Abstract
Blood-brain barrier (BBB) breakdown, inflammatory and immune cell activation, and chronic cerebral hypoperfusion are features of multiple sclerosis (MS). The aim is to determine the influence of endothelin-1 (ET1) and asymmetric dimethylarginine (ADMA) on cerebral circulation time (CCT) in patients with MS. In all, 64 patients with MS (39 relapsing-remitting [RR]-MS; 25 secondary progressive [SP]-MS subtype) and 37 controls (C) were studied. Cerebral circulation time was obtained by angiography. Plasmatic ET1 and ADMA were measured by enzyme-linked immunosorbent assay. Lesion load (LL) and brain volume (BV) were obtained by magnetic resonance imaging. Cerebral circulation time was correlated to ET1, ADMA, LL, BV, disease duration (DD), and Expanded Disability Status Scale (EDSS). In MS, both ET1 and ADMA were significantly higher than C (P < .0001); CCT was approximately 2 times lower than C (P < .0001) and significantly slower in SP than in RR-MS (P = .0215). Cerebral circulation time significantly correlated with ET1 in SP-MS (r = 0.38), whereas in RR-MS CCT significantly correlated with DD (r = 0.75). The LL, BV, and EDSS did not correlate with CCT. Endothelin-1 significantly influences CCT delay in SP-MS. Diversely, CCT in RR-MS is independent of ET1 and correlates significantly with DD. We conclude that in RR-MS, DD responds to neurovascular damage accumulation. It is supposed that high ET1 and ADMA levels stem from a protective response to early insults, aimed at opposing nitric oxide overproduction, whereas persistent pathological ET1 and ADMA levels translate into detrimental long-term effects, due to increased brain micro-vessel resistance.
Keywords: ET1, ADMA, multiple sclerosis, progressive, brain circulation time
Introduction
Multiple sclerosis (MS) lesions are characterized by the breakdown of blood-brain barrier (BBB), multifocal inflammation, demyelination, oligodendrocyte loss, reactive gliosis, and axonal degeneration. Critical features of MS lesions include (a) activated immune cells from the peripheral vascular compartment through dysfunctional BBB; (b) extensive immune cell infiltration into the perivascular space, oligodendrocyte death, demyelination, and axonal damage; and (c) vascular-derived insults initiating and/or contributing to neuronal degeneration.1–4 The hypothesis is that changes in the neurovascular unit (ie, the anatomical substrate of the BBB which includes microvascular endothelium, astrocytes, pericytes, neurons, and extracellular matrix) contribute to the disruption of tight junctions and increased BBB permeability, leading to neuroinflammation, neuronal dysfunction, and damage.5,6
Accumulating evidence indicates that vascular-derived insults result in decreased cerebral perfusion in MS.7–9 The effect of this reduction has been studied extensively at the molecular and cellular levels. Briefly, it may affect (a) protein synthesis that is required for the synaptic plasticity mediating learning and memory10; (b) adenosine triphosphate synthesis, diminishing (Na++K+) adenosine triphosphatase activity and the ability of neurons to generate action potentials11; and (c) electrolyte balance and water gradients, leading to the development of edema and white matter lesions, and the accumulation of glutamate and toxins in the brain.12
Various pathophysiological mechanisms underlying the decreased cerebral perfusion in MS have been hypothesized: (1) primary vascular pathology in the context of the perivascular inflammation of MS lesions13; (2) deficiency in β2-adrenergic receptors14 compromising cerebral blood flow (CBF) by astrocytes15; (3) overproduction of vasoconstrictor molecules, such as endothelin-1 (ET1)16,17; and, finally, (4) impaired vasodilation through the inhibition of endothelium-derived relaxing factors, such as nitric oxide (NO).18
As vascular endothelium plays a key role in the local regulation of vascular tone and vascular architecture, altered production of endothelium-derived vasoconstricting factors, such as endothelin, and endothelium-derived relaxing factors, such as NO, may play an influential role in changing vascular resistance.
In fact, overproduction of ET1 has been documented in MS,16,17 which may induce severe and prolonged cerebral vasoconstriction via endothelin receptor type A.19 On the contrary, elevation of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of both constitutive (endothelial nitric oxide synthase [eNOS]) and inducible (inducible nitric oxide synthase [iNOS]) isoforms,20 is associated with impaired vasodilation.21 It is worth noting that during ischemic events, neurovascular dysfunction is characterized by the alteration of CBF, seemingly caused by the overproduction of reactive oxygen (ROS) and nitrogen species at the level of the vascular endothelium.22 The resulting NO depletion and peroxynitrite production disrupt the normal regulation of vessel tone.23 Both the depletion of NO and increased production of ROS initiate a proinflammatory trait in the vascular wall, characterized by leukocyte adhesion to the endothelium and vessel remodeling and damage.24
The main purpose of this study was to assess the relationship between brain hypoperfusion (achieved by digital subtracted angiography) and ET1 and ADMA plasma levels in MS patients with relapsing-remitting (RR) and secondary-progressive (SP) forms. Perfusion parameters can be mathematically extrapolated from many different technical approaches (computed tomography [CT], single-photon emission CT, magnetic resonance imaging [MRI] perfusion). However, the most accurate technique to obtain a direct measure of cerebral perfusion is the analysis of cerebral circulation time (CCT) of the whole brain, achieved by digital subtracted angiography (DSA). It has already been demonstrated that this perfusion parameter is consistently compromised in patients with MS.25 Animal studies by direct microscopy have shown that CCT values depend mainly on capillary flow patterns. Increased capillary transit time reduces tissue oxygen tension, leading to capillary dysfunction and then cerebral hypoperfusion.26,27
Here, the whole-brain CCT was measured in MS patients with RR and SP forms. The former is characterized by clearly defined attacks of new or increasing neurologic symptoms, followed by periods of partial or complete recovery. During remission, there is no apparent progression of the disease. Secondary-progressive form (ie, a progressive worsening of neurologic function over time) follows an initial RR form and therefore occurs as a second phase of the disease. It is unknown why people progress from RR to SP.6 Furthermore, the so called disease-modifying drugs, which make relapses happen less often and symptoms less severe, do not work in SP patients.
