Abstract
More than 2 billion people are infected with parasites globally, and the majority have coinfections. Intestinal protozoa and helminths induce polarizing CD4+ T-helper cell 1 (Th1) mediated cytokine responses within the host. Such immune polarization may inhibit the ability of the host to mount an adequate immune response for pathogen clearance to concurrent pathogens. The current study evaluated the plasma cytokine profile in Ascaris and Giardia coinfected children compared with Giardia- and Ascaris-only infected children. Fecal samples and blood samples were collected from asymptomatic 3-year-old children living in the district of Quininde, Ecuador. Stool samples that tested positive for Giardia lamblia-only, Ascaris lumbricoides-only, or G. lamblia and A. lumbricoides coinfections were confirmed by quantitative real-time polymerase chain reaction. Plasma samples from the study subjects were used to quantitate cytokines. A total of 39 patients were evaluated. Children with coinfection had a significant decrease in Th1 cytokine production, interleukin 2 (IL-2) (P < 0.05), IL-12 (P < 0.05), and tumor necrosis factor alpha (P < 0.05) compared with Giardia-only infected children. Coinfected children had an increase in IL-10/interferon gamma (IFN-γ) ratio compared with uninfected (P < 0.05) and Ascaris alone (P < 0.05). The increased IL-10/IFN-γ ratio in the setting of decreased Th1 cytokine response indicates Th2 polarization in the coinfected group. Reduced Th1 cytokines in children coinfected with Ascaris and Giardia may impair the host's ability to eradicate Giardia infection leading to chronic giardiasis.
Introduction
More than 2 billion people are infected with parasitic infections worldwide and, not uncommonly, many have coinfections with more than one parasite.1–3 Coinfections with soil-transmitted helminths (STHs) and protozoal parasites are particularly prevalent in young children.4 However, until recently, diagnosis of coinfection has been limited by the poor sensitivity of conventional microscopic diagnostic techniques.5 As diagnostic technologies have evolved with improved throughput and increased sensitivity, the ability to detect coinfections within an individual host has become more feasible.5 Multiparallel quantitative real-time polymerase chain reaction (qPCR) has allowed for concurrent detection of not only STHs, including Ascaris lumbricoides, but also intestinal protozoa, such as Giardia lamblia.4–6 Coinfection specifically with A. lumbricoides and G. lamblia is common due to overlapping geographic prevalence and similar transmission dynamics in resource-limited areas.6 We have observed within a birth cohort in rural Ecuador that 213 children at 3 years of age were 16.4% infected with A. lumbricoides and 52.1% infected with G. lamblia. Of those with ascariasis, 77.4% were coinfected with G. lamblia while 34.3% were only infected with G. lamblia (R. Mejia, unpublished data), raising the statistical possibility that the immunomodulatory effects of ascariasis may play a role in host susceptibility to giardiasis.7 Alternative explanations could include changes in the host intestinal environment as a result of mucosal integrity breakdown or shifts in the commensal microbiome allowing for secondary infection.
