiated germ cells was then divided by the total number of STs presented in whole section. When the slide did not contain an entire cross-section of the testis fragments, only the STs with germ cell differentiation were counted because it was not possible to count the number of all STs. As previously reported (Sato et al., 2011a), STs without germ cell differentiation or with vacuoles (indicating germ cell death/degeneration) were observed. STs without differentiated germ cells were excluded from analysis (asterisks in Supplementary Fig. S1, which is available online). When the STs have differentiated germ cells and vacuoles, we counted the STs as STs with germ cell differentiation.
FLOW CYTOMETRY
Flow cytometry is a good tool to distinguish testicular cells (Sertoli and Leydig cells and different germ cells types) by measuring their DNA contents quantitatively (Suresh et al., 1992). The DNA contents of Sertoli and Leydig cells, spermatogonia and early spermatocytes are diploid (2N); pachytene spermatocytes are tetraploid (4N); and spermatids are haploid (1N). To determine the percentages of differentiated spermatids quantitatively in testis fragments, we measured germ cell DNA content (ploidy) with a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) as previously described (Sato et al., 2011a). Briefly, germ cells from one or two pieces of testis tissue were incubated with 2.5 mg/ml collagenase type I (Sigma-Aldrich) at 37°C for 15 min. An equal volume of 0.5% trypsin-1 mM ethylenediaminetetraacetic acid solution (Sigma-Aldrich) was then added, followed by incubation at 37°C for 10 min. The dissociated germ cell solution was passed through 100-μm mesh strainers, an equal volume of 2% paraformaldehyde in phosphate-buffered saline was then added, followed by incubation on ice for 15 min. Isolated germ cells were obtained by centrifugation at 300 × g for 5 min at room temperature. The cells were suspended in 2 ml of 70% ethanol and stored at −20°C for 24–48 h. After centrifugation, cells were stained with 0.02 mg/ml propidium iodide in phosphate-buffered saline containing 0.2 mg/ml RNase A and 0.1% Triton X-100 at 37°C for 15 min. After passage through a 40-μm mesh strainer, DNA ploidy was measured by flow cytometry.
STATISTICAL ANALYSIS
Flow cytometry data were analyzed using the Student’s t test. Values are presented as the mean ± SD, with the significance level set at P < 0.05. Image processing and statistical analyses were performed using Microsoft Excel 2010 (Redmond, WA) and GraphPad Prism (GraphPad Inc., La Jolla, CA) software.
Results and Discussion
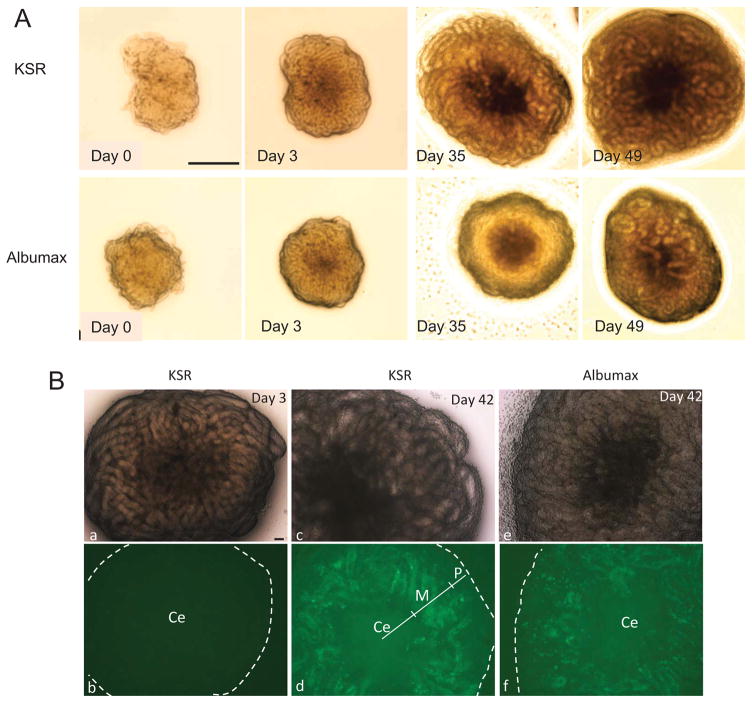
We evaluated the in vitro testis culture system using Acrv1tg mice, and two different culture media (KSR and Albumax). Testis fragments, in general, grew larger in size in KSR as compared to Albumax (Fig. 1A); testis fragments in KSR were observed to be flattened, while testis fragments in Albumax appeared to be more rounded. EGFP expression was observed starting from day 22 of culture (corresponding to PND 27) in both media. This suggests that germ cell differentiation of cultured testis fragments is obviously delayed in vitro relative to in vivo development (PND 21) (Reddi et al., 1999). EGFP expression was predominantly observed in the middle region (M) of all cultured testis fragments collected from Acrv1tg pups on day 42 of culture (Fig. 1B, d and f). EGFP was also detected in the peripheral region of testis fragments cultured in KSR or Albumax (Fig. 1B, panel f); however, this EGFP expression disappeared with time and correlates with germ cell degeneration independent of the culture media used. In some testis fragments, over 90% of the area excluding the center region (Ce) in the culture testis fragments was EGFP-positive, while only 50%–60% of the area was positive in others (Supplementary Fig. S2). Thus, different testis fragments from the same pup exhibited different levels of spermatid differentiation.