Materials and Methods
Subject recruitment
The proposed study has undergone the evaluation by the ethical board of the AOUS General Hospital Santa Maria alle Scotte, Siena, and written informed consent was obtained from all the subjects. The degree of patient disability has been assessed using the Expanded Disability Status Scale (EDSS), arm/hand dexterity has been tested by the nine-hole peg test (NHPT), and leg function has been tested by timed 8-meter walk test (T8), prior to the angiographic and MRI studies. Pregnant or nursing women, heavy smokers, and patients within 30 days of previous therapy have been excluded from the study. A total of 64 patients with proven MS have been included: 39 (15 men and 24 women) with RR-MS form and 25 (11 men and 14 women) with SP-MS disease. The primary-progressive MS form has been excluded from the study. A group of 37 (13 men and 24 women) age- and sex-pair-matched healthy control subjects (C) has been included in the study. In 64 patients with MS and 37 control subjects, peripheral blood samples were collected after 30 minutes of rest in a supine position from the brachial vein, and circulating ET1 and ADMA levels were measured. Cerebral circulation time was calculated in all the subjects recruited. In 25 patients with MS, MRI acquisitions were evaluated to calculate the lesion load (LL). A parallel group of 44 age- and sex-pair-matched subjects with nonrelated disease to MS was enrolled for the evaluation of CCT.
Biochemical assessment
From the same patient/subject, only 1 sample was obtained. Plasma was aliquoted in 500 μL/vial (3–4/patient) at −80°C until the measurement of ET1, ADMA, and other vasoactive factors. Residual samples are stored at −80°C for further evaluation, if required.
Plasma samples were aliquoted and stored at −80°C soon after blood collection and centrifugation at 2500 rpm for 15 minutes. Samples with evident hemolysis were discarded. Commercially available enzyme-linked immunosorbent assay kits for the measurement of vasoactive mediators (ET1, ADMA) were run according to the manufacturers’ instructions (R&D Systems, Space Import Export Srl, Milan, Italy, and Alexis, Vinci-Biochem, Vinci, Firenze, Italy, respectively). Each sample was run in duplicate, and measurements were extrapolated by standard curve performed with the reference compound. Data were expressed as picogram per milliliter and micromolar for ET1 and ADMA, respectively.
Imaging techniques
Control group and patients with MS underwent DSA of the neck and cerebral vessels.
A standardized DSA protocol (a 2-dimensional DSA acquired at a variable frame rate between 2 and 4 frames/s, with a 4 mL/s contrast injection rate via power injector) was performed. Intravascular contrast medium injection was done in the internal carotid artery. Images were analyzed to determine CCT, as previously described.25 Cerebral circulation time is the frame time between the beginning (arterial inflow) and the end (venous outflow) of the whole cerebral blood flow. Cerebral circulation time measurement on the right carotid injection was considered conventionally because no statistical difference was demonstrated between CCT of right and left internal carotid arteries. Two neuroradiologists with considerable expertise in clinical neuroimaging visually inspected all scans. Class membership of the subjects was unknown to the observers as a non-related MS disease control group. All DSAs were obtained from anteroposterior, lateral, and working view acquisitions. In case of discrepancies, a third neuroradiologist independently reviewed examinations in a blinded fashion.
As a control group, 44 age-matched patients with sine materia subarachnoid hemorrhage (mean age: 50.73 years; 10 men and 34 women), who had undergone 6 to 8 months of DSA follow-up, were retrospectively evaluated to confirm the absence of small vessel disease or other pathologic vascular patterns. In particular, these control subjects did not show pathological MRI, clinical, and DSA findings. To assume normality of cardiac output and systemic vascular resistance, all participants (patients with MS and controls) underwent a physical examination, including cardiovascular and respiratory evaluations.
Lesion loads (LL) were analyzed in a subsample of 25 patients with MS, as previously described.25 The subsample was defined on the basis of the same MRI parameters, such as intensity fields, technical approach, and image quality. Two-dimensional fluid attenuation inverse recovery images were obtained in the axial plain (echo time [TE] = 104 ms, repetition time [TR] = 9000 ms, slice thickness = 5 mm, slices = 26, matrix = 256 × 256, voxel size = 1 × 0.9 × 5, phase field of view = 87.5). Whole-brain magnetization-prepared rapid gradient-echo T1 images were obtained in the sagittal plane (TE = 3.61 ms, TR = 2400 ms, flip angle = 8°, voxel size = 1.3 mm × 1.3 mm × 1.2 mm). A neuroradiologist visually inspected all scans with relevant expertise in clinical neuroimaging.
