Abstract
Toxoplasma gondii infects a broad range of warm-blooded hosts, including humans. Important clinical manifestations include encephalitis in immunocompromised patients as well as miscarriage and fetal damage during early pregnancy. Toxoplasma gondii dense granule antigen 2 and 5 (GRA2 and GRA5) are essential for parasitophorous vacuole development of the parasite. To evaluate the potential of GRA2 and GRA5 as recombinant DNA vaccine candidates, these antigens were cloned into eukaryotic expression vector (pcDNA 3.1C) and evaluated in vaccination experiments. Recombinant DNA vaccines constructed with genes encoding GRAs were validated in Chinese hamster ovary cells before evaluation using lethal challenge of the virulent T. gondii RH strain in BALB/c mice. The DNA vaccines of pcGRA2 and pcGRA5 elicited cellular-mediated immune response with significantly higher levels of interferon-gamma, interleukin-2 (IL-2), IL-4, and IL-10 (P < 0.05) compared with controls. A mixed T-helper cell 1 (Th1)/Th2 response was associated with slightly prolonged survival. These findings provide evidence that DNA vaccination with GRA2 and GRA5 is associated with Th1-like cell-mediated immune responses. It will be worthwhile to construct recombinant multiantigen combining full-length GRA2 or/and GRA5 with various antigenic proteins such as the surface antigens and rhoptry antigens to improve vaccination efficacy.
Introduction
Toxoplasma gondii is a ubiquitous and obligate intracellular protozoan parasite which infects a broad range of warm-blooded hosts,1 causing a disease known as toxoplasmosis. Toxoplasmosis is globally distributed and affects up to one-third of the world's human population.2 Acute toxoplasmosis is correlated with intracellular growth of the rapidly replicating tachyzoites, causing the death of infected host cell by rupturing to liberate more tachyzoites to continue invading neighboring cells.3 Chronic toxoplasmosis is related to the formation of tissue cysts containing bradyzoites as a result of the parasite's response to the host immune mechanism.4 Tissue cysts are found predominantly in the brain and skeletal muscle of the host. The cysts do not trigger any inflammation and remain dormant throughout the entire life of the host.5 Encystation of the bradyzoites protects them from being detected by the host's immune system.
Interconversion between tachyzoites and bradyzoites is a reversible process. Suppressed level of nitric oxide, T lymphocytes, interferon-gamma (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α), especially in immunocompromised patients, can cause reactivation of T. gondii infection through the rupture of tissue cysts. This releases bradyzoites that will convert into active tachyzoites.4 Disease reactivation, toxic effects, and possibility of drug resistance in the parasites makes drug treatment unreliable for long term.3,6 As a result, there is a need to develop vaccines that confer lifelong protection against T. gondii primary infection (during pregnancy), reactivation (immunocompromised patients), and reinfection.6
One of the approaches to produce safe vaccines is through DNA vaccine technology. In this approach, a DNA plasmid encoding the protein of interest is taken up by muscle cells of the host. Protein expression is then driven by the host's cellular machinery. The expressed protein may then be degraded by host proteases into smaller peptides before being transported into the endoplasmic reticulum and binds to major histocompatibility complex (MHC) class I molecules. The peptides are then presented on the cell surface for recognition by CD8+ cytotoxic T cells, thereby inducing cellular-mediated immunity. The expressed proteins may also be directly delivered out from the muscle cell via exocytosis and taken up by antigen presenting cells (APCs) such as macrophage. The protein will then be processed into peptide-MHC class II complex within the APC before being presented on the cell surface to CD4+ helper T cells for stimulation of humoral-mediated immunity.7 DNA plasmid vaccine has been observed to elicit mechanism of immune responses similar to that triggered in natural T. gondii infection.
The key element in protection against T. gondii in the infected host is the triggering of T-helper cell-1 (Th1) cellular-mediated immune response via the production of pro-inflammatory cytokines such as IL-12, TNF-α, and IFN-γ. However, an overwhelming production of these cytokines may lead to severe inflammation at the infected sites causing severe tissue damages. Therefore, anti-inflammatory cytokines such as IL-10 and transforming growth factor-beta have to be secreted at the same time to ensure equilibrium.8,9
When the T. gondii tachyzoite invades a cell, a parasitophorous vacuole (PV) will be formed to enclose and protect the parasite within the infected cell. The T. gondii dense granules (GRAs) are specialized secretory organelles involved in PV development. The GRA-related proteins help in the maturation and modification of the PV and its membrane.10 These proteins are found in the vacuole which surrounds the tachyzoite and encysted bradyzoites.11,12 Some of these proteins have been identified as potential vaccines.13–15 Two of the potential vaccines are GRA2 and GRA5. GRA2 is involved in the formation of intravacuolar network in PV, whereas GRA5 helps to inhibit apoptosis of the infected cells, thereby protecting the parasite during cell invasion.10,16 Both GRA2 and GRA5 are expressed throughout the whole intermediate host life cycle of T. gondii, thus preventing stage-limited protection against toxoplasmosis.17,18
Several studies have been conducted to evaluate multicomponent vaccines, which incorporate GRA2 or GRA5 with other proteins.19–22 However, only limited number of studies have been performed using GRA2 or GRA5 as single antigen vaccine. The objective of this study was to evaluate the protective effect of DNA vaccines encoding GRA2 and GRA5 against acute toxoplasmosis in a mouse model.
Materials and Methods
Mice and ethics statement.
Six- to eight-week old female BALB/c mice were purchased from Monash University Sunway Campus. The mice were maintained in a pathogen-free environment and were fed ad lib with commercial food pellets and water. Experiments were carried out in compliance with the animal ethics approved by Institutional Animal Care and Use Committee of the University of Malaya, Faculty of Medicine (2014-06-03/PARA/R/CXT).
Parasites propagation and harvest.
Toxoplasma gondii tachyzoites of the virulent wild-type RH strain were provided by the Department of Parasitology, University of Malaya, Kuala Lumpur, Malaysia. They were maintained according to the procedures described in our previous study.23
DNA plasmid transfection.
Six-well flat-bottom microplate was seeded with of 0.8–2.4 × 105 Chinese hamster ovary (CHO) cells in 4 mL of DMEM (Dulbecco's modified Eagle's medium) complete medium 24 hours before transfection. When the cells reached 70–90% confluence, 4 μg of the isolated endotoxin-free DNA plasmid was diluted in 400 μL of serum-free DMEM. Diluted DNA plasmid was then mixed immediately with 6 μL Turbofect™ (Thermo Scientific, Waltham, MA) Protein Transfection reagent through vortexing. The Turbofect/DNA mixture was incubated for 20 minutes at room temperature before adding 400 μL of the mixture into each well containing CHO cells. The cells were then incubated in carbon dioxide (CO2) incubator at 37°C for 24–48 hours before harvested for the analysis of recombinant protein expressions by western blot assay.24
Immunization regimen.
Six- to eight-week old female inbred BALB/c mice were divided into four immunization groups with 13 mice in each group. Four different groups of BALB/c mice were given intramuscular injection at tibialis anterior muscle of both leg with 100 μL (50 μL in each leg) of phosphate-buffered saline (PBS) (negative control), empty vector (negative control), 100 μg of pcGRA2, and 100 μg of pcGRA5. A total of three injections were carried out at 3 weeks interval. Blood samples (50–100 μL) were collected from the injected mice through tail-bleeding on day 0, 21, 42, and 63.
Evaluation of humoral response.
Mice serum samples harvested were analyzed by western blot assay24 and in-house enzyme-linked immunosorbent assay (ELISA) against Toxoplasma total lysate antigen (TLA) to detect the presence of antigen-specific immunoglobulin G (IgG) antibodies. IgG antibody titers and subclass determination were carried out as described previously with TLA as the coating protein.23
Evaluation of cellular response.
In vitro splenocyte proliferation assay and cytokines (IFN-γ, IL-2, IL-4, and IL-10) assay were performed according to the protocols described previously.23 Cultured mice splenocytes were induced with culture medium alone (negative control), 10 μg/mL TLA, or 5 μg/mL con A (positive control) before incubated at 37°C in a 5% CO2 incubator for 24, 72, and 96 hours.
Mice challenge.
The remaining vaccinated and control mice were injected intraperitoneally with 1,000 live tachyzoites. Mortality rate of the mice was monitored and recorded twice daily whereby the infected mice were observed for end-point criteria; heavily infected with symptoms of sluggish movement, hunched back posture, ruffled, and thinning hair coat as well as obvious reduced food and water consumption. The heavily infected mice that reached end-point criteria were humanely killed by exposure to a gradually increasing concentration of CO2 inside a closed chamber.
Statistical analysis.
Significance levels of the differences between groups of mice were analyzed through Student's t test or analysis of variance. P < 0.05 indicates statistical significance. The survival rate was calculated based on χ2 test, whereas the survival graph was drawn based on Kaplan–Meier method.25
Results
Mammalian cell expression of pcDNA 3.1C constructs.
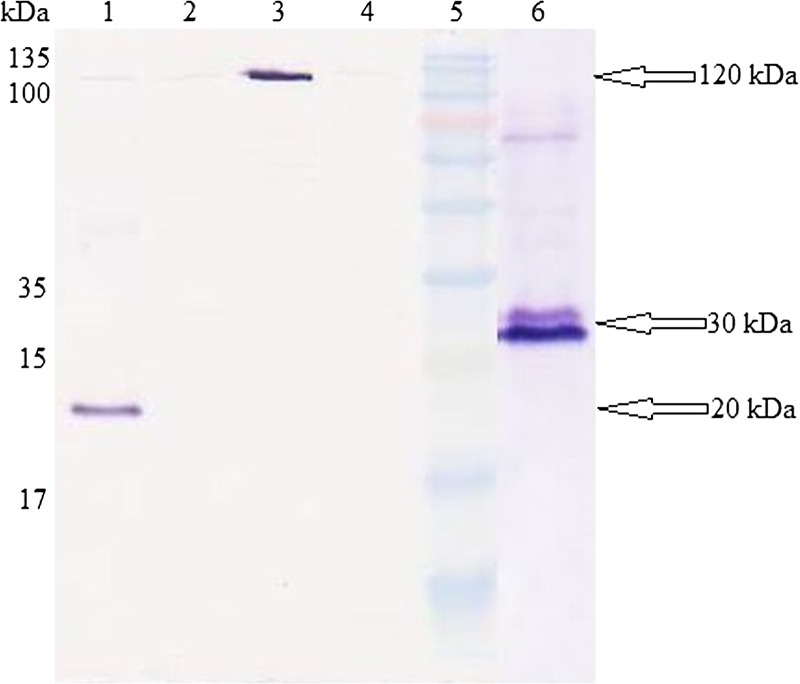
Transfection study involved two negative controls: pcDNA 3.1C empty vector and nontransfected cells, one positive control: pcDNA 3.1/His/lacZ, and two target genes: pcGRA2 and pcGRA5. Protein expressions of the constructs were analyzed through western blot assay using Xpress mouse monoclonal antibody as illustrated in Figure 1 . Results obtained showed that pcGRA2 and pcGRA5-transfected CHO cells produced antigenic proteins with their respective expected protein sizes of 30 and 20 kDa. Positive control–transfected CHO cells produced β-galactosidase protein with approximate protein size of 120 kDa. Meanwhile, no protein bands were detected in both the negative control-transfected and nontransfected CHO cells.