FIGURE 1.
Culture of testis fragments from Acrv1tg mice in KSR and Albumax media. (A) Upper and lower panels show images of testis fragments cultured in KSR and Albumax, respectively, on days 0, 3, 35, and 49 of culture. Scale bar = 0.5 mm. (B) EGFP signals in cultured testis fragments from Acrv1tg mice on days 3 and 42. Upper panels show light micrographs; lower panels show EGFP fluorescence. On day 3 (a,b), no EGFP signals were observed. On day 42, EGFP signals were detected within STs in KSR (c,d) and Albumax (e,f). Ce, M, and P: center, middle, and peripheral regions of testis fragments, respectively. Scale bar = 0.1 mm.
HISTOLOGY AND FLOW CYTOMETRY ANALYSES FOR SPERMATID DIFFERENTIATION
Cultured testis fragments were collected at 35, 42, 45, or 49 days of culture and analyzed. To investigate the detailed level of spermatid differentiation in the testis culture system, we carried out a histological analysis using H&E staining as well as immunohistochemical staining using antibodies against ACR and AKAP4, markers for the acrosome and step 15–16 spermatids, respectively. To quantify spermatid differentiation, the number of STs in immunostained testis sections was counted (Table 1), and DNA ploidy was also measured by flow cytometry (Table 2).
TABLE 1.
Percentage of STs with Germ Cell Differentiation, Round, Elongated, or AKAP4-Positive Spermatids
| Percentage of STs with germ cell differentiationa | Percentage of STs with round spermatidsb | Percentage of STs with elongated spermatidsb | Percentage of STs with AKAP4-positive spermatidsb | |
|---|---|---|---|---|
| KSR | ||||
| Day 35 of culture | 58.63 ± 4.97 (n = 7) | 50.13 ± 4.91 (n = 13) | 4.12 ± 0.76 (n = 13) | 1.33 ± 2.00 (n = 12) |
| Day 42 of culture | 62.09 ± 4.90 (n = 3) | 60.38 ± 9.43 (n = 10) | 7.78 ± 2.01 (n = 10) | 6.47 ± 3.10 (n = 6) |
| Day 45 of culture | 35.70 ± 5.91 (n = 5) | 46.62 ± 13.01 (n = 6) | 11.89 ± 6.64 (n = 6) | 0.67 ± 0.67 (n = 6) |
| Day 49 of culture | 28.53 ± 7.31 (n = 8) | 61.78 ± 14.23 (n = 10) | 8.39 ± 4.02 (n = 10) | 1.65 ± 1.14 (n = 7) |
| Albumax | ||||
| Day 35 of culture | 87.44 ± 2.04 (n = 3) | 30.46 ± 8.52 (n = 12) | 0.90 ± 0.60 (n = 11) | 0.00 ± 0.00 (n = 12) |
| Day 42 of culture | 64.68 ± 4.45 (n = 3) | 48.56 ± 11.11 (n = 8) | 4.64 ± 2.11 (n = 8) | 1.17 ± 0.96 (n = 9) |
| Day 45 of culture | 69.66 ± 6.25 (n = 9) | 68.66 ± 3.67 (n = 10) | 21.84 ± 3.54 (n = 10) | 10.65 ± 4.30 (n = 8) |
| Day 49 of culture | 50.21 ± 5.33 (n = 7) | 78.14 ± 6.45 (n = 8) | 45.06 ± 8.78 (n = 8) | 28.57 ± 6.22 (n = 7) |
Values represent mean ± SEM; n equals the total number of testis fragments evaluated.
