Abstract
Articular cartilage lacks the ability to self-repair and a permanent solution for cartilage repair remains elusive. Hydrogel implantation is a promising technique for cartilage repair; however for the technique to be successful hydrogels must interface with the surrounding tissue. The objective of this study was to investigate the tunability of mechanical properties in a hydrogel system using a phenol-substituted polymer, tyramine-substituted hyaluronate (TA-HA), and to determine if the hydrogels could form an interface with cartilage. We hypothesized that tyramine moieties on hyaluronate could crosslink to aromatic amino acids in the cartilage extracellular matrix. Ultraviolet (UV) light and a riboflavin photosensitizer were used to create a hydrogel by tyramine self‐crosslinking. The gel mechanical properties were tuned by varying riboflavin concentration, TA-HA concentration, and UV exposure time. Hydrogels formed with a minimum of 2.5 min of UV exposure. The compressive modulus varied from 5–16 kPa. Fluorescence spectroscopy analysis found differences in dityramine content. Cyanine-3 labelled tyramide reactivity at the surface of cartilage was dependent on the presence of riboflavin and UV exposure time. Hydrogels fabricated within articular cartilage defects had increasing peak interfacial shear stress at the cartilage-hydrogel interface with increasing UV exposure time, reaching a maximum shear stress 3.5× greater than a press‐fit control. Our results found that phenol-substituted polymer/riboflavin systems can be used to fabricate hydrogels with tunable mechanical properties and can interface with the surface tissue, such as articular cartilage.
Keywords: photocrosslinking, riboflavin, hyaluronic acid, cartilage, hydrogel, biomaterials
1. Introduction
Articular cartilage was among the first targets for tissue regeneration, however due to its avascularity, complex structure, and low metabolic activity, repair and regeneration of this tissue has remained elusive.[1] Cartilage has a limited ability to self-repair, most often requiring surgical intervention to address damage to this tissue.[2–5] While small fissure, < 2 cm, focal defects in cartilage are asymptomatic, they can slowly grow in size and ultimately lead to osteoarthritis if left untreated.[3] Currently, small defects are treated using techniques such as microfracture and mosaicplasty; however, these techniques often result in the formation of fibrous tissue rather than hyaline cartilage and suffer from poor lateral integration with the surrounding native tissue. [3, 4, 6, 7] As an alternative, synthetic materials are currently being explored for cartilage repair and among these materials, hydrogels are one of the most studied.[8, 9]
Hydrogels can be biopolymer-based, such as chitosan and hyaluronic acid (HA), or synthetic, poly(vinyl alcohol) for example.[2, 5, 8–20] Hydrogels can be formed through a variety of methods and recent reviews discuss these strategies.[9, 21, 22] Injectable hydrogel systems, which can be gelled in situ, are advantageous since their applications can be minimally invasive and directly delivered to the damage site.[9] Such injectable hydrogel systems include those based on poly(ethylene glycol),[23–25] gelatin,[26, 27] chitosan,[27, 28] alginate,[29] chondroitin sulfate,[30] carboxymethylcellulose,[31] poly(vinyl alcohol),[32, 33] and HA.[34–39] While implanted materials must be biocompatible and meet the mechanical needs of the tissue,[5, 8, 9] they must also integrate with the surrounding tissue, in this case forming a cartilage-hydrogel interface. Without the formation of a stable interface upon or shortly after repair, the implanted hydrogel may loosen within the defect and eventually fail, requiring further surgical intervention.
To address the interface problem, Wang et al. designed a chondroitin sulfate adhesive for use as an adhesion interlayer between cartilage and a chondroitin sulfate hydrogel. The chondroitin sulfate adhesive was fabricated by functionalizing chondroitin sulfate with methacrylate and aldehyde moieties, which form a cross-linked network with the application of light and a photosensitizer. In addition, the adhesive reacts with amines at the cartilage tissue surface in the extracellular matrix (ECM) via Schiff-base reaction, thereby activating the cartilage surface to interface with a chondroitin sulfate hydrogel. This two-component construct formed an interface to the tissue in an in vivo rabbit model,[30] and was also used with a poly(ethylene glycol) hydrogel for cartilage repair.[40] While the use of an adhesive interlayer is unique, it requires an extra step and itself creates an additional interface that may need to be optimized. To circumvent this problem we sought to develop a polymer/photosensitizer system based on phenolic cross-coupling to simultaneously gel and interface with the tissue.
We recently reported a system utilizing tyramine-substituted sodium hyaluronate (TA-HA) with a riboflavin photosensitizer that gels when irradiated by UV light (365 nm) through the formation of dityramines (Figure 1), and demonstrated that it could be used to resurface damaged articular cartilage.[41] TA-HA hydrogels are generally formed through reaction with hydrogen peroxide and horseradish peroxidase (HRP).[36, 39, 42] The mechanical properties of TA‐HA hydrogels fabricated through this enzymatic route are tunable by changing the concentrations of HRP and hydrogen peroxide.[35] While use of HRP/H2O2 is the most common method used to crosslink TA in TA-substituted polymers, others have reported TA-hydrogel fabrication using light and tris(2,2′-bipyridyl)dichlororuthenium(II) hexahydrate, [Ru(bpy)3]Cl2. [32, 33, 43, 44] Phenols, including TA, have been incorporated into other polymeric materials, such as dextran and chitosan, and gelled using HRP/H2O2 in studies for tissue engineering applications.[42, 45–51]
Figure 1.
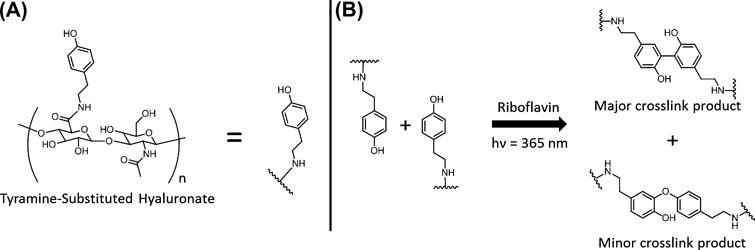
(A) Structure of TA-HA and short hand representation. (B) Crosslinking reaction showing tyramine dimerization to form the C-C bonded dityramine (major product) and the C-O bonded dityramine (minor product).
The objective of this study was to determine if TA-HA could interface with cartilage through a photochemical process initiated by riboflavin and to characterize hydrogels fabricated through this photochemical process. Tyramine-substituted polymers have the ability to react with aromatic side chains on ECM amino acid residues, such as tyrosine, and thus form a crosslink between the polymer to the tissue.[32, 33, 45] We hypothesized that tyramine moieties on hyaluronate could crosslink to aromatic amino acids in the cartilage extracellular matrix via riboflavin photoinitiated phenolic cross-coupling. We investigated how varying the hydrogel fabrication parameters (riboflavin concentration, UV exposure time, and TA-HA concentration) would affect the mechanical properties by unconfined compression testing, and characterized the dityramine crosslinks in the hydrogels using fluorescence spectroscopy. We also mechanically loaded the hydrogels to failure to determine their maximum failure stress and strain. After exploring how the synthetic parameters affected TA cross‐coupling and hydrogel properties, we examined if tyramide-labelled cyanine-3 (Cy3-TA) could react to the surface of articular cartilage in the presence of UV light. Finally, to determine if the hydrogel-cartilage interface strength could be increased with UV exposure, we performed push-out tests on TA-HA gels fabricated within articular cartilage defects, and examined the cell toxicity of the final gel constructs using chondrocytes.