Segmentation and volume estimation of white matter lesions have been performed using an in-house pipeline based on Statistical Parametric Mapping software (SPM, Wellcome Department of Cognitive Neurology, Institute of Neurology, University College London; www.fil.ion.ucl.ac.uk/spm/) and ad hoc scripts developed in the MATLAB scientific computing environment (www.mathworks.com; MathWorks, Natick, MA, USA). Our approach allows for lesion identification and evaluation directly in each patient’s native space, providing increased accuracy compared with those procedures implying image spatial normalization to a normative space (eg, Montreal National Institute).
Statistical analysis
The entire data set was initially analyzed by sex and by group (control vs patients). The homogeneity of the sample composition by sex between the control group and the patient group, and between the MS forms was verified through the χ2 test (with 1 degree of freedom). The assumptions of normal distribution were checked by the Shapiro-Wilk test. Accordingly, unpaired t test (with Welch correction for unequal standard deviation [SD]) or Mann-Whitney test was used to compare 2 samples. The 1-way analysis of variance test (or nonparametric Kruskal-Wallis test) was used to verify the null hypothesis of equal levels of variables. The Holm-Šidak multiple comparison was used as a post hoc test. After testing whether the values come from a Gaussian distribution, analysis of correlation by 2-tailed Pearson coefficients or nonparametric Spearman test was performed among the following variables: CCT, ET1, ADMA, disease duration (DD), brain volume (BV), LL, and EDSS.
Results
Sample composition
The sample under analysis included 64 patients with MS and 37 C subjects (13 men and 24 women) (Table 1). The composition of the MS sample by sex resulted in 40.63% of men (59.37% of women) in the control group and 35.14% of men (64.86% of women) in the patient group. The difference in the proportions was not significant: χ2 = 0.924 (degrees of freedom = 1); P = .3365.
Table 1.
Summary of relevant clinical and anagraphic data (reported as means ± SD).
The MS group composition was 39 RR-MS patients (15 men and 24 women) and 25 SP-MS patients (11 men and 14 women).
Pattern of clinical and biochemical data in patients with MS and healthy controls
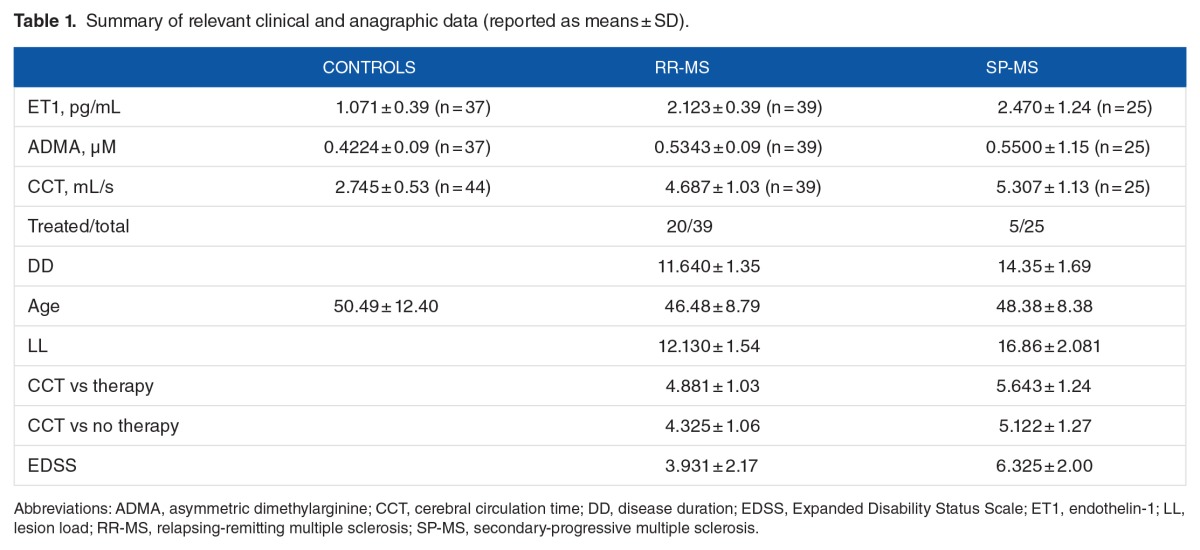
Figure 1A reports CCT values (mean ± SD) in control subjects (2.745 ± 0.53) and in RR (4.687 ± 1.03) and SP (5.307 ± 1.13) MS subgroups. Both CCT-RR and CCT-SP were significantly higher than control value (F = 72.94, P < .0001), and CCT values in SP were significantly higher than in RR subgroup (P = .0215).
Figure 1.
(A–D) Box-plot analysis of mean values of CCT, ET1, ADMA, and DD among controls and MS subgroups (RR and SP). The figure represents the distribution of CCT measured in seconds in controls and in RR-MS and SP-MS patients. The control group showed significantly lower CCT. (A) The difference of the CCT mean between MS subgroups is statistically significant (P = .0215): CCT values in SP were significantly higher than in RR subgroup. (B) Distribution of the ET1 titration is statistically different between controls and patients with MS (F = 28.8, P < .0001), whereas the Holm-Šidak post hoc test specified that there was no significant difference between RR and SP patients (P = .5729). (C) ADMA titration is significantly different between controls and patients with MS (mean 0.4224 ± 0.09 SD; F = 11.95, P < .0001), but is not significantly different (P = .6671) between the MS forms (RR mean 0.5343 ± 0.09 SD; SP mean 0.5500 ± 1.15 SD). (D) No significant difference has been demonstrated between RR-MS and SP-MS patients when DD is considered (t = 1.234, P = .222). ADMA indicates asymmetric dimethylarginine; CCT, cerebral circulation time; ET1, endothelin-1; MS, multiple sclerosis; RR, relapsing-remitting; SP, secondary-progressive.