Percentages are based on the total number of STs in whole testis fragment sections. Only the slides containing an entire cross section of the testis fragments were used.
Percentages are based on the number of STs with germ cell differentiation.
TABLE 2.
DNA Ploidy (1N) Analysis of Dissociated Germ Cells from Cultured Testis Fragments
| Medium | Day 35 | Percentage of haploid germ cells | ||
|---|---|---|---|---|
| Day 42 | Day 45 | Day 49 | ||
| KSR | 2.73 ± 0.31 (n = 5) | 8.42 ± 0.98 (n = 6) | 2.75 ± 0.63 (n = 6) | 5.12 ± 0.74 (n = 9) |
| Albumax | 1.73 ± 1.11 (n = 5) | 3.69 ± 0.90** (n = 7) | 7.04 ± 1.16* (n = 8) | 9.04 ± 0.73** (n = 13) |
Values represent mean ± SEM of the total number (n) of testis fragments analyzed.
p < 0.01 vs. KSR.
p < 0.005.
On day 35, round spermatids were present in the STs of testis fragments, but the number was higher in samples cultured in KSR (Fig. 2A,B, arrows) as compared to Albumax medium (Fig. 2F,G; Table 1). Acrosome formation in round spermatids, as evidenced by ACR immunoreactivity, was seen in samples cultured in KSR as early as day 35 (Fig. 3A) but did not occur in samples cultured in Albumax until at least day 42 (Fig. 3B–E). Similarly, elongated spermatids were observed as early as day 35 in KSR (Table 1; Fig. 2A–D, arrowhead). In contrast, elongated spermatids were observed at day 42 in Albumax (Table 1; Fig. 2H–K, arrowheads). Immunohistochemistry was performed using an antibody against AKAP4; AKAP4-positive step 15–16 spermatids were mainly observed in testes cultured in KSR on day 42 (Fig. 3F) and in Albumax on day 49 (Fig. 3G).
FIGURE 2.
Histological analysis of testis fragments by H&E staining. The images in A–E represent fragments cultured in KSR, while the images in F–K represent growth in Albumax. Testis fragments on days 35 (A,B in KSR; F,G in Albumax), 42 (C,D in KSR; H,I in Albumax), and 49 (E in KSR; J,K in Albumax) of culture. B, D, G, I, and K are higher magnification images of STs indicated by the white boxes in A, C, F, H, and J, respectively. On day 35 of culture, round spermatids were observed in the STs of testis fragments cultured in KSR (arrows in A and B). On day 42, elongated spermatids were detected in STs (arrowheads in A, B, C, and D) cultured in the same media. In Albumax, round spermatids were not observed until day 42 (arrows in H and I), and elongated spermatids (arrowheads in H, I, J, and K) were only observed at day 49. Scale bar = 50 μm.
FIGURE 3.
Histological analysis of testis fragments by immunohistochemistry. (A–E) Testis fragment sections were incubated with anti-ACR antibody. (A) An image of STs on day 35 of culture in KSR, while other panels show images of ACR-expressing cells on days 35 (B,C) and 42 (D,E), in Albumax. C and E are higher magnification images of STs indicated in the white boxes in B and D, respectively. The acrosome in spermatids as demonstrated by staining (brown) with the anti-ACR antibody was observed on days 35 (A) in KSR or 42 of culture in Albumax (D,E). However, on day 35 of culture in Albumax, some acrosomes were observed that did not stain with anti-ACR (arrows in B and C). (F,G) Testis fragment sections cultured in KSR (F) or Albumax (G) medium were incubated with anti-AKAP4 antibody on days 42 and 49 of culture, respectively. The sperm flagella that reacted anti-AKAP4 antibody were observed in KSR and Albumax (arrows). Scale bar = 50 μm.