2. Materials and Methods
2.1 General
Riboflavin, Safranin-O, Fast Green FCF, dimethyl sulfoxide (Sigma-Aldrich); tyramine‐substituted sodium hyaluronate (873 kDa, 5.5 % substitution, w/w; LifeCore Biomedical); 1× phosphate buffered saline (PBS) and Dublecco’s Modified Eagle Medium (DMEM, LifeTechnologies); fetal bovine serum (FBS, Atlanta Biologicals); 10% formalin, xylene (Pharmco-Aaper); paraffin (Electron Microscopy Sciences); cyanine-3 tyramide (Perkin Elmer); LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells and Penicillin-Streptomycin (ThermoFisher Scientific). All chemicals were used without further purification. Immature bovine knees were obtained from a local abattoir.
2.2 TA-HA Gel Fabrication
Riboflavin solutions were prepared by dissolution in 1× PBS at 50 μg/mL, sonicated for 30 sec at 30 W using a Misonix Sonicator 3000, and then diluted to concentrations between 6 to 35 μg/mL in 1× PBS. TA-HA was dissolved to concentrations between 1.0 and 3.0 % (w/v) in the riboflavin solutions while protected from light; this constituted the pre-gel solution. The bottom surface of hollow, cylindrical steel molds (10 mm diameter × 12 mm) were coated with a thin layer of vacuum grease and placed onto a clean glass slide; 500 μL of pre-gel solution was pipetted into the molds. The slide was placed on top of the window of a UV light source (4 W, 365 nm) and exposed to UV for 2.5 to 120 min. After exposure the gels were removed and placed into 1× PBS overnight, Figure 2. TA-HA gels for load to failure and cytocompatibility testing were fabricated under the same conditions in cylindrical glass molds (6 mm diameter × 8 mm) with 20 min of UV exposure.
Figure 2.
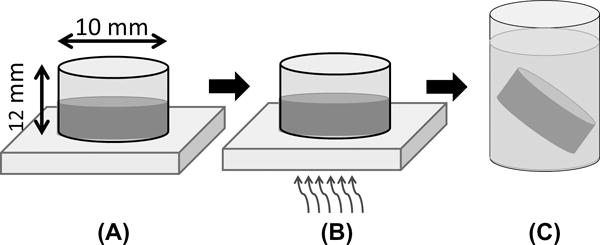
Fabrication of TA-HA hydrogels. (A) Pre-gel solution is pipetted into hollow, cylindrical steel molds with bottoms thinly coated with vacuum grease and placed on a clean glass slide; (B) the pre-gel solution is irradiated with UV light from the bottom through the glass slide; (C) the TA-HA hydrogels are removed and placed in 1× PBS.
2.3 Mechanical Testing
Unconfined compression testing was performed as described by Toh et al.[35] In brief, TA-HA gels (n = 3 per group) were sectioned on a sledge microtome to form flat, parallel top and bottom surfaces. The gels were then pre-loaded to 5 g with a non-porous platen (50 mm diameter) using an EnduraTEC ELF32000 (Bose Corporation) mechanical test system. The gels were compressed at 0.5% strain/sec to 30 % strain and the load recorded using a 250 g load cell (Model WMCP‐250G-567, Bose Corporation). The compressive modulus was calculated as the linear slope of the stress-strain curve between 20–30 % strain. Load to failure testing was performed on TA-HA gels (n = 3 per group) using this protocol with strain continually applied until gel failure, defined as the maximum stress.
2.4 Fluorescence Spectroscopy
Dissolved salts within the fabricated TA-HA gels were removed by soaking the hydrogels in water with gentle rocking for 2 days; the water was changed at least 6 times. The gels were frozen to −80 °C and then lyophilized for 2 weeks. Samples were prepared by homogenizing 12 mg of dry, salt-free TA-HA gel (approximately 2–3 whole gels) in 3 mL of 1× PBS on the highest setting for 30 sec with an Omni TH homogenizer; this yielded a homogeneous, opaque solution. Emission spectra (200–800 nm) were collected using a Perkin-Elmer LS55 fluorescence spectrometer: excitation = 280 nm, slit width = 3.5 nm, average of 10 scans at 500 nm/min; this experiment was performed in duplicate. Gaussian curves were fit to the emission peaks and integrated using OriginLab 8.5 to calculate the fraction of total peak area for tyramine and dityramine within the homogenized hydrogel samples.
2.5 Tyramide-Labelled Cyanine-3 Crosslinking with Articular Cartilage
Articular cartilage was harvested from immature bovine knees, incubated overnight in DMEM/10 % fetal bovine serum, and cut into discs (10 mm diameter × 2.5 mm thick). Cy3-TA was dissolved in DMSO according to the manufacturer’s specifications. Dilutions were made (1:50) in 1× PBS and 50 μg/mL riboflavin in 1× PBS, the former serving as a control and the latter as the experimental group. The cartilage explants were placed on a clean glass slide, 20 μL of the Cy3-TA experimental solutions applied to the surface, and immediately exposed to UV light (365 nm, 10 mW/cm2) for 5–20 min from the top using an EFOS Novacure system. The controls were exposed to UV light for 20 min; these included Cy3-TA (without riboflavin), 50 μg/mL riboflavin in 1× PBS, and 1× PBS only. Three explants were tested for each group. The irradiated explants were placed in 1× PBS and protected from light. The explants were washed with 1× PBS while being agitated on a rocking table for 4 days to remove unreacted Cy3-TA before analysis.
The explant surfaces exposed to UV were visualized by fluorescence microscopy to quantify the intensity of Cy3 brightness. All surfaces were digitally imaged with the same exposure time and analyzed using the same brightness scale (0–255). ImageJ was used to find the average brightness of each explant based on the methods used by Moreira Teixeira et al.[45]
2.6 Interfacial Shear Testing
Articular cartilage was harvested from immature bovine knees and incubated overnight in DMEM/10 % FBS. The cartilage was cut into discs 10 mm diameter × 2.5 mm thick based on a study by Ng et al.[12] A 3.5 mm diameter defect was created in the center of the cartilage explants with a biopsy punch. A glass microscope slide was coated with a thin layer of vacuum grease and the explants placed on the slide. The defect was filled with approximately 25 μL of pre-gel solution (1% TA-HA, 50 μg/mL riboflavin) and allowed to rest for 5 min to provide ample time for the pre-gel solution to coat and potentially penetrate the surface of the explant. The TA-HA was then exposed to UV light (365 nm, 10 mW/cm2) for 5–20 min, light was directed at the gel from the top (not through the slide/grease) using an EFOS Novacure system. The explants were removed from the slide and placed in 1× PBS for 2 hr before mechanical testing. Control samples were prepared by press-fitting a pre-formed 4 mm diameter TA-HA gel into the 3.5 mm hole.
The interfacial shear strength between the TA-HA gel and the cartilage explant was measured by pushing the gels out of the explants using a 2.75 mm flat-bottom, non-porous indenter at a constant displacement rate of 10 μm/s; load was recorded using a 250 g load cell (Model WMCP-250G-567, Bose Corporation) on an EnduraTEC ELF32000 (Bose Corporation). The interface shear strength was calculated by dividing the maximum force by the contact surface area between the TA-HA gel and cartilage explant. After measuring the interfacial shear strength, the gels and explants were placed in 10% formalin for histological analysis. At least seven explants were tested for each group.
2.7 Histological Analysis
Explants were removed from 10% formalin after 3 days of fixation and washed in deionized water for 1 day before dehydrating the explants through a series of ethanol washes, 70% (v/v) to 100% (v/v) in deionized water. After ethanol dehydration the explants were washed in xylene and finally embedded in molten paraffin. Explants were longitudinally cut in half, embedded cut‐face down in paraffin, and 5 μm slices were removed and stained using Saffranin‐O/Fast Green for negatively charged moieties.
