ABSTRACT
mTORC1 is the major homeostatic nutrient sensor for the cell. As such, it is integrated into diverse signaling networks and co-factor interactions that determine its activity. Our recent work implicates the mTORC1 co-factor and PI3K-related Kinase (PIKK) stabilizer, TELO2, in regulating mTORC1 activity in a MAPK-Interacting Kinase (MNK) responsive manner during mitogenic stimulation of cancer cells and T cell activation.
keywords: DDB1, DEPTOR, MNK, mTOR, TELO2
Complex regulation of mTORC1 involves several co-factors that influence its catalytic output. Components of mTORC1 include mTOR, the catalytic subunit; Raptor, the substrate adaptor; GβL, which regulates mTOR catalytic activity; DEPTOR and Pras40, endogenous inhibitors of mTORC1; and TELO2, which was previously implicated in binding to and regulating the protein stability of PIKKs including mTOR.1,2 Collectively, these accessory proteins integrate multifaceted signals to influence basic homeostatic processes and growth programs.
mTORC1 suppression with the prototypical inhibitor rapamycin and its derivatives, the rapalogs, have found clinical utility for immunosuppression after organ transplantation, but disappointed as cancer therapeutics. The lacking efficacy of the rapalogs against cancer is evident in the form of compensatory feedback signals, including through MAPK activation, that sustain—or even promote—cancer cell growth.3 We previously discovered that MNK1/2, a downstream substrate of the ERK1/2 (MNK1 and 2) and p38α MAPK (MNK1) kinases, stimulates mTORC1 signaling to control viral IRES-mediated translation.4–6 Earlier reports have linked MNK signaling to mTORC1 inhibitor resistance in cancer.7 More recently, we discovered that the mechanism of MNK-mediated mTORC1 regulation occurs through inverse coordination of DEPTOR and TELO2 binding with mTORC1, which ultimately determines mTORC1:substrate interactions.8 Our findings implicate MNK in an mTORC1-centered feedback loop, wherein mTORC1 inhibition activates MAPK signaling, which induces MNK activity to reactivate mTORC1. Thus, our work uncovers deep functional integration of the principal Raf-ERK1/2 and PI3K-mTOR signal transduction pathways via MNK. Deciphering these integrated signaling networks is required to understand the implications of protein kinase inhibition in disorders with unhinged mitogenic signaling pathways, e.g. advanced, treatment-refractory cancers.
MNK is best known for its association with translation initiation machinery. It binds the “ribosome adapter,” eukaryotic initiation factor (eIF) 4G, to phosphorylate the cap binding protein, eIF4E. Despite 20 years of research, MNK's influence on protein synthesis control remains obscure. The functional intricacies resulting from MNK's involvement in multiple signaling systems with roles in protein synthesis regulation may be to blame. Also, MNK's broader functional role in oncogenic signaling remains elusive.9 Therefore, after describing MNK's apparent role in regulating mTORC1 activity,5 we sought to identify the mechanism responsible. Phenotypically, MNK inhibition synergizes with rapamycin in tempering mTORC1 phosphorylation of the rapamycin-resistant substrates ULK1 and 4EBP in cancer cells and T cells.8 Surprisingly, MNK accomplishes such regulation independent of its canonical role in phosphorylating eIF4E.4,8
To identify potential binding partners through which MNK might control mTORC1, we tested whether MNK associates with known mTORC1 co-factors. This analysis uncovered MNK associations with mTOR, Raptor, TELO2, and the E3 ubiquitin ligase DNA Damage Binding protein 1 (DDB1). Aside from its involvement in DNA damage responses, DDB1 regulates mTORC1 signaling through ubiquitination of Raptor.10 Intriguingly, MNK binds this complex through a mechanism distinct from eIF4G, because deleting MNK's eIF4G binding domain did not abolish formation of the complex.8 These associations depend on upstream MAPK signals, because PKC-Raf-ERK1/2 stimuli led to increased binding of MNK with mTORC1:TELO2/DDB1.8 Interestingly, MNK inhibition reduces MNK binding to TELO2/DDB1, but did not affect its association with mTORC1.8 These findings suggest that MNK most prominently interacts with mTORC1 to facilitate mTORC1:TELO2/DDB1 binding. Indeed, MNK inhibition reduces mTORC1 co-IP with both TELO2 and DDB1, indicating that MNK signaling stabilizes mTORC1:TELO2/DDB1 associations.8
Co-regulation of TELO2 and DDB1 has not previously been documented. DDB1 and TELO2 binding to mTORC1 responds to MNK inhibition in tandem,8 indicating functional coordination of these factors. This is a particularly interesting possibility because TELO2 and DDB1 are both linked to the DNA damage response (DDR); TELO2 binds to the DDR regulators and PIKKs: ATM, ATR, and DNA-PK; and DDB1 has numerous roles in the DDR including initiating DNA damage repair. Whether or not MNK might play a role in TELO2 and/or DDB1 biology aside from mTORC1 remains to be determined.