On day 49, germ cells in STs of testis fragments had degenerated to a greater degree in KSR than in Albumax medium (Fig. 2E). To quantify spermatid differentiation, we counted the number of STs with round or elongated spermatids as well as AKAP4-positive step 15 to 16 spermatids per total number of STs with differentiated germ cells (Table 1), excluding STs that lacked differentiated germ cells (as depicted in Supplementary Fig. S1). The percentage of STs with round spermatids was higher in testis fragments cultured in KSR as compared to Albumax on days 35 and 42 (corresponding to day 40 and 47 in vivo, respectively). However, on day 45 and later, the number of STs with round and late spermatids was higher in testis fragments cultured in Albumax as compared to KSR. This difference was also reflected in DNA ploidy measurements obtained by flow cytometry analysis of germ cells dissociated from cultured testis fragments (Table 2). There were more haploid germ cells (DNA amount = 1N) present when fragments were cultured in KSR than in Albumax on day 35, although the difference was not statistically significant. However, on day 45 and later, DNA ploidy (1N) was higher in testis fragments cultured in Albumax as compared to KSR (Table 2). The number of STs with elongated spermatids was higher and there were more haploid cells on and after day 42 in Albumax medium.
The percentage of STs with elongated spermatids was higher in Albumax as compared to KSR medium from day 45 to 49 of culture. Similarly, the percentage of STs with AKAP4-positive cells was higher in Albumax, likely reflecting the greater degeneration of STs in testis fragments cultured in KSR (Table 1). The percentage of STs with differentiated germ cells in whole testis fragments was 58.63% on day 35 and decreased to 28.53% on day 49 in KSR, while the percentage of STs with differentiated germ cells in whole testis fragments was 87.44% on day 35 and 50.21% on day 49 in Albumax. However, it remains unclear when the first wave of spermatogenesis occurs and how long the duration of the first wave of spermatogenesis lasts in cultured testis fragments. Spermatogenesis is the stepwise process of germ cell differentiation from spermatogonia to mature sperm, and it is well known when unique germ cells types are differentiated during the first wave of spermatogenesis in vivo (Leblond and Clermont, 1952). If we know when the unique germ cells (e.g., spermatocytes or spermatids) are differentiated in cultured testis fragments, it will be useful to determine which steps drugs or chemicals affect the first wave of spermatogenesis in in vitro toxicity testing like in vivo testing; further experiments are necessary to investigate this.
EFFECTS OF CONSTANT ROTATION ON SPERMATID DIFFERENTIATION
We found, using histological analysis, that STs at the center of the testis fragments commonly degenerated or had altered vacuolization when cultured in KSR and Albumax medium, as previously reported (Sato et al., 2011a) (Supplementary Fig. S1). In addition, this was more apparent in fragments cultured over a longer period of time, suggesting that germ cells had started to degenerate due to a lack of oxygen at the center of the fragments (Gangal, 2010).
We examined whether cultures that were rotated constantly until sample collection would have a greater number of STs with differentiated germ cells, because more oxygen would be available for the testis fragments under this condition (Klöckner and Büchs, 2012). We examined testis fragments collected on day 45 with or without constant rotation by histology and flow cytometry and found that the percentage of STs with round spermatids and 1N DNA ploidy was higher in cultures that were subjected to rotation (Tables 3 and 4, respectively) in the KSR and Albumax medium compared with non-rotation (Tables 1 and 2). However, the percentage of STs with elongated and AKAP-positive spermatids was only increased in Albumax cultures (Table 3).
TABLE 3.
Effects of Constant Rotation on Round, Elongated and AKAP4-Positive Spermatid Differentiation
| Medium (endpoint) | % of STs with germ cell differentiationa | % of STs with round spermatidsb | % of STs with elongated spermatidsb | % of STs with AKAP4-positive spermatidsb |
|---|---|---|---|---|
| KSR (45 days) | ||||
| With constant rotation | 23.37 ± 3.01 (n = 4) | 72.16 ± 8.21 (n = 6) | 4.55 ± 2.61 (n = 6) | 0.00 ± 0.00 (n = 3) |
| Without constant rotation | 35.70 ± 5.91 (n = 5) | 46.62 ± 13.01 (n = 6) | 11.89 ± 6.64 (n = 6) | 0.67 ± 0.67 (n = 6) |
| Albumax (45 days) | ||||
| With constant rotation | 69.45 ± 3.00 (n = 3) | 89.73 ± 4.91 (n = 6) | 46.16 ± 14.43 (n = 5) | 34.13 ± 23.02 (n = 2) |
| Without constant rotation | 69.66 ± 6.25 (n = 9) | 68.66 ± 3.67 (n = 10) | 21.84 ± 3.54 (n = 10) | 10.65 ± 4.30 (n = 8) |
Values represent mean ± SEM; n equals the total number of testis fragments evaluated. The data without constant rotation was from Table 1.
