Abstract
Streptococcus suis is an important zoonotic pathogen in swine and humans that causes sepsis and meningitis. Our previous study in Thailand showed that the prevalence of S. suis infection in humans, especially in northern areas of Thailand, and the transmission of the pathogen occurred mainly through the consumption of traditional raw pork products. Considering the high incidence proportion and mortality rate of the disease as an important public health problem, we implemented a food safety campaign in the Phayao Province in northern Thailand in 2011. We evaluated the effects of a food safety campaign by comparing the sociodemographic, clinical, and bacteriological characteristics of cases before and after the campaign. The follow-up study showed a marked decrease of the incidence proportion in the first 2 years, indicating the effectiveness of the campaign. In the third year, however, the incidence proportion slightly increased again, indicating the existence of deep-rooted cultural behaviors and the necessity of continuous public health intervention. Furthermore, epidemiological analysis of the cases made it possible to estimate the infectivity of the pathogen via the oral route of infection. In the present study, we showed the effectiveness of the food safety campaign for controlling the S. suis infection, and we present a role model public health intervention for prevalent areas affected by S. suis infection in humans.
Introduction
Streptococcus suis is an important zoonotic pathogen that causes invasive infections in swine and humans. Among 29 serotypes identified to date,1 serotype 2 strains are the most prevalent and pathogenic in human cases, followed by serotype 14 strains.2 Streptococcus suis infection causes bacteremia, sepsis, septic arthritis, and meningitis with severe neurological sequelae, including permanent hearing loss.3,4 In North America and European countries, the S. suis infection is a kind of occupational disease, and it occurs mostly among abattoir workers, pig breeders, and butchers who have close contact with pigs or pork-derived products.2 In Asian countries, however, it commonly affects people who consume traditional raw pork products and those who raise pigs in their backyard, as there is a high annual incidence proportion among the general population. Our previous study in northern Thailand in 2010 showed the prevalence of the disease with annual incidence proportions of 6.4/100,000 persons (total population) and 6.2/100,000 persons (general population), which were higher than that in other areas and countries.2,5 The high mortality rate of 16.5% demonstrated that the disease is an important social problem. The study also showed that the majority of patients had a history of consuming raw pork products (71%) and a history of close contact with pigs, pork, or raw pork products (6.5%), indicating the two major routes of infection in the general population. They were closely associated with several traditional festivals and parties held in the area and the possible effectiveness of public health interventions was suggested. In the present study, we aimed to evaluate the effects of a food safety campaign by comparing the sociodemographic, clinical, and bacteriological characteristics of cases before and after the campaign.
Materials and Methods
Study design and population.
The study was performed in the Phayao Province of Thailand, which is located in northern Thailand and consists of nine districts, including Muang Phayao, Chiang Kham, Mae Chai, Phu Kamyoo, Dok Khamtai, Chun, Chiang Muan, Pong, and Phu Sang (Figure 1 ). A food safety campaign was implemented between January 2011 and December 2011, and the incidence of S. suis infections in humans in the Phayao Province was prospectively recorded between January 2010 and September 2013. The results of our previous study in 2010 were included in the results of the present study for comparison.5 In addition, the data in 2008 and 2009 were retrospectively collected for further assessment. The population of the Phayao Province in each year was 487,386 (in 2008), 487,120 (in 2009), 486,304 (in 2010), 486,472 (in 2011), 488,120 (in 2012), and 486,744 persons (in 2013). Data of the population were collected from the Thailand Provincial Administration Department, Ministry of Interior.6
Figure 1.
Location of the study site. (A) Location of the Phayao Province (black) in northern Thailand (brown). (B) Location of the nine districts in the Phayao Province.
Process of the food safety campaign.