2.8 TA-HA Hydrogel Toxicity towards Chondrocytes
Immortalized TC-28a2 chondrocytes[52] were cultured and maintained in DMEM/10% FBS and 1% Penicillin-Streptomycin. TA-HA hydrogels (6 mm diameter) were prepared and placed in 1× PBS with 1% Penicillin-Streptomycin over night after fabrication to reduce the risk of infection when introduced to the cells. TC-28a2 cells were seeded into wells of a 24-well plate at 20,000 cells/cm2 and allowed to attach and proliferate overnight, next TA-HA hydrogels were placed on top of the attached TC-28a2 cells; the total volume of media in the wells was 1.5 mL. Cells were cultured without hydrogels on top to serve as controls. The cells and hydrogels were placed into a 37°C incubator and groups were removed for after 1,3, and 7 days when the hydrogels were removed and the cell were stained using a LIVE/DEAD® Viability/Cytotoxicity kit following the manufacturer’s protocol. The cells were imaged using a fluorescence microscope and the percentage of live cells was calculated as the number of live cells divided by the total number of cells in each micrograph (6 micrographs/group) using ImageJ. All groups were evaluated in duplicate.
2.9 Statistical Analysis
Data was statistically analyzed using one-way analysis of variance (ANOVA) with Holm‐Sidak post-hoc testing and regression analysis at the α = 0.05 significance level.
3. Results and Discussion
3.1 Characterization and Mechanical Properties of TA-HA Hydrogels
Irradiation of TA-HA in the presence of riboflavin results in the formation of hydrogels through dityramine crosslinks (Figure 1). The mechanism presumably follows phenolic radical dimerization as in the HRP/H2O2 system, where more stable ring residing phenoxy radicals dimerize to form the C-C bonded dimer and a ring residing radical dimerizes with an oxygen residing radical to form the less common C-O dimer.[36, 37, 42, 53–55] While it has been suggested that other photosensitizers, that operate through a similar mechanism, can be used to photodynamically generate dityramine only riboflavin is considered in this work.[53]
We explored the effect of three treatment variables in the TA-HA gel fabrication process on the mechanical properties of the resulting hydrogels: riboflavin concentration, UV exposure time, and TA-HA concentration. TA-HA gels formed from a 2.5% TA-HA pre-gel solution after 20 min of UV exposure with riboflavin concentrations ranging from 6 to 50 μg/mL had a compressive modulus ranging from 5.1 ± 1.6 kPa to 11.5 ± 2.0 kPa (Figure 3A). There was no statistical difference for the compressive modulus between the 25, 35, and 50 μg/mL riboflavin groups. These data fit, with statistical significance, a saturation regression model, further supporting that the mechanical properties were not different after 25 μg/mL riboflavin in the pre-gel solution (see supplemental information (SI) Figure S1). Concentrations below 6 μg/mL riboflavin did not result in gel formation for a 2.5% TA-HA solution within 20 min.
Figure 3.
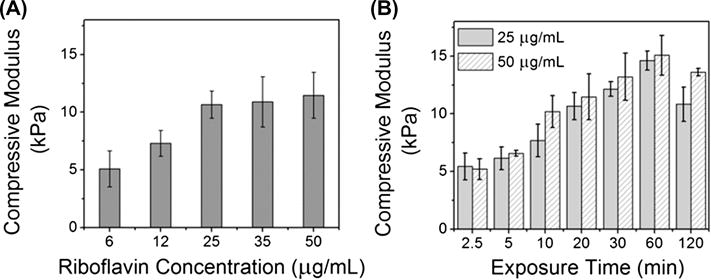
(A) Compressive modulus of TA-HA gels fabricated from a 2.5% TA-HA solution with varying riboflavin concentration after 20 min UV exposure. (B) Compressive modulus of TA-HA gels fabricated from a 2.5% TA-HA solution with 25 μg/mL (solid bars) and 50 μg/mL (patterned bars) riboflavin after various UV exposure times. Bars represent the mean moduli and error bars ± 1standard deviation (n = 3).
The effect of UV exposure time on the compressive modulus was studied by fabricating 2.5% TA-HA gels over 2.5 to 120 min of UV exposure (Figure 3B); gels were fabricated using 25 μg/mL and 50 μg/mL riboflavin. After 2.5 min of UV exposure, gels made with both concentrations of riboflavin had a compressive modulus of 5.4 ± 1.2 kPa for 25 μg/mL riboflavin and 5.2 ± 0.9 kPa for 50 μg/mL riboflavin. This result corroborates the data from Figure 3A in that both riboflavin concentrations yielded gels with similar mechanical properties. As the UV exposure time increased the compressive modulus increased, and a maximum compressive modulus of about 15 kPa was reached at 60 min. Linear regression analysis from 2.5–60 min found that the increase in mechanical properties was statistically significant with respect to UV exposure time and that the fits for both riboflavin concentrations were not different from each other (SI Figure S2). As compared to 60 min UV exposure, exposure to UV for 120 min resulted in a decrease in the compressive modulus from 14.6 ± 0.8 kPa to 10.8 ± 1.5 kPa for the 25 μg/mL riboflavin group. We attributed this observation to degradation of HA by UV light, which has been previously reported;[56] and indeed photobleaching of riboflavin was visually observed after 20 min of UV exposure, which became more pronounced with time. The 50 μg/mL riboflavin group also showed a decrease in compressive modulus from 60 min to 120 min UV exposure but to a lesser extent compared to the 25 μg/mL group, 15.1 ± 1.7 kPa to 13.6 ± 0.3 kPa. This observation is attributed to the difference in riboflavin concentration between the groups: the greater concentration of riboflavin may be acting sacrificially; attenuating the decrease in compressive properties.
TA-HA gels from the UV exposure time analysis were used to make samples for spectrofluorometric analysis. Figure 4 shows the emission spectra for the 5 and 60 min UV exposure TA-HA gels with 50 μg/mL riboflavin (see SI Figure S3 for the 25 μg/mL riboflavin group). Tyramine has two characteristic emission peaks centered around 306 nm and 605 nm (left and right peaks in Figure 4, see SI Figure S4 for a tyramine emission spectrum).[53] The sharp peaks at 280 nm and 560 nm are artefactual due to scattering and second‐order transmission, respectively, and were not included in peak area analysis. The formation of dityramine results in a new, broad emission peak centered around 410 nm.[36, 53] It can be seen in Figure 4 that as UV exposure time increased the total peak area of the tyramine emissions decreased relative to the dityramine peak, which grew slightly. The fraction of total peak area for tyramine, the sum of emission peaks at 306 nm and 605 nm over total area, decreased from 0.66 ± 0.03 at 5 min UV exposure to 0.48 ± 0.04 at 60 min; an increase in dityramine total area fraction was also observed from 0.34 ± 0.03 to 0.52 ± 0.04. We have also shown in model reactions with free tyramine that increasing UV exposure time in the presence of riboflavin increases the yield of dityramine.[41] Taken together these data support the formation of more dityramine crosslinks within the TA-HA gel as UV exposure time increases, resulting in a greater compressive modulus as observed in Figure 3B. No direct comparisons are implied from this analysis since tyramine and dityramine do not have equal molecular extinction coefficients at 280 nm. In addition, due to the homogenization process to prepare the samples, the amount of dissolved solute in each sample is likely unequal, thus resulting in the total peak area also being unequal. However, relative comparisons between the fractions of total peak areas are valid.
Figure 4.