Ascariasis and giardiasis cause significant chronic morbidity in children.7 Ascariasis, although often asymptomatic in lightly infected children, is a common cause of abdominal pain in endemic areas and occasionally causes intestinal and biliary obstruction.4,7 Chronic infections can lead to significant malabsorption, nutrient deficiencies, growth delays, and cognitive impairment.4,7 Helminth infections such as ascariasis have also been associated with increased susceptibility to malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome.8–10 Giardiasis causes acute symptoms of diarrhea, abdominal pain, flatulence, and nausea. In over 50% of infected individuals the illness is self-limiting.11–13 However, chronic, persistent infection with Giardia can occur and lead to chronic wasting syndrome or failure to thrive.13,14 Persistent giardiasis has been most commonly described in children with altered immune responses, including hypogammaglobulinemia, common variable acquired immunodeficiency, nephrotic syndrome, and protein-calorie malnutrition.12,15,16
The interaction between the host immune response and STH and protozoal coinfections is complex involving both the innate and acquired immune responses.3 Protozoa stimulate a predominantly CD4+ T-helper cell 1 (Th1) mediated cytokine profile. Th1 pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin 2 (IL-2), and IL-12, may play an important role in the elimination of protozoa from the human host.3,13,17 Previous studies have shown that CD4+ T cells and certain Th1 cytokines, specifically TNF-α and IFN-γ, determine parasite load and parasite clearance, whereas the absence of such an immunologic response contributes to the establishment of chronic giardiasis.18 Conversely, STHs such as A. lumbricoides stimulate a polarized Th2-mediated cytokine profile.19 Th2 anti-inflammatory cytokines, IL-4, IL-5, and IL-13, promote the activation of mast cells, eosinophils, basophils, and B-cell-mediated immunoglobulin and IgG4 production. Ascariasis has also been associated with expansion of monocytes and regulatory T cells (Tregs) leading to increased levels of the regulatory cytokines, transforming growth factor (TGF-β) and IL-10.8,9 Immunomodulation through upregulation of IL-10 and TGF-β allows for persistence of helminth infection.8,17,20 Murine studies suggest that helminths induce a profound Th2 response at the expense of the Th1 response leading to downregulation of serum pro-inflammatory cytokines.8,9,20,21 Th2 polarization and Th1 downregulation during helminth infection may protect the host from significant tissue pathology but may reduce the host capacity to respond effectively to infections with intracellular organisms.8,9,20,21
We hypothesized that children coinfected with the STH infection, A. lumbricoides, and the intestinal protozoa, G. lamblia, have increased immune regulation associated with a reduction in Th1 plasma cytokine concentrations compared with children infected with either parasite only, allowing for persistent giardiasis.
Methods
Sample and data collection and study design.
This was a cross-sectional analysis of 39 asymptomatic 3-year-old children analyzed within an Ecuadorian birth cohort, the Ecuador Life cohort of children living in the district of Quininde, Esmeraldas Province, Ecuador.22 Participants were selected from an initial random subsample of 400 children providing stool samples at 13 months of age,5 of whom 213 provided a stool sample at 3 years of age. Blood samples for plasma were collected at 3 years of age. The final sample for analysis of 39 children selected based on having a plasma sample for analysis and the presence of DNA in stool for G. lamblia, A. lumbricoides, both parasites, and absence of any other common helminth (Ancylostoma duodenale, Necator americanus, Strongyloides stercoralis, and Trichuris trichiura) or protozoal infection (Cryptosporidium hominis/parvum and Entamoeba histolytica)23 was as follows: 11 patients with Giardia only, seven children with Ascaris only, and 13 children with Ascaris and Giardia coinfection. Additionally, eight children, negative for all parasites by qPCR, were used as an uninfected control group. Stools were also examined using a combination of microscopic methods including direct saline mounts, Kato–Katz, and formol-ethyl acetate concentration methods at the time of collection for helminth and protozoal parasites. Results of stool microscopy and appropriate antiparasite medicines were given to the guardian of each child. Aliquots of stool and plasma samples were frozen without fixatives at −80°C until analysis. Data on baseline characteristics were collected using a questionnaire administered in Spanish to the child's primary guardian around the time of birth of the child.22
DNA extraction and multiparallel qPCR.
DNA was extracted from 50 mg stool using the FastPrep® Spin Kit for Soil (MP Biomedicals, Santa Ana, CA),6 according to the manufacturer's instructions for all parasites except T. trichiura. An additional step required for the extraction of T. trichiura DNA was performed as previously described.5 Multiparallel qPCR was performed as previously described5 with the species-specific primers to identify eight gastrointestinal parasite pathogens including A. lumbricoides, A. duodenale, N. americanus, S. stercoralis, T. trichiura, C. parvum, E. histolytica, and G. lamblia. Samples that tested positive by qPCR for G. lamblia, A. lumbricoides, and G. lamblia plus A. lumbricoides were used in this study.
Cytokine assay.
Plasma collected from the study subjects at 3 years of age were used to quantitate cytokines using Bio-Rad Luminex Magpix® (Hercules, CA). Bio-Plex Pro™ Human Cytokine Th1/Th2 Assay (Bio-Rad) were used per the manufacturer's protocol to detect Th1 and Th2 dominant cytokines including IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, granulocyte macrophage colony stimulating factor, TNF-α, and IFN-γ. Cytokine values below the detection limit of the assays were assigned a nominal value of 0 pg/mL. Standard curves for each cytokine were measured along with the samples.