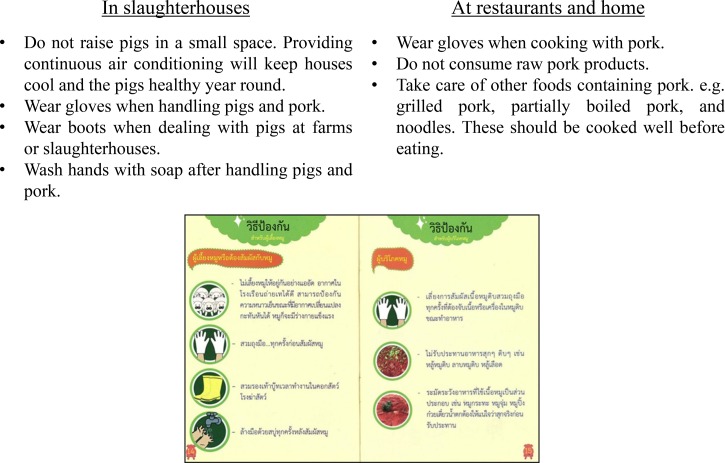
The objectives of the campaign were to increase knowledge about the disease and change people's behaviors related with the transmission routes. The recommended behavior changes are described in Figure 2 . The Phayao Province had already established and used a unique public health network to distribute updated medical information to local residents, and our food safety campaign was implemented with the help of this public health network. The process of the campaign is outlined as a flow diagram in Figure 3 . With the help of the Ministry of Public Health in Thailand, Bureau of Food Safety Extension and Support, and Department of Medical Sciences, the Phayao Public Health Office held a lecture with all health-care volunteers in the Phayao Province, and these volunteers returned to their respective villages to conduct educational lectures for local residents. Prior to the campaign, several campaign materials were created (Figure 4 ): pamphlets showing the transmission route of the pathogen and related risk behaviors, symptoms of the disease, and the prophylactic methods to prevent infection with humorous cartoon characters intended for easier understanding; public display banners depicting the former minister of the Ministry of Public Health in Thailand explaining that consuming raw pork products, which is a common side dish for alcohol in the region, is ultimately associated with hearing loss and death (drawing a relationship to death was intended to work as an emotional driver to evoke disgust at the consumption of raw pork products); and posters explaining the transmission route and major symptoms of the disease.
Figure 2.
Behavior changes recommended by the food safety campaign. The two pages of the pamphlet are shown at the bottom of the figure, and the recommendation is described in Thai. Recommendation is translated in English above the pamphlet.
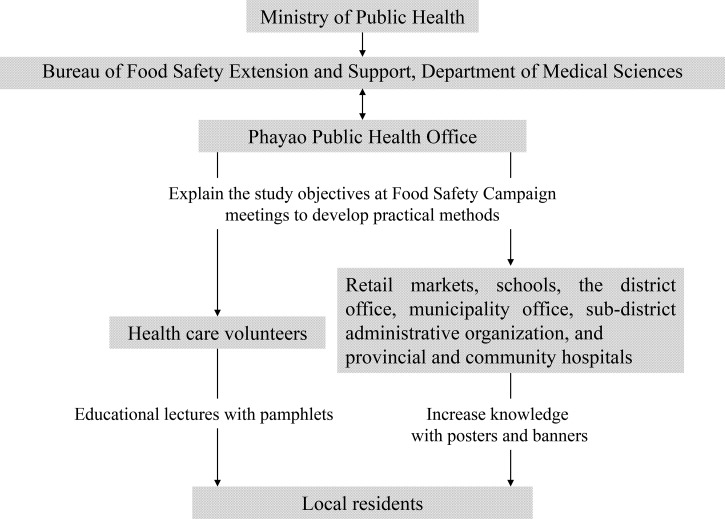
Figure 3.
Flow diagram of the food safety campaign. The preexisting public health network established by the Phayao Public Health Office was used to distribute knowledge to the local people.
Figure 4.
Examples of the campaign materials. Pamphlets were used in educational lectures for the local people, and the banner and poster were distributed and displayed in every district in the Phayao Province.
The pamphlets were distributed to every health-care volunteer as tools during educational lectures for local residents. They were also widely distributed to the local residents. The banners were distributed to every district in the Phayao Province and displayed at district and municipality offices, retail markets, schools, and some villages. The posters were displayed inside of two central hospitals.
The total cost of the campaign was around $1,500 (50,000 Thai Baht). The cost included the preparation and distribution of banners, posters, and pamphlets, and the lecture with health-care volunteers.
Collection and analysis of the data on S. suis infection cases.