Figure 1.
Chinese hamster ovary cells expression of recombinant proteins. Western blot analysis of mammalian cell expressions of pcDNA 3.1C constructs using Xpress mouse monoclonal antibody. Lane 5 contained Prestained Broad Range Protein Marker. Lane 1 contained cell pellet fraction transfected with pcGRA5. Lane 2 contained cell pellet fraction transfected with negative control, pcDNA 3.1C empty vector. Lane 3 contained cell pellet fraction transfected with positive control, pcDNA 3.1/His/lacZ. Lane 4 contained non-transfected cell pellet fraction. Lane 6 contained cell pellet fraction transfected with pcGRA2. The GRA2 and GRA5 protein bands of interest were observed at molecular weights of 30 and 20 kDa (arrow), respectively, compared with the negative control. The positive control expressed β-galactosidase protein with expected size of 120 kDa (arrow).
IgG antibody detection.
Specific anti-TLA IgG antibody was at an undetectable level in the sera collected from all the injected mice at 0, 3, 6, and 9 weeks after first injections (data not shown). The cutoff (mean + 2 standard deviation) optical density (OD)450 value of the IgG level in the sera of pcGRA2- and pcGRA5-immunized mice groups was approximately the same as that of both PBS- and pcDNA 3.1C-injected mice groups (data not shown). On the other hand, antibody titers and IgG isotypes were unable to determine as well.
In vitro splenocytes proliferation assay.
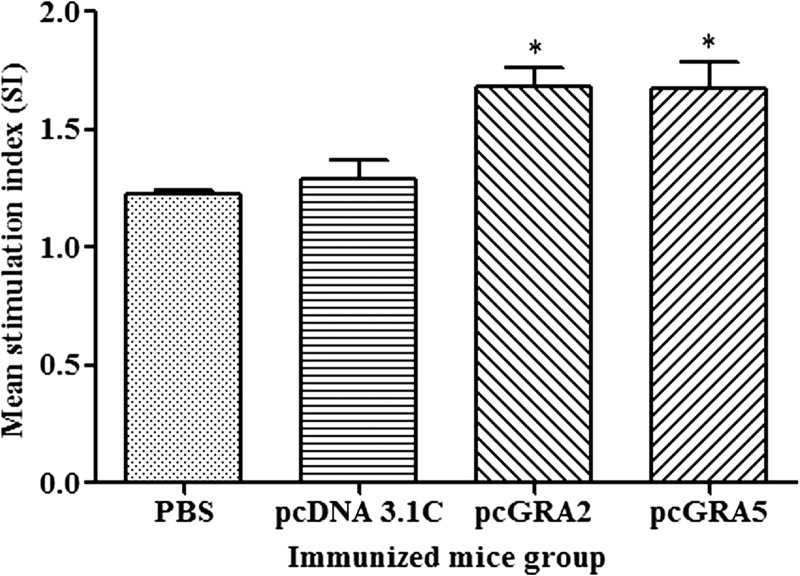
Significantly higher SI value was observed in the recombinant DNA plasmid–vaccinated groups compared with the control groups (P < 0.05) (Figure 2 and Table 1). There was no statistical difference between two vaccinated groups (P > 0.05) and also between two control groups (P > 0.05). These results indicated that T lymphocytes of the vaccinated mice were successfully stimulated.
Figure 2.
In vitro splenocytes proliferation response in mice. Spleen lymphocytes were harvested from mice immunized with pcGRA2, pcGRA5, pcDNA 3.1C, and phosphate-buffered saline (PBS) 3 weeks after last injection. The splenocytes were cultured and stimulated with total lysate antigen. Proliferative response was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data are expressed as mean stimulation index (SI) ± standard deviation (N = 3). Statistical difference is represented by * (P < 0.05) in comparison with the control groups (PBS or pcDNA 3.1C).
Table 1.
Characterization of cellular-mediated immunity in the vaccinated mice
| Group (N = 3) | Proliferation (SI) | Cytokine level (pg/mL) | |||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-10 | ||
| pcGRA2 | 1.679 ± 0.0828* | 5341 ± 79.7* | 359.1 ± 74.51* | 21.51 ± 11.28* | 57.27 ± 25.29* |
| pcGRA5 | 1.672 ± 0.1136* | 4669 ± 453.2* | 360 ± 63.48* | 15.12 ± 2.738 | 51.47 ± 20.06* |
| pcDNA3.1C | 1.289 ± 0.0812 | 757.5 ± 365.4 | 179.9 ± 42.14 | Undetectable | Undetectable |
| PBS | 1.222 ± 0.0189 | 625.1 ± 367.8 | 143.1 ± 34.39 | Undetectable | Undetectable |
IFN-γ = interferon-gamma; IL = interleukin; PBS = phosphate-buffered saline; SI = stimulation index. IFN-γ activity was assayed at 96 hours, IL-2 and IL-4 activities were assayed at 24 hours, and IL-10 activity was assayed at 72 hours. Undetectable IL-4 and IL-10 levels were observed in the stimulated splenocytes culture supernatant of the negative control mice groups. Data are expressed as mean ± standard deviation (N = 3). Statistical difference is represented by * (P < 0.05) in comparison with the control groups (PBS or pcDNA 3.1C).