Figure 1B shows ET1 plasma levels in control (1.071 ± 0.39) and in RR (2.123 ± 0.39) and SP (2.470 ± 1.24) subjects. The results indicated a significant difference between control and patients with MS (F = 28.8, P < .0001). The Holm-Šidak post hoc test specified that there was no significant difference between ET1 plasma levels in RR-MS and SP-MS patients (P = .5729).
Asymmetric dimethylarginine plasma levels (Figure 1C) were significantly higher (F = 11.95, P < .0001) in patients with MS (0.5343 ± 0.09 in RR; 0.5500 ± 1.15 in SP) than in control subjects (0.4224 ± 0.09). Multiple comparisons demonstrated no difference in ADMA levels between RR and SP patients (P = .6671).
Figure 1D shows DD (in years) in RR (11.640 ± 1.35) and SP (14.35 ± 1.69), and no significant difference between RR and SP mean values was observed (t = 1.234, P = .222).
Correlation analysis in control subjects and patients with MS
Analysis of the distribution of whole BV, LL, and CCT in MS subgroups has not demonstrated any difference in BV (mL) between RR (1120 ± 176.1) and SP (1227 ± 160.8) and in LL values (ml) between RR (12.130 ± 1.54) and SP (16.86 ± 2.081) (t = 1.868, P = .0847). Moreover, comparison among data of treated and nontreated RR-MS and SP-MS subgroups shows no significant differences in CCT values (F = 1.873, P = .1484). Indeed, the effect of interferon β treatment (20 RR-MS patients and 5 SP-MS patients) has been evaluated on CCT values: no significant difference (F = 1.873, P = .1484) was observed between nontreated (4.325 ± 1.06) and treated(4.881 ± 1.03) RR patients and between nontreated (5.122 ± 1.27) and treated (5.643 ± 1.24) SP patients. Considering a clinical score such as EDSS, this was significantly higher in SP (6.325 ± 2.00) than in RR (3.931 ± 2.17) patients (t = 3.791, P = .0006).
Correlation analysis among biological parameters, such as age, has not demonstrated a significant relationship with CCT, ADMA, and ET1 in C group (F = 0.063, P = .385; F = 0.773, P = .802; F = 2.974, P = .092, respectively) and in patients with MS (F = 0.480, P = .492; F = 3.007, P = .088; F = 0.278, P = .599, respectively).
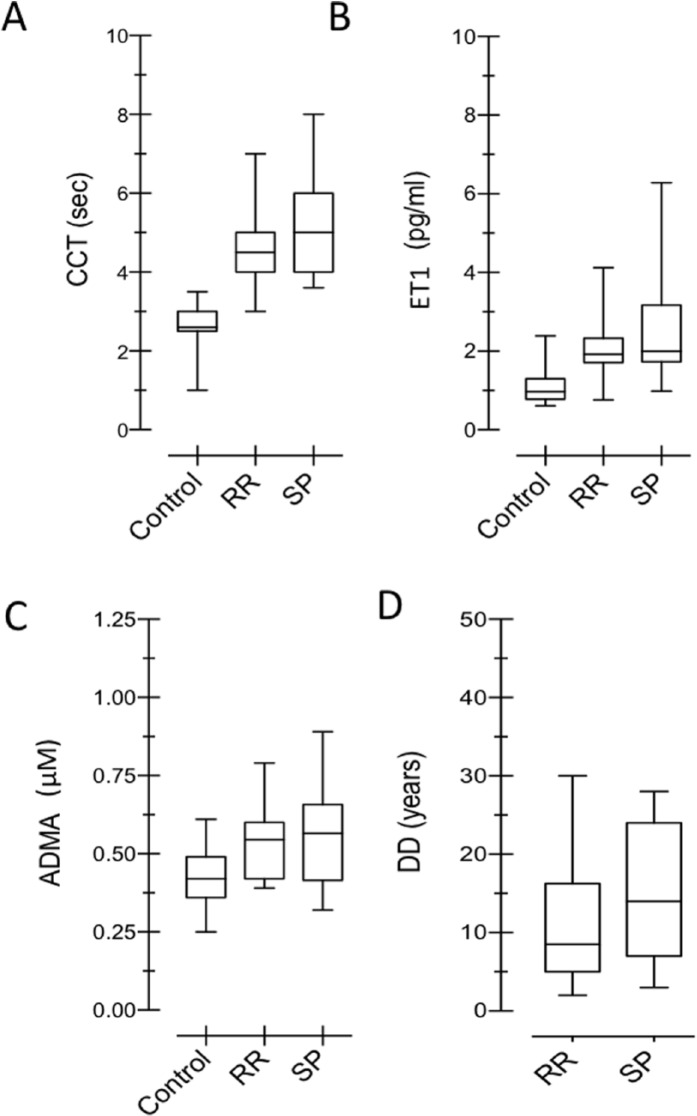
When ADMA and ET1 values were considered with respect to DD (years) in all patients with MS, no relation among these variables (RR: r = 0.0903, P = .6349; SP: r = 0.1586, P = .5042) was demonstrated in RR-MS and SP-MS patients. Therefore, ADMA and ET1 production were independent of DD (Figure 2C and D).