Data on the S. suis infected human cases were collected as described in our previous report.5 Briefly, we established a S. suis surveillance network among the Public Health Office, two tertiary hospitals, and five district hospitals. By using this surveillance network, patients were enrolled in our study when S. suis was isolated from blood or cerebrospinal fluid, and patients' clinical record forms were collected and analyzed. The possibility of cluster cases and outbreaks was assessed according to the same criteria as previously described.5
Based on the collected data, the incidence proportions before and after the food safety campaign, sociodemographic and clinical characteristics of the cases, locations where the patients consumed raw pork products, and number of people who consumed raw pork products with the patient were analyzed. It should be noted that all the data from 2013 were underestimated because the project was terminated at the end of September.
To analyze the locations where the patient consumed raw pork products, the locations were categorized as public (e.g., restaurants and markets) or private (e.g., homes and village parties) to assess the propagation of the campaign.
To analyze the number of people who consumed raw pork products at the same place with the patient, the cases were divided into three groups according to the size of the group: 1 (only the patient), 2–9 (2–9 persons including the patient), and ≥ 10 (large groups such as village parties of ≥ 10 persons including the patient).
Microbiological study.
The microbiological study was performed as described previously.5 Briefly, the bacteriological identification of the clinical isolates and the confirmation of S. suis serotypes were performed by a biochemical test, S. suis-specific polymerase chain reaction (PCR), and coagglutination test with rabbit antisera. All confirmed S. suis clinical isolates were subjected to further analysis by multilocus sequence typing (MLST) and pulsed-field gel electrophoresis. Regarding the retrospectively collected clinical isolates in 2008 and 2009, API Strep test (bioMérieux, Durham, NC) and S. suis-specific PCR methods were used for the confirmation.
Climate information.
Climate information of the Phayao Province between 2010 and 2013 was collected from the Phayao Meteorological Station, Thai Meteorological Department, Ministry of Information and Communication Technology.7
Statistical analysis.
Poisson's regression analysis and Dunnett's multiple comparison were used to compare the annual incidence proportion in each year after the campaign with that in 2010. Generalized estimating equation (GEE) regression model was used to analyze the change in trend of incidence proportion before and after the campaign. Mann–Whitney's U test and Fisher's exact test were used to compare the sociodemographic, clinical, and bacteriological characteristics between the study periods before and after implementing the food safety campaign.
Ethics.
The present study was reviewed and approved by the ethics committees of the Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand, and Research Institute for Microbial Diseases, Osaka University. This study was conducted according to the principles expressed in the Declaration of Helsinki. Patients or their guardians provided written informed consent for all cases. This study was registered in the University hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000006449).
Results
Changes in the incidence proportion and seasonal distribution of S. suis infection cases with climate information.
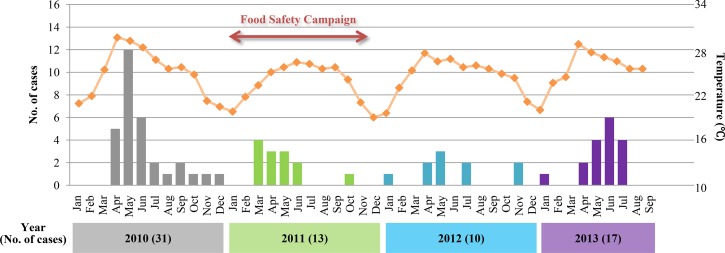
Compared with 31 cases in 2010, the number of S. suis infection cases in 2011 and 2012 markedly decreased to 13 and 10 cases, respectively. Additionally, the annual incidence proportion significantly decreased from 6.4/100,000 persons in 2010 to 2.7/100,000 persons in 2011 (P = 0.0248) and to 2.0/100,000 persons in 2012 (P = 0.0055) (Table 1). However, in 2013, the number of cases slightly increased to 17, showing the peak incidence in June 2013 (Figure 5 ). The annual incidence proportion of 3.5/100,000 persons in 2013 was not significantly different from that in 2010 (P = 0.1274) (Table 1).
Table 1.
Summary of the statistics in Poisson's regression analysis and Dunnett's multiple comparison of the annual incidence proportion
| Year | Year compared | Estimate | Standard error | Wald 95% confidence intervals | Pr > |Z| | Adjusted P value |
|---|---|---|---|---|---|---|
| 2011 | 2010 | −0.8690 | 0.3304 | −1.5167 to −0.2214 | 0.0085 | 0.0248 |
| 2012 | 2010 | −1.1314 | 0.3637 | −1.8442 to −0.4186 | 0.0019 | 0.0055 |
| 2013 | 2010 | −0.6008 | 0.3018 | −1.1923 to −0.0093 | 0.0465 | 0.1274 |
Figure 5.
