Abstract
Background
Alveolar echinococcosis (AE) is caused by the metacestode stage of Echinococcus multilocularis. The inflammatory response to this infection is influenced by the interaction of the parasite with the host. We aimed to analyze human liver lesions infected with Echinococcus multilocularis and the changes of the cellular infiltrates during albendazole (ABZ) treatment.
Methodology/Principal findings
We analyzed liver tissue samples from 8 untreated patients, 5 patients treated with two daily doses of 400 mg ABZ for up to two months and 7 patients treated for more than two months with the same ABZ therapy. A broad panel of monoclonal antibodies was used to characterize the lesion by immunohistochemistry. A change in the cellular infiltrate was observed between the different chemotherapy times. During the initial phases of treatment an increase in CD15+ granulocytes and CD68+ histocytes as well as in small particles of Echinococcus multilocularis (spems) was observed in the tissue surrounding the metacestode. Furthermore, we observed an increase in CD4+ T cells, CD20+ B cells and CD38+ plasma cells during a longer duration of treatment.
Conclusions/Significance
ABZ treatment of AE leads to morphological changes characterized by an initial, predominantly acute, inflammatory response which is gradually replaced by a response of the adaptive immune system.
Author summary
Alveolar echinococcosis (AE) is a life-threatening disease in humans caused by the larval stages of E. multilocularis. It has been shown that the infection in humans is associated with a modulated immune response. Depending on multiple factors, such as the stage of disease, total or partial surgical resection and albendazole (ABZ) therapy are treatments of choice. ABZ is known as a parasitostatic drug that has to be administered for years to suppress metacestode development. Here we compared human liver lesions before and after short and long term treatment with ABZ by immunohistochemistry using a broad panel of antibodies. We found a change in the cellular infiltrate, characterized by a shift to an infiltrate rich in T cells, B cells and plasma cells during long-term treatment with ABZ, including a pronounced detection of small particles of E. multilocularis (spems). We argue that ABZ treatment is likely to change the cellular infiltrate, leading to an enhancement of the host immune response during treatment.
Introduction
Human alveolar echinococcosis (AE), caused by the tapeworm Echinococcus multilocularis, is considered one of the most pathogenic zoonosis in humans with endemic areas in the Northern hemisphere and also in Western China [1,2]. The adult worms live in the intestine of canids, such as the red fox (Vulpes vulpes). Eggs are released into the environment through the feces of canids. In humans who accidentally ingest the eggs, the multi-vesicular metacestode shows a tumor-like growth pattern predominantly in the liver. However, the disease may spread to other organs [3]. If possible, first-line treatment is radical surgery, accompanied by treatment with benzimidazole derivatives. Lifelong treatment is necessary if the patient has non-resectable lesions.
The metacestode stage of E. multilocularis consists of a cellular germinal layer surrounded by an acellular laminated layer. The laminated layer synthesized by the germinal layer is the histological hallmark of the lesion [4]. Since the laminated layer is rich in polysaccharide protein complexes, these fragments have a high affinity to PAS staining and are well recognized on histological examination. The central core of the lesion is necrotic and may contain particles of protoscoleces and fragments of the laminated layer [5]; this zone is surrounded by a cellular infiltrate [6].
The monoclonal antibody Em2G11 is specific for the Em2 antigen of the E. multilocularis metacestode and exclusively stains the laminated layer as well as the cyst content in tissue sections. Additionally, the antibody marks acellular Em2-antigen-positive small particles of E. multilocularis (spems) inside and outside the main lesion [7]. These particles are probably shed due to the growth of the metacestode and/or the inflammatory response [8] and may play a modulatory role in the immunological process during the infection [9].
Infection with E. multilocularis in humans is characterized by modulation of the immune response, which allows the parasite to escape the immune response of the host [10],[11]. This phenomenon is reflected by changes in the cytokine profile and the T-helper cell response. During the course of inflammation, the acute inflammatory Th1 response is gradually converted into a Th2 response in mice, reflecting the chronic phase of AE [12,13].
The severity of the disease may depend on the genetic background of the host and on the acquired disturbances of the Th1-related immunity [12,14,15]. The laminated layer of the metacestode, particularly its carbohydrate components, plays a major role in the evasion of cellular and humoral immunomechanisms and, furthermore, in tolerance induction and immunomodulation [16]. The Em2 antigen is a T cell-independent antigen and the response against Em2 antigen has been shown to lack antibody maturation [9]. Moreover, in contrast to Em492 antigen, the Em2 antigen does not lead to anti-CD3 apoptosis. Em492 stimulates peritoneal macrophages to produce high levels of nitric oxide leading to an inhibition of murine splenocyte proliferation [11], therefore acting as an immunosuppressant [17].