Figure 2.
(A–D) Correlation analysis of evaluated biological parameters in MS and controls. In (A) and (B), correlation between ET1 and ADMA in C and MS groups: no significant correlations are demonstrated between ADMA and ET1 in both groups (MS and control). Note that the slope of fitting lines for ADMA and ET1 remained about the same in C and MS subjects. In (C) and (D), correlation between DD and ADMA, DD, and ET1: DD is not significantly correlated with these vasoactive factors. Different cohorts of patients have been highlighted: RR-MS (°) and SP-MS (•). ET1 and ADMA normal line value has been introduced and time (years) in x-axis has been set. Note that there is no difference between 2 MS subgroups over time. If the higher values of ET1 and ADMA were the late expression of disease, we should have demonstrated increasing values over the course of the disease. ADMA indicates asymmetric dimethylarginine; DD, disease duration; ET1, endothelin-1; LL, lesion load; MS, multiple sclerosis; RR, relapsing-remitting; SP-MS, secondary-progressive.
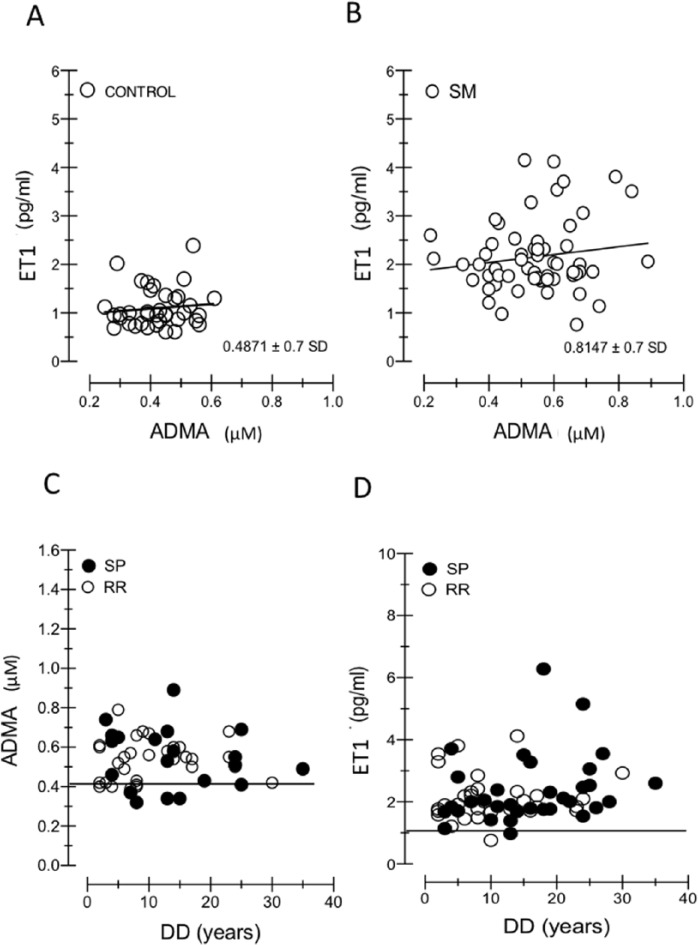
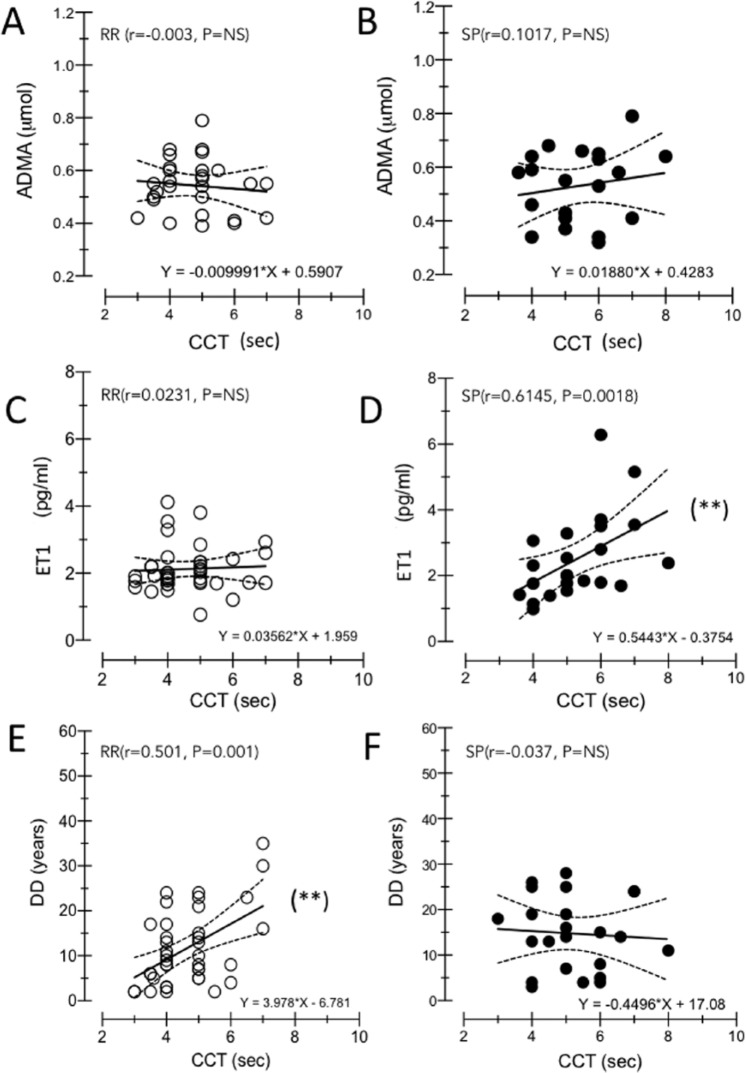
Figure 2A and B shows the relation between ET1 and ADMA levels. They proved to be independent, both in control subjects (r = 0.013) and in MS patients (RR: r = 0.115, P = .2507; SP: r = −0.0076, P = .8820). The slope of their regression lines fitted to experimental data (control = 0.1797; MS = 0.1797: 1.524 and 0.2848 in RR and SP, respectively) demonstrated that their ratio remained relatively constant in patients with MS compared with healthy subjects. The absence of correlation among CCT and ADMA is shown in Figure 3A to B. Figure 3C to F illustrates the correlation between CCT and ET1 in RR (r = 0.0231, P = .8919) and SP (r = 0.6145, P = .0018) forms and shows the correlation between CCT and DD in RR (r = 0.5001, P = .0012) and SP (r = 0.0372, P = .7570).