Seasonal distribution of the cases and temperature changes from 2010 to 2013 in the Phayao Province. The period of the food safety campaign is shown by a bidirectional arrow.
To confirm the decrease of incidence was not a result of normal variation, the trends of incidence proportion before (2008, 2009, and 2010) and after (2011, 2012, and 2013) the campaign were analyzed by GEE regression model. It revealed a 3.94/100,000 persons decrease in the trend of incidence proportion after campaign (P < 0.001) (Supplemental Table 1). Considering the total cost of the campaign ($1,500), the cost to reduce an incidence proportion of 1.0/100,000 persons was around $380.
According to the three seasons in Thailand (rainy, hot, and dry), temperature changed in a similar way each year. There were no characteristic changes of temperature that may have influenced the annual incidence proportion of the disease. In addition, we could not obtain any information on outbreaks of diseases in pigs, which may also have an influence on the incidence proportion in humans during the study period.
Sociodemographic, clinical, and bacteriological characteristics of S. suis infection cases.
The sociodemographic and clinical characteristics of cases after the food safety campaign (2011–2013) were not significantly different from those in 2010 (Table 2). During and after the campaign, men were more susceptible to the infection than women. The mean age of patients was 59.1 years; 85% of cases had a history of recently consuming raw pork products, and 25% had a history of recent contact with pigs or raw pork products. The median days from exposure to the onset of illness were 2 days in both estimated routes of infection; 45% of cases developed meningitis, and the characteristic symptom of hearing loss was found in 22.5%. Although the fatality rate was 5% and was smaller than that in 2010, it was not statistically significant. The combined data from 2010 to 2013 are presented in Table 2.
Table 2.
Summary of the sociodemographic, clinical, and bacteriological characteristics
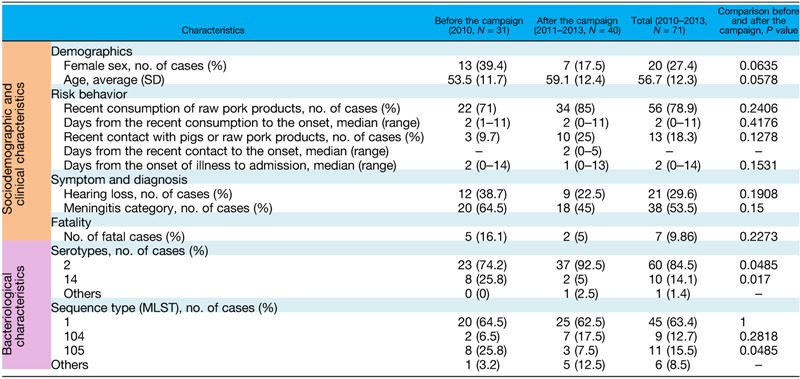
MLST = multilocus sequence typing; N = number; SD = standard deviation.
In microbiological analysis, the prevalence of serotype 2 strains followed by serotype 14 strains was confirmed. In MLST analysis, ST1 strains were the most prevalent followed by the ST105 and ST104 strains. All ST1 and ST104 strains belong to serotype 2 strains, and all ST105 strains belong to serotype 14 strains. The comparison of the prevalent serotype showed a significant increase in the proportion of serotype 2 strains and a significant decrease of serotype 14 strains after the food safety campaign. In MLST analysis, ST105-infected cases decreased after the campaign.
All cases between 2011 and 2013 were sporadic without epidemiological and bacteriological associations according to our definition of cluster cases and outbreaks.
Locations where the patients consumed raw pork products.