In testing the effects of MNK activity on mTORC1 associations, we also determined that MNK inhibition increased mTORC1:DEPTOR binding, coinciding with a reduction in mTORC1:substrate binding.8 Overexpression and depletion studies revealed that MNK controls mTORC1:substrate proximity through TELO2, but not DDB1.8 Also, MNK does not regulate mTORC1:DEPTOR association through its enhancement of TELO2:mTORC1 binding, as TELO2 overexpression stimulates mTORC1:substrate association without reducing DEPTOR:mTORC1 binding.8 Rather, DEPTOR overexpression reduced TELO2: mTORC1 binding while inhibiting mTORC1:substrate association. Given the lack of a MNK:DEPTOR interaction, our findings suggest that MNK acts on mTORC1 to displace DEPTOR to facilitate TELO2 binding (Fig. 1).
Figure 1.
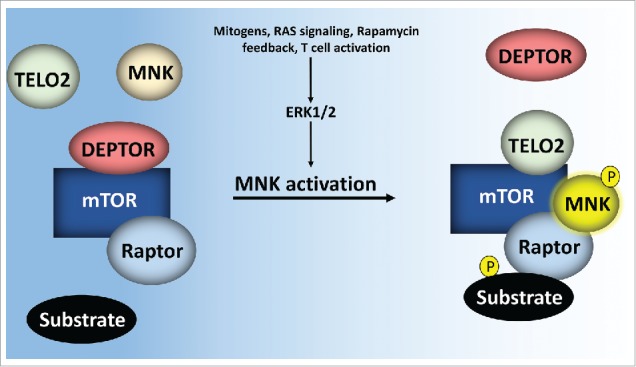
MNK activity displaces DEPTOR from mTORC1 and facilitates TELO2 binding to enhance mTORC1 substrate association. MNK is phosphorylated by ERK1/2 and p38α MAPKs downstream of mitogenic/stress stimuli. This enables MNK binding with mTORC1, along with simultaneous displacement of DEPTOR and recruitment of TELO2. The resulting TELO2:mTORC1 binding stabilizes mTORC1:substrate association.
Overall, our work defines MNK as an inhibitor of DEPTOR:mTORC1 binding and stabilizer of TELO2:mTORC1 binding. The finding that TELO2:mTORC1 association enables mTORC1:substrate association demonstrates that TELO2 is involved in mTOR biology beyond its role in stabilizing nascent mTOR protein.2 It remains to be determined whether TELO2 similarly influences other PIKKs. From our studies, we also hypothesize that DEPTOR's inhibitory role in mTORC1 signaling, and possibly mTORC2, might be accomplished though the displacement of TELO2 from mTOR. DEPTOR and TELO2 clearly serve as inversely regulated, counterbalancing forces that modulate mTORC1 signaling. It is clear that the critical lever in controlling these forces is MNK, an ERK1/2 and p38α MAPK substrate: the profound functional integration of MAPK- with the mTOR signaling network may make therapeutic targeting of mTOR in cancer intractable.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; https://doi.org/ 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takai H, Wang RC, Takai KK, Yang H, de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell 2007; 131:1248-59; PMID:18160036; https://doi.org/ 10.1016/j.cell.2007.10.052 [DOI] [PubMed] [Google Scholar]
- 3.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al.. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 2008; 118:3065-74; PMID:18725988; https://doi.org/ 10.1172/JCI34739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MC, Bryant JD, Dobrikova EY, Shveygert M, Bradrick SS, Chandramohan V, Bigner DD, Gromeier M. Induction of viral, 7-methyl-guanosine cap-independent translation and oncolysis by mitogen-activated protein kinase-interacting kinase-mediated effects on the serine/arginine-rich protein kinase. J Virol 2014; 88:13135-48; PMID:25187541; https://doi.org/ 10.1128/jvi.01883-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MC, Dobrikov MI, Gromeier M. Mitogen-activated protein kinase-interacting kinase regulates mTOR/AKT signaling and controls the serine/arginine-rich protein kinase-responsive type 1 internal ribosome entry site-mediated translation and viral oncolysis. J Virol 2014; 88:13149-60; PMID:25187540; https://doi.org/ 10.1128/JVI.01884-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MC, Gromeier M. Oncolytic immunotherapy through tumor-specific translation and cytotoxicity of poliovirus. Discov Med 2015; 19:359-65; PMID:26105699 [PMC free article] [PubMed] [Google Scholar]
- 7.Grzmil M, Huber RM, Hess D, Frank S, Hynx D, Moncayo G, Klein D, Merlo A, Hemmings BA. MNK1 pathway activity maintains protein synthesis in rapalog-treated gliomas. J Clin Invest 2014; 124:742-54; PMID:24401275; https://doi.org/ 10.1172/JCI70198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MC, Gromeier M. MNK Controls mTORC1:Substrate Association through Regulation of TELO2 Binding with mTORC1. Cell Rep 2017; 18:1444-57; PMID:28178522; https://doi.org/ 10.1016/j.celrep.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K Fukunaga R, Mak TW. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci U S A 2010; 107:13984-90; PMID:20679220; https://doi.org/ 10.1073/pnas.1008136107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain S, Feldman AL, Das C, Ziesmer SC, Ansell SM, Galardy PJ. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol 2013; 33:1188-97; PMID: 23297343; https://doi.org/ 10.1128/MCB.01389-12 [DOI] [PMC free article] [PubMed] [Google Scholar]


