Abstract
Several epidemiological studies have indicated the presence of asymptomatic infections with Leishmania donovani in the Indian subcontinent, where parasite transmission is considered anthroponotic. In India, such asymptomatic Leishmania cases have been identified in the state of Bihar. We explored here, the presence of asymptomatic Leishmania infection among healthy individuals living in two districts in the state of West Bengal, India, using serological and molecular tests. Blood samples of 246 healthy individuals were collected from nine villages of Malda and Murshidabad districts in West Bengal, considered endemic for visceral leishmaniasis (VL). Real-time quantitative polymerase chain reaction (qPCR) was performed for the quantification of parasite load in the blood. In addition, two serological tests were carried out to demonstrate anti-Leishmania antibodies: rK39 strip test and anti-total soluble Leishmania antigen IgG using enzyme-linked immunosorbent assay method. Nearly one-fifth (53/246) of the screened population was positive in qPCR as against 10.97% (27/246) positive in rK39 strip test. A range of parasite load was observed in the blood of identified asymptomatic Leishmania cases with a median value of 7.7 parasites/mL (range = 1–65). There was poor agreement between qPCR and serological tests (κ = 0.089, P = 0.13), and 29.62% and 20.54% of the population were qPCR positive in seropositive and seronegative groups, respectively. Combined molecular and serological tests enhanced the capacity to detect asymptomatic Leishmania infection in healthy individuals residing in the endemic areas of VL. A significant proportion of asymptomatic Leishmania individuals was detected in the examined endemic regions of West Bengal that might play a role in promoting VL transmission.
Introduction
Visceral leishmaniasis (VL) or kala-azar is the severest form of leishmaniasis, characterized by irregular bouts of fever, splenomegaly with or without hepatomegaly, anemia, and weight loss. VL is highly endemic in the Indian subcontinent and East Africa, and over 90% of VL incidence have been reported from six countries: India, Sudan, South Sudan, Brazil, Ethiopia, and Nepal.1 Of approximately 0.2–0.4 million new cases of VL that occur annually, nearly 10% succumb to the disease.1 Besides, in the areas endemic for Leishmania donovani, post-kala-azar dermal leishmaniasis (PKDL), a dermal sequel of VL, develops in 5–15% of recovered VL individuals in the Indian subcontinent, as against 50–60% cases in Sudan.2–5
In India, VL has been one of the major health issues in the state of Bihar, and the adjoining states such as West Bengal, Uttar Pradesh, and Jharkhand.6 The most affected region for VL is the state of Bihar where 31 of 38 districts, followed by West Bengal with 12 of 20 districts, are considered endemic for VL.7 Overall, 52 districts in India, 45 in Bangladesh, 12 in Nepal, and four in Bhutan are affected.8
In the Indian subcontinent, the transmission of VL is anthroponotic.9,10 Hence, the cases of active VL and PKDL could serve as a reservoir of L. donovani, especially during the inter-epidemic period11; therefore, a better management of the disease is warranted. In addition, a majority of the Leishmania-infected human population do not develop into full-blown VL cases and are categorized as asymptomatic Leishmania cases12–14 and such cases may have a role in maintaining transmission dynamics of Leishmania infection.15,16 This threat is further supported by fact that parasites could be cultured from the blood of healthy donors17 and such cases could be infective. However, the knowledge of the magnitude of the asymptomatic Leishmania cases present in an endemic area is difficult to assess. Few studies have reported the presence of asymptomatic Leishmania cases in the VL-endemic areas of the state of Bihar, India,14,18,19 in the range of 11–34%. Similarly, there could be a high probability of the presence of asymptomatic Leishmania infection in the state of West Bengal, another VL-endemic region in India. VL elimination from the Indian subcontinent has been a long-term goal with three major time frames documented. The first VL elimination time frame that ended in 2015 was to monitor the progress that has been made. The second time frame has been set until 2017 to eliminate VL as a public health problem with commitment from governments of Indian subcontinent and managers of various VL elimination programs; and the third, as part of the London declaration on neglected tropical diseases, is to eliminate tropical diseases including VL as a public health problem in India and Bangladesh by 2020.20,21 Hence, given the national goal of VL elimination by the year 2020, the identification and estimation of asymptomatic Leishmania infections in VL-endemic areas are of paramount importance for formulating appropriate public health policies.
An important challenge toward the identification of asymptomatic Leishmania cases is the deployment of the appropriate, cost-effective diagnostic method that could be deployed for the large scale screening of Leishmania infection in the healthy human population residing in the VL-endemic zones. Serology-based detection tests (e.g., DAT, rk39 strip/enzyme-linked immunosorbent assay [ELISA], and western blot assays) is of limited use as it cannot differentiate individuals with present and past episode of VL, PKDL, and asymptomatic Leishmania cases.22,23 On the other hand, molecular diagnostic methods are highly sensitive, reliable, and rapid for the diagnosis of Leishmania infection, and polymerase chain reaction (PCR)-based assays currently constitute the main molecular diagnostic approach of researchers and health professionals.24,25 Previous studies on asymptomatic Leishmania population were done utilizing ITS-1-based PCR,14,18 whereas one study was performed using TaqMan-based quantitative PCR (qPCR).19 Here, we performed SYBR Green-based qPCR assay for the detection and quantification of Leishmania parasites in blood samples. The present study, to best of our knowledge, is the first report from the state of West Bengal, India, on serological and molecular analysis of Leishmania parasites in blood samples of healthy individuals living in VL-endemic regions, to understand the magnitude of asymptomatic Leishmania infection prevalent in the region.