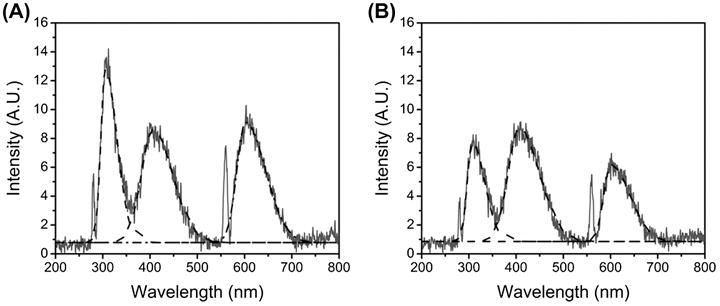
Emission spectra of 2.5% TA-HA gels fabricated with 50 μg/mL riboflavin after 5 min UV exposure(A) and 60 min UV exposure(B). Best fit curves used for peak area calculations are shown for tyramine (right and left) and dityramine (center) as dashed lines.
The fraction of the total peak area for tyramine and dityramine was calculated from the emission spectra to compare the relative extent of dityramine formation as a function of UV exposure time (Figure 5). The fraction of dityramine increased with increasing UV exposure time for both riboflavin concentrations. After 5 min of UV exposure the 25 and 50 μg/mL riboflavin samples had dityramine fractions of 0.25 ± 0.09 and 0.34 ± 0.03, respectively, which increased with increasing UV exposure time. A concomitant drop in tyramine fraction was also observed with time. These trends were found to be statistically significant, and there was no difference found between riboflavin concentrations (SI Figure S5). These results corroborate compressive modulus data in Figure 3B since the formation of more dityramine suggests more crosslinks within the hydrogel and therefore greater compressive modulus. Further, plotting compressive modulus versus dityramine fraction resulted in a linear increase that was statistically significant (SI Figure S6). After 120 min the dityramine fraction was 0.50 ± 0.03 and 0.54 ± 0.02 for the 25 μg/mL and 50 μg/mL riboflavin concentrations, respectively, suggesting that the loss in mechanical compressive modulus after 60 min of UV exposure (Figure 3B) was mainly due to HA degradation, which was expected as UV irradiation has been shown to degrade HA.[56]
Figure 5.

Fraction of total peak area for tyramine (black) and dityramine (grey) from fluorospectroscopic measurements of 2.5% TA-HA gels fabricated with 25 (solid lines and squares) and 50 (dashed lines and circles) μg/mL riboflavin with increasing UV exposure time (n = 2, error bars ± 1 standard deviation).
TA-HA concentration was varied to determine its effect on hydrogel mechanical properties. TA-HA hydrogels were fabricated from a pre-gel solution of 1.0 % to 3.0 % TA-HA and 25 μg/mL or 50 μg/mL riboflavin with 20 min UV exposure (Figure 6). The greatest compressive modulus of 16.1 ± 2.7 kPa was found for 1.0% TA-HA gels and 50 μg/mL riboflavin. The 3.0% TA-HA group had the lowest modulus. The decrease in compressive modulus with increasing TA-HA concentration was observed for both concentrations of riboflavin. Linear regression analysis found the trend to be statistically significant with no difference between the concentrations (SI Figure S7). The trend of decreasing compressive modulus with increasing to TA-HA concentration was attributed differences in dityramine content measured by fluorescence spectroscopy.
Figure 6.
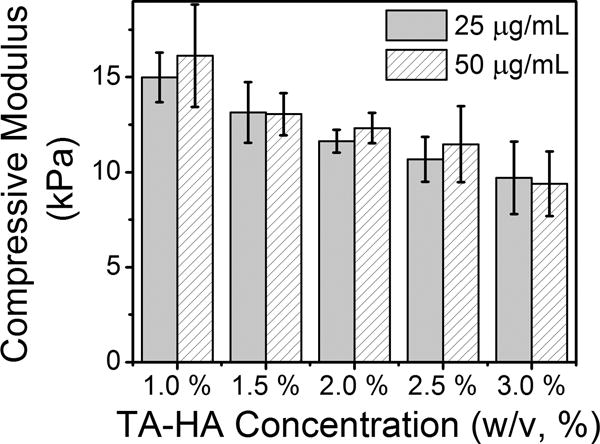
Compressive modulus of 1.0–3.0% TA‐HA hydrogels fabricated with 25 μg/mL (solid bars) and 50 μg/mL (patterned bars) riboflavin after 20 min UV exposure. Bars represent the mean moduli and error bars ± 1standard deviation (n = 3).
The fraction of tyramine and dityramine within the 1% and 3% TA-HA gels was also different: 0.46 ± 0.06 tyramine and 0.54 ± 0.06 dityramine in the 1% gels; and 0.67 ± 0.03 tyramine and 0.33 ± 0.03 dityramine in the 3% gels (analysis performed in duplicate using fluorescence spectroscopy). It would appear counterintuitive that increasing the amount of tyramine would not result in an increased yield of dityramine and therefore a gel with greater compressive modulus; however, the reaction conditions did not change, i.e. riboflavin concentration and UV exposure time. The number of crosslinks formed in the 1% and 3% TA-HA gels studied in Figure 6 depends on UV exposure time and not on the initial amount of tyramine. Longer UV exposure would result in a gel with a greater fraction of dityramine, and therefore a gel with a greater compressive modulus, as observed in Figure 3B for the 2.5% gels. The fluorescence measurements of tyramine and dityramine found that 3% gels were less crosslinked (smaller dityramine fraction) than the 1% gels, explaining the decrease in mechanical properties.
To determine gel failure stress and strain, we fabricated 6 mm diameter 1%–3% TA-HA hydrogels with 20 mins of UV exposure and loaded them in compression to failure at 0.5% strain/sec. All of the hydrogels failed between 72–76% strain; the 1% hydrogels had the highest fail stress of 40.6 ± 4.2 kPa and 41.7 ± 2.1 kPa for 25 and 50 μg/mL riboflavin, respectively. The failure stress decreased as TA-HA concentration increased to 29.1 ± 2.4 kPa and 28.6 ± 8.5 kPa for 3% hydrogels fabricated with 25 and 50 μg/mL riboflavin, respectively (see SI Figure S8 for failure stress versus TA-HA concentration, and Figure S9 for representative stress-strain failure curves, and Table S1 for summary data). This trend is similar to that observed in Figure 6 for the compressive moduli.
The mechanical properties of TA-HA gels fabricated using the HRP/H2O2 system can also be modulated by varying the amount of HRP and/or H2O2 in the pre-gel mixture. In a study by Toh et al.[35] 2.0% TA-HA gels had a compressive modulus that varied between 5.4 to 11.8 kPa after 30 min of reaction by varying the concentration of H2O2 in the fabrication solutions with a constant HRP concentration. The TA-HA gel mechanical properties described herein using the riboflavin photochemical system achieved and exceeded those described by Toh et al. in less than 30 min of UV exposure time, evaluated using the same mechanical testing parameters. For example, 2.0% TA-HA gels fabricated with 25 and 50 μg/mL riboflavin and 20 min of UV exposure had an average compressive modulus of 11.6 ± 0.6 kPa and 12.3 ± 0.3 kPa, respectively. Moreover, 1% TA-HA gels fabricated with 50 μg/mL riboflavin and 20 min of UV exposure had an average compressive modulus of 16.1 ± 2.7 kPa. The riboflavin system also produced TA-HA gels with a compressive modulus of 5.44 ± 1.2 kPa in only 2.5 min of UV exposure as compared to 30 min with the enzymatic system.