High-sensitivity C-reactive protein assay.
Levels of C-reactive protein (CRP), a biomarker of systemic inflammation, were measured in plasma samples from study children. High-sensitivity C-reactive protein (hsCRP) ELISA (MP Biomedicals) was used according to the manufacturer's instructions.
Statistical analysis.
Sample size was based on cost considerations. The primary analysis was comparisons in levels of Th1, Th2, T regulatory (i.e., IL-10), and IL-10/IFN-g ratios between coinfected and singly infected children with the uninfected group included as the control. The Kruskal–Wallis test was used to compare the four groups with two-group analyses performed using the Mann–Whitney test. Statistical analyses were performed using Prism v 5.0 d (GraphPad, La Jolla, CA). P values less than 0.05 were considered significant.
Ethical approval and consent.
Informed written consent was obtained from each participant or from a parent/guardian. Antiparasitic treatment, based on microscopy findings, was provided per standard of care in the region. The subjects were preschool children and did not receive school-based mass drug administration with anthelmintics. Study protocols were approved by the bioethics committee of Hospital Pedro Vicente Maldonado, Pichincha Province, and by the institutional review board of Baylor College of Medicine.
Results
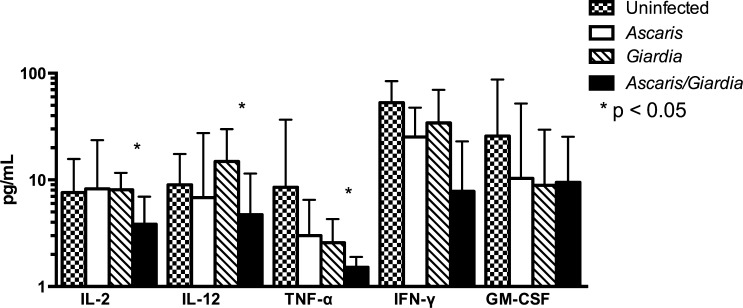
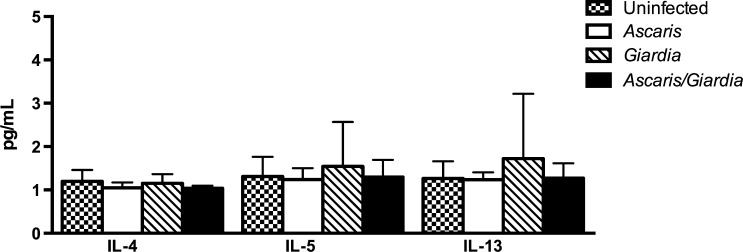
Baseline characteristics of the children in the four infection groups are shown in Table 1. The distributions of characteristics between the four groups were comparable and none showed significant intergroup heterogeneity. Coinfection with A. lumbricoides and G. lamblia was found to be associated with reduced Th1 cytokine production, specifically TNF-α (P = 0.0148), when the groups were compared. Further subset analysis of A. lumbricoides and G. lamblia coinfected group compared with G. lamblia-only infected children demonstrated reduction in IL-2 (3.834 versus 8.082 pg/mL; P < 0.05), IL-12 (4.719 versus 14.89 pg/mL; P < 0.05), and TNF-α (1.518 versus 2.579 pg/mL; P < 0.05) (Figure 1 ). Ascaris lumbricoides and G. lamblia coinfection was not associated with an increase in serum Th2-mediated cytokines including IL-4, IL-5, or IL-13 compared with participants with monoparasitic infection (Figure 2 ). In addition, there was no significant difference in immune regulatory IL-10 level between A. lumbricoides and G. lamblia coinfected children and A. lumbricoides-only infection, and G. lamblia-only infection (Figure 3 ).
Table 1.