Th2-type and anti-inflammatory cytokines, IL-10 and TGF-β, as well as nitric oxide, are involved in the maintenance of tolerance and partial inhibition of cytotoxic mechanisms [12,18]. The complex immune response during infection is characterized by an abnormal production of various interleukins, such as interleukin-5 [19,20], IL-27 [21], high levels of IL-10 and TGF-beta [18,22]. Simultaneously, insufficient production of IL-12 [23], IL-31, IL-33 [21], IFN-gamma and cytotoxic T cells leads to an enhanced tolerance towards the parasite. It has been shown that the Fibrinogen-like-protein 2 (FGL2), a CD4+CD25+ regulatory T cell effector molecule secreted by T regulatory cells, plays a crucial role in the immune response by suppressing Th1 and Th17 responses [24]. In line with this observation, mice infected with E. multilocularis eggs showed up-regulation of FGL2 in the liver [25].
Long-term treatment with ABZ has improved the 10-year survival rate in comparison with untreated historical controls from 6–25% to 80–83% [26][27].
ABZ binds to beta-tubulin and inhibits absorptive functions in E. multilocularis [28]. ABZ also binds to beta-tubulin in the human host, which is very similar with more than 90% identical amino acids between the parasite and humans [29]. Treatment results in an inhibition of metacestode proliferation, and leads to destruction of protoscoleces; it inhibits formation of the germinal layer and therefore of the metacestode [27]. ABZ treatment is regarded as parasitostatic [30], [31]; in some cases benzimidazoles show a parasitocidal action [32] in vitro.
Here, we hypothesized that treatment with ABZ may have an influence on the cellular infiltrate of E. multilocularis in infected human liver tissue. Therefore, we conducted a morphological and immunohistological analysis of 20 human liver tissue lesions (12 treated/8 untreated with ABZ) using a broad panel of antibodies to characterize the lesions.
Materials and methods
Ethics statement
The study has been approved; see: Zentrale Ethikkommission bei der Bundesärztekammer. Mitteilungen: Die (Weiter-) Verwendung von menschlichen Körpermaterialien für Zwecke medizinischer Forschung. Dtsch Arztebl. 2003: 1632.
Patients and tissue samples
Human liver tissue samples from 20 patients were retrieved from the paraffin archives of the Institute of Pathology, University of Ulm. The samples were anonymized according to German law for correct usage of archival tissue for clinical research [33]. Of the 20 liver specimens with histologically confirmed E. multilocularis infection, 8 samples are from untreated patients (patients #1–8), 5 samples from patients treated with 2 x 400 mg ABZ (Eskazole) per day for up to two months (patients #9–13) and 7 patients treated for more than two months with 2 x 400 mg ABZ (Eskazole) per day (patients # 14–20). 17 cases were resection samples and 5 cases were cutting needle biopsies with more than 90% of representative tissue of the lesion. Cutting needle biopsies were performed as a diagnostic step regarding a liver lesion of unknown significance. The clinical characteristics of the patients are shown in Table 1. The cohort was divided into three groups: group 1 = no therapy, group 2 = treatment of up to two months with a range between 4 and 37 days’ treatment, group 3: treatment of more than two months. These groups were formed on the basis of samples of patients available for the analysis.
Table 1. Characteristics of the patients.
| No. | Age at surgery | Sex | Organ/Site | Type of lesion | Material | Medication/dose | Albendazole treatment time before surgery |
|---|---|---|---|---|---|---|---|
| 1 | 82 | F | liver | 4–5 lesions, largest lesion 79x61x70mm | biopsy | / | / |
| 2 | 56 | M | liver | not specified, incidental finding during surgery inguinal hernia | partial hepatectomy | / | / |
| 3 | 65 | F | peritoneum | 5 lesions, largest lesion 34x30mm | biopsy | / | / |
| 4 | 54 | M | liver | 1 lesion, size 37x28x32mm | biopsy | / | / |
| 5 | 66 | M | liver | 1 lesion, size 96x88mm | biopsy | / | / |
| 6 | 61 | F | liver | n.a. | partial hepatectomy | / | / |
| 7 | 60 | F | liver | n.a. | partial hepatectomy | / | / |
| 8 | 47 | F | liver | n.a. | partial hepatectomy | / | / |
| 9 | 68 | F | liver | 1 lesion, size 28x32mm | partial hepatectomy | Albendazole/800 mg | 4 days |
| 10 | 63 | F | liver | 1 lesion, size 148x136mm | partial hepatectomy | Albendazole/800 mg | 19 days |
| 11 | 49 | F | liver | multiple lesions, largest lesion 80x90mm | partial hepatectomy | Albendazole/800 mg | 27 days |
| 12 | 41 | M | liver | 1 lesion, size 100x60mm | partial hepatectomy | Albendazole/800 mg | 36 days |
| 13 | 29 | F | liver | 1 lesion, size 37x34x72mm | partial hepatectomy | Albendazole/800 mg | 37 days |
| 14 | 74 | F | liver | 1 lesion, size 83x70x77mm | partial hepatectomy | Albendazole/800 mg | intermittent 3 months (total approximately 60 days) |
| 15 | 53 | M | liver | 2 lesions, largest lesion 54x40mm | partial hepatectomy | Albendazole/800 mg | 105 days |
| 16 | 47 | F | liver | 1 lesion, size 53x44mm | partial hepatectomy | Albendazole/800 mg | 125 days |
| 17 | 20 | F | liver | 3 lesions, largest lesion 45x32mm | partial hepatectomy | Albendazole, 107 days 800 mg, 57 days 400 mg | 164 days |
| 18 | 72 | M | liver | 3 lesions, largest lesion 37x30x25mm | partial hepatectomy | Mebendazole/ 1500 mg 85 days, Albendazole, 800mg 132 days | intermittent 9 months (total approximately 217 days) |
| 19 | 60 | F | liver | 2 lesions, largest lesion 66x59x40mm | partial hepatectomy | Albendazole/800 mg | 2 years |
| 20 | 48 | F | liver | 1 lesion, size 97x60x70mm | partial hepatectomy | Mebendazole, Albendazole/800 mg | intermittent 18 years |
Staining procedures
Hematoxylin and eosin (HE) and Periodic Schiff staining (PAS) staining, as well as immunohistochemistry, were performed according to standard protocols [7].