3.2 TA-HA Hydrogel Cytotoxicity
The cytotoxicity of complete TA-HA hydrogels was examined by placing 1% and 3% TA-HA gels, synthesized with both 25 μg/mL and 50 μg/mL riboflavin, on top of immortalized TC-28a2 chondrocytes in monolayer cell culture. Upon placing the hydrogels on top of the chondrocytes no noticeable changes were observed over the course of the study (i.e. the pH indicator in the DMEM did not change color). Control groups were defined as chondrocytes in monolayer that did not come into contact with the hydrogels. The hydrogels were in contact with the chondrocytes for 7 days total and cell viability of examined by LIVE/DEAD® staining after 1, 3, and 7 days. After 24 hrs, > 99% of the chondrocytes were alive for all groups; at the end of the study > 98% of the chondrocytes were alive and there was no statistical difference between the control cells and the cells that were in contact with the hydrogels at any time point in the study (see SI Figure S10 for images of the cells, and SI Table S2 for a summary of viability data). The chondrocytes also proliferated similarly in all of the groups, reaching confluence by day 7.
The results of this study support that TA-HA hydrogels synthesized through riboflavin-mediated photocrosslinking are not toxic to cells (i.e. chondrocytes). However, the process of synthesizing the hydrogels using this photochemical method relies on dosing the pre-gel solution with UV light. We experimented with adding chondrocytes to the pre-gel solution and then forming hydrogels through UV light exposure to determine if the synthesis was cytotoxic. Hydrogels were successfully assembled with cells encapsulated inside, but almost all of the encapsulated cells did not survive the process (data not shown). This is not unexpected since the encapsulated cells were receiving full UV intensity and were in an environment rich with reactive oxygen species (free radicals), which catalyze the tyramine crosslinking but are also cytotoxic even in short periods of exposure.
In a study using TA-HA hydrogels to resurface articular cartilage, Grenier et al. examined cell death after TA-HA was applied to and gelled onto the surface of cartilage by UV exposure with riboflavin.[41] Some cell death was observed on the surface of the cartilage as a result of specimen preparation, but also with the highest riboflavin concentration of 0.1%, suggesting that high concentrations of riboflavin are toxic towards cell. In our study the highest concentration of riboflavin was 50 μg/mL or 0.005%. The Grenier et al. study supports that these TA-HA gels and the method to fabricate them are cytocompatible when used in this manner.[41]
3.3 Riboflavin Mediated Cy3-TA Crosslinking to Articular Cartilage
To test our hypothesis that tyramine can link to the surface of tissue through a photochemical reaction with riboflavin, we measured the reactivity of Cy3-TA toward the surface of articular cartilage with and without riboflavin. Fluorescence microscopy images of the cartilage explant surfaces after UV irradiation for all experimental and control groups are shown in Figure 7A. The fluorescence intensity increased (images were brighter) with increasing UV exposure time for the experimental groups (riboflavin + Cy3-TA) indicating more Cy3-TA is linked to the cartilage surface with time. Controls (Cy3-TA, riboflavin, and 1× PBS) all appear black in the images, indicating no reaction at the surface. Quantification of intensity images (n = 3) for the riboflavin and 1× PBS control groups was < 0.001. Although the Cy3-TA control surface appeared black in the image the average intensity was 16 ± 5 (Figure 7B). The experimental groups had an average intensity of 105 ± 9 for 5 min of UV exposure that increased to 183 ± 17 after 20 min of UV exposure. Statistical analysis found all experimental groups different from the Cy3-TA control. These data support that TA reacts with moieties present in ECM, such as tyrosine residues, and that riboflavin is needed to initiate the cross-coupling.
Figure 7.
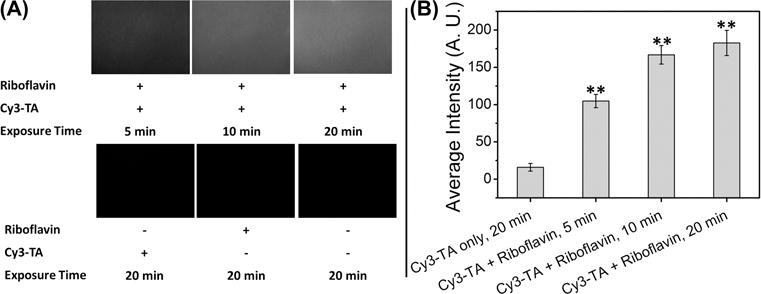
Results from the Cy3-TA crosslinking to articular cartilage experiment. (A) Representative fluorescence microscopy images for experimental groups (riboflavin + Cy3-TA; top row) and control groups (Cy3-TA only, riboflavin only, and 1× PBS; bottom row); n = 3 explant surfaces for each group. Images were taken in the center of the explant. (B) Average fluorescence intensity for the experimental and Cy3-TA articular cartilage surfaces, (0–255; 0 = pure black, 255 = pure white). The riboflavin and 1× PBS controls were not included since the average intensity was < 0.001. Bars are average intensity, error bars ± 1 standard error; (**) denotes statistical difference compared to the Cy3‐TA group by the Holm-Sidak post-hoc test following ANOVA, α = 0.05.
3.4 Interfacing TA-HA to Articular Cartilage
We tested the ability of TA-HA with riboflavin to form an immediate interface with articular cartilage following UV exposure by performing push-out tests on gels fabricated within a cartilage defect. We hypothesized that the TA-HA gel would form crosslinks between the substituted tyramines and amino acid residues, such as tyrosine, on ECM proteins within the tissue when exposed to UV light, thus increasing the interfacial peak failure stress. Press-fit TA‐HA gels were used as controls. As UV exposure time increased the average peak interfacial stress increased for all TA-HA gels fabricated within the cartilage defect; all were greater than the press-fit control. The average peak interfacial failure stress for 1% TA-HA gels at different UV exposure times is shown in Figure 8A. The greatest interfacial failure stress was found for the 20 min UV exposure group at 750 ± 140 Pa, almost 3.5× the control value of 220 ± 28 Pa. These results suggested that the TA-HA gels crosslinked to the tissue. After testing, no gel remnants were observed at the edge of the cartilage explants by visual inspection, suggesting that the shear resistance occurred at the cartilage-hydrogel interface. However, longitudinal explant-gel histological sections did show some hydrogel remnants at the interface (Figure 8B–E).
Figure 8.
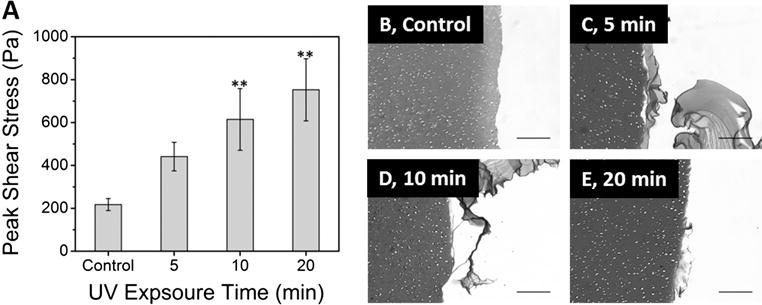
(A) Average peak failure shear stress for 1% TA-HA gelled within cartilage defects and a press-fit control. Both 10 and 20 min UV exposure times are statistically different from the control. Bars are the average (n = 7) and error bars ± standard error; (**) denotes statistical difference compared to the control group by the Holm-Sidak post-hoc test following ANOVA, α = 0.05. (B‐E) Representative histological images of the cartilage-hydrogel interface after interfacial shear stress testing. (B) press-fit control; (C) 5 min UV exposure; (D) 10 min UV exposure; (E) 20 min UV exposure. The cartilage is located on the left side of each micrograph image. Note the remnants of TA-HA gels left on the edges of the cartilage in B, C and D. See SI Figure S11 for an image of the cartilage edge that was not in contact with a TA-HA gel. Images taken at 10× (scale bar = 200 μm).