Baseline characteristics of 39 children aged 3 years by infection group
| Characteristic | Uninfected (n = 8) | Giardia only (n = 11) | Ascaris only (n = 7) | Ascaris and Giardia (n = 13) | P value |
|---|---|---|---|---|---|
| Sex (%) | |||||
| Male | 37.5 | 36.4 | 28.6 | 61.5 | 0.440 |
| Female | 62.5 | 63.6 | 71.4 | 38.5 | |
| Nutritional status, mean (SD) | |||||
| Weight (kg) | 16.3 (3.1) | 16.8 (3.6) | 16.7 (2.5) | 16.6 (1.1) | 0.983 |
| Height (cm) | 105.3 (7.3) | 104.8 (6.5) | 106.2 (4.7) | 106.6 (4.3) | 0.885 |
| SES (%) | |||||
| Low | 25.0 | 27.3 | 28.6 | 30.8 | |
| Medium | 25.0 | 54.6 | 57.1 | 23.1 | |
| High | 50.0 | 18.2 | 14.3 | 46.2 | 0.488 |
| Residence (%) | |||||
| Urban | 87.5 | 81.8 | 71.4 | 69.2 | |
| Rural | 12.5 | 18.2 | 28.6 | 30.8 | 0.752 |
| Overcrowding (%) | |||||
| No | 50.0 | 54.6 | 42.9 | 46.2 | |
| Yes | 50.0 | 45.4 | 57.1 | 53.9 | 0.963 |
| Birth order, mean (SD) | 2.6 (1.9) | 3.6 (1.8) | 3.0 (1.6) | 2.5 (1.8) | 0.470 |
| Maternal factors | |||||
| Educational status (%) | |||||
| Illiterate | 12.5 | 9.1 | 14.3 | 30.8 | |
| Complete primary | 62.5 | 63.6 | 71.4 | 46.2 | |
| Complete secondary | 25 | 27.3 | 14.3 | 23.1 | 0.835 |
| Maternal ethnicity (%) | |||||
| Afro-Ecuadorian | 37.5 | 9.1 | 42.9 | 30.8 | |
| Other | 62.5 | 90.9 | 57.1 | 69.2 | 0.375 |
SD = standard deviation; SES = socioeconomic status. Household overcrowding defined by ≥ 3 persons per sleeping room. P values represent comparisons between the three infection groups using the Kruskal–Wallis or χ2 tests as appropriate.
Figure 1.
Th1-mediated cytokine repertoire in Ascaris and Giardia coinfection, Ascaris-only infection, Giardia-only infection, and uninfected. Decrease in IL-2, IL-12, and TNF-α in Ascaris and Giardia coinfected children compared with Giardia-only infected children. IL = interleukin; Th1 = T-helper cell 1; TNF-α = tumor necrosis factor alpha.
Figure 2.
Th2-mediated cytokine repertoire in Ascaris and Giardia coinfection, Ascaris-only infection, Giardia-only infection, and uninfected. No difference was detected in Ascaris and Giardia coinfected children compared with Giardia- or Ascaris-only infected children. Th2 = T-helper cell 2.
Figure 3.
Th2-mediated cytokine repertoire in Ascaris and Giardia coinfection, Ascaris-only infection, Giardia-only infection, and uninfected children. No difference was detected in Ascaris and Giardia coinfected children compared with Giardia- or Ascaris-only infected children.
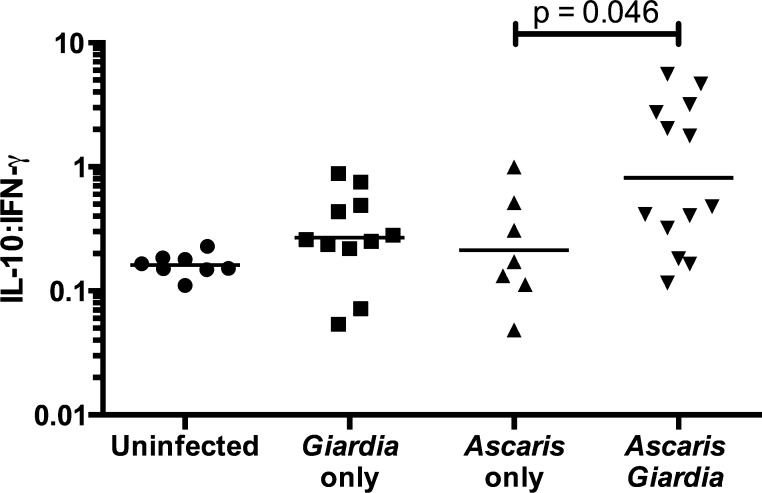
However, there was a significant difference in the IL-10/IFN-γ ratio between the groups (P = 0.0136). In post hoc subset analysis, the A. lumbricoides and G. lamblia coinfected children were found to have a significant increase in IL-10/IFN-γ ratio compared with children with A. lumbricoides-only infection (0.4761 and 0.1724 pg/mL; P < 0.05) (Figure 4 ). There was no significant difference in A. lumbricoides DNA levels signifying burden of disease between coinfected and A. lumbricoides-only infected children (26.21 versus 29.94 fg/μL).