The resection specimens and biopsies were fixed in 4% buffered formaldehyde for at least 36 hours. Serial sections of about 3 μm from paraffin blocks with representative tissue were performed with a microtome. Paraffin was dissolved with xylol and ethanol.
For antigen retrieval, different pretreatment methods were used according to the companies’ recommendation. As primary antibodies, the following antibodies were used: monoclonal antibody CD3 (F7.2.38, 1:100 dilution, DAKO, Glostrup, DK), CD4 (4B12, 1:200 dilution, DAKO), CD8 (C8/144B, 1:200, DAKO), CD15 (MMA, 1:300, BD; Erembodegem, BEL), CD20 (L26, 1:500, DAKO), CD38 (SPC32, 1:100, Menarini, Florence, IT)), CD68 (PG-M1, 1:100, DAKO), eosinophil major basic protein (EMBP) antibody (BMK-13, 1:25, Zytomed Systems; Berlin, GER), Em2G11 (1:100; kind gift of Peter Deplazes, Institute of Parasitology, University of Zürich, Switzerland) and FGL2 (1:4000, Abnova; Taipeh, TW).
The primary antibody was diluted in Antibody Dilution solution (DAKO) and each slide was incubated with 50 μl in a humid chamber at room temperature for 30 min. The DAKO REAL Detection System, Alkaline Phosphatase/Red (DAKO, Carpintera, CA, USA) was used as the detection system according to the manufacturer’s protocols. As negative controls, staining was performed without the primary antibody.
The evaluation of the immunohistological stainings was carried out in a blinded fashion by three observers (TFEB; FJR; JN) at a multihead microscope. Five different high-power observation fields of one section centering on the inflammation zone between the normal liver parenchyma and the necrosis were analyzed and the stained cells counted (400x magnifications). The average for each section was calculated. Regarding the possible sample error, we stained representative sections of two different blocks of tissue of three cases with the whole antibody panel. In sections of two cutting needle biopsies, only four positions were evaluated. To measure the quantity of spems, we determined the percentage of the whole necrotic area with a typical pattern of spem staining using a 25x magnification. To register the lymphatic aggregates, we counted all lymphatic aggregates larger than 1 mm within an area of 1.2 cm in diameter.
The average and the standard deviation for each evaluation, was calculated. Furthermore, we performed a two-sided t-test type 3 with unequal variance with Excel (Microsoft Office 2007) and IBM SPSS (Statistic Version 21, IBM Corp.). The result was regarded as significant for p-values p< 0.05.
Results
Using HE staining, we first defined the microscopical parameters of the lesion. All lesions, with and without treatment with ABZ, had a central necrosis of varying diameters in common; next followed an inner circle close to the necrotic zone, characterized by epithelioid cells and granulocytes, and an outer circle with lymphocytes followed by hepatic tissue. Between the outer and the inner zone, a fibrotic layer of varying diameter was found. Of note, fragments of protoscoleces were found only once in 20 samples (case # 10).
On analysis of the different parameters of the inflammatory infiltrate, histological differences and similarities were noted.
All lesions had the following immunohistological characteristic in common: In a CD68 staining, the macrophages and epithelioid cells were highlighted in the inner zone. CD15+ granulocytes were intermingled with the CD68+ cells in the inner circle but not in the outer circle. Some positive EMBP eosinophilic granulocytes were resident in the inner zone.
The outer circle was characterized by a mixture of CD8+ and CD4+ T cells; CD8+ cells were generally more frequent than CD4+ cells. CD20+ B cells were mixed with the T cells. CD38+ plasma cells were interspersed predominantly in the outer zone.