The edge of the cartilage defect in control explants did not contain residual HA, Figure 8B, indicating no integration between the TA-HA gels and surrounding cartilage. In two of four explants with TA-HA gelled within the defect at each UV exposure time, there were remnants of the gel on the cartilage surface after the gels were pushed out (Figure 8C–E). We interpret these findings as the TA-HA gel forming a stronger interface with the tissue as UV exposure time increased through the formation of more crosslinks. These data also corroborate the peak interfacial shear stress data in Figure 8A. The Cy3-TA cross-coupling data (Figure 7) also suggests greater phenolic cross-coupling with increasing UV exposure time. However, we did not observe a difference in the amount of TA-HA gel left on the cartilage surface between the groups; the 20 min UV exposure group did not have more or larger gel remnants than the 5 min group. Longer UV exposure time implies more dityramine linkages and therefore a stronger bond between the two materials, which was observed as an increase in peak shear stress. However the amount of, or presence of, gel remnants does not necessarily relate to interfacial strength.
Our findings corroborate previous work by Moreira Teixeira et al.[45], where TA‐dextran with HRP/H2O2 was used for cartilage repair and micro-Raman spectroscopy was used to investigate the interface between the dextran hydrogel and cartilage. They found that collagen, polysaccharides, and amino acids, in particular tyrosine, reorganized at the interface between the hydrogel and cartilage, suggesting the formation of crosslinks between the polymer and tissue. They also applied cyanine-5 labelled tyramide to the cartilage surface with HRP/H2O2 and found that the dye indeed crosslinked to the articular cartilage.
While HRP/H2O2 gelation methods can also achieve chemical integration between polymers and tissue, an important advantage of the photochemical treatment of TA-substituted polymers is the rapid gelation time. A study by Elvin et al.[57] used gelatin with [Ru(bpy)3]Cl2 as a tissue sealant and found that it reached its maximum adhesion strength in 30 sec of light exposure. This study reported that high adhesion strength was almost instantaneously obtained using a light emitting diode lamp.[57] The mechanism of adhesion was hypothesized to be through dityrosine crosslinks, analogous to the linkages described herein. Fast gelation (< 2 min) was also reported using [Ru(bpy)3]Cl2 to crosslink materials through phenolic amino acids.[44] It should be noted that [Ru(bpy)3]Cl2 is generally used in conjugation with white or visible light, while in our study we used longwave UV. A disadvantage of using UV light is that it is known to cause damage to cells, proteins, and DNA, especially at long light exposure times.[58] We previously assessed the viability of chondrocytes within the tissue when exposed to the riboflavin/UV treatment. Almost all chondrocytes survived the UV exposure, save a few dead cells at the articular surface and in the superficial zone.[41] Our current cytocompatibility study also supported that TA-HA hydrogels are cytocompatible, but that prolonged UV exposure in an environment rich with reactive oxygen species is cytotoxic. Thus, one shortcoming for this method is that it is not appropriate for encapsulation of cells as described in this report. However, we envision this method and material to be utilized in a cell-free manner, such as resurfacing damaged soft tissue, as described by Grenier et al.[41], or to fill small cartilage defects, where cells would not be directly exposed to full UV irradiation.
While our results indicate that TA-HA gel mechanical properties can be varied via photosensitizer concentration, UV light exposure time, and TA-HA concentration, the mechanical properties of the TA-HA are not well matched for applications in high load bearing tissues. However, TA-HA may be useful for other applications, such as replacing intervertebral disc nucleus pulpous,[59] resurfacing damaged articular cartilage,[41] and sealing the interface between osteochondral grafts and host tissue to prevent edema and to enhance the integration between the graft and the host.[60] We also envision that this material or a similar TA-polymer adduct might be used for meniscal repair,[61] to fill small fissure type defects on the articular cartilage surface,[30] and to facilitate healing of bone tunnels after anterior cruciate ligament repair as a cell-free matrix.[62] Filling small fissures on the surface of articular cartilage would be advantageous since it could potentially slow the growth of these defects to delay the onset of osteoarthritis.
One limitation of our study is that mechanical testing was performed at a single strain rate. To fully characterize the viscoelastic mechanical properties of TA-HA gels for use in soft tissue applications, the rheometric properties will need to be characterized. In a study by Bian et al.[63], the storage modulus of self-crosslinking thiol substituted HA gels were measured. Gels with < 38% substitution had less than a 0.6 kPa change in the storage modulus between frequencies of 0.5 to 10 Hz. Only in gels with > 50% substitution was there large increases in the storage modulus with frequency. As the crosslinking mechanism is similar in our TA‐HA gels, we would expect similar trends in storage modulus. However, since our TA-HA was only 5.5% substituted we would not expect a significant difference in the storage modulus with frequency.
Dityrosine formation between proteins within cartilage, such as collagen, has been observed using the HRP/H2O2 system to bond TA-dextran to the tissue through micro-Raman analysis.[45] Dityrosine formation has also been observed to occur in proteins exposed to 365 nm UV light with riboflavin,[64] and in this manuscript, but also with high energy (254 nm) UV light alone, too high for use with biologics.[65] Further, riboflavin has been used to crosslink collagen, particularly in the eye to treat keratoconus.[66–68] This method relies on the same photochemistry used in this report to crosslink tyrosine residues in native collagen, and was determined to be a safe and effective method to treat keratoconus in a 1 year follow-up study.[66] While we did not directly observe the formation of collagen-collagen dityrosine crosslinks in our studies, it is likely that these protein crosslinks are forming in the tissue where both riboflavin is present and UV photons can penetrate.
The attachment of TA-HA to cartilage through protein crosslinking is beneficial since it provides fast fixation of the hydrogel to provide mechanical and functional stability.[45] In doing so, it will cause changes in protein conformation at the interface as once free protein residues are attached to a hydrogel. There would also be dityrosine formation between proteins within the cartilage since dityramine crosslinks between polymer chains are not specific towards reaction with only polymer moieties. This would create an interface between the hydrogel and tissue, as well as hydrogel-tissue stiffening. This has been examined in a theoretical study and experimentally in corneas treated with UV light and riboflavin for 30 min, resulting in an increase in mechanical stiffness.[69, 70] The use of the riboflavin/UV systems to treat damaged tissues, such as crosslinking cornea or resurfacing cartilage, would suggest that dityrosine protein crosslinks within the tissue could be considered beneficial since it would improve tissue mechanical properties and improve functionality. However, exposing healthy tissue to UV for prolonged periods of time may produce negative outcomes, as oxidative damage will occur to tissue proteins and potentially cells within the tissue.[58] A method using riboflavin and 30 min UV light exposure was successfully used on patients to treat keratoconus and was found to be safe and effective,[66] thus suggesting that careful optimization of methods can be safely used in medical applications. In addition, we have only investigated the use of riboflavin as a photosensitizer. Other photosensitizers may be more efficient and should be investigated; for example, [Ru(bpy)3]Cl2.[32, 33, 43, 44] Finally, the procedures used in our experiments were for scientific purposes only and are not optimized for use with patients.
We attributed the increase in average peak interfacial shear stress to bonding of tyramine on HA to amino acid residues on ECM proteins, such as tyrosine. However, it may be that interdigitation/entanglement of the HA polymer chains to surface polypeptide chains of the tissue contributed to the increase in the peak shear stress. Although, it would be unlikely that the increase in peak interfacial shear stress observed would be UV dose dependent if simple interdigitation/entanglement was the only mechanism of integration. Further, the Cy3-TA experiment provides more direct chemical evidence that TA can link to the surface of cartilage through a reaction photochemically initiated by riboflavin. Finally, our study only used 5.5% substituted TA-HA; increasing the percent substitution on the polymer chain should result in increased mechanical properties and may further strengthen the interface between the hydrogel and tissue since more sites on the polymer will be available to crosslink to the tissue. It would also be advantageous to investigate methods to increase available aromatic amino acid residues in order to improve the chances of forming crosslinks to phenol-substituted polymers, thus increasing the strength of the hydrogel-tissue interface further.