Figure 4.
IL-10/IFN-γ ratio depicting increased Th2–Th1 cytokine polarization in Ascaris and Giardia coinfected children and Ascaris-only infected children. IL = interleukin; Th = T-helper cell; IFN = interferon.
There was no significant difference of systemic inflammation across the groups by measurement of plasma hsCRP (G. lamblia–only, 1.62 mg/L; A. lumbricoides-only, 1.53 mg/L; and A. lumbricoides and G. lamblia coinfections, 3.28 mg/L; P = 0.5283, graph not shown). Mean levels of hsCRP in the uninfected group were 3.06 mg/L.
Discussion
Parasitic infections, including STHs such as A. lumbricoides and intestinal protozoa such as G. lamblia, are a major cause of morbidity worldwide particularly in children.24 Because of the epidemiologic overlap and the complex interactions of helminths and protozoa with the host immune system, children in endemic regions commonly have coinfections with Ascaris and Giardia.4,7 Coinfections with multiple parasites are associated with reduced treatment efficacy, increased treatment costs, and overall worse health outcomes.25
In this study, we compared serum Th1 and Th2 cytokine production in four groups of asymptomatic children living in an Ascaris- and Giardia-endemic area of Ecuador: uninfected, Ascaris-only infected, Giardia-only infected, and Ascaris and Giardia coinfected children. Children coinfected with Ascaris and Giardia were noted to have decreased serum Th1 cytokine concentrations compared with children with Giardia-only infection. Th1 cytokines are necessary for expansion of humoral and cellular effector cells responsible for Giardia clearance.13,16 Reduction of plasma Th1 cytokines as a result of the immunomodulatory effects of ascariasis may impede the eradication of protozoa and permit persistent infections with Giardia in coinfected children.
IL-10, a product of innate immune cells, Tregs and B cells, is a potent anti-inflammatory cytokine.17 IL-10 has been shown to inhibit IFN-γ-induced killing potential of macrophages against both intra- and extracellular parasites as well as prevent immune-mediated host tissue destruction in response to infectious agents.17 The present study observed that children coinfected with Ascaris and Giardia had an increased IL-10/IFN-γ ratio (Th2/Th1 cytokines) compared with Ascaris-only infected children suggesting Th2 polarization. In a recent study of 1,060 children aged 4–11 years living in Brazil, those chronically infected with A. lumbricoides also demonstrated Th2 predominance with increased levels of Th2 cytokines including IL-5 and IL-10.26 In a study by Sanchez and others, children coinfected with STHs in Honduras also had increased IL-10 concentrations compared with negative controls.8 Previous studies have suggested that Th2 polarization and immunomodulation may be secondary to worm burden such that children with heavier helminth burden also have the highest levels of IL-10 concentrations in serum.7,17 Furthermore, studies have shown that heavy Ascaris worm burden is associated with increased prevalence of Giardia coinfections.7 However, despite demonstrating Th2 cytokine polarization in the current study, there was no significant difference in Ascaris burden between the Ascaris and Giardia coinfected group and the Ascaris-only group. There was also no statistically significant difference in concentrations of Th2 cytokines and IL-10 in plasma from children with Ascaris and Giardia coinfections compared with children infected with Ascaris only or Giardia only. Interestingly, there was no significant difference in hsCRP between the groups, and in fact hsCRP levels were comparable to those observed in U.S. children aged 3–16 years from the National Health and Nutrition Exam Survey study (mean value = 1.22 mg/mL).27
The complex interaction between parasitic coinfection and the immune system remains inconclusive. In a recent study by Blackwell and others, helminth infections and giardiasis were shown to have a negative association. Giardia infection was associated with lower odds of infection with A. lumbricoides (odds ratio [OR] = 0.63), and A. lumbricoides infection was associated with lower odds of Giardia (OR = 0.65). Interestingly, Blackwell and others did show that recovery from helminth infection after appropriate antihelminthic therapy was much less likely if the patient was coinfected with Giardia suggesting that the immunomodulation is occurring.2 Recent studies have also shown the importance not only of CD4+ producing Th1 cytokines, IFN-γ, and TNF-α, but also of CD4+ producing IL-17A cells (Th17) in acute giardiasis. IL-17A is known to contribute to innate cell recruitment at the mucosal surface and, as a result, elevated levels of IL-17A during acute giardiasis may provide protection against development of chronic giardiasis.18 Future studies evaluating the role of Th17 cells and IL-17A may provide additional knowledge on parasitic coinfections.