Percentages are based on the total number of STs in whole testis fragment sections. Only slides containing an entire cross section of the testis fragments were used.
Percentages are based on the number of STs with germ cell differentiation.
TABLE 4.
DNA Ploidy (1N) Analysis of Dissociated Germ Cells from Testis Fragments Cultured with Constant Rotation for 45 Days
| Medium | Percentage of haploid germ cells | |
|---|---|---|
| With constant rotation | Without constant rotation | |
| KSR | 8.05 ± 2.21 (n = 5) | 2.75 ± 0.63 (n = 6) |
| Albumax | 10.76 ± 2.85 (n = 5) | 7.04 ± 1.16 (n = 8) |
Values represent mean ± SEM of the total number (n) of testis fragments analyzed. The data without constant rotation are shown in Table 2.
Previously, constant rotation has been shown to be beneficial for in vitro organ culture systems (Klöckner and Büchs, 2012), and in this system shaking appears to promote greater spermatid differentiation in Albumax. It is unclear why elongated spermatid production was not increased in KSR medium; a possible reason is that the different media composition for elongated spermatid production may be present in Albumax medium, but not in KSR medium. In addition, the rotating effect on elongated spermatid production may be limited in KSR medium due to pH differences in KSR and Albumax medium. The pH of KSR was 7.25 ± 0.1, while the pH of Albumax was 6.95 ± 0.1; thus, further experiments are necessary to determine the optimal endpoints in each medium. Nevertheless, constant rotation may improve the rate of spermatid production.
Table 5 summarizes the differences in results between KSR and Albumax medium in this study. Overall, Albumax medium induces better morphology than KSR medium in this in vitro organ culture system. The rounded shape of testis fragments in Albumax medium seems to show better spermatid production; many Sertoli cells in the testis fragments may produce testicular fluid (Richburg et al., 1994) that may be trapped in the tubules, so that testis fragments may develop a more rounded shape by the expanding tubules. However, there is a large amount of variability in spermatid production from one isolation to the next in both media (Supplementary Table S1). Further studies are necessary to improve the methods for more consistent spermatid production in each testis fragment.
TABLE 5.
Summary of the Differences between KSR and Albumax
| Parameters | KSR | Albumax |
|---|---|---|
| Testis fragments size | +++ | ++ |
| Testis fragments shape | Flattened | Rounded |
| ACR formation | Day 35 | Day 42 |
| Elongated spermatids observed | Day 35 | Day 42 |
| AKAP4-positive Step 15–16 spermatids observed | Day 42 | Day 49 |
| Percent of STs with AKAP4-positive spermatids (%) | ||
| Day 35 | 1.3 | 0 |
| Day 42 | 6.5 | 1.2 |
| Day 49 | 1.7 | 28.6 |
Size was categorized into three levels: +, small; ++, medium; +++, large.
CONCLUSIONS
Our results demonstrate that a previously developed in vitro testis culture system can be used to produce spermatids from more than one mouse strain; testis fragments containing spermatids can be collected on days 35–42 when cultured in KSR and on days 45–49 when cultured in Albumax medium. Increased oxygen availability to the cells by rotating the culture plate (Klöckner and Büchs, 2012) also was shown to increase spermatid production. An advantage of this in vitro testis culture system is that it allows observation of the morphological benchmarks of seminiferous tubular organization including germ cell differentiation and other testicular cells (Sertoli and Leydig cells). From the view of testis morphology and germ cell differentiation in this study, Albumax medium may be superior to KSR medium. Although some aspects of the methodology need to be improved and criteria for a paradigm to assess reproductive toxicity and risk must be established, our findings provide a basis for the continued development of effective in vitro testis culture systems which may be useful as a supplement to current male reproductive toxicity testing or an alternative model in cases where in vivo testing may not be feasible.
Supplementary Material
Acknowledgments
The authors thank Drs. Yang Xi and Xuan Zhang for useful comments to this manuscript. The authors thank Ms. Kahrin Prince, Ms. Melanie Dumas, and the National Center for Toxicological Research animal care technicians for animal care. The author (N.N.) thanks Ms. Lisa D. Freeman and Dr. Kelly Davis for assistance in the preparation of paraffin sections, and Ms. Sherry Smith and Ms. Kathy Caroll for preparing cage cards. The authors declare that there are no conflicts of interest.