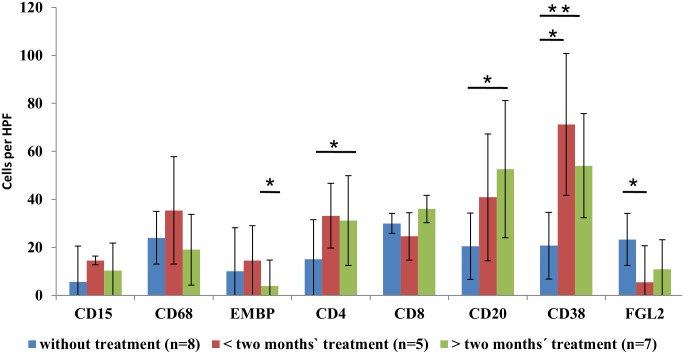
Regarding the time course of ABZ treatment, we noted differences in the composition of the cellular infiltrate, which varied in relation to the duration of treatment. In the lesions of patients treated up to two months, CD68+ and CD15+ cells were more prominent in the inner zone compared to non-treated lesions and lesions treated for over two months. With respect to T cells, CD4+ cells increased significantly and the number of CD8+ cells remained largely stable during the course of treatment. CD20+ B cells and CD38+ plasma cells increased significantly (Figs 1 and 2, S1 Table) with plasma cells outnumbering B cells. Along with the increased number of T and B cells, we noted an increase in lymph follicles larger than 1 mm consisting of CD4+/CD8+ T cells and CD20+ B cells. Small particles of E. multilocularis (spems) increased during the course of treatment. Spem staining was most prominent during the initial treatment phase up to two months (Fig 1). Spems were detected in the necrotic area, in sinusoids, vessels and lymph follicles around the lesion. In contrast, FGL2+ cells showed a tendency to decrease under therapy, being lowest in patients having a short duration of treatment (Fig 1).
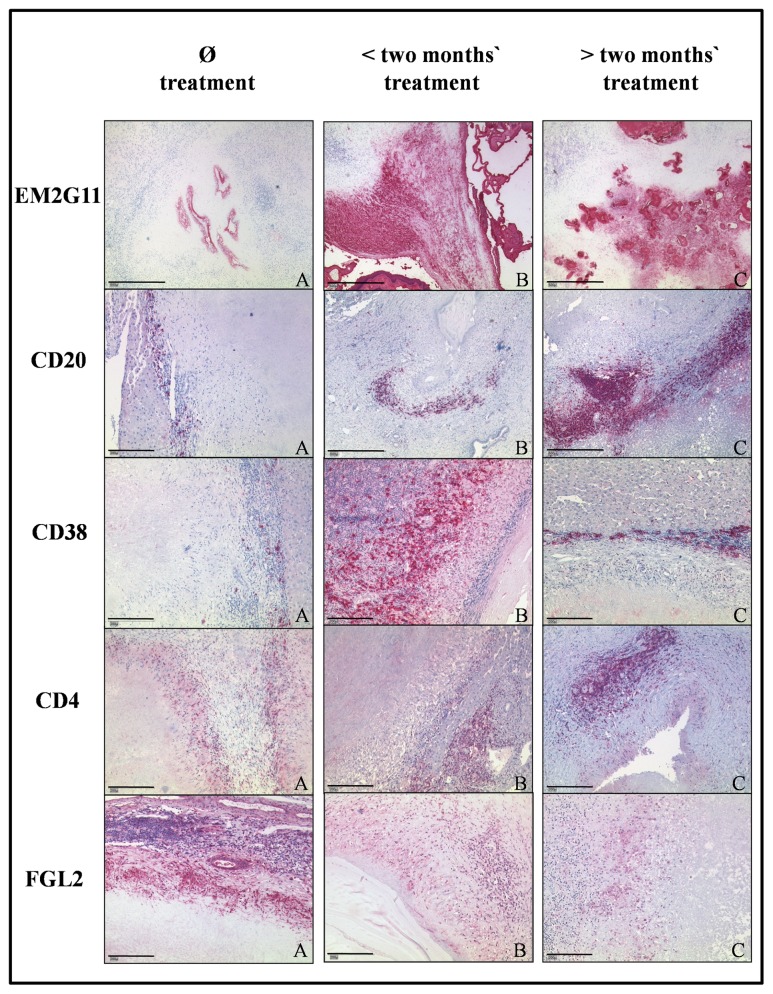
Fig 1. Immunohistochemical analysis of sections of liver tissue from patients with E. multilocularis infection without ABZ treatment (n = 8), < two months’ (n = 5) and > two months’ treatment with ABZ (n = 7).
EM2G11 staining shows an increase in spems from sections without treatment as compared to stainings of samples with treatment up to two months and a decrease in > two months’ treatment. (A: Case #3, B: Case #11, C: Case #19, bar = 500μm) CD20 staining illustrates an increase in B cells during treatment. (A: Case #7, B: Case #12, C: Case #19, bar = A: 200μm, B, C: 500μm) CD38 staining shows an increase in plasma cell content with a maximum in < two months of ABZ treatment. (A: Case #6, B: Case #9, C: Case #17, bar = 200μm) CD4 staining reveals an increase in the number of CD4+ T cells in the tissue after treatment for > two months. (A: Case #3, B: Case #13, C: Case #18, bar = 200μm) FGL2 staining shows a decrease in the number of FGL2+ cells with a minimum at < two months of treatment. (A: Case #7, B: Case #12, C: Case #18, bar = 200μm).