Cytokine production assay.
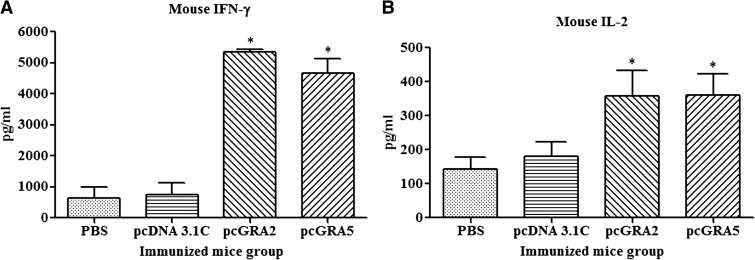
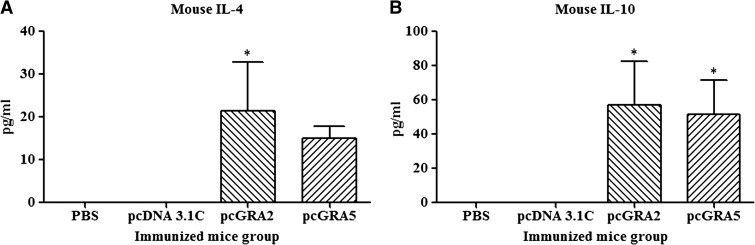
Results showed that the vaccinated mice produced significantly higher level of IFN-γ and IL-2 compared with the control groups (P < 0.05) (Figure 3 and Table 1). No statistical difference was observed between two vaccinated groups (P > 0.05) and between two control groups (P > 0.05). Relatively low levels of IL-4 and IL-10 were secreted by the stimulated splenocytes of the mice immunized with pcGRA2 and pcGRA5 (Figure 4 and Table 1). In contrast, these two cytokines levels were undetectable in the control groups. These results showed that predominantly Th1 immune response was favored in the vaccinated mice.
Figure 3.
Interferon-gamma (IFN-γ) and interleukin-2 (IL-2) production by the stimulated splenocytes of the immunized mice. Culture supernatants from the total lysate antigen–stimulated immunized mice splenocytes were collected at 96 and 24 hours postincubation for the evaluation of (A) IFN-γ and (B) IL-2 production, respectively, via enzyme-linked immunosorbent assay. Data are expressed as mean ± standard deviation (N = 3). Statistical difference is represented by * (P < 0.05) in comparison with the control groups (phosphate-buffered saline [PBS] or pcDNA 3.1C).
Figure 4.
Interleukin-4 (IL-4) and IL-10 production by the stimulated splenocytes of the immunized mice. Culture supernatants from the total lysate antigen–stimulated immunized mice splenocytes were collected at 24 and 72 hours postincubation for the evaluation of (A) IL-4 and (B) IL-10 production, respectively, via enzyme-linked immunosorbent assay. Data are expressed as mean ± standard deviation (N = 3). Statistical difference is represented by * (P < 0.05) in comparison with the control groups (phosphate-buffered saline [PBS] or pcDNA 3.1C).
Protective efficacy of DNA plasmid vaccination in BALB/c mice.
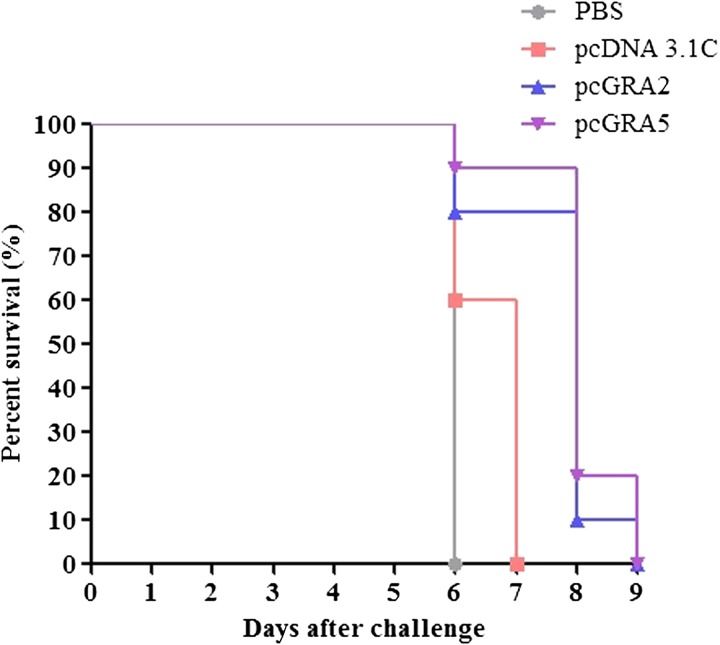
Results (Figure 5 ) indicated that two vaccinated mice groups were only managed to prolong the survival days up to 2–3 days as compared with the two control mice groups (PBS and pcDNA 3.1C) (P < 0.05). All PBS-injected mice died on day 6 (median survival of 6 days), whereas pcDNA 3.1C-injected mice died within 6–7 days (median survival of 7 days). On the other hand, pcGRA2- and pcGRA5-immunized mice succumbed to the parasite infection on 6–9 days postinfection with the median survival of 8 days.