4. Conclusions
Injectable hydrogel systems that gel and interface directly with tissue provide a promising treatment for soft tissue repair. To this end, we formed TA-HA hydrogels by irradiation with UV light in the presence of riboflavin as a photosensitizer. The resulting TA-HA hydrogel is assembled through the formation of dityramine crosslinks, observed in the emission spectrum of the gelled materials. The hydrogels have tunable mechanical properties, with the compressive modulus varying between 5–16 kPa with increasing riboflavin concentration, TA-HA concentration, and UV exposure time. Cy3-TA was found to react with the surface of cartilage in a manner dependent on UV exposure time and dependent on the presence of riboflavin. TA-HA gels fabricated within articular cartilage defects had greater interfacial peak shear stress compared to a TA-HA gel press-fit into the defect. Histological analysis found remnants of the TA-HA gels on the cartilage after being pushed out for TA-HA gels fabricated in cartilage defects. In conclusion, our results suggest that tyramine-substituted polymer/riboflavin systems can be used to fabricate hydrogels with tunable mechanical properties and create an immediate interface between the hydrogel and tissue. This chemistry may be particularly useful for soft tissue repair applications, such as in articular cartilage.
Supplementary Material
Acknowledgments
The authors thank Dr. Adele Boskey and Prof. Jeffrey Schwartz for their support and assistance with this work, and Dr. Chitra Dahia for use of equipment. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers AR007281 (P.E.D.) and AR059203 (P.A.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Statement
Tony Chen is an option holder at Hydro-Gen LLC. Suzanne Maher is a co-founder of Hydro-Gen LLC.
References
- 1.Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–21. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21(5):303–11. doi: 10.5435/JAAOS-21-05-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomoll AH, Minas T. The quality of healing: articular cartilage. Wound Repair Regen. 2014;22(Suppl 1):30–8. doi: 10.1111/wrr.12166. [DOI] [PubMed] [Google Scholar]
- 4.Vaquero J, Forriol F. Knee chondral injuries: clinical treatment strategies and experimental models. Injury. 2012;43(6):694–705. doi: 10.1016/j.injury.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Leone G, Volpato MD, Nelli N, Lamponi S, Boanini E, Bigi A, et al. Continuous multilayered composite hydrogel as osteochondral substitute. Journal of biomedical materials research. 2014 doi: 10.1002/jbm.a.35389. Part A. [DOI] [PubMed] [Google Scholar]
- 6.Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren K, He C, Xiao C, Li G, Chen X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials. 2015;51:238–49. doi: 10.1016/j.biomaterials.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng. 2011;17(4):281–99. doi: 10.1089/ten.teb.2011.0077. Part B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balakrishnan B, Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem Rev. 2011;111(8):4453–74. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 10.Shokrgozar MA, Bonakdar S, Dehghan MM, Emami SH, Montazeri L, Azari S, et al. Biological evaluation of polyvinyl alcohol hydrogel crosslinked by polyurethane chain for cartilage tissue engineering in rabbit model. J Mater Sci Mater Med. 2013;24(10):2449–60. doi: 10.1007/s10856-013-4995-1. [DOI] [PubMed] [Google Scholar]
- 11.Scholten PM, Ng KW, Joh K, Serino LP, Warren RF, Torzilli PA, et al. A semi-degradable composite scaffold for articular cartilage defects. J Biomed Mater Res. 2011;97(1):8–15. doi: 10.1002/jbm.a.33005. Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng KW, Wanivenhaus F, Chen T, Hsu HC, Allon AA, Abrams VD, et al. A novel macroporous polyvinyl alcohol scaffold promotes chondrocyte migration and interface formation in an in vitro cartilage defect model. Tissue Eng. 2012;18(11–12):1273–81. doi: 10.1089/ten.tea.2011.0276. Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez JS, Alvarez VA. Mechanical properties of polyvinylalcohol/hydroxyapatite cryogel as potential artificial cartilage. J Mech Behav Biomed Mater. 2014;34:47–56. doi: 10.1016/j.jmbbm.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. 2010;16(8):2675–85. doi: 10.1089/ten.tea.2009.0798. Part A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levett PA, Hutmacher DW, Malda J, Klein TJ. Hyaluronic acid enhances the mechanical properties of tissue-engineered cartilage constructs. PLoS One. 2014;9(12):e113216. doi: 10.1371/journal.pone.0113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degoricija L, Bansal PN, Sontjens SH, Joshi NS, Takahashi M, Snyder B, et al. Hydrogels for osteochondral repair based on photocrosslinkable carbamate dendrimers. Biomacromolecules. 2008;9(10):2863–72. doi: 10.1021/bm800658x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoichet MS. Polymer Scaffolds for Biomaterials Applications. Macromolecules. 2010;43(2):581–91. [Google Scholar]
- 18.Allison DD, Grande-Allen KJ. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12(8):2131–40. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 19.Nimmo CM, Owen SC, Shoichet MS. Diels-Alder Click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules. 2011;12(3):824–30. doi: 10.1021/bm101446k. [DOI] [PubMed] [Google Scholar]
- 20.Oka M. Biomechanics and repair of articular cartilage. J Orthop Sci. 2001;6(5):448–56. doi: 10.1007/s007760170014. [DOI] [PubMed] [Google Scholar]
- 21.Hunt JA, Chen R, van Veen T, Bryan N. Hydrogels for tissue engineering and regenerative medicine. J Mater Chem B. 2014;2(33):5319–38. doi: 10.1039/c4tb00775a. [DOI] [PubMed] [Google Scholar]
- 22.Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–85. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 23.Hermann CD, Wilson DS, Lawrence KA, Ning X, Olivares-Navarrete R, Williams JK, et al. Rapidly polymerizing injectable click hydrogel therapy to delay bone growth in a murine re-synostosis model. Biomaterials. 2014;35(36):9698–708. doi: 10.1016/j.biomaterials.2014.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Meng H, Konst S, Sarmiento R, Rajachar R, Lee BP. Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl Mater Interfaces. 2014;6(19):16982–92. doi: 10.1021/am504566v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlichting KE, Copeland-Johnson TM, Goodman M, Lipert RJ, Prozorov T, Liu X, et al. Synthesis of a novel photopolymerized nanocomposite hydrogel for treatment of acute mechanical damage to cartilage. Acta Biomater. 2011;7(8):3094–100. doi: 10.1016/j.actbio.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JW, Kim BY, Lih E, Choi JH, Lee Y, Park KD. In situ formation of enzyme-free hydrogels via ferromagnetic microbead-assisted enzymatic cross-linking. Chem Commun. 2014;50(89):13710–3. doi: 10.1039/c4cc04436c. [DOI] [PubMed] [Google Scholar]
- 27.Han F, Yang X, Zhao J, Zhao Y, Yuan X. Photocrosslinked layered gelatin-chitosan hydrogel with graded compositions for osteochondral defect repair. J Mater Sci Mater Med. 2015;26(4):5489. doi: 10.1007/s10856-015-5489-0. [DOI] [PubMed] [Google Scholar]
- 28.Park H, Choi B, Hu J, Lee M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013;9(1):4779–86. doi: 10.1016/j.actbio.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Balakrishnan B, Joshi N, Jayakrishnan A, Banerjee R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10(8):3650–63. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nature Mater. 2007;6(5):385–92. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varma DM, Gold GT, Taub PJ, Nicoll SB. Injectable carboxymethylcellulose hydrogels for soft tissue filler applications. Acta Biomater. 2014;10(12):4996–5004. doi: 10.1016/j.actbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Lim KS, Alves MH, Poole-Warren LA, Martens PJ. Covalent incorporation of non-chemically modified gelatin into degradable PVA-tyramine hydrogels. Biomaterials. 2013;34(29):7097–105. doi: 10.1016/j.biomaterials.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Lim KS, Roberts JJ, Alves M-H, Poole-Warren LA, Martens PJ. Understanding and tailoring the degradation of PVA-tyramine hydrogels. J Appl Polym Sci. 2015;132(26) [Google Scholar]
- 34.Baier Leach J, Bivens KA, Patrick CW, Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82(5):578–89. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 35.Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33(15):3835–45. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 36.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20(1):33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 37.Lee F, Chung JE, Kurisawa M. An injectable enzymatically crosslinked hyaluronic acid-tyramine hydrogel system with independent tuning of mechanical strength and gelation rate. Soft Matter. 2008;4(4):880–7. doi: 10.1039/b719557e. [DOI] [PubMed] [Google Scholar]
- 38.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32(3):391–7. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 39.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma B, Fermanian S, Gibson M, Unterman S, Herzka DA, Cascio B, et al. Human cartilage repair with a photoreactive adhesive-hydrogel composite. Sci Transl Med. 2013;5(167):167ra6. doi: 10.1126/scitranslmed.3004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grenier S, Donnelly PE, Gittens J, Torzilli PA. Resurfacing damaged articular cartilage to restore compressive properties. J Biomech. 2015;48(1):122–9. doi: 10.1016/j.jbiomech.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1917. [DOI] [PubMed] [Google Scholar]
- 43.Lim KS, Ramaswamy Y, Roberts JJ, Alves MH, Poole-Warren LA, Martens PJ. Promoting Cell Survival and Proliferation in Degradable Poly(vinyl alcohol)-Tyramine Hydrogels. Macromol Biosci. 2015 doi: 10.1002/mabi.201500121. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y, Li Y, Qin M, Cao Y, Wang W. Photo-cross-linking approach to engineering small tyrosine-containing peptide hydrogels with enhanced mechanical stability. Langmuir. 2013;29(43):13299–306. doi: 10.1021/la4029639. [DOI] [PubMed] [Google Scholar]
- 45.Moreira Teixeira LS, Bijl S, Pully VV, Otto C, Jin R, Feijen J, et al. Self-attaching and cell-attracting in-situ forming dextran-tyramine conjugates hydrogels for arthroscopic cartilage repair. Biomaterials. 2012;33(11):3164–74. doi: 10.1016/j.biomaterials.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2544–51. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Jin R, Moreira Teixeira LS, Dijkstra PJ, Zhong Z, van Blitterswijk CA, Karperien M, et al. Enzymatically crosslinked dextran-tyramine hydrogels as injectable scaffolds for cartilage tissue engineering. Tissue Eng. 2010;16(8):2429–40. doi: 10.1089/ten.TEA.2009.0764. Part A. [DOI] [PubMed] [Google Scholar]
- 48.Correia CR, Moreira-Teixeira LS, Moroni L, Reis RL, van Blitterswijk CA, Karperien M, et al. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. 2011;17(7):717–30. doi: 10.1089/ten.tec.2010.0467. Part C. [DOI] [PubMed] [Google Scholar]
- 49.Wang LS, Du C, Chung JE, Kurisawa M. Enzymatically cross-linked gelatin-phenol hydrogels with a broader stiffness range for osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2012;8(5):1826–37. doi: 10.1016/j.actbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Qu T, Ding C, Ma C, Sun H, Li S, et al. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015;13:88–100. doi: 10.1016/j.actbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang R, Leber N, Buhl C, Verdonschot N, Dijkstra PJ, Karperien M. Cartilage adhesive and mechanical properties of enzymatically crosslinked polysaccharide tyramine conjugate hydrogels. Polym Adv Technol. 2014;25(5):568–74. [Google Scholar]
- 52.Goldring MB. Culture of immortalized chondrocytes and their use as models of chondrocyte function. Methods Mol Med. 2004;100:37–52. doi: 10.1385/1-59259-810-2:037. [DOI] [PubMed] [Google Scholar]
- 53.Kanwar R, Balasubramanian D. Structural studies on some dityrosine-cross-linked globular proteins: stability is weakened, but activity is not abolished. Biochemistry. 2000;39(48):14976–83. doi: 10.1021/bi0008579. [DOI] [PubMed] [Google Scholar]
- 54.Malencik DA, Anderson SR. Dityrosine formation in calmodulin: conditions for intermolecular cross-linking. Biochemistry. 1994;33(45):13363–72. doi: 10.1021/bi00249a024. [DOI] [PubMed] [Google Scholar]
- 55.Gross AJ, Sizer IW. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959;234(6):1611–4. [PubMed] [Google Scholar]
- 56.Lapcik L, Jr, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem Rev. 1998;98(8):2663–84. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 57.Elvin CM, Vuocolo T, Brownlee AG, Sando L, Huson MG, Liyou NE, et al. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials. 2010;31(32):8323–31. doi: 10.1016/j.biomaterials.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 58.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11(5):439–57. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 59.Silva-Correia J, Correia SI, Oliveira JM, Reis RL. Tissue engineering strategies applied in the regeneration of the human intervertebral disk. Biotechnol Adv. 2013;31(8):1514–31. doi: 10.1016/j.biotechadv.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Link TM, Mischung J, Wortler K, Burkart A, Rummeny EJ, Imhoff AB. Normal and pathological MR findings in osteochondral autografts with longitudinal follow-up. Eur Radiol. 2006;16(1):88–96. doi: 10.1007/s00330-005-2818-6. [DOI] [PubMed] [Google Scholar]
- 61.Ishimura M, Ohgushi H, Habata T, Tamai S, Fujisawa Y. Arthroscopic meniscal repair using fibrin glue. Part II: Clinical applications. Arthroscopy. 1997;13(5):558–63. doi: 10.1016/s0749-8063(97)90180-8. [DOI] [PubMed] [Google Scholar]
- 62.Soon MY, Hassan A, Hui JH, Goh JC, Lee EH. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med. 2007;35(6):962–71. doi: 10.1177/0363546507300057. [DOI] [PubMed] [Google Scholar]
- 63.Bian S, He M, Sui J, Cai H, Sun Y, Liang J, et al. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surface B. 2016;140:392–402. doi: 10.1016/j.colsurfb.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Kato Y, Uchida K, Kawakishi S. Aggregation of collagen exposed to UVA in the presence of riboflavin: a plausible role of tyrosine modification. Photochem Photobiol. 1994;59(3):343–9. doi: 10.1111/j.1751-1097.1994.tb05045.x. [DOI] [PubMed] [Google Scholar]
- 65.Kristo E, Hazizaj A, Corredig M. Structural changes imposed on whey proteins by UV irradiation in a continuous UV light reactor. J Agr Food Chem. 2012;60(24):6204–9. doi: 10.1021/jf300278k. [DOI] [PubMed] [Google Scholar]
- 66.Arbelaez MC, Sekito MB, Vidal C, Choudhury SR. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: One-year results. Oman J Ophthalmol. 2009;2(1):33–8. doi: 10.4103/0974-620X.48420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30(3):689–95. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 68.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–7. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 69.Chen YC, Chen M, Gaffney EA, Brown CP. Effect of crosslinking in cartilage-like collagen microstructures. J Mech Behav Biomed Mater. 2016;66:138–43. doi: 10.1016/j.jmbbm.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29(9):1780–5. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