Fig 2. Detection values of different antigens tested without (n = 8), < two months (n = 5) and > two months of treatment (n = 7) with ABZ in human alveolar echinococcosis liver lesions (* = p < 0.05, ** = p < 0.01, HPF = High power field).
These results show that the inflammatory infiltrate changes during early and late treatment with an increase in macrophages and granulocytes during the first six weeks of treatment followed by a shift to a specific cellular response with an increase in CD4+ T cells during early response; by contrast, the number of CD8+ T cells remains stable during treatment. Furthermore, CD20+ B cells, plasma cells and lymph follicles generally increase during late treatment (Fig 3). Immunohistological analysis of sections of two different paraffin blocks from one resection specimen from the same patient showed almost identical immunohistological results. This was repeated for two samples from three patients and therefore confirmed the method. The cohorts were also analyzed with a cut-off level of one month. The results showed the same trends as described for the two-months threshold (S1 Fig).
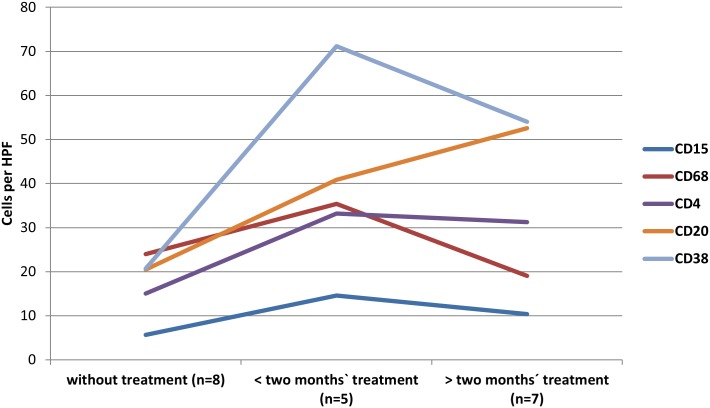
Fig 3. Summary of expression of various antigens in human liver lesions with E. multilocularis.
There is an increase in CD15+ and CD68+ cells during the first two months of treatment, which then diminishes. CD38+ and CD4+ cells increase during the first two months and then reach a plateau. CD20+ cells show a constant increase during treatment.
Discussion
To date, only very few histological/immunohistological studies characterizing the inflammatory infiltrates around the human lesions of E. multilocularis before and after treatment have been performed [34,35]. We detected a morphological spectrum of lesions caused by an infection with E. multilocularis which is characterized by irregular sized metacestodes [36] and the lamellar layer, which is the hallmark of these lesions [7]. We detected protoscolex remnants in only one sample, which is in agreement with the literature (less than 10%) [3,37]. Based on our morphological and immunohistological data, the lesion is characterized by an inner layer with a cellular composition typical of a non-specific response consisting of macrophages and granulocytes and an outer layer consisting of T and B cells.
This confirms data from mice infected with E. multilocularis, which showed an intense granulomatous infiltration in the periparasitic area of the lesions [37].
Peripheral blood mononuclear cells and polymorph nuclear granulocytes are activated after stimulation in vitro with E. multilocularis vesicles and synthesize interleukin-8 (IL-8) [38] and monocyte chemo attractant protein-1 (MCP-1) [39]. IL-8 leads to neutrophil migration and activation [40] and MCP-1 attracts and activates macrophages [41] and is an attractant for CD4+ and CD8+ T cells. These findings are reflected by our data showing that T cells are present in the lesions in situ and are increased during ABZ therapy.
In mice infected with E. multilocularis, the number of CD4+ and CD8+ cells is reduced, probably due to the diminished ability of antigen-presenting cells to present conventional antigens [42]. Furthermore, there is an elevation in CD4+ T cells in abortive or died-out lesions and active metacestodes are indicated by higher levels of CD8+ T cells [34]. ABZ acts as an intracellular tubulin inhibitor [28] and prevents metacestode formation. In mice, treatment with ABZ leads to loss of integrity in the germinal layer and a reduction in tumor mass [43]. Liance et al. [37] showed that rodents inoculated with E. multilocularis material from treated human patients have a decreased larval development in contrast to inoculation with samples from untreated patients. At a high concentration, ABZ leads to a collapse of the alveolar architecture of the parasite, partially dissolving the laminated layer followed by an invasion of the lesion with host inflammatory cells, such as histocytes, lymphocytes, neutrophils and eosinophils [44].
Reduction of the width of the laminated layer upon therapy [45] was confirmed in our study and degradation of the laminated layer may contribute to the observed increase of spems in and around the lesion, such as sinusoids, vessels and lymph follicles, which may influence the immune reaction [7]. In support of our immunohistological finding ABZ treatment has been shown to affect differentiated cells of E. multilocularis including the tegument, which is responsible for the production of the laminated layer [46]; therefore, it might be hypothesized that by this mechanism ABZ treatment leads to an increased immunohistological detection of spems.