Figure 5.
Survival rate of the immunized mice. All four groups of the immunized mice (phosphate-buffered saline [PBS], pcDNA 3.1C, pcGRA2, and pcGRA5) were subjected to lethal challenge with 1,000 live tachyzoites of Toxoplasma gondii-virulent RH strain 3 weeks after the last immunization. Mice immunized with pcGRA2 and pcGRA5 demonstrated a minor increase in their survival days (median survival of 8 days) in comparison to that of the control mice injected with phosphate-buffered saline and pcDNA 3.1C (median survival of 6 and 7 days, respectively). Each group consisted of 10 mice.
Discussion
Mammalian cells such as CHO cells have been widely used for expression of Toxoplasma recombinant protein,26 functional validation of Toxoplasma recombinant DNA plasmid27–29 as well as growth investigation of the parasite.30 In this study, CHO cell was used to validate the expression of GRA2 and GRA5 by the recombinant DNA construct prior to DNA vaccination study. Heterologous expression of recombinant protein in mammalian cell involves gene transfection into the host cell, delivery into host cell nucleus and subsequently protein translation in the cytoplasm.31 The TurboFect Transfection Reagent used in this study is a water-based cationic polymer, which forms a positively charged complex with the recombinant DNA plasmid. This stable complex is essential for the uptake by cell through endocytosis against the negatively charged cell membrane. Apart from providing the positive charge, the transfection reagent also helps to protect the DNA from degradation.31
The successful transfection and expression of pcGRA2 and pcGRA5 in CHO cells were confirmed in the western blot analysis. However, protein expression levels were too low for detection in Coomassie-stained sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. The transfection was transient as the plasmid DNA was not integrated into the CHO cell genome. The GRA genes would not be propagated but occurred in the host cell for only a few days. The genes would eventually be diluted on host cell divisions and degradation by host nucleases.32
Transient transfection usually serves as a preliminary experiment before the actual DNA vaccination in live animal. A previous study reported pGRA2- and pGRA5-transfected HEK 293-T cells produced 28 kDa GRA2 and 18 kDa GRA5,33,34 which were almost similar to that obtained in this study. Another study reported the in vitro expression of pVAX1-GRA2 in HFF mammalian cells, producing an antigenic protein at 20 kDa.35 Expression of several other T. gondii genes in mammalian cells has also been evaluated.13,22,35–43
In this study, DNA vaccination of BALB/c mice with pcGRA2 and pcGRA5 triggered mixed Th1/Th2-like cellular immune responses, predominantly of Th1. Humoral immunity was not successfully elicited as anti-TLA IgG was not detected either in western blot and ELISA analyses. Furthermore, the vaccinated mice were not protected against lethal challenge of T. gondii infection.
These results demonstrated stimulation and proliferation of T lymphocytes in the vaccinated mice. High level of the pro-inflammatory cytokines IFN-γ and IL-2 was achieved. However, relatively low levels of anti-inflammatory cytokines IL-4 and IL-10 were obtained. Collectively, it suggested that a predominantly Th1 immune response was elicited, which prolonged median survival days of the injected mice from 1 to 2 days.
The predominant Th1-like response in the vaccinated mice was most likely attributed to the presence of unmethylated CpG motifs in the vaccine vector pcDNA 3.1C.15,44 The immunostimulatory CpG motifs possess Th1-directing adjuvant activity and are associated with IFN-γ secretion as well as enhancing the immunogenicity of DNA vaccines.45–48
The DNA vaccines in this study were constructed without signal peptide at the N-terminal to ensure the translated proteins remained in the cytosol for antigen processing and presentation through the MHC class I complex to trigger cellular-mediated immune response.49 The exclusion of signal peptide from these two DNA vaccine constructs might be the reason contributing to the failure to develop antigen-specific antibody response (humoral immune response) as the translated proteins were not signaled out from the cells for the formation of peptide-MHC class II complex.7 A similar phenomenon has been observed in previous studies in which DNA vaccine candidates encoding T. gondii MIC2-MIC3-SAG1 and GRA3-GRA7-M2AP without the signal peptides stimulated only weak antibody production.44 No antibody production was elicited by DNA vaccines expressing MIC2 and MIC3 antigens.50 In contrast, full-length recombinant DNA vaccine constructs have been shown to produce strong antigen-specific antibody response.51
Several earlier investigations reported GRA2 DNA vaccine success against acute T. gondii infection. Multi- or single antigen elicited IgG antibody production with high ratio of IgG2a/IgG1, and conferred partial protection to the vaccinated BALB/c mice.19,20,22,35 Nonetheless, the pcGRA2 in present study triggered Th1-favored immunity associated with significant lymphocytes proliferation, increased IFN-γ secretion but low production of IL-4 and IL-10. These results were similar to those reported in previous investigations.19,20,22,35 Cao and others (2015)19 combined T- and B-cell epitopes of GRA2 with those from SAG1, GRA7, and ROP16, whereas Liu and others (2009)20 incorporated small segment of GRA2 with other small segments of SAG1, GRA1, and GRA4. Xue and others (2008)22 used GRA2 fragment in combination with SAG1-ROP2 plus DNA plasmid encoding IL-12 as the additional adjuvant. Zhou and others (2012)35 used plasmid vector pVAX in place of pcDNA 3.1.