Figure 3.
(A–F) Correlation analysis of evaluated biological parameters in RR-MS and SP-MS subgroups. In MS subgroups, CCT and ADMA correlation is not significant (A,B). When CCT is considered, different significance of DD and ET1 between RR-MS and SP-MS patients is obtained: comparing (C) and (E), RR-MS group shows a significant correlation between CCT with DD, but not with ET1, whereas an opposite trend is demonstrated in SP-MS group (D,F). ADMA indicates asymmetric dimethylarginine; DD, disease duration; ET1, endothelin-1; MS, multiple sclerosis; RR, relapsing-remitting; SP-MS, secondary-progressive.
Figure 3C to F illustrates opposite trend between RR-MS and SP-MS patients when DD and ET1 are considered. Comparing (E) and (C), RR-MS group shows a significant correlation with DD but not with ET1, whereas an opposite trend is demonstrated in SP-MS group. In (F) CCT in not correlated with DD, whereas in (D) ET1 is correlated with CCT.
Discussion
It is known that when cerebral blood flow value is greater than 20 mL/100 g/min, the increased value of CCT depends on the capillary patency. It has been shown that when CCT is prolonged beyond 4 seconds, corresponding to 21 mL/100 g/min, cerebral blood flow, capillary patency, and oxygen availability in tissues change, leading to tissue hypoperfusion.27,28 In our sample, 87% RR-MS and 97% SP-MS subjects exhibited a CCT above the threshold value. Therefore, it is highly likely that capillary dysfunction with increased brain microcirculation resistance was the main factor responsible for CCT increase in patients with MS. Accordingly, this article focuses on the possible role of vasoconstrictors, such as ET1 and ADMA, in contributing to the increased brain vessel resistance in MS. Before approaching the results related to the main aim, there are aspects that require comment. First, in control subjects, CCT, ET1, and ADMA values remained constant with age, and therefore, their possible changes in patients with MS were not influenced by age. Second, ADMA and ET1 plasma levels in MS were independent of DD, so their increase had to be in the very early stages of the disease (Figure 2C and D). This is coherent with the hypothesis that their overproduction may be a response to the initial, altered integrity of the BBB.29 Also, as there is abundant evidence that the production of NO is significantly raised within MS lesions,30 the possibility that NO-induced vasodilation was a trigger for ET1 and ADMA overproduction cannot be discarded (see below). Third, as can be deduced from the slope of the fitting line of the ratio between ET1 and ADMA, this did not change in patients with MS with respect to controls (Figure 2A and B). It is suggested that their high levels (about 2 times higher in MS than in controls) (Figure 1B and C) can be interpreted as a resetting, rather than a dysregulation, of the mechanisms governing cerebral hemodynamics. Theoretically, these results strengthen the hypothesis of a common factor triggering ET1 and ADMA upregulation. Whole-brain circulation time correlated significantly with ET1 levels only in SP subtype (Figure 3D). The CCT values rose with increasing ET1 plasma levels, the average rate being 0.16-second delay per 1 pg/mL ET1. Contrastingly, in RR subtype, CCT values correlated with DD: the former showed a 0.2-second CCT delay per year of disease. Therefore, while in the SP subtype a specific factor (ie, ET1) emerged in conditioning the CCT delay, in the RR subgroup the CCT correlated with a surrogate (DD) of a shadowy subliminal factor. In view of the observed difference in CCT and its conditioning factors between RR and SP subtypes, all characteristics shared by these 2 forms of MS could be excluded as factors responsible for CCT delay. Accordingly, CCT values were unrelated to both LL and brain atrophy. Although it is known that brain volume decreases with increasing DD,31 it is unlikely to have been the conditioning factor of CCT delay in RR patients. Indeed, it has been shown that the rate of tissue loss in patients with MS is independent of course and MS subtypes.32 It follows that if brain atrophy was a conditioning factor for CCT delay, then the relationship between CCT delay and DD had to be observed in both RR and SP subtypes. Actually, we found that CCT values and DD were independent variables in SP patients.