Materials and Methods
Study population.
The study area covers regions from both north and south Bengal. The regions selected were the villages of Malda and Murshidabad districts, based on the high VL incidence as per government records during the 3-years period 2011–2013. These regions are considered highly endemic for VL. Individuals living in these high-endemic zones were taken as the study population. Although enrolling for the study, we randomly selected resident healthy individuals from different villages of these two highly VL-endemic districts and recorded their clinico-epidemiological data. During the period between January 2014 and December 2014, a team of physician, technician, and trained field investigators made nine field trips to different villages and clinically examined individuals for the symptoms of VL such as fever for more than 2 weeks, an enlarged spleen and/or liver and general physical conditions. A house-to-house survey was undertaken for the identification of a cohort of asymptomatic Leishmania individuals. A set of questionnaire was given to each to obtain basic information such as age, gender, nativity, history of VL and/or PKDL, and history of VL/PKDL in the family and/or neighborhood. Of 248 individuals examined, one had active VL and one active PKDL. These two cases were referred to the primary health center for their treatment and were excluded from the study; the rest 246 individuals were included in the present study. Peripheral blood samples (2–3 mL) were collected in heparinized tubes for serological and molecular analysis. A flow chart of the enrolment and the outcomes of the study population are shown in Figure 1 .
Figure 1.
Schematic diagram for enrollment and outcomes of the study populations. H/VL = cases with a history of VL; +ve = cases with a positive test result; −ve = cases with the negative test result.
Case definition.
Visceral leishmaniasis.
A case of VL is an individual who presents clinical signs such as prolonged irregular fever, splenomegaly, anemia, and weight loss with serological and/or parasitological confirmation.
Post-kala-azar dermal leishmaniasis.
An individual from a VL-endemic area with or without a history of VL who presents clinical features such as hypopigmented macules, indurated papular or nodular lesions on face that often disseminate to the other body parts without loss of sensation and confirmed through visualization of Leishmania amastigotes by direct microscopy in skin tissue/slit aspirates samples and/or a qPCR test for Leishmania DNA.
Asymptomatic leishmania case.
Asymptomatic Leishmania cases refer to the healthy individuals from VL-endemic regions who test positive by rK39 strip test (for cases with no history of VL) and/or by qPCR assay.
The rK39 test using plasma sample.
The rK39 strip test (Inbios International Inc., Seattle, WA) is an immunochromatographic test used for screening individuals for the Leishmania infection. It is specific for antibodies to L. donovani complex in patients with VL.26 The sensitivity of the test for detection of VL in India is 99% (95% confidence interval [CI] = 95–100%), and its specificity is 89% (95% CI = 86–92%).26 The test is cost-effective, easy, and reliable diagnostic tool for VL, but fails to discriminate between healed cases of VL, asymptomatic Leishmania, and active cases of VL and PKDL. The test was carried out as per the manufacturer's instructions. Two drops of plasma were placed at the tip of the rK39 strip, and two drops of chase buffer were added. The result of the test was observed after 10 minutes. Development of two visible (control and test) bands indicated the presence of anti-rK39 IgG.
DNA isolation.
DNA was isolated from the heparinized blood using QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany) as per the manufacturer's instructions. To obtain high yield, the sample was digested overnight with proteinase K in lysis solution (Qiagen). DNA was isolated from 200 μL blood and eluted in 50 μL distilled water. All samples were processed, and DNA was stored at −80°C until use.
Real-time PCR assay.
SYBR Green I-based real-time PCR was used for the quantification of the target DNA sequence as described earlier.27 Briefly, the assay was performed using an absolute quantification method on 7500 Fast Real-Time PCR machine (Applied Biosystems, Carlsbad, CA). All samples were analyzed in triplicates. A 10 μL PCR reaction mixture was performed, containing 1 μL DNA sample, 5 pmol each of forward and reverse primer, and 1X SYBR Green I PCR Master Mix (Applied Biosystems). Cycling parameters were 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Results were analyzed using 7500 Software v2.0.6 (Applied Biosystems) by comparing the cycle threshold (Ct) values from the samples to a standard curve, which was constructed using serial 10-fold dilutions from 103 to 0.1 parasite DNA per reaction. If the standard deviation (SD) between triplicates was > 0.38, the sample set was reanalyzed. The Ct value for each sample was calculated by determining the point at which the fluorescence exceeded the threshold limit. For minimizing the variability between plates, values from each plate was normalized using a common fluorescence detection baseline as obtained from standard curve analysis with the default setting. For confirmatory positive results, the samples with Ct values < 30 were considered positive.
Preparation of total soluble Leishmania antigen.
Total soluble Leishmania antigen (TSLA) was prepared as described in our earlier study.28 Briