Abstract
Although the use of ultrasound as a potential therapeutic modality in the brain has been under study for several decades, relatively few neuroscientists or neurologists are familiar with this technology. Stereotactic brain lesioning had been widely used as a treatment for medically refractory patients with essential tremor (ET), Parkinson disease (PD), and dystonia but has been largely replaced by deep brain stimulation (DBS) surgery, with advantages both in safety and efficacy. However, DBS is associated with complications including intracerebral hemorrhage, infection, and hardware malfunction. The occurrence of these complications has spurred interest in less invasive stereotactic brain lesioning methods including magnetic resonance imaging–guided high intensity–focused ultrasound (FUS) surgery. Engineering advances now allow sound waves to be targeted noninvasively through the skull to a brain target. High intensities of sonic energy can create a coagulation lesion similar to that of older radiofrequency stereotactic methods, but without opening the skull, recent Food and Drug Administration approval of unilateral thalamotomy for treatment of ET. Clinical studies of stereotactic FUS for aspects of PD are underway. Moderate intensity, pulsed FUS has also demonstrated the potential to safely open the blood-brain barrier for localized delivery of therapeutics including proteins, genes, and cell-based therapy for PD and related disorders. The goal of this review is to provide basic and clinical neuroscientists with a level of understanding to interact with medical physicists, biomedical engineers, and radiologists to accelerate the application of this powerful technology to brain disease
Keywords: Focused ultrasound, FUS, essential tremor, Parkinson disease, blood-brain barrier, HIFU
Introduction
Although diagnostic ultrasound is a well-established method, there is only growing awareness of ultrasound as a potential therapeutic modality for neurologic disease. With the very recent Food and Drug Administration (FDA) approval (July 2016) of essential tremor (ET) as the first neurologic condition for focused ultrasound (FUS) treatment, both preclinical and clinical research are expanding rapidly for several neurologic indications.1 Much of this progress is due to improving technology to provide controlled levels of ultrasonic energy that can be focused to a brain target, noninvasively through the skull and guided by magnetic resonance imaging (MRI). High-intensity FUS (HIFU) is sufficient to create a coagulation lesion in the brain with the goal of developing a substantially less invasive way to create stereotactic brain lesions. Moderate levels of FUS energy, provided in pulsed (p) mode, can be employed to safely open the blood-brain barrier (BBB) for localized delivery of large therapeutics such as protein, genes, and cells as a potentially restorative treatment of neurodegenerative diseases such as the Parkinson disease (PD).2–4
A Brief Summary of Stereotactic Surgery for Movement Disorders
Stereotactic brain lesioning has been explored for decades as an effective treatment for medically refractory patients with movement disorders. Only recently, however, MRI-guided high intensity–focused ultrasound (MRgHIFU) has been evaluated as a viable treatment option for generating these targeted lesions. Although effective, lesioning for movement disorder has been largely replaced by deep brain stimulation (DBS) surgery. Deep brain stimulation is similar in strategy to stereotactic lesioning but has 2 significant advantages. Unlike lesional surgery, DBS does not create any intentional brain injury. Suppression of motor abnormalities such as tremor is accomplished through continuous high-frequency (130–180 Hz) stimulation, although the mechanism of its lesion-like inhibitory effect is still debated.5
An issue with stereotactic lesions has been the association with unexpected neurological difficulties when performed on both hemispheres. In particular, bilateral thalamotomy for tremor related to PD was found to be strongly associated with dysarthria in early studies.6 Unlike stereotactic lesions, bilateral DBS can be safely performed to improve the larger number of patients with bilateral motor symptoms. Adjustability of these devices is a major advantage of this approach over lesional surgery. When side effects of bilateral stimulation such as dysarthria occur, these can usually be mitigated by lowering the intensity of stimulation.7
Deep brain stimulation is not, however, without its own complications, which include surgical complications such as intracerebral hemorrhage (0.5%–2.0%) and infection (1%–3%), as well as DBS-specific issues such as lead migration and fracture (1%–3%) and device malfunction (1%–3%).8–10 Deep brain stimulation also introduces added procedures and costs over surgical lesioning, including surgical implantation and periodic replacement of the programmable pulse generator, as well as device programming visits.
These limitations of DBS provided a rationale for investigating less invasive surgical methods. Radiosurgery uses contemporary stereotactic methods to localize the brain target and a focused array of emitters that have been extensively used to treat brain tumors (Gamma Knife).11 This less invasive method has also been applied to relieve symptoms of both ET and PD, with the best results seen in lesioning of ventral intermediate nucleus (VIM) for ET.12–14 A major issue preventing widespread acceptance of this method for functional neurosurgery is the delayed effect of ionizing radiation–based lesioning. Extent of treatment is determined solely by a calculation of dose because the effects of radiation occur with a variable delay. Although the rate of off-target effects for radiosurgery is relatively low, they can occur with a delay of days to months.15 These previous studies of radiosurgery along with the real-time effects of sonic energy have added to the rationale for the study of MRgHIFU as a treatment of movement disorders.
FUS: Principles of Operation
Clinically approved applications of ultrasound include low-intensity exposures for healing in physical therapy16 and higher intensity FUS for noninvasively ablating a variety of benign and malignant tumors.17 The former includes the treatment of uterine fibroids,18 breast cancer,19 and bone metastasis20 and the latter being for palliative purposes.
Similar to light waves, ultrasound waves can be focused using either single-element concave transducers or electronically controlled phased arrays of many smaller piezoelectric transducers (somewhat analogous in principle to a Gamma Knife). The energy can be concentrated up to 3 orders of magnitude into a small and elongated ellipsoid volume, typically on the order of 2 × 7 mm at the focus. As a result, the rates of energy deposition generally used clinically are capable of raising tissue temperature of the tissues within seconds to 60°C or greater to induce denaturation of cell proteins and ultimately coagulative necrosis. In the intervening tissues of the pre- and postfocal region, lower intensities of sonic energy are found. As a result, energy absorption is also lower, and the deleterious effects of the exposures (i.e. thermal damage) do not occur.17,21,22
The current standard for image-guided HIFU for minimally invasive nonincisional treatments in the brain employs MRI (MRgHIFU) as the imaging modality. Magnetic resonance imaging enables high-resolution soft tissue imaging for treatment planning, whereas magnetic resonance (MR) thermometry also allows for quasi real-time brain temperature monitoring. This allows for validating that the region of treatment has received the designated thermal dose, while also ensuring that regions outside of the treatment zone are not adversely affected. These devices employ multielement, phased array transducers for fast accurate electronic beam steering. Current technology allows for more precise correction of aberrations in the sonic beam path that occurs as it passes through the skull.23 This capacity to create a targeted thermal lesion within brain through the intact skull allows for the application of FUS to a variety of neurologic conditions including movement disorders.
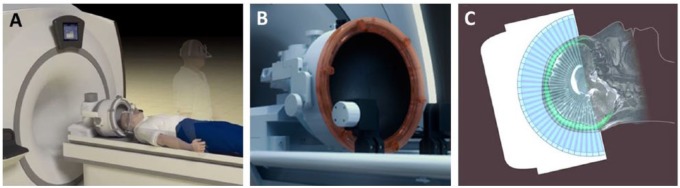
Similar to DBS and open surgical lesioning methods, MRgHIFU-treated patients begin their day with shaving of the head followed by placement of a stereotactic frame using a local anesthetic at the pin sites, where bruising and bleeding of the scalp can occur. They then spend approximately an hour in the MRI scanner during the alignment process of the ultrasound array and the MRI. As with DBS, patients remain off medication for their movement disorder on that day to maximize these symptoms as a target for the treatment end point. Current studies for movement disorders have used a device possessing 1024 ultrasound elements (Exablate; INSIGHTEC Ltd, Tirat Carmel, Israel; Figure 1). Along with the stereotactic frame, the head is covered with silicone rubber bag filled with chilled degassed circulating water. This both improves coupling of the ultrasound array to the head and is important to reduce sonication-related heating of the skull and scalp, the major technical limitation for current devices.
Figure 1.
Transcranial magnetic resonance imaging (MRI)–guided focused ultrasound. (A) A schematic representation of a patient to be treated with a transcranial MRI-guided focused ultrasound system. The upright patient in the background has already been fitted with a stereotactic frame. The patient’s shaved headed is coupled with the (B) phase-array transducer, which possesses 1024 ultrasound elements for electronic steering of the ultrasound beam. Coupling of the head with the transducer occurs through an acoustically transparent, flexible bladder fitted over the patient’s head. Chilled, degassed water is circulated between the bladder and the face of the transducer array to maximize coupling and reduce heating effects. (C) A schematic 2-dimensional representation of the multiple ultrasound beams focused noninvasively through the skull (bright green) to a single target. The image of the skull is obtained from a prior computed tomographic scan that is mechanically registered to the magnetic resonance image. Information from the skull is used by the planning software to correct for aberrations to the beam paths and accurately position the focus at the desired target. Images obtained and adapted with permission from INSIGHTEC Ltd, Tirat Carmel, Israel.
The effects of MRgHIFU, DBS, and radiofrequency surgical lesioning can be observed during the procedure. During both DBS and surgical lesioning, the intensity of electrical stimulation or thermal energy is gradually increased, and the response of the awake patient (relief of symptoms or intrusion of other neurologic symptoms) is used to functionally validate accuracy of the anatomic target and determine the treatment end point. The goal of all forms of surgery for movement disorders is maximal relief of motor symptoms (tremor, bradykinesia, rigidity, and dystonia) without intrusion of symptoms associated with damage or stimulation of adjacent (off-target) brain regions such as dysarthria, paresthesia, weakness, diplopia, or visual field defects.
MRgHIFU for Movement Disorders
Essential tremor was the first neurological disorder evaluated for treatment with MRgHIFU for several reasons. (1) ET is a common disorder where medical therapy is frequently inadequate for patients with severe disability tremor.24 (2) The VIM of the thalamus is a well-established target for both lesioning and DBS for reduction in tremor in medically refractory patients with either ET or PD. (3) The anatomical target—the VIM—is centrally located within the brain, which reduces the distortional effects of the skull on focusing the ultrasound energy. (4) Treatment of the VIM in ET not only results in tremor reduction but also substantially reduces disability in selected patients with only unilateral treatment, such as patients with severe tremor in the dominant hand. This approach has been validated by several published clinical studies that have shown significant improvement after treatment using standardized scales rating both tremor amplitude and tremor-related disability.
An initial study treated 4 medication-resistant patients with ET resulting in more than 80% reduction on tremor scores for at least 3 months with associated functional improvement. As expected from earlier surgical studies, the only adverse neurological effect was paresthesias on the treated hand.25 The University of Virginia group treated 15 patients with ET with similar characteristics and sonication parameters.26 Treatment resulted in more than 60% reduction in hand tremor compared with baseline, with associated improvement in tremor-related disabilities in activities such as writing, drinking, and eating and was persistent for at least 1 year. Adverse effects related to MRgHIFU treatment included head pain, light-headedness, nausea, and a sensation of movement.27 Thalamotomy-related adverse events included sensory changes seen in most of the patients but persisted only in 3 patients. Transient unsteadiness, weakness, and dysarthria were also observed. Magnetic resonance imaging–related abnormalities were observed within 24 hours of sonication at the target location predicted by the real-time MR thermography. Treated patients also showed alterations in thalamic connectivity on MRI diffusion tensor imaging sequences.28
Earlier observations have been validated in a recent larger multicenter double-blind sham-controlled pivotal study.1 Attempts to treat patients with the current device (650-kHz frequency of sonic energy), designed for a high level of accuracy of target sonication, sometimes fail to attain sufficient thermal doses at the target for lesioning. This is usually due to skull characteristics that can raise sonic energy attenuation, which ultimately limits energy deposition at the target.29,30 It is also due to the fact that the frequency is lower than typical therapeutic ultrasound devices (~1 MHz), being optimized for transmission through the skull. Higher frequencies generate proportionally higher energy deposition due to greater energy absorption, similar to ultrasound imaging, creating larger temperature elevations.
The VIM has also been targeted with MRgHIFU for relief of tremor associated with PD. An initial study of 7 patients with severe refractory tremor associated with PD demonstrated immediate abolition of contralateral arm tremor that persisted for at least 3 months, with mild neurology deficits that did not persist.31 Benefit of MRgHIFU has been confirmed in a recent blinded sham-controlled study for PD tremor.32
Targets other than VIM have been treated with MRgHIFU for relief of other aspects of PD besides tremor. Unilateral lesions were created with MRgHIFU in 13 consecutive patients with PD targeting where the fiber tracts exiting the pallidum on route to thalamus (pallidothalamic tract), including the fasciculus lenticularis and ansa lenticularis.33 Although initial patients treated had rapid return of PD symptoms associated with insufficient increases in target temperature, increasing the ultrasound energy resulted in a 60% reduction in unified Parkinson disease rating scale (UPDRS) scores in 9 subsequent patients. This group of patients was heterogeneous regarding motor signs of PD (tremor, bradykinesia, rigidity, and dyskinesias) but showed improvement that persisted during the 3-month follow-up period. Patients with PD have also begun to be treated with MRgHIFU targeted to the globus pallidus interna (GPi), a well-established target for both surgical treatment and DBS.34–36 Lesional surgery to the GPi has not only been shown to improve cardinal signs of PD, such as tremor, bradykinesia, and rigidity, but also is particularly effective in reducing the abnormal movements that develop after years of treatment with l-Dopa (l-Dopa–induced dyskinesias or LIDs).37 In the first reported case, a patient with PD and intrusive dyskinesias was successfully treated with an MRgHIFU unilateral pallidotomy. This patient experienced a 76% reduction in the severity of motor signs in the “off ” medication state, as well as a 53% reduction in dyskinesia ratings even 6 months after the procedure. As with surgical pallidotomy, some improvement was even seen ipsilateral to the treated hemisphere, without any off-target neurological adverse effects.38 Our center is currently part of a safety and feasibility study of MRgHIFU pallidotomy with plans for a multicenter phase 2 study. Treated patients have highly asymmetric motor signs, are L-Dopa responsive, but have significant disability from LIDs.
Although the subthalamic nucleus (STN) is the most common target for DBS in PD, it was rarely targeted for stereotactic surgery in the past. The reluctance is based on lesion studies in primates and experience in patients after stroke, where destruction of the STN results in dramatic involuntary movements such as hemiballismus.39 A large study, where stereotactic surgical lesions were created in the STN, resulted in improvement in PD motor symptoms, but a significant incidence of hemiballismus.40 Whether a more controlled approach using MRgHIFU will allow for safe and effective lesioning of the STN remains to be determined.
Stereotactic surgery including DBS placement commonly uses microelectrode recording to validate target location by the firing patterns of neuronal units followed by test stimulation of the putative target for patient responses. The minimally invasive strategy of MRgHIFU does not allow for physiologic recording, but both neurons and myelinated axons can be stimulated by nonlethal ultrasound energy with responses similar to electrical stimulation.41 This allows for targeting in MRgHIFU studies of relevant gray matter nuclei as well as white matter tracts. The group that targeted the pallidothalamic tract in patients with PD also targeted the cerebellothalamic tract (CTT) in a group of 21 consecutive patients with severe refractory ET, with comparable improvement in tremor severity and disability. Adverse events of treatment were relatively mild and nonserious. Notably, this study included the first 3 patients to receive bilateral (staged) MRgHIFU brain lesions for a movement disorder.42 A desire to reduce complications associated with thalamic damage was the rationale for targeting the CTT with MRgHIFU. Bilateral surgical lesioning for movement disorders has rarely been performed after early experiences in the 1960s. Unexpected severe dysarthria or imbalance was part of the rationale for the development of DBS, where most of the treated patients have undone bilateral implantations without these adverse effects. The observation that these MRgHIFU-treated patients had a low level of worsening of preexisting gait instability (4/21 transient 1/21 permanent), without reported dysarthria, is consistent with results of a large study of bilateral thalamotomy using radiosurgery.43 Ventral intermediate nucleus lesions were created bilaterally to treat patients with ET with both bilateral appendicular and axial tremor. The incidence of dysarthria was far lower than older lesional surgery and similar to that of DBS.
Essential tremor and especially PD are progressive conditions where motor symptoms worsen over time. Patients treated with DBS are seen at regular intervals where the parameters of stimulation are adjusted to compensate for worsening symptoms. Unfortunately, disease progression may eventually result in worsening symptoms in many patients in spite of reprogramming.44 When significant worsening occurs after lesional surgery, repeat surgery may be performed, which has usually been successful in regaining the original clinical response.45,46 The potential efficacy and safety of retreatment of any aspect of a movement disorder with MRgHIFU remain to be determined.
FUS-Enhanced Delivery: Opening the BBB
All neurodegenerative diseases including PD lack any therapy that can slow, halt, or reverse their underlying pathology that includes neuronal loss. This situation has resulted in an explosion in research attempting to develop so-called restorative therapies that include recombinant proteins, genes, and cell-based therapy. Despite the advances in molecular and cell biology, delivery of large therapeutics in both animal models and patients with PD still requires needle injection directly into the brain. Not only is this highly invasive method associated with serious risks such as bleeding and infection but its efficacy is also frequently limited by inadequate distribution of the injected therapeutics.47,48 Human stem cells in particular show limited migration after transplantation. Limited distribution from a needle injection site may contribute to the failure of such large therapeutics to translate from promising results in small animal models to successful clinical trials.49 Although the vasculature is the most effective route to distribute therapeutics, the BBB prevents entry of cell-based therapy into brain. The specialized endothelia of the brain have continuous tight junctions which form the BBB, limiting the movement of therapeutics from the bloodstream into brain. Strategies that have been developed to open or bypass the BBB include hyperosmotic solutions of mannitol and carrier molecules that are transported across brain endothelia.50–53
BBB disruption using MRgFUS
Although FUS exposures for ablation are conducted in continuous mode for tissue destruction, FUS exposures in pulsed mode (pFUS) are nondestructive due to their lower temporal averaged intensities.54,55 FUS exposures in pulsed mode also allow for cooling to occur between pulses, further reducing temperature increases.56 Instead of heat generation, these exposures are capable of creating mechanical effects, most notably for nondestructively increasing vascular permeability to improve the delivery of therapeutic agents. This has been demonstrated in a variety of solid tumor models57–60
Many pFUS studies have involved increasing the permeability of the BBB to enhance or enable the delivery of agents to the brain. Studies by Hynynen, McDannold, and colleagues initially demonstrated that pFUS applied during the circulation of microbubble suspensions (FDA-approved ultrasound contrast agents) can create an MRI-targeted region of transient and safe disruption of the BBB.61–64 The lower intensity pFUS exposures activate the microbubbles into a state of stable oscillations (i.e. noninertial cavitation), causing transient separation of endothelial tight junctions—the basis for the BBB.63,65,66 The procedure can create transient (hours) opening of the BBB sufficient to allow extravasation of large therapeutics without pathology or entry blood components.67–70 This allows large therapeutics to enter the brain from the systemic circulation including antibodies, growth factors, nanoparticles, nucleic acids, viral vectors, and cells.71–77
The first direct application of this strategy to neurological disease was in brain tumor therapy. In preclinical models of brain metastatic breast cancer, pFUS-mediated BBB opening substantially improved the efficacy of the anti-HER2 monoclonal antibody trastuzumab.78 Preclinical studies have used pFUS to improve the delivery of growth factors and their genes in the treatment of PD. The delivery of glial-derived growth factor (GDNF) and the related factor neurturin from the blood was improved in rodents with the use of this procedure.2,74 Gene therapy with GDNF has been successful in restoring dopamine metabolism and reversing motor abnormalities in a toxin-induced rat model of PD.3 In this study, a plasmid expressing GDNF was preloaded into the microbubbles to enhance its concentration in the region of pFUS-mediated BBB opening. Viral vectors carrying potentially beneficial genes can also be delivered to brain from an intravenous injection after pFUS-mediated opening of the BBB.73,79 After more than a decade of experience in animals that include nonhuman primates, this method is accumulating substantial data supporting its safety, including the use of repeated treatments, an approach essential to the continued treatment of chronic progressive neurologic disease.80,81
Treatment of the Alzheimer disease (AD) is also a potential goal with pFUS-mediated opening of the BBB. Studies in mouse models of AD have demonstrated both reduction in brain amyloid burden and behavioral improvement using this strategy coupled with either injected or endogenous anti-amyloid antibodies.82–86 The development of clinical amyloid-based nuclear medicine scans makes a planned pilot study in humans feasible, with the aim of determining whether pFUS can reduce the amyloid burden in a local brain region. A similar approach may be useful to deliver anti-alpha-synuclein antibodies that are under development as a disease-modifying therapy for PD.
Using pFUS to open the BBB has even been applied to therapeutics as large as cells in experimental animals. Stem cells have been found in brain regions that have FUS opening of the BBB after intracarotid injection, whereas lymphocytes will enter treated brain regions even after intravenous injections.4,87 Stem cell therapy could also be advanced through another novel use of pFUS reported to stimulate endogenous brain stem cell proliferation.88
Delivery of large therapeutics across the BBB has always been limited by the inefficiency of the transfer where accumulation of 1% to 2% of the total injected agent/cell into the blood in brain is a true accomplishment.52 Studies of molecular or cellular therapies usually find that less than 0.1% of the injected agent can be detected in the sonicated region of brain after pFUS-mediated opening of the BBB.4,71 Our group has attempted to address this issue by combining a pFUS-based method with a complimentary strategy known as magnetic targeting or attraction. This method is based on attracting super paramagnetic iron oxide–containing nanoparticles (SPIONs) to an applied magnetic field.88 Molecular therapeutics such as beneficial genes can be coupled with the particles, or in the case of our own work, stem cells can be loaded with SPIONs that they engulf in culture.89,90 Our recent work indicates that stem cells loaded with SPIONs have a much greater likelihood of entering brain from the blood after pFUS-mediated opening of the BBB combined with the application of a powerful external magnet.91
Delivery of molecular and cellular therapeutic through opening the BBB has the potential to be both safer and more effective than the current method in both humans and experimental animals of intracerebral needle injection. A major limitation of the current approach in the case of cell-based therapy for PD is the poor migration of stem cells from the injection site into the large and unfavorable environment of the adult human brain.47–49,92 Although inefficient, opening the BBB allows the cells to be widely distributed throughout the target region using the brains’ natural route of delivery—the microvasculature.
Focused ultrasound is also being investigated to further improve convection-enhanced delivery (CED) by intracerebral injection, which is the current standard for delivery of protein and gene therapy to the brain. Applying energy to brain tissue with FUS improves the spread of injectates including nanoparticles after CED.93,94 We recently demonstrated that pulsed ultrasound exposures can safely enlarge both the extracellular and perivascular spaces in ex vivo brain tissue. Generating these effects was subsequently shown to significantly enhance the diffusion of densely pegylated nanoparticles as large as 500 nm when injected directly into the cortex following FUS exposures.95 Similar mechanisms may be involved in the enhancement of transnasal delivery of proteins after sonication of the brain.96
As a therapy for movement disorders, FUS has both advantages and limitations. The MRgHIFU creates a coagulation-based brain lesion with little risk of the open surgical complications of intracerebral bleeding or infection. At this point, MRgHIFU is a onetime procedure with risk of long-lasting side effects from off-target brain injury as well as concern regarding the permanency of its symptomatic benefit. The use of pFUS to open the BBB as an alternative to direct brain injection for delivery of molecular and cellular therapeutics has great potential. Currently, this strategy is in the experimental stage, and concerns about safety, including risk of intracerebral hemorrhage, still need to be addressed. The cost of this technology is substantial and requires a highly skilled multidisciplinary team.
Over the last decade, the number of publications and animal and human studies using some form of therapeutic ultrasound has expanded exponentially. This progress is a reflection of a growing understanding of this new technology among a community of investigators that include medical physicists, biomedical engineers, neuroradiologists, neurophysiologists, neurosurgeons, psychiatrists, and neurologists. The need for translating new technologies and concepts into clinical therapies and the growing appreciation of a “team science” approach will bring the application of therapeutic ultrasound to neurological disease into focus.
Footnotes
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 600 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
PSF was the major contributor for clinical aspects related to movement disorder. VF was the major contributor for historical and technical aspects of the article. Both authors contributed equally in aspects related to the blood-brain barrier.
REFERENCES
- 1.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375:730–739. doi: 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- 2.Samiotaki G, Acosta C, Wang S, Konofagou EE. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood—brain barrier opening in vivo. J Cereb Blood Flow Metab. 2015;35:611–622. doi: 10.1038/jcbfm.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan CH, Ting CY, Lin CY, et al. Non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson’s disease. Sci Rep. 2016;6:19579. doi: 10.1038/srep19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: restoring function or functionality? Neurotherapeutics. 2016;13:264–283. doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selby G. Stereotactic surgery for the relief of Parkinson’s disease. 2. An analysis of the results in a series of 303 patients (413 operations) J Neurol Sci. 1967;5:343–375. doi: 10.1016/0022-510x(67)90140-2. [DOI] [PubMed] [Google Scholar]
- 7.Tasker RR. Deep brain stimulation is preferable to thalamotomy for tremor suppression. Surg Neurol. 1998;49:145–153. doi: 10.1016/s0090-3019(97)00459-x. discussion 153–154. [DOI] [PubMed] [Google Scholar]
- 8.Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse events associated with deep brain stimulation for movement disorders: analysis of 510 consecutive cases. Neurosurgery. 2015;11:190–199. doi: 10.1227/NEU.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 9.Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery. 2008;62:360–366. doi: 10.1227/01.neu.0000316002.03765.33. discussion 366–367. [DOI] [PubMed] [Google Scholar]
- 10.Fenoy AJ, Simpson RK., Jr Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120:132–139. doi: 10.3171/2013.10.JNS131225. [DOI] [PubMed] [Google Scholar]
- 11.Specht HM, Combs SE. Stereotactic radiosurgery of brain metastases. J Neurosurg Sci. 2016;60:357–366. [PubMed] [Google Scholar]
- 12.Campbell AM, Glover J, Chiang VL, Gerrard J, Yu JB. Gamma knife stereotactic radiosurgical thalamotomy for intractable tremor: a systematic review of the literature. Radiother Oncol. 2015;114:296–301. doi: 10.1016/j.radonc.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Young RF, Li F, Vermeulen S, Meier R. Gamma Knife thalamotomy for treatment of essential tremor: long-term results. J Neurosurg. 2010;112:1311–1317. doi: 10.3171/2009.10.JNS09332. [DOI] [PubMed] [Google Scholar]
- 14.Hua Z, Guodong G, Qinchuan L, Yaqun Z, Qinfen W, Xuelian W. Analysis of complications of radiofrequency pallidotomy. Neurosurgery. 2003;52:89–99. doi: 10.1097/00006123-200301000-00011. discussion 99–101. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto M, Kawabe T, Higuchi Y, et al. Delayed complications in patients surviving at least 3 years after stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:53–60. doi: 10.1016/j.ijrobp.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Warden SJ, Fuchs RK, Kessler CK, Avin KG, Cardinal RE, Stewart RL. Ultrasound produced by a conventional therapeutic ultrasound unit accelerates fracture repair. Phys Ther. 2006;86:1118–1127. [PubMed] [Google Scholar]
- 17.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 18.Kong CY, Meng L, Omer ZB, et al. MRI-guided focused ultrasound surgery for uterine fibroid treatment: a cost-effectiveness analysis. AJR Am J Roentgenol. 2014;203:361–371. doi: 10.2214/AJR.13.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roubidoux MA, Yang W, Stafford RJ. Image-guided ablation in breast cancer treatment. Tech Vasc Interv Radiol. 2014;17:49–54. doi: 10.1053/j.tvir.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Napoli A, Anzidei M, Marincola BC, et al. MR imaging-guided focused ultrasound for treatment of bone metastasis. Radiographics. 2013;33:1555–1568. doi: 10.1148/rg.336125162. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien WD., Jr Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol. 2007;93:212–255. doi: 10.1016/j.pbiomolbio.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huisman M, van den Bosch MA. MR-guided high-intensity focused ultrasound for noninvasive cancer treatment. Cancer Imaging. 2011;11:S161–S166. doi: 10.1102/1470-7330.2011.9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med. 2005;24:1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- 24.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 25.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013;12:462–468. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 26.Elias WJ, Huss D, Voss T, et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2013;369:640–648. doi: 10.1056/NEJMoa1300962. [DOI] [PubMed] [Google Scholar]
- 27.Huss DS, Dallapiazza RF, Shah BB, Harrison MB, Diamond J, Elias WJ. Functional assessment and quality of life in essential tremor with bilateral or unilateral DBS and focused ultrasound thalamotomy. Mov Disord. 2015;30:1937–1943. doi: 10.1002/mds.26455. [DOI] [PubMed] [Google Scholar]
- 28.Wintermark M, Huss DS, Shah BB, et al. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: in vivo fiber tracking by using diffusion-tensor MR imaging. Radiology. 2014;272:202–209. doi: 10.1148/radiol.14132112. [DOI] [PubMed] [Google Scholar]
- 29.Chang WS, Jung HH, Zadicario E, et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg. 2016;124:411–416. doi: 10.3171/2015.3.JNS142592. [DOI] [PubMed] [Google Scholar]
- 30.Jung HH, Chang WS, Rachmilevitch I, Tlusty T, Zadicario E, Chang JW. Different magnetic resonance imaging patterns after transcranial magnetic resonance-guided focused ultrasound of the ventral intermediate nucleus of the thalamus and anterior limb of the internal capsule in patients with essential tremor or obsessive-compulsive disorder. J Neurosurg. 2015;122:162–168. doi: 10.3171/2014.8.JNS132603. [DOI] [PubMed] [Google Scholar]
- 31.Schlesinger I, Eran A, Sinai A, et al. MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson’s disease. Parkinsons Dis. 2015;2015:219149. doi: 10.1155/2015/219149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond AE, Dallapiazza R, Huss D, et al. 132 A randomized, Sham-controlled trial of transcranial magnetic resonance-guided focused ultrasound thalamotomy trial for the treatment of tremor-dominant, idiopathic Parkinson disease. Neurosurgery. 2016;63:154. [Google Scholar]
- 33.Magara A, Bühler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J Ther Ultrasound. 2014;2:11. doi: 10.1186/2050-5736-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson’s disease. N Engl J Med. 1997;337:1036–1042. doi: 10.1056/NEJM199710093371503. [DOI] [PubMed] [Google Scholar]
- 35.Munhoz RP, Cerasa A, Okun MS. Surgical treatment of dyskinesia in Parkinson’s disease. Front Neurol. 2014;5:65. doi: 10.3389/fneur.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitek JL. Deep brain stimulation for Parkinson’s disease. A critical re-evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg. 2002;78:119–131. doi: 10.1159/000068959. [DOI] [PubMed] [Google Scholar]
- 37.Iacono RP, Shima F, Lonser RR, Kuniyoshi S, Maeda G, Yamada S. The results, indications, and physiology of posteroventral pallidotomy for patients with Parkinson’s disease. Neurosurgery. 1995;36:1118–1125. doi: 10.1227/00006123-199506000-00008. discussion 1125–1127. [DOI] [PubMed] [Google Scholar]
- 38.Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neurology. 2015;85:549–551. doi: 10.1212/WNL.0000000000001826. [DOI] [PubMed] [Google Scholar]
- 39.Lee MS, Marsden CD. Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord. 1994;9:493–507. doi: 10.1002/mds.870090502. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry. 2009;80:979–985. doi: 10.1136/jnnp.2008.154948. [DOI] [PubMed] [Google Scholar]
- 41.Tufail Y, Matyushov A, Baldwin N, et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron. 2010;66:681–694. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Gallay MN, Moser D, Rossi F, et al. Incisionless transcranial MR-guided focused ultrasound in essential tremor: cerebellothalamic tractotomy. J Ther Ultrasound. 2016;4:5. doi: 10.1186/s40349-016-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young RF, Hesselgesser RD, Ahn E, Vermeulen S, Li F, Lee J. Bilateral Gamma Knife thalamotomy for treatment of axial tremor. Transl Cancer Res. 2014;3:525–529. [Google Scholar]
- 44.Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 45.Nagaseki Y, Shibazaki T, Hirai T, et al. Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg. 1986;65:296–302. doi: 10.3171/jns.1986.65.3.0296. [DOI] [PubMed] [Google Scholar]
- 46.Fine J, Duff J, Chen R, et al. Long-term follow-up of unilateral pallidotomy in advanced Parkinson’s disease. N Engl J Med. 2000;342:1708–1714. doi: 10.1056/NEJM200006083422304. [DOI] [PubMed] [Google Scholar]
- 47.Emborg ME, Zhang Z, Joers V, et al. Intracerebral transplantation of differentiated human embryonic stem cells to hemiparkinsonian monkeys. Cell Transplant. 2013;22:831–838. doi: 10.3727/096368912X647144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silvestrini MT, Yin D, Coppes VG, et al. Radially branched deployment for more efficient cell transplantation at the scale of the human brain. Stereotact Funct Neurosurg. 2013;91:92–103. doi: 10.1159/000343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daadi MM, Grueter BA, Malenka RC, Redmond DE, Jr, Steinberg GK. Dopaminergic neurons from midbrain-specified human embryonic stem cell-derived neural stem cells engrafted in a monkey model of Parkinson’s disease. PLoS ONE. 2012;7:e41120. doi: 10.1371/journal.pone.0041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzales-Portillo GS, Sanberg PR, Franzblau M, et al. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant. 2014;23:531–539. doi: 10.3727/096368914X678337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doolittle ND, Muldoon LL, Culp AY, Neuwelt EA. Delivery of chemotherapeutics across the blood-brain barrier: challenges and advances. Adv Pharmacol. 2014;71:203–243. doi: 10.1016/bs.apha.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012;503:269–292. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- 53.Hersh DS, Wadajkar AS, Roberts NB, et al. Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des. 2016;22:1177–1193. doi: 10.2174/1381612822666151221150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hancock HA, Smith LH, Cuesta J, et al. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med Biol. 2009;35:1722–1736. doi: 10.1016/j.ultrasmedbio.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neill BE, Vo H, Angstadt M, Li KP, Quinn T, Frenkel V. Pulsed high intensity focused ultrasound mediated nanoparticle delivery: mechanisms and efficacy in murine muscle. Ultrasound Med Biol. 2009;35:416–424. doi: 10.1016/j.ultrasmedbio.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv Drug Deliv Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dittmar KM, Xie J, Hunter F, et al. Pulsed high-intensity focused ultrasound enhances systemic administration of naked DNA in squamous cell carcinoma model: initial experience. Radiology. 2005;235:541–546. doi: 10.1148/radiol.2352040254. [DOI] [PubMed] [Google Scholar]
- 58.Ziadloo A, Xie J, Frenkel V. Pulsed focused ultrasound exposures enhance locally administered gene therapy in a murine solid tumor model. J Acoust Soc Am. 2013;133:1827–1834. doi: 10.1121/1.4789390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poff JA, Allen CT, Traughber B, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology. 2008;248:485–491. doi: 10.1148/radiol.2482071674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S, Shin IS, Hancock H, et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Release. 2012;162:218–224. doi: 10.1016/j.jconrel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hynynen K, McDannold N, Vykhodtseva J, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 62.Hynynen K. Focused ultrasound for blood-brain disruption and delivery of therapeutic molecules into the brain. Expert Opin Drug Deliv. 2007;4:27–35. doi: 10.1517/17425247.4.1.27. [DOI] [PubMed] [Google Scholar]