This study was limited by a sample size of 39 children and by high cost of the Luminex multiplex assays. Such a sample size would only have power to detect relatively large differences between infection groups, more likely to be biologically meaningful. Further analyses of the ECUAVIDA birth cohort will provide the opportunity to follow the children longitudinally to evaluate changes in cytokine production in acute versus chronic infection, cytokine production after treatment, and cytokine production upon reinfection. Figueiredo and others demonstrated that the production of IL-10 was greater in chronically infected children with A. lumbricoides than noninfected children or those in whom infection was detected at one observation time only.26 Additionally, the study does not take into account the possibility of other infectious agents causing coinfection, such as viruses and bacteria, which may have significant Th1 and Th2 immunomodulatory effects. Both the Ascaris monoparasitic infected and the uninfected children did have elevated Th1 cytokine concentrations compared with the coinfected children suggesting that these children had additional inflammatory influence on the Th1 versus Th2 balance. However, these children were asymptomatic, with no reported diarrhea or abdominal symptoms at the time of stool collection. They did not have significantly elevated levels of the systemic inflammatory marker hsCRP compared with the uninfected group, also indicative of lack of clinically relevant concurrent bacterial or viral infections. There were no significant differences in baseline characteristics between the groups making confounding a less likely alternative explanation for our findings. These characteristics were chosen as factors known to be associated with the risk of infection at 3 years of age in a previous analysis of STH infections in the cohort.28 However, as the effects of these potential confounders were not controlled or included in the analysis because of limited power, confounding cannot be excluded. Unfortunately, the measurement of plasma cytokines does not provide information regarding the specific cytokine-producing cells nor the tissue source. Previous studies of peripheral blood mononuclear cells have shown that STHs and intestinal protozoa may induce other types of host immune response such as Treg or Th17 cells during infection which have direct effects on both Th1 and Th2 responses.13,29 Further investigation of the influence of antigen-specific Th17 cells, including IL-17A production, Tregs, including TGF-β, and additional Th2 cytokines such as IL-6 will contribute more depth to the peripheral cytokine profile landscape during Ascaris and Giardia coinfection in children.
Conclusions
Parasitic coinfections in children remain a significant health problem around the world. STHs and intestinal protozoa have complex interactions with the host immune system that allow for persistent infection and subsequent chronic morbidity. In a similar study conducted in Venezuela, children with moderate A. lumbricoides infection had diminished antibody response and diminished pro-inflammatory cytokine production (Th1) when coinfected with Giardia duodenalis indicating ascariasis as a strong immunomodulating agent.7 Additional studies evaluating the role of cytokines within the ECUAVIDA birth cohort suggest that chronic infection with STHs leads to cytokine hyporesponsiveness and a tolerized Th2 response further supporting the immunomodulatory role of STHs such as ascariasis.30 Gaining a better understanding of this complex interaction between parasitic coinfection and the host immune system is essential to improve the long-term health outcome of children living in endemic areas through identification of therapeutic targets including vaccine development, alterations in environmental strategies, and shifts in public health policy.