Two previous studies using GRA5 DNA vaccine as single or multiantigen obtained little increase in the survival rate of the vaccinated mice against acute T. gondii infection.21,52 Induction of Th1 immunity coupled with significant production of IFN-γ and IgG2a isotype was observed. In contrast to the present study, the previous studies used the complete GRA5 sequence or combination of full length GRA5 + SAG1 + ROP2.21,52
The results of this study suggest that cell-mediated immunity alone may not be able to combat acute T. gondii infection effectively. Hence, mortality of the vaccinated mice might be attributed to the immunopathology exerted by overwhelming levels of IFN-γ and IL-2 followed by lethal parasitic infection. It is believed that deficiency in B cells increased Th1-related cytokines and the cells eventually succumbed to the inflammatory effect of the cytokine.53,54 The importance of humoral immunity against T. gondii infection has also been demonstrated in studies reporting increased survival of infected mice on receiving antigen-specific antibody.55,56 Induction of strong humoral and cellular responses is therefore essential for effective protection against T. gondii infection.55
Our previous studies using subunit vaccines GRA2 and GRA5 showed partial protection in infected mice.23 In these instances, both humoral and cellular immunity were successfully mounted with relatively lower IFN-γ and higher IL-10 levels in comparison with DNA vaccination. This observation is supported further by the various investigations that observed increased mortality and susceptibility rates toward T. gondii infection in mice lacking anti-inflammatory cytokines (IL-4 and IL-10) but high level of pro-inflammatory cytokine (IFN-γ).57–59
Conclusion
Intramuscular vaccination of mice with DNA vaccine triggered Th1/Th2 response with predominant Th1-directed response associated with significant elevation of IFN-γ and IL-2 levels, but relatively low levels of IL-4 and IL-10. Humoral immunity was not elicited in the vaccinated mice, which subsequently succumbed against Toxoplasma challenge. Despite the successful induction of cell-mediated immunity, failure to trigger humoral immunity has become one of the limitations in this study. Therefore, in the future studies, it will be worthwhile to evaluate the efficacy of recombinant multi-antigen incorporating full-length GRA2 or/and GRA5 with several other antigenic proteins such as the surface and rhoptry antigens.
ACKNOWLEDGMENTS
We express our gratitude to the Department of Parasitology, University of Malaya, for providing T. gondii tachyzoites of RH strain.
Footnotes
Financial support: This research project was supported by University of Malaya High Impact Research (HIR) Grant UM-MOHE (UM.C/HIR/MOHE/MED/16) from the Ministry of Higher Education, Malaysia.
Authors' addresses: Xiao Teng Ching, Mun Yik Fong, and Yee Ling Lau, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: cxteng85@yahoo.com, fongmy@um.edu.my, and lauyeeling@um.edu.my.
References
- 1.Dubey JP. Toxoplasmosis of Animals and Humans. Boca Raton, FL: CRC Press; 2010. p. 313. [Google Scholar]
- 2.Jackson MH, Hutchison WM. The prevalence and source of Toxoplasma infection in the environment. Adv Parasitol. 1989;28:55–105. doi: 10.1016/s0065-308x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- 3.Bhopale GM. Development of a vaccine for toxoplasmosis: current status. Microbes Infect. 2003;5:457–462. doi: 10.1016/s1286-4579(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 4.Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18:198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 5.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kur J, Holec-Gasior L, Hiszczynska-Sawicka E. Current status of toxoplasmosis vaccine development. Expert Rev Vaccines. 2009;8:791–808. doi: 10.1586/erv.09.27. [DOI] [PubMed] [Google Scholar]
- 7.Corr M, Tighe H. Plasmid DNA vaccination: mechanism of antigen presentation. Springer Semin Immunopathol. 1997;19:139–145. doi: 10.1007/BF00870264. [DOI] [PubMed] [Google Scholar]
- 8.Bessieres MH, Swierczynski B, Cassaing S, Miedouge M, Olle P, Seguela JP, Pipy B. Role of IFN-gamma, TNF-alpha, IL4 and IL10 in the regulation of experimental Toxoplasma gondii infection. J Eukaryot Microbiol. 1997;44:87S. doi: 10.1111/j.1550-7408.1997.tb05800.x. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli RT, Hakim FT, Hieny S, Shearer GM, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 10.Nam HW. GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane. Korean J Parasitol. 2009;47((Suppl)):S29–S37. doi: 10.3347/kjp.2009.47.S.S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capron A, Dessaint JP. Vaccination against parasitic diseases: some alternative concepts for the definition of protective antigens. Ann Inst Pasteur Immunol. 1988;139:109–117. doi: 10.1016/0769-2625(88)90135-3. [DOI] [PubMed] [Google Scholar]
- 12.Cesbron-Delauw MF, Capron A. Excreted/secreted antigens of Toxoplasma gondii–their origin and role in the host-parasite interaction. Res Immunol. 1993;144:41–44. doi: 10.1016/s0923-2494(05)80096-3. [DOI] [PubMed] [Google Scholar]