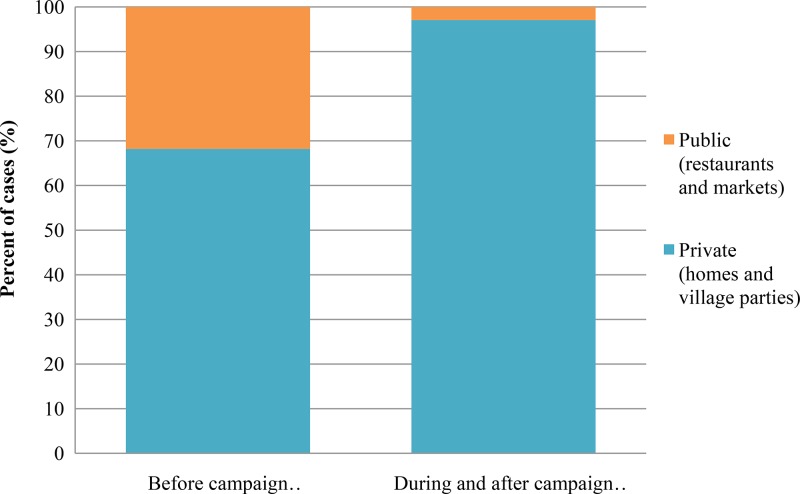
The locations where patients consumed raw pork products were investigated (Figure 6 ). Compared with the cases in 2010, the proportion of consumption at public places, such as restaurants and markets, markedly decreased from 32% to 3% during and after the campaign (P = 0.0039), respectively. However, the proportion of patients who consumed homemade raw pork products (made at home or at a village party) increased during and after the campaign.
Figure 6.
Places where the patients consumed raw pork products. The places were divided into two groups: public (e.g., restaurants and markets) and private (e.g., homes and village parties). The Fisher's exact test was used to compare the data before and after the campaign.
The number of people who consumed raw pork products at the same place with the patient.
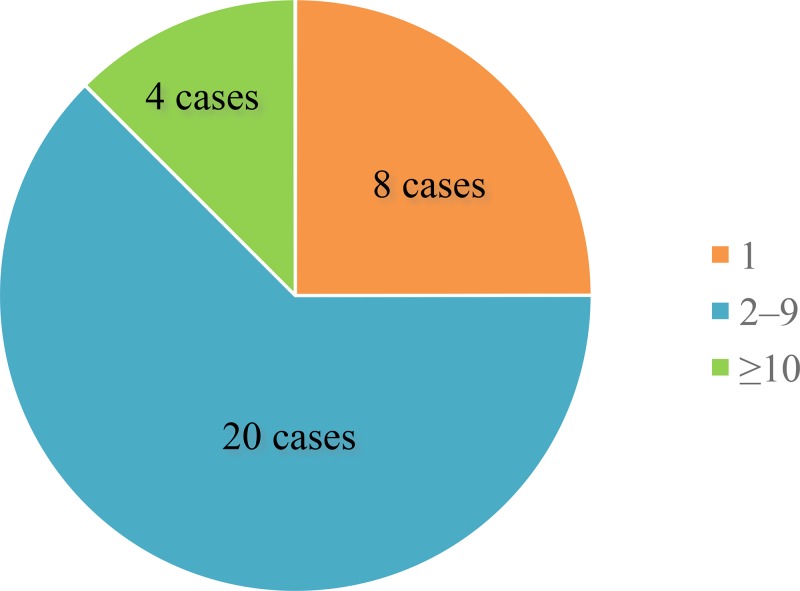
The number of people who consumed the same contaminated food at the same place with the patient was investigated between 2011 and 2013 to estimate the infectivity of S. suis via the oral route of infection (Figure 7 ). Twenty-two cases sporadically occurred in the group with 2–9 participants, and four cases occurred in the relatively larger groups with ≥ 10 participants. In eight cases, the patient solely consumed raw pork products.
Figure 7.
The number of people who consumed raw pork products at the same place with the patient. The cases were divided into three groups: 1 (only the patient), 2–9 (2–9 persons including the patient), and ≥ 10 (large groups such as village parties of ≥ 10 persons including the patient).
Discussion
In our previous population-based study in 2010, S. suis infection in humans was found to be prevalent in northern Thailand.4 These results prompted us to implement a food safety campaign in the Phayao Province to prevent infections and evaluate its impact. The food safety campaign seemed to be effective in 2011 and 2012 with a marked reduction of the number of cases compared with that in 2010 (Figure 5). The significant decrease in the trend of incidence proportion after the campaign also supported the effectiveness of it (Supplemental Table 1). The successful control of the disease seemed to be achieved by the successful distribution of knowledge to local residents through the active public health network in the Phayao Province (Figure 3). Since the network had continuously contributed to the education of local residents on various health problems, local residents were familiar with this system and exhibited good compliance. During the final step of the campaign, health-care volunteers established small educational groups with local residents to discuss prophylactic behavioral changes (Figure 2). This face-to-face education with small groups seemed to be effective.