Footnotes
Authors' addresses: Jill Weatherhead, Department of Pediatrics, Baylor College of Medicine, Houston, TX, E-mail: weatherh@bcm.edu. Andrea Arévalo Cortés, Carlos Sandoval, Maritza Vaca, Martha Chico, Sophia Loor, and Philip J. Cooper, Facultad de Ciencias Medicas, Universidad International del Ecuador, Quito, Ecuador, E-mails: aarevalocortes@gmail.com, sandoval_acarlos@hotmail.com, marimar_ecq@yahoo.com, marthachico6@yahoo.es, sofy_loor@hotmail.es, and pcooper@sgul.ac.uk. Rojelio Mejia, National School of Tropical Medicine, Baylor College of Medicine, Houston, TX, E-mail: rojelio.mejia@bcm.edu.
References
- 1.Supali T, Verweij JJ, Wiria AE, Djuardi Y, Hamid F, Kaisar MMM, Wammes LJ, van Lieshout L, Luty AJF, Sartono E, Yazdanbakhsh M. Polyparasitism and its impact on the immune system. Int J Parasitol. 2010;40:1171–1176. doi: 10.1016/j.ijpara.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proc Biol Sci. 2013;280:20131671. doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox FE. Concomitant infections, parasites and immune responses. Parasitology. 2001;122((Suppl)):S23–S38. doi: 10.1017/s003118200001698x. http://www.ncbi.nlm.nih.gov/pubmed/11442193 Available at. Accessed March 21, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Lamberton PHL, Jourdan PM. Human ascariasis: diagnostics update. Curr Trop Med Rep. 2015;2:189–200. doi: 10.1007/s40475-015-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mejia R, Vicuña Y, Broncano N, Sandoval C, Vaca M, Chico M, Cooper PJ, Nutman TB. A novel, multi-parallel, real-time polymerase chain reaction approach for eight gastrointestinal parasites provides improved diagnostic capabilities to resource-limited at-risk populations. Am J Trop Med Hyg. 2013;88:1041–1047.. doi: 10.4269/ajtmh.12-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cimino RO, Jeun R, Juarez M, Cajal PS, Vargas P, Echazú A, Bryan PE, Nasser J, Krolewiecki A, Mejia R. Identification of human intestinal parasites affecting an asymptomatic peri-urban Argentinian population using multi-parallel quantitative real-time polymerase chain reaction. Parasit Vectors. 2015;8:380. doi: 10.1186/s13071-015-0994-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagel I, Cabrera M, Puccio F, Santaella C, Buvat E, Infante B, Zabala M, Cordero R, Di Prisco MC. Co-infection with Ascaris lumbricoides modulates protective immune responses against Giardia duodenalis in school Venezuelan rural children. Acta Trop. 2011;117:189–195. doi: 10.1016/j.actatropica.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez AL, Mahoney DL, Gabrie JA. Interleukin-10 and soil-transmitted helminth infections in Honduran children. BMC Res Notes. 2015;8:55. doi: 10.1186/s13104-015-1019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafi W, Ribeiro-Rodrigues R, Ellner JJ, Salgame P. Coinfection-helminthes and tuberculosis. Curr Opin HIV AIDS. 2012;7:239–244. doi: 10.1097/COH.0b013e3283524dc5. [DOI] [PubMed] [Google Scholar]
- 10.Mouser EEIM, Pollakis G, Paxton WA. Effects of helminths and Mycobacterium tuberculosis infection on HIV-1: a cellular immunological perspective. Curr Opin HIV AIDS. 2012;7:260–267. doi: 10.1097/COH.0b013e3283521144. [DOI] [PubMed] [Google Scholar]
- 11.Nash TE, Herrington DA, Losonsky GA, Levine MM. Experimental human infections with Giardia lamblia. J Infect Dis. 1987;156:974–984. doi: 10.1093/infdis/156.6.974. http://www.ncbi.nlm.nih.gov/pubmed/3680997 Available at. Accessed March 21, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Stark D, Barratt JLN, van Hal S, Marriott D, Harkness J, Ellis JT. Clinical significance of enteric protozoa in the immunosuppressed human population. Clin Microbiol Rev. 2009;22:634–650. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Romero G, Quintero J, Astiazarán-García H, Velazquez C. Host defences against Giardia lamblia. Parasite Immunol. 2015;37:394–406. doi: 10.1111/pim.12210. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira FS, Baptista-Fernandes T, Oliveira D, Rodrigues R, Neves E, Lima A, Garrido E, Afonso G, Zaky A, Telles de Freitas P, Atouguia J, Centeno-Lima S. Giardia duodenalis and soil-transmitted helminths infections in children in São Tomé and Príncipe: do we think Giardia when addressing parasite control? J Trop Pediatr. 2015;61:106–112. doi: 10.1093/tropej/fmu078. [DOI] [PubMed] [Google Scholar]