NCTR; contract grant number: E0758901.
Footnotes
Disclaimer: The views expressed are those of the authors and do not represent the views of the Food and Drug Administration.
Additional Supporting information may be found in the online version of this article.
References
- De Vries JW, Willemsen R, Geuze HJ. Immunocytochemical localization of acrosin and hyaluronidase in epididymal and ejaculated porcine spermatozoa. Eur J Cell Biol. 1985;37:81–88. [PubMed] [Google Scholar]
- Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–115. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- Eddy EM. Knobil and Neill’s physiology of reproduction. Amsterdam: Elsevier; 2006. The spermatozoon; pp. 3–54. [Google Scholar]
- Fulcher KD, Mori C, Welch JE, et al. Characterization of Fsc1 cDNA for a mouse sperm fibrous sheath component. Biol Reprod. 1995;52:41–49. doi: 10.1095/biolreprod52.1.41. [DOI] [PubMed] [Google Scholar]
- Gangal S. Principles and practice of animal tissue culture. 2. Telangana, India: Universities Press; 2010. [Google Scholar]
- Hadley MA, Byers SW, Suárez-Quian CA, et al. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH guideline S5(R2) [Accessed January 17, 2017];Detection of toxicity to reproduction for medicinal products & toxicity to male fertility: ICH Harmonised Tripartite Guideline. 2005 Available at: http://www.ich.org/filead-min/Public_Web_Site/ICH_Products/Guidelines/Safety/S5/Step4/S5_R2__Guideline.pdf.
- Klöckner W, Büchs J. Advances in shaking technologies. Trends Biotech. 2012;30:307–314. doi: 10.1016/j.tibtech.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Miki K, Willis WD, Brown PR, et al. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248:331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Reddi PP, Flickinger CJ, Herr JC. Round spermatid-specific transcription of the mouse SP-10 gene is mediated by a 294-base pair proximal promotoer. Biol Reprod. 1999;61:1256–1266. doi: 10.1095/biolreprod61.5.1256. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Redenbach DM, Boekelheide K. Seminiferous tubule fluid secretion is a Sertoli cell microtubule-dependent process inhibited by 2,5-hexanedione exposure. Toxicol Appl Pharmacol. 1994;128:302–309. doi: 10.1006/taap.1994.1210. [DOI] [PubMed] [Google Scholar]
- Sasaki JC, Chapin RE, Hall DG, et al. Incidence and nature of testicular toxicity findings in pharmaceutical development. Birth Defects Res B Dev Reprod Toxicol. 2011;92:511–525. doi: 10.1002/bdrb.20338. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011a;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Yokonishi T, et al. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011b;2:472. doi: 10.1038/ncomms1478. [DOI] [PubMed] [Google Scholar]
- Sato T, Yokonishi T, Komeya M, et al. Testis tissue explantation cures spermatogenic failure in c-Kit ligand mutant mice. Proc Natl Acad Sci USA. 2012;109:16934–16938. doi: 10.1073/pnas.1211845109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh R, Aravindan GR, Moudgal NR. Quantitation of spermatogenesis by DNA flow cytometry: comparative study among six species of mammals. J Biosci. 1992;17:413–419. [Google Scholar]
- Steinberger A, Steinberger E. In vitro growth and development of mammalian testes. In: Johnson AD, Gomes WR, Vandemark NL, editors. The testis: biochemistry. New York: Academic Press; 1970. pp. 363–391. [Google Scholar]
- Stokes WS, Schechtman LM, Hill RN. The Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM): a review of the ICCVAM test method evaluation process and current international collaborations with the European Centre for the Validation of Alternative Methods (ECVAM) Altern Lab Anim. 2002;30(Suppl 2):23–32. doi: 10.1177/026119290203002S04. [DOI] [PubMed] [Google Scholar]
- Yokonishi T, Sato T, Katagiri K, Ogawa T. In vitro spermatogenesis using an organ culture technique. Methods Mol Biol. 2013;927:479–488. doi: 10.1007/978-1-62703-038-0_41. [DOI] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: an improved in vitro model for assessment of male reproductive toxicity. Toxicol Sci. 2009;84:378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]
- Yu X, Hong S, Moreira EG, Faustman EM. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol Appl Pharmacol. 2009;239:325–336. doi: 10.1016/j.taap.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.