Taken together, we found an overall increase in the number of immune cells during the course of treatment with ABZ. This effect was enhanced in the first weeks of treatment with ABZ. Our findings support the view that the non-specific immune reaction is activated at the beginning of treatment with an increase in macrophages and granulocytes, which then reduce during later treatment; our data suggest that this response is shifted towards the specific immune response, dominated by B and plasma cells which, however, do not eliminate the infection. Therefore, ABZ treatment supports the activation of the host immune system by reducing the immunosuppressive functions of the parasite. Our data suggest that, by reducing the metabolism of the metacestode during ABZ treatment and dissolution of the laminated layer, more parasite antigens are exposed and detected by the immune system and that this may lead to a more specific immune response. Supportive of this finding is that protoscoleces not protected by the laminated layer are killed by macrophages [47]. Furthermore, there are several parasite excretory/secretory products with suppressive effects on the immune system of the host [48]; by damaging the tegument, the function of these products may be reduced and may, in turn, lead to an increase in the immune response of the host.
FGL2, secreted by macrophages and T regulatory cells, leads by various mechanisms to an suppressed immune status of the host and to a progression of the metastatic growth [49]. It has been shown in mice that FGL2 suppresses the Th1 and Th17 immune response and supports the Th2 response [24]. Our finding that the FGL2 effector molecule is reduced during ABZ treatment corresponds to these observations. This indicates that treatment with ABZ may lead to a change in the immune response towards a Th1-shifted immune response by down-regulation of FGL2.
To summarize, our histological study confirms and extends findings of in vitro and in vivo studies in mice and humans infected with E. multilocularis and may help to explain the mechanism of action of ABZ during the course of treatment of patients with an initial acute inflammatory response that is gradually replaced by the adaptive immune system. The finding that spems are increased during early treatment may point to a role of spems as mediators of this inflammatory response.
Supporting information
(The mean value and in brackets the range of values).
(TIF)
(TIF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was partly supported by a grant from the German Research Foundation (DFG) to Peter Kern (Grant No. KE 282-7/8/9). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006; 55 Suppl: S187–91. 10.1016/j.parint.2005.11.028 [DOI] [PubMed] [Google Scholar]
- 2.Thompson RCA, Deplazes P, Lymbery AJ. Echinococcus and echinococcosis. London, Oxford, Cambridge, MA, San Diego, CA: Academic Press is an imprint of Elsevier; 2017. [Google Scholar]
- 3.Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996; 25: 655–689. [DOI] [PubMed] [Google Scholar]
- 4.Craig P. Echinococcus multilocularis. Curr Opin Infect Dis. 2003; 16: 437–444. 10.1097/01.qco.0000092815.64370.39 [DOI] [PubMed] [Google Scholar]
- 5.Marty AM, Johnson LK, Neafie RC. Hydatidosis (echinococcosis). Pathology of Infectious Diseases; 2000: 145–164. [Google Scholar]
- 6.Wang J, Lin R, Zhang W, Li L, Gottstein B, Blagosklonov O, et al. Transcriptional profiles of cytokine/chemokine factors of immune cell-homing to the parasitic lesions: a comprehensive one-year course study in the liver of E. multilocularis-infected mice. PLoS ONE. 2014; 9: e91638 10.1371/journal.pone.0091638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth, Thomas F E, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K, et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis. 2012; 6: e1877 10.1371/journal.pntd.0001877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz Á, Fernández C, Pittini Á, Seoane PI, Allen JE, Casaravilla C. The laminated layer: Recent advances and insights into Echinococcus biology and evolution. Exp Parasitol. 2015; 158: 23–30. 10.1016/j.exppara.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Dai WJ, Hemphill A, Waldvogel A, Ingold K, Deplazes P, Mossmann H, et al. Major carbohydrate antigen of Echinococcus multilocularis induces an immunoglobulin G response independent of alphabeta+ CD4+ T cells. Infect Immun. 2001; 69: 6074–6083. 10.1128/IAI.69.10.6074-6083.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuitton DA, Zhang SL, Yang Y, Godot V, Beurton I, Mantion G, et al. Survival strategy of Echinococcus multilocularis in the human host. Parasitol Int. 2006; 55 Suppl: S51–5. 10.1016/j.parint.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 11.Gottstein B, Hemphill A. Echinococcus multilocularis: the parasite-host interplay. Exp Parasitol. 2008; 119: 447–452. 10.1016/j.exppara.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Vuitton DA, Gottstein B. Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. J Biomed Biotechnol. 2010; 2010: 923193 10.1155/2010/923193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery I, Liance M, Deriaud E, Vuitton DA, Houin R, Leclerc C. Characterization of T-cell immune responses of Echinococcus multilocularis-infected C57BL/6J mice. Parasite Immunol. 1996; 18: 463–472. [DOI] [PubMed] [Google Scholar]