However, in 2013, the number of cases slightly increased again to 17 (Figure 5). Considering that there were not any possible cluster cases, climatic changes, disease outbreaks in pigs, or newly emerging bacterial strains that may show unexpected routes of infection, the increase in the number of cases in 2013 seemed to be attributable to the increase of contact with raw pork products and consumption of them by local residents. As these raw pork products are strongly related with cultural behaviors,8,9 it may be difficult to maintain behavioral changes throughout a 1-year campaign. A continuous campaign or introducing educational programs in childhood may be effective for further behavior changes. Moreover, a direct approach may be effective to improve the sanitation of pork processing and eliminate the disease.
One of the factors to consider for the peak incidence between April to June in 2010 and 2013 is, as previously discussed in our report,5 an increase in the frequency of raw pork products consumed during these months because of the existence of a traditional Thai festival (i.e., the Songkran festival), agricultural parties, and farewell parties for people leaving for the military service. The characteristic peak of the incidence in 2013, which reemerged along with an increase of the number of cases, is suggestive of the increased consumption of raw pork products at these parties. Another possible reason for the peak incidence is the influence of climatic factors, which are commonly reported in several kinds of infectious diseases.10 In a previous study, a hot and humid climate was considered to contribute to an increase in bacterial density in pork samples at retail markets.11 Hence, it was conceivable that the hot and rainy season (from May to July) in northern Thailand also influences bacterial concentrations in pork and raw pork products; thus, the incidence between April and June increased.
In addition to the decrease in the number of cases after the food safety campaign was implemented in the first 2 years, we found a relatively significant decrease in the proportion of female cases (Table 2). This may be related to the gender difference in the acceptance of new knowledge associated with health risk. Additionally, we found a change in places where patients consumed raw pork products (Figure 6). The significant decrease in the proportion of public places such as restaurants and markets is preferable for visitors from other areas, since these places are where they consume raw pork products without knowledge on the risk of raw pork consumption. However, the changes does not mean the decrease of a total risk of the infection in the province as is shown by the increase of the proportion of those that consumed raw pork at home and at village parties. Raw pork consumed at these private places pose a risk of cluster cases and outbreaks among local residents. The persistent consumption at private places indicated the deep-rooted culture of consuming raw pork products in northern Thailand.
In the present study, we obtained new interesting knowledge about the infectivity of S. suis. The fact that infections sporadically occurred at parties with more than one participant who consumed the same contaminated food (Figure 7) seemed to indicate that the infectivity of S. suis via the oral route is not so high. Additionally, this result also suggests the importance of host factors in establishing infection, such as alcohol abuse or liver cirrhosis.12,13 However, it is too hasty to draw a conclusion about the low infectivity of the pathogen in all human infection cases. Indeed, we confirmed a cluster case in the previous study in 2010.5 Moreover, considering the past outbreaks in which a higher proportion of raw pork consumers were infected with the same strain from the same contaminated food,14 infectivity may differ with each strain, and some extra virulence factors exist in outbreak-causing strains to acquire higher infectivity. To determine the bacterial virulence factor and the mechanism of the S. suis infection, it is necessary to closely monitor the incidence to detect outbreaks and analyze the responsible strains.
In microbiological analysis, the prevalence of serotype 2 strains and the clonal dissemination of serotype 14 strains were confirmed as previously described.5,15 Since all ST105 strains belonged to serotype 14 strains and all serotype 14 strains, except for one strain, belonged to ST105 strains, the decreased isolation rate of ST105 strains after the food safety campaign seemed to reflect the decreased isolation rate of serotype 14 strains. The reason for the decreased isolation rate of serotype 14 strains (and ST105) after the campaign is unclear. This may have resulted from the relatively lower infectivity of serotype 14 strains, or it may have resulted from the shift of prevalent strains in the natural environment, although no evidence of them exists. A continuous investigation on the clinical isolates and further study on the prevalent strains in pigs in the field will elucidate the answer to this question.