Focusing on the unshared features of RR and SP subtypes, the most immediate differential characteristic is that the former is subject to compensatory mechanisms that cause the remitting phases. It is generally believed that once a threshold is surpassed, compensatory mechanisms fail and progressive neurologic damage may ensue. In this sense, the SP subtype can be regarded as the failure of compensatory mechanisms.33 Although in RR the compensatory mechanisms are still effective, patients who are in the quiescent phase, however, accumulate tissue injuries caused by relapsing phases. For example, lesion of BBB persists in chronic plaques, although on a more limited level than in the active ones.34 Therefore, it seems plausible that the observed neurovascular alteration in patients with MS may be seen as the damage summation/accumulation over time, given as DD. It could be argued that if the DD in RR actually reflected the accumulation of neurovascular injuries, then a relationship between LL and CCT also had to be observed. In fact, although vessel wall damage was found in all acute plaques, it has been demonstrated that microvascular injuries also occur without white matter involvement.35 It follows that the level of vascular damage cannot be deduced by LL values.
As mentioned above, despite the fact that the ET1 plasma level was similar in RR and SP subtypes, it correlated with CCT values in SP, but not in RR patients. One possible explanation may be found in the evidence that the activity of the same ET1 plasma level is increased when the BBB is severely altered. This is indirectly confirmed in this study, showing a significantly 40-second higher CCT delay in SP-MS than in RR-MS forms.
Therefore, we conclude that the statistical correlation between ET1 plasma level and CCT values is conditioned by the level of neurovascular unit dysfunction. If the relationship between CCT and ET1 is confirmed in SP form, though not in RR-MS form, this CCT-ET1 coupling could be the biomarker able to identify when the RR became the SP form or to differentiate RR versus progressive form ab initio. The strength of this finding is to ameliorate clinical assessment. The prognosis accuracy could be improved, leading to precision medicine, and could modify therapeutic approaches.
On the contrary, CCT in both RR and SP subgroups was shown to be statistically independent of ADMA plasma levels. It is known that vessel reactivity is the vasodilation and vasoconstriction balance among multiple vascular factors, and the exact molecular interaction is not yet known in MS, as well as in other neurodegenerative diseases. The absence of significant correlation of ADMA with any parameters chosen in this study could be covered and mediated by other vascular factors and not be enhanced using clinical laboratory titration. However, it does not rule out having a role in MS. Based on the evidence that endothelial NOS is highly expressed in intraparenchymal vascular endothelial cells of patients with MS,36 here we recall the above-mentioned hypothesis that NO-induced vasodilation was the common trigger for ET1 and ADMA overproduction. Nitric oxide has 2 major effects on cerebral vessels, both of which may be involved in the pathogenesis of MS lesions, namely, vasodilation and alteration of the BBB. Vasodilation by itself may facilitate inflammation by decreasing the velocity of blood flow, thereby aiding leukocyte transmigration—the latter facilitated by NO-induced BBB breakdown. In addition, NO may cause conduction block, perhaps by impairing the function of sodium channels, and demyelinated axons are particularly vulnerable to this effect.37 Furthermore, raised concentrations of NO and related reactive species may impair synaptic transmission which, in addition to compromising transmission in motor and sensory pathways, may contribute to the loss of function in patients with MS. Due to this, it seems plausible that production of ADMA and ET1 can be seen as a compensatory response aimed at opposing the effects of NO overproduction: ADMA by inhibiting both eNOS and iNOS and ET1 by opposing the NO-induced vasodilation. The response effectiveness to NO overproduction could be one of the factors contributing to the compensatory mechanisms causing the remission phase in RR patients.
It might be argued that ET1-induced vasoconstriction response may cause hypoperfusion and therefore tissue hypoxic/ischemic stress. However, it has been shown that ET1 has an important function of protecting the astrocytes against hypoxic/ischemic stress so that these cells can either repair their neighboring damaged neurons or participate in forming a protective boundary of the injured cells of the brain. In fact, astrocytes regulate glutamate uptake, preventing excitotoxic neuronal injury, control the levels of critical extracellular ions such as K+ and H+, and promote antioxidant defense in the brain.15
Therefore, we suggest that ET1 and ADMA overproduction reflects a protective response to the early vascular-derived insults in MS, possibly contributing to the remitting phase in RR patients. Later, due to their persistent high levels, the detrimental effects of these vasoactive molecules could prevail and contribute to functional/structural abnormalities of the brain microvasculature.38 When it begins to interfere with basic laws of fluid dynamics, hemorheological compromise will result in cerebral capillary resistance, abnormal flow patterns, and changes in shear stress and shear rate in vessel walls.
Acknowledgments
The authors thank Dr Emiliano Santernecchi, Pietro Piu, and Martina Monti for data analysis and biochemical assays, respectively.