- 14.Godot V, Harraga S, Beurton I, Tiberghien P, Sarciron E, Gottstein B, et al. Resistance/susceptibility to Echinococcus multilocularis infection and cytokine profile in humans. II. Influence of the HLA B8, DR3, DQ2 haplotype. Clin Exp Immunol. 2000; 121: 491–498. 10.1046/j.1365-2249.2000.01309.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eiermann TH, Bettens F, Tiberghien P, Schmitz K, Beurton I, Bresson-Hadni S, et al. HLA and alveolar echinococcosis. Tissue Antigens. 1998; 52: 124–129. [DOI] [PubMed] [Google Scholar]
- 16.Gottstein B, Felleisen R. Protective immune mechanisms against the metacestode of Echinococcus multilocularis. Parasitol Today (Regul Ed). 1995; 11: 320–326. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y. Strategies of Echinococcus species responses to immune attacks: implications for therapeutic tool development. Int Immunopharmacol. 2013; 17: 495–501. 10.1016/j.intimp.2013.07.022 [DOI] [PubMed] [Google Scholar]
- 18.Kilwinski J, Jenne L, Jellen-Ritter A, Radloff P, Flick W, Kern P. T lymphocyte cytokine profile at a single cell level in alveolar Echinococcosis. Cytokine. 1999; 11: 373–381. 10.1006/cyto.1998.0432 [DOI] [PubMed] [Google Scholar]
- 19.Sturm D, Menzel J, Gottstein B, Kern P. Interleukin-5 is the predominant cytokine produced by peripheral blood mononuclear cells in alveolar echinococcosis. Infect Immun. 1995; 63: 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenne L, Kilwinski J, Scheffold W, Kern P. IL-5 expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin Exp Immunol. 1997; 109: 90–97. 10.1046/j.1365-2249.1997.4031299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Grüner B, Lechner CJ, Kern P, Soboslay PT. Distinctive cytokine, chemokine, and antibody responses in Echinococcus multilocularis-infected patients with cured, stable, or progressive disease. Med Microbiol Immunol. 2014; 203: 185–193. 10.1007/s00430-014-0331-8 [DOI] [PubMed] [Google Scholar]
- 22.Vuitton DA. The ambiguous role of immunity in echinococcosis: protection of the host or of the parasite. Acta Trop. 2003; 85: 119–132. 10.1016/S0001-706X(02)00230-9 [DOI] [PubMed] [Google Scholar]
- 23.Hübner MP, Manfras BJ, Margos MC, Eiffler D, Hoffmann WH, Schulz-Key H, et al. Echinococcus multilocularis metacestodes modulate cellular cytokine and chemokine release by peripheral blood mononuclear cells in alveolar echinococcosis patients. Clin Exp Immunol. 2006; 145: 243–251. 10.1111/j.1365-2249.2006.03142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Vuitton DA, Müller N, Hemphill A, Spiliotis M, Blagosklonov O, et al. Deletion of Fibrinogen-like Protein 2 (FGL-2), a Novel CD4+ CD25+ Treg Effector Molecule, Leads to Improved Control of Echinococcus multilocularis Infection in Mice. PLoS Negl Trop Dis. 2015; 9: e0003755 10.1371/journal.pntd.0003755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottstein B, Wittwer M, Schild M, Merli M, Leib SL, Müller N, et al. Hepatic gene expression profile in mice perorally infected with Echinococcus multilocularis eggs. PLoS ONE. 2010; 5: e9779 10.1371/journal.pone.0009779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010; 114: 1–16. 10.1016/j.actatropica.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ. 1996; 74: 231–242. [PMC free article] [PubMed] [Google Scholar]
- 28.Dayan A. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003; 86: 141–159. 10.1016/S0001-706X(03)00031-7 [DOI] [PubMed] [Google Scholar]
- 29.Brehm K, Kronthaler K, Jura H, Frosch M. Cloning and characterization of beta-tubulin genes from Echinococcus multilocularis. Mol Biochem Parasitol. 2000; 107: 297–302. [DOI] [PubMed] [Google Scholar]
- 30.Hemphill A, Stadelmann B, Rufener R, Spiliotis M, Boubaker G, Müller J, et al. Treatment of echinococcosis: albendazole and mebendazole—what else. Parasite. 2014; 21: 70 10.1051/parasite/2014073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuter S, Merkle M, Brehm K, Kern P, Manfras B. Effect of Amphotericin B on Larval Growth of Echinococcus multilocularis. Antimicrobial Agents and Chemotherapy. 2003; 47: 620–625. 10.1128/AAC.47.2.620-625.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ammann RW, Stumpe Katrin D M, Grimm F, Deplazes P, Huber S, Bertogg K, et al. Outcome after Discontinuing Long-Term Benzimidazole Treatment in 11 Patients with Non-resectable Alveolar Echinococcosis with Negative FDG-PET/CT and Anti-EmII/3-10 Serology. PLoS Negl Trop Dis. 2015; 9: e0003964 10.1371/journal.pntd.0003964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zentrale Ethikkommission bei der Bundesärztekammer. Mitteilungen: Die (Weiter-) Verwendung von menschlichen Körpermaterialien für Zwecke medizinischer Forschung. Dtsch Arztebl. 2003: 1632.