Our study had several limitations. This is a pre-post intervention design and the lack of random assignment is the major weakness of it. Therefore, there are several limitations in the assessment including causal inference and transferability of the intervention. It is needed to increase the number of measurements pre and post intervention or use a control group for an improvement in the study design. Although campaign materials, such as pamphlets, banners, and posters, have been used in the region as generally acceptable tools in public health interventions, they should have been field tested before implementation. In addition, intervention may have been based on a theory of behavior change to evaluate the outcome more efficiently. Regarding the data collection, the number of cases in 2008 and 2009 were possibly underestimated, because the surveillance network was established in 2010 and the data were collected retrospectively. And we could not collect the information on the population of pigs and the amount of pig consumption in the region, hence we could not evaluate their influence on the incidence proportion. In addition, data on the movement of general population in and out of intervention area, Phayao Province, was not available during the study period. Incomplete enrollment of target population due to unknown border movement may account for the limited change seen in the incidence proportion after the campaign.
In conclusion, we demonstrated the effectiveness of a food safety campaign for controlling the S. suis infection in humans. However, the effect seemed to last for only a few years because there was a strong connection between raw pork products and the culture of northern Thailand. Considering these results, a continuous campaign or additional and alternative public health interventions are needed to eliminate the disease. The results of our study would be applicable to other regions where the disease is prevalent, and this information may help clarify the nature of the infectious disease related with traditional food and cultures.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the support from Jonkolnee Vithayarungruangsri and Suwat Lertchayanti, who helped manage the food safety campaign. We would also like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Financial support: We thank the Department of Medical Sciences, Ministry of Public Health of Thailand, Japan Society for the Promotion of Science (KAKENHI 21406027), and Japan Initiative for a Global Research Network on Infectious Diseases launched by the Ministry of Education, Science, and Culture of Japan for providing financial support for this work.
Authors' addresses: Dan Takeuchi and Shigeyuki Hamada, Section of Bacterial Drug Resistance Research, Thailand-Japan Research Collaboration Center on Emerging and Re-emerging Infections, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan, E-mails: dantake@biken.osaka-u.ac.jp and hamadas@biken.osaka-u.ac.jp. Anusak Kerdsin, Faculty of Public Health, Kasetsart University Chalermphrakiat Sakon Nakhon Campus, Sakon Nakhon, Thailand, E-mail: noksak99@gmail.com. Yukihiro Akeda, Department of Infection Control and Prevention, Graduate School of Medicine, Osaka University, Osaka, Japan, E-mail: akeda@hp-infect.med.osaka-u.ac.jp. Piphat Chiranairadul, Phacharaphan Loetthong, and Nutchada Tanburawong, Phayao Provincial Hospital, Phayao, Thailand, E-mails: ayapiphat@yahoo.com, phacha0037@gmail.com, and wnithita@yahoo.com. Prasanee Areeratana, Panarat Puangmali, Kasean Khamisara, Wirasinee Pinyo, and Rapeepun Anukul, Chiang Kham General Hospital, Phayao, Thailand, E-mails: lekprass2@hotmail.com, puangli_1@hotmail.com, kasian04@hotmail.com, wirasinee_loy@hotmail.com, and ra.peepan111@hotmail.com. Sutit Samerchea, Phayao Public Health Office, Phayao, Thailand, E-mail: sutit2007@hotmail.com. Punpong Lekhalula, Food Safety Operation Center, Nonthaburi, Thailand, E-mail: punpong.fsoc@gmail.com. Tatsuya Nakayama, Global Collaboration Center, Osaka University, Osaka, Japan, E-mail: t.nakayama-glocol@outlook.com. Kouji Yamamoto, Department of Clinical Epidemiology and Biostatistics, Graduate School of Medicine, Osaka University, Osaka, Japan, E-mail: yamamoto-k@stat.med.osaka-u.ac.jp. Masayo Hirose, Institute of Statistical Mathematics, Tokyo, Japan, E-mail: masayo@ism.ac.jp. Surang Dejsirilert, National Institute of Health, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand, E-mail: surang_dej@yahoo.com. Kazunori Oishi, Infectious Disease Surveillance Center, National Institute of Infectious Disease, Tokyo, Japan, E-mail: oishik@nih.go.jp.