- 13.Hiszczynska-Sawicka E, Oledzka G, Holec-Gasior L, Li H, Xu JB, Sedcole R, Kur J, Bickerstaffe R, Stankiewicz M. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Vet Parasitol. 2011;177:281–289. doi: 10.1016/j.vetpar.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 14.Scorza T, D'Souza S, Laloup M, Dewit J, De Braekeleer J, Verschueren H, Vercammen M, Huygen K, Jongert E. A GRA1 DNA vaccine primes cytolytic CD8(+) T cells to control acute Toxoplasma gondii infection. Infect Immun. 2003;71:309–316. doi: 10.1128/IAI.71.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun XM, Zou J, Saeed EAA, Yan WC, Liu XY, Suo X, Wang H, Chen QJ. DNA vaccination with a gene encoding Toxoplasma gondii GRA6 induces partial protection against toxoplasmosis in BALB/c mice. Parasit Vectors. 2011;4:213. doi: 10.1186/1756-3305-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng P, Park J, Lee BS, Lee SH, Bram RJ, Jung JU. Kaposi's sarcoma-associated herpesvirus mitochondrial K7 protein targets a cellular calcium-modulating cyclophilin ligand to modulate intracellular calcium concentration and inhibit apoptosis. J Virol. 2002;76:11491–11504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilley M, Fichera ME, Jerome ME, Roos DS, White MW. Toxoplasma gondii sporozoites form a transient parasitophorous vacuole that is impermeable and contains only a subset of dense-granule proteins. Infect Immun. 1997;65:4598–4605. doi: 10.1128/iai.65.11.4598-4605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Gu Q, Zhao Q, Zhang J, Cong H, Li Y, He S. Toxoplasma gondii: expression and characterization of a recombinant protein containing SAG1 and GRA2 in Pichia pastoris. Parasitol Res. 2007;100:829–835. doi: 10.1007/s00436-006-0341-6. [DOI] [PubMed] [Google Scholar]
- 19.Cao A, Liu Y, Wang J, Li X, Wang S, Zhao Q, Cong H, He S, Zhou H. Toxoplasma gondii: vaccination with a DNA vaccine encoding T- and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015;33:6757–6762. doi: 10.1016/j.vaccine.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Shi L, Cheng YB, Fan GX, Ren HX, Yuan YK. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitol Res. 2009;105:267–274. doi: 10.1007/s00436-009-1393-1. [DOI] [PubMed] [Google Scholar]
- 21.Naserifar R, Ghaffarifar F, Dalimi A, Sharifi Z, Solhjoo K, Hosseinian Khosroshahi K. Evaluation of immunogenicity of cocktail DNA vaccine containing plasmids encoding complete GRA5, SAG1, and ROP2 antigens of Toxoplasma gondii in BALB/C mice. Iran J Parasitol. 2015;10:590–598. [PMC free article] [PubMed] [Google Scholar]
- 22.Xue M, He S, Cui Y, Yao Y, Wang H. Evaluation of the immune response elicited by multi-antigenic DNA vaccine expressing SAG1, ROP2 and GRA2 against Toxoplasma gondii. Parasitol Int. 2008;57:424–429. doi: 10.1016/j.parint.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Ching XT, Fong MY, Lau YL. Evaluation of immunoprotection conferred by the subunit vaccines of GRA2 and GRA5 against acute toxoplasmosis in BALB/c mice. Front Microbiol. 2016;7:609. doi: 10.3389/fmicb.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ching X-T, Lau Y-L, Fong M-Y. Heterologous expression of Toxoplasma gondii dense granule protein 2 and 5. Southeast Asian J Trop Med Public Health. 2015;46:375–387. [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Kim K, Bulow R, Kampmeier J, Boothroyd JC. Conformationally appropriate expression of the Toxoplasma antigen SAG1 (p30) in CHO cells. Infect Immun. 1994;62:203–209. doi: 10.1128/iai.62.1.203-209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdizadeh R, Maraghi S, Ghadiri AA, Tavalla M, Shojaee S. Cloning and expression of major surface antigen 1 gene of Toxoplasma gondii RH strain using the expression vector pVAX1 in Chinese hamster ovary cells. Jundishapur J Microbiol. 2015;8:e22570. doi: 10.5812/jjm.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonaimuthu P, Ching XT, Fong MY, Kalyanasundaram R, Lau YL. Induction of protective immunity against toxoplasmosis in BALB/c mice vaccinated with Toxoplasma gondii Rhoptry-1. Front Microbiol. 2016;7:808. doi: 10.3389/fmicb.2016.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parthasarathy S, Fong MY, Ramaswamy K, Lau YL. Protective immune response in BALB/c mice induced by DNA vaccine of the ROP8 gene of Toxoplasma gondii. Am J Trop Med Hyg. 2013;88:883–887. doi: 10.4269/ajtmh.12-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ihara F, Nishikawa Y. Starvation of low-density lipoprotein-derived cholesterol induces bradyzoite conversion in Toxoplasma gondii. Parasit Vectors. 2014;7:248. doi: 10.1186/1756-3305-7-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunert R, Vorauer-Uhl K. Strategies for efficient transfection of CHO-cells with plasmid DNA. Methods Mol Biol. 2012;801:213–226. doi: 10.1007/978-1-61779-352-3_14. [DOI] [PubMed] [Google Scholar]
- 32.Hartley JL. Why proteins in mammalian cells? Methods Mol Biol. 2012;801:1–12. doi: 10.1007/978-1-61779-352-3_1. [DOI] [PubMed] [Google Scholar]
- 33.Babaie J, Sadeghiani G, Golkar M. Construction and in vitro expression analyses of a DNA plasmid encoding dense granule GRA5 antigen of Toxoplasma gondii. Avicenna J Med Biotechnol. 2011;3:135–141. [PMC free article] [PubMed] [Google Scholar]
- 34.Golkar M, Shokrgozar MA, Rafati S, Sadaie MR, Assmar M. Construction, expression and preliminary immunological evaluation of a DNA plasmid encoding the GRA2 protein of Toxoplasma gondii. Iran Biomed J. 2005;9:1–8. [Google Scholar]
- 35.Zhou H, Min J, Zhao Q, Gu Q, Cong H, Li Y, He S. Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant. Vaccine. 2012;30:1800–1806. doi: 10.1016/j.vaccine.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Huang SY, Li ZY, Yuan ZG, Zhou DH, Petersen E, Zhang NZ, Zhu XQ. Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine. 2013;31:1734–1739. doi: 10.1016/j.vaccine.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Hiszczynska-Sawicka E, Li H, Xu JB, Oledzka G, Kur J, Bickerstaffe R, Stankiewicz M. Comparison of immune response in sheep immunized with DNA vaccine encoding Toxoplasma gondii GRA7 antigen in different adjuvant formulations. Exp Parasitol. 2010;124:365–372. doi: 10.1016/j.exppara.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Ismael AB, Sekkai D, Collin C, Bout D, Mevelec MN. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infect Immun. 2003;71:6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu D, Han J, Du A. Evaluation of protective effect of multiantigenic DNA vaccine encoding MIC3 and ROP18 antigen segments of Toxoplasma gondii in mice. Parasitol Res. 2013;112:2593–2599. doi: 10.1007/s00436-013-3425-0. [DOI] [PubMed] [Google Scholar]
- 40.Tao Q, Fang R, Zhang W, Wang Y, Cheng J, Li Y, Fang K, Khan MK, Hu M, Zhou Y, Zhao J. Protective immunity induced by a DNA vaccine-encoding Toxoplasma gondii microneme protein 11 against acute toxoplasmosis in BALB/c mice. Parasitol Res. 2013;112:2871–2877. doi: 10.1007/s00436-013-3458-4. [DOI] [PubMed] [Google Scholar]
- 41.Wu XN, Lin J, Lin X, Chen J, Chen ZL, Lin JY. Multicomponent DNA vaccine-encoding Toxoplasma gondii GRA1 and SAG1 primes: anti-Toxoplasma immune response in mice. Parasitol Res. 2012;111:2001–2009. doi: 10.1007/s00436-012-3047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan ZG, Zhang XX, Lin RQ, Petersen E, He S, Yu M, He XH, Zhou DH, He Y, Li HX, Liao M, Zhu XQ. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine. 2011;29:6614–6619. doi: 10.1016/j.vaccine.2011.06.110. [DOI] [PubMed] [Google Scholar]
- 43.Yuan ZG, Zhang XX, He XH, Petersen E, Zhou DH, He Y, Lin RQ, Li XZ, Chen XL, Shi XR, Zhong XL, Zhang B, Zhu XQ. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin Vaccine Immunol. 2011;18:119–124. doi: 10.1128/CVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg C, De Craeye S, Jongert E, Gargano N, Beghetto E, Del Porto P, Vorup-Jensen T, Petersen E. Induction of partial protection against infection with Toxoplasma gondii genotype II by DNA vaccination with recombinant chimeric tachyzoite antigens. Vaccine. 2009;27:2489–2498. doi: 10.1016/j.vaccine.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 45.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 47.Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 48.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 49.York IA, Goldberg AL, Mo XY, Rock KL. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol Rev. 1999;172:49–66. doi: 10.1111/j.1600-065x.1999.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 50.Beghetto E, Nielsen HV, Del Porto P, Buffolano W, Guglietta S, Felici F, Petersen E, Gargano N. A combination of antigenic regions of Toxoplasma gondii microneme proteins induces protective immunity against oral infection with parasite cysts. J Infect Dis. 2005;191:637–645. doi: 10.1086/427660. [DOI] [PubMed] [Google Scholar]
- 51.Dautu G, Munyaka B, Carmen G, Zhang G, Omata Y, Xuenan X, Igarashi M. Toxoplasma gondii: DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Exp Parasitol. 2007;116:273–282. doi: 10.1016/j.exppara.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Ghaffarifar F, Naserifar R, Jafari Madrak M. Eukaryotic plasmids with Toxoplasma gondii dense granule antigen (GRA 5) and Microneme 3 (MIC3) genes as a cocktail DNA vaccine and evaluation of immune responses in BALB/C Mice. J Clin Med Genom. 2014;3:2. [Google Scholar]
- 53.Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000;68:1026–1033. doi: 10.1128/iai.68.3.1026-1033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson LL, Sayles PC. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect Immun. 2002;70:185–191. doi: 10.1128/IAI.70.1.185-191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frenkel JK, Taylor DW. Toxoplasmosis in immunoglobulin M-suppressed mice. Infect Immun. 1982;38:360–367. doi: 10.1128/iai.38.1.360-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson AM, McDonald PJ, Neoh SH. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J Protozool. 1983;30:351–356. doi: 10.1111/j.1550-7408.1983.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 57.McLeod R, Eisenhauer P, Mack D, Brown C, Filice G, Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989;142:3247–3255. [PubMed] [Google Scholar]
- 58.Roberts CW, Ferguson DJ, Jebbari H, Satoskar A, Bluethmann H, Alexander J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect Immun. 1996;64:897–904. doi: 10.1128/iai.64.3.897-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]