- 34.Vuitton DA, Bresson-Hadni S, Laroche L, Kaiserlian D, Guerret-Stocker S, Bresson JL, et al. Cellular immune response in Echinococcus multilocularis infection in humans. II. Natural killer cell activity and cell subpopulations in the blood and in the periparasitic granuloma of patients with alveolar echinococcosis. Clin Exp Immunol. 1989; 78: 67–74. [PMC free article] [PubMed] [Google Scholar]
- 35.Gillet M, Bresson-Hadni S. L'échinococcose alvéolaire hépatique. Rev Prat. 1991; 41: 1805–1811. [PubMed] [Google Scholar]
- 36.Kern P. Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis. 2010; 23: 505–512. 10.1097/QCO.0b013e32833d7516 [DOI] [PubMed] [Google Scholar]
- 37.Liance M, Bresson-Hadni S, Vuitton D, Bretagne S, Houin R. Comparison of the viability and developmental characteristics of Echinococcus multilocularis isolates from human patients in France. Int J Parasitol. 1990; 20: 83–86. [DOI] [PubMed] [Google Scholar]
- 38.Eger A, Kirch A, Manfras B, Kern P, Schulz-Key H, Soboslay PT. Pro-inflammatory (IL-1beta, IL-18) cytokines and IL-8 chemokine release by PBMC in response to Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 2003; 25: 103–105. [DOI] [PubMed] [Google Scholar]
- 39.Dreweck CM, Soboslay PT, Schulz-Key H, Gottstein B, Kern P. Cytokine and chemokine secretion by human peripheral blood cells in response to viable Echinococcus multilocularis metacestode vesicles. Parasite Immunol. 1999; 21: 433–438. [DOI] [PubMed] [Google Scholar]
- 40.Reali E, Spisani S, Gavioli R, Lanza F, Moretti S, Traniello S. IL-8 enhances antibody-dependent cellular cytotoxicity in human neutrophils. Immunol Cell Biol. 1995; 73: 234–238. 10.1038/icb.1995.38 [DOI] [PubMed] [Google Scholar]
- 41.Izumi S, Hirai K, Miyamasu M, Takahashi Y, Misaki Y, Takaishi T, et al. Expression and regulation of monocyte chemoattractant protein-1 by human eosinophils. Eur J Immunol. 1997; 27: 816–824. 10.1002/eji.1830270404 [DOI] [PubMed] [Google Scholar]
- 42.Mejri N, Gottstein B. Intraperitoneal Echinococcus multilocularis infection in C57BL/6 mice affects CD40 and B7 costimulator expression on peritoneal macrophages and impairs peritoneal T cell activation. Parasite Immunol. 2006; 28: 373–385. 10.1111/j.1365-3024.2006.00836.x [DOI] [PubMed] [Google Scholar]
- 43.Taylor DH, Morris DL, Reffin D, Richards KS. Comparison of albendazole, mebendazole and praziquantel chemotherapy of Echinococcus multilocularis in a gerbil model. Gut. 1989; 30: 1401–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abulaihaiti M, Wu X-W, Qiao L, Lv H-L, Zhang H-W, Aduwayi N, et al. Efficacy of Albendazole-Chitosan Microsphere-based Treatment for Alveolar Echinococcosis in Mice. PLoS Negl Trop Dis. 2015; 9: e0003950 10.1371/journal.pntd.0003950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Küster T, Hermann C, Hemphill A, Gottstein B, Spiliotis M. Subcutaneous infection model facilitates treatment assessment of secondary Alveolar echinococcosis in mice. PLoS Negl Trop Dis. 2013; 7: e2235 10.1371/journal.pntd.0002235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koziol U, Rauschendorfer T, Zanon Rodríguez L, Krohne G, Brehm K. The unique stem cell system of the immortal larva of the human parasite Echinococcus multilocularis. Evodevo. 2014; 5: 10 10.1186/2041-9139-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanazawa T, Asahi H, Hata H, Mochida K, Kagei N, Stadecker MJ. Arginine-dependent generation of reactive nitrogen intermediates is instrumental in the in vitro killing of protoscoleces of Echinococcus multilocularis by activated macrophages. Parasite Immunol. 1993; 15: 619–623. [DOI] [PubMed] [Google Scholar]
- 48.Nono JK, Pletinckx K, Lutz MB, Brehm K. Excretory/secretory-products of Echinococcus multilocularis larvae induce apoptosis and tolerogenic properties in dendritic cells in vitro. PLoS Negl Trop Dis. 2012; 6: e1516 10.1371/journal.pntd.0001516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Gottstein B. Immunoregulation in larval Echinococcus multilocularis infection. Parasite Immunol. 2016; 38: 182–192. 10.1111/pim.12292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(The mean value and in brackets the range of values).
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.