References
- 1.Kerdsin A, Akeda Y, Hatrongjit R, Detchawna U, Sekizaki T, Hamada S, Gottschalk M, Oishi K. Streptococcus suis serotyping by a new multiplex PCR. J Med Microbiol. 2014;63:824–830. doi: 10.1099/jmm.0.069757-0. [DOI] [PubMed] [Google Scholar]
- 2.Huong VT, Ha N, Huy NT, Horby P, Nghia HD, Thiem VD, Zhu X, Hoa NT, Hien TT, Zamora J, Schultsz C, Wertheim HF, Hirayama K. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis. 2014;20:1105–1114. doi: 10.3201/eid2007.131594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010;5:371–391. doi: 10.2217/fmb.10.2. [DOI] [PubMed] [Google Scholar]
- 4.Kerdsin A, Dejsirilert S, Puangpatra P, Sripakdee S, Chumla K, Boonkerd N, Polwichai P, Tanimura S, Takeuchi D, Nakayama T, Nakamura S, Akeda Y, Gottschalk M, Sawanpanyalert P, Oishi K. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg Infect Dis. 2011;17:835–842. doi: 10.3201/eid1705.100754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi D, Kerdsin A, Pienpringam A, Loetthong P, Samerchea S, Luangsuk P, Khamisara K, Wongwan N, Areeratana P, Chiranairadul P, Lertchayanti S, Petcharat S, Yowang A, Chaiwongsaen P, Nakayama T, Akeda Y, Hamada S, Sawanpanyalert P, Dejsirilert S, Oishi K. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One. 2012;7:e31265. doi: 10.1371/journal.pone.0031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thailand Provincial Administration Department, Ministry of Interior http://www.dopa.go.th Available at. Accessed October 27, 2015.
- 7.Phayao Meteorological Station, Thai Meteorological Department, Ministry of Information and Communication Technology http://www.cmmet.tmd.go.th/station/phayao/ Available at. Accessed October 27, 2015.
- 8.Nakai S. Analysis of pig consumption by smallholders in a hillside swidden agriculture society of northern Thailand. Hum Ecol. 2009;37:501–511. [Google Scholar]
- 9.Wangsomboonsiri W, Luksananun T, Saksornchai S, Ketwong K, Sungkanuparph S. Streptococcus suis infection and risk factors for mortality. J Infect. 2008;57:392–396. doi: 10.1016/j.jinf.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Semenza JC, Menne B. Climate change and infectious diseases in Europe. Lancet Infect Dis. 2009;9:365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 11.Cheung PY, Lo KL, Cheung TT, Yeung WH, Leung PH, Kam KM. Streptococcus suis in retail markets: how prevalent is it in raw pork? Int J Food Microbiol. 2008;127:316–320. doi: 10.1016/j.ijfoodmicro.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Kerdsin A, Dejsirilert S, Sawanpanyalert P, Boonnark A, Noithachang W, Sriyakum D, Simkum S, Chokngam S, Gottschalk M, Akeda Y, Oishi K. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet. 2011;378:960. doi: 10.1016/S0140-6736(11)60923-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama T, Takeuchi D, Matsumura T, Akeda Y, Fujinaga Y, Oishi K. Alcohol consumption promotes the intestinal translocation of Streptococcus suis infections. Microb Pathog. 2013;65:14–20. doi: 10.1016/j.micpath.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Khadthasrima N, Hannwong T, Thammawitjawa P, Pingsusean D, Akkanij B, Jaikhar A, Paungmali P, Yudee P, Wongyai S, Samerchea S, Tipsriraj S, Pruksakorn S, Sutdan D, Noimoh T, Chalamaat M, Samitsuwan P, Chuxnum T, Areechokchai D. Human Streptococcus suis outbreak in Phayao Province, Thailand, 2007. OSIR. 2008;1:4–7. [Google Scholar]
- 15.Kerdsin A, Oishi K, Sripakdee S, Boonkerd N, Polwichai P, Nakamura S, Uchida R, Sawanpanyalert P, Dejsirilert S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J Med Microbiol. 2009;58:1508–1513. doi: 10.1099/jmm.0.013656-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.