Footnotes
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1580 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
LM, LM, LB and AR conceived and designed the experiments. AR and LM analyzed the data. LM and AR wrote the first draft of the manuscript. LM, AR, and LM contributed to the writing of the manuscript. LM, AR, LB, and LM agree with manuscript results and conclusions. LM, AR, LB, and LM jointly developed the structure and arguments for the paper. LM, LM, and AR made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics
As a requirement of publication, the author(s) has/have provided the publisher signed confirmation of compliance with legal and ethical obligations, including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
REFERENCES
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.McFarland HF Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 3.Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–31. doi: 10.1212/01.wnl.0000275229.13012.32. discussion S43–S54. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leech S, Kirk J, Plumb J, McQuaid S. Persistent endothelial abnormalities and blood-brain barrier leak in primary and secondary progressive multiple sclerosis. Neuropathol Appl Neurobiol. 2007;33:86–98. doi: 10.1111/j.1365-2990.2006.00781.x. [DOI] [PubMed] [Google Scholar]
- 7.De Keyser J, Steen C, Mostert JP, Koch MW. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab. 2008;28:1645–1651. doi: 10.1038/jcbfm.2008.72. [DOI] [PubMed] [Google Scholar]
- 8.D’haeseleer M, Hostenbach S, Peeters I, et al. Cerebral hypoperfusion: a new pathophysiologic concept in multiple sclerosis? J Cereb Blood Flow Metab. 2015;35:1406–1410. doi: 10.1038/jcbfm.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhya S, Johnson G, Herbert J, et al. Pattern of hemodynamic impairment in multiple sclerosis: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage. 2006;33:1029–1035. doi: 10.1016/j.neuroimage.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 11.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68:S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemiya T, Yamagata K. Intercellular signaling pathway among endothelia, astrocytes and neurons in excitatory neuronal damage. Int J Mol Sci. 2013;14:8345–8357. doi: 10.3390/ijms14048345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge Y, Law M, Herbert J, et al. Prominent perivenular spaces in multiple sclerosis as a sign of perivascular inflammation in primary demyelination. AJNR Am J Neuroradiol. 2005;26:2316–2319. [PMC free article] [PubMed] [Google Scholar]
- 14.De Keyser J, Wilczak N, Leta R, et al. Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology. 1999;53:1628–1633. doi: 10.1212/wnl.53.8.1628. [DOI] [PubMed] [Google Scholar]
- 15.Pekny M, Pekna M, Messing A, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 16.Pache M, Kaiser HJ, Akhalbedashvili N, et al. Extraocular blood flow and endothelin-1 plasma levels in patients with multiple sclerosis. Eur Neurol. 2003;49:164–168. doi: 10.1159/000069085. [DOI] [PubMed] [Google Scholar]
- 17.D’haeseleer M, Beelen R, Fierens Y, et al. Cerebral hypoperfusion in multiple sclerosis is reversible and mediated by endothelin-1. Proc Natl Acad Sci U S A. 2013;110:5654–5658. doi: 10.1073/pnas.1222560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauer V, Sotníková R. Nitric oxide—the endothelium-derived relaxing factor and its role in endothelial functions. Gen Physiol Biophys. 2010;29:319–340. [PubMed] [Google Scholar]
- 19.Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006;13:627–638. doi: 10.2174/092986706776055652. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448–460. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kielstein JT, Donnerstag F, Gasper S, et al. ADMA increases arterial stiffness and decreases cerebral blood flow in humans. Stroke. 2006;37:2024–2029. doi: 10.1161/01.STR.0000231640.32543.11. [DOI] [PubMed] [Google Scholar]
- 22.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland PR, Searcy JL, Salvadores N, et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J Cereb Blood Flow Metab. 2015;35:1005–1014. doi: 10.1038/jcbfm.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monti L, Donati D, Menci E, et al. Cerebral circulation time is prolonged and not correlated with EDSS in multiple sclerosis patients: a study using digital subtracted angiography. PLoS ONE. 2015;10:e0116681. doi: 10.1371/journal.pone.0116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Østergaard L, Jespersen SN, Mouridsen K, et al. The role of the cerebral capillaries in acute ischemic stroke: the extended penumbra model. J Cereb Blood Flow Metab. 2013;33:635–648. doi: 10.1038/jcbfm.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Østergaard L, Engedal TS, Aamand R, et al. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. J Cereb Blood Flow Metab. 2014;34:1585–1598. doi: 10.1038/jcbfm.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund H, Krakauer M, Skimminge A, et al. Blood-brain barrier permeability of normal appearing white matter in relapsing-remitting multiple sclerosis. PLoS ONE. 2013;8:e56375. doi: 10.1371/journal.pone.0056375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 31.Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- 32.Kalkers NF, Ameziane N, Bot JC, et al. Longitudinal brain volume measurement in multiple sclerosis: rate of brain atrophy is independent of the disease subtype. Arch Neurol. 2002;59:1572–1576. doi: 10.1001/archneur.59.10.1572. [DOI] [PubMed] [Google Scholar]
- 33.Trapp BD, Bö L, Mörk S, et al. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochem Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Wakefield AJ, More LJ, Difford J, et al. Immunohistochemical study of vascular injury in acute multiple sclerosis. Clin Pathol. 1994;47:129–133. doi: 10.1136/jcp.47.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill KE, Zollinger LV, Watt HE, et al. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: distribution, cellular expression and association with myelin damage. J Neuroimmunol. 2004;151:171–179. doi: 10.1016/j.jneuroim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Rossi A, Rossi S, Ginanneschi F. Activity-dependent changes in intrinsic excitability of human spinal motoneurones produced by natural activity. J Neurophysiol. 2012;108:2473–2480. doi: 10.1152/jn.00477.2012. [DOI] [PubMed] [Google Scholar]
- 38.Faraco G, Moraga A, Moore J, et al. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766. doi: 10.1161/HYPERTENSIONAHA.113.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]