- 63.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 64.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol. 2007;33:584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu WY, Wang ZB, Zhang LC, Wei X, Li L. Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther. 2012;18:609–615. doi: 10.1111/j.1755-5949.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nhan T, Burgess A, Cho EE, Stefanovic B, Lilge L, Hynynen K. Drug delivery to the brain by focused ultrasound induced blood-brain barrier disruption: quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J Control Release. 2013;172:274–280. doi: 10.1016/j.jconrel.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbit. Ultrasound Med Biol. 2005;31:1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Marquet F, Tung YS, Teichert T, Ferrera VP, Konofagou EE. Noninvasive, transient and selective blood-brain barrier opening in non-human primates in vivo. PLoS ONE. 2011;6:e22598. doi: 10.1371/journal.pone.0022598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72:3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics. 2012;2:1223–1237. doi: 10.7150/thno.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 72.Etame AB, Diaz RJ, O’Reilly MA, et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine. 2012;8:1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thévenot E, Jordão JF, O’Reilly MA, et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23:1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Shi Y, Lu L, et al. Targeted delivery of GDNF through the blood-brain barrier by MRI-guided focused ultrasound. PLoS ONE. 2012;7:e52925. doi: 10.1371/journal.pone.0052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Q, Deng J, Wang F, et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233:350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 76.Jordão JF, Thévenot E, Markham-Coultes K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan CH, Ting CY, Lin HJ, et al. SPIO-conjugated, doxorubicin-loaded micro-bubbles for concurrent MRI and focused-ultrasound enhanced brain-tumor drug delivery. Biomaterials. 2013;34:3706–3715. doi: 10.1016/j.biomaterials.2013.01.099. [DOI] [PubMed] [Google Scholar]
- 78.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. 2006;103:11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alonso A, Hennerici MG, Reinz E, et al. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol Ther Nucleic Acids. 2013;2:e73. doi: 10.1038/mtna.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Downs ME, Buch A, Sierra C, et al. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE. 2015;10:e0125911. doi: 10.1371/journal.pone.0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum. PLoS ONE. 2015;10:e0130860. doi: 10.1371/journal.pone.0130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobus T, Vykhodtseva N, Pilatou M, Zhang Y, McDannold N. Safety validation of repeated blood-brain barrier disruption using focused ultrasound. Ultrasound Med Biol. 2016;42:481–492. doi: 10.1016/j.ultrasmedbio.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason Imaging. 2008;30:189–200. doi: 10.1177/016173460803000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burgess A, Dubey S, Yeung S, et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. 2014;273:736–745. doi: 10.1148/radiol.14140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leinenga G, Gotz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model. Sci Transl Med. 2015;7(278):278ra33. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 85.Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer’s disease. PLoS ONE. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leinenga G, Langton C, Nisbet R, Götz J. Ultrasound treatment of neurological diseases–current and emerging applications. Nat Rev Neurol. 2016;12(3):161–174. doi: 10.1038/nrneurol.2016.13. Epub ahead of print 19 February 2016. [DOI] [PubMed] [Google Scholar]
- 87.Alkins R, Burgess A, Kerbel R, Wels WS, Hynynen K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro Oncol. 2016;18:974–981. doi: 10.1093/neuonc/nov318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arbab AS, Jordan EK, Wilson LB, Yocum GT, Lewis BK, Frank JA. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]
- 89.Shen WB, Plachez C, Chan A, et al. Human neural progenitor cells retain viability, phenotype, proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. Int J Nanomedicine. 2013;8:4593–4600. doi: 10.2147/IJN.S53012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen WB, Plachez C, Tsymbalyuk O, et al. Cell-based therapy in TBI: magnetic retention of neural stem cells in vivo. Cell Transplant. 2015;25:1085–1099. doi: 10.3727/096368915X689550. [DOI] [PubMed] [Google Scholar]
- 91.Shen W-B, Anastasiadis P, Nguyen B, Yarnell D, Yarowsky P, Frenkel V, Fishman PS. Magnetic enhancement of stem cell targeted delivery into the brain following MR guided focused ultrasound for opening the blood brain barrier. Cell Transplantation. doi: 10.1177/0963689717715824. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Munter JP, Melamed E, Wolters EC. Stem cell grafting in parkinsonism—why, how and when. Parkinsonism Relat Disord. 2014;20:S150–S153. doi: 10.1016/S1353-8020(13)70036-1. [DOI] [PubMed] [Google Scholar]
- 93.Mano Y, Saito R, Haga Y, et al. Intraparenchymal ultrasound application and improved distribution of infusate with convection-enhanced delivery in rodent and nonhuman primate brain. J Neurosurg. 2015;124:1490–1500. doi: 10.3171/2015.3.JNS142152. [DOI] [PubMed] [Google Scholar]
- 94.Wang S, Karakatsani ME, Fung C, Sun T, Acosta C, Konofagou E. Direct brain infusion can be enhanced with focused ultrasound and microbubbles. J Cereb Blood Flow Metab. 2016;37:706–714. doi: 10.1177/0271678X16637881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hersh D, Nguyen BA, Perez JG, et al. Pulsed ultrasound expands the extracellular and perivascular spaces of the brain. Brain Res. 2016;1646:543–550. doi: 10.1016/j.brainres.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen H, Chen CC, Acosta C, Wu SY, Sun T, Konofagou EE. A new brain drug delivery strategy: focused ultrasound-enhanced intranasal drug delivery. PLoS ONE. 2014;9:e.108880. doi: 10.1371/journal.pone.0108880. [DOI] [PMC free article] [PubMed] [Google Scholar]