- 15.Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, Moore TA. Treatment of patients with refractory giardiasis. Clin Infect Dis. 2001;33:22–28. doi: 10.1086/320886. [DOI] [PubMed] [Google Scholar]
- 16.Heyworth MF. Immunological aspects of Giardia infections. Parasite. 2014;21:55. doi: 10.1051/parasite/2014056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite Immunol. 2014;36:233–252. doi: 10.1111/pim.12113. [DOI] [PubMed] [Google Scholar]
- 18.Saghaug CS, Sørnes S, Peirasmaki D, Svärd S, Langeland N, Hanevik K. Human memory CD4+ T cell immune responses against Giardia lamblia. Clin Vaccine Immunol. 2016;23:11–18. doi: 10.1128/CVI.00419-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper PJ, Chico ME, Sandoval C, Espinel I, Guevara A, Kennedy MW, Urban JF, Jr, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–1213. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 20.Ezenwa VO, Jolles AE. From host immunity to pathogen invasion: the effects of helminth coinfection on the dynamics of microparasites. Integr Comp Biol. 2011;51:540–551. doi: 10.1093/icb/icr058. [DOI] [PubMed] [Google Scholar]
- 21.Curry AJ, Else KJ, Jones F, Bancroft A, Grencis RK, Dunne DW. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J Exp Med. 1995;181:769–774. doi: 10.1084/jem.181.2.769. http://www.ncbi.nlm.nih.gov/pubmed/7836929 Available at. Accessed March 21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper PJ, Chico ME, Platts-Mills TA, Rodrigues LC, Strachan DP, Barreto ML. Cohort profile: the Ecuador Life (ECUAVIDA) study in Esmeraldas Province, Ecuador. Int J Epidemiol. 2015;44:1517–1527. doi: 10.1093/ije/dyu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper PJ, Chico ME, Guadalupe I, Sandoval CA, Mitre E, Platts-Mills TAE, Barreto ML, Rodrigues LC, Strachan DP, Griffin GE. Impact of early life exposures to geohelminth infections on the development of vaccine immunity, allergic sensitization, and allergic inflammatory diseases in children living in tropical Ecuador: the ECUAVIDA birth cohort study. BMC Infect Dis. 2011;11:184. doi: 10.1186/1471-2334-11-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet (London, England) 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths EC, Pedersen AB, Fenton A, Petchey OL. Analysis of a summary network of co-infection in humans reveals that parasites interact most via shared resources. Proc Biol Sci. 2014;281:20132286. doi: 10.1098/rspb.2013.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, Alcantara-Neves NM. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78:3160–3167. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menzies SK, Rodriguez A, Chico M, Sandoval C, Broncano N, Guadalupe I, Cooper PJ. Risk factors for soil-transmitted helminth infections during the first 3 years of life in the tropics; findings from a birth cohort. PLoS Negl Trop Dis. 2014;8:e2718. doi: 10.1371/journal.pntd.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner JD, Jackson JA, Faulkner H, Behnke J, Else KJ, Kamgno J, Boussinesq M, Bradley JE. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197:1204–1212. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo CA, Amorim LD, Vaca M, Chico ME, Campos AC, Barreto ML, Cooper PJ. Effects of poor hygiene on cytokine phenotypes in children in the tropics. World Allergy Organ J. 2016;9:34. doi: 10.1186/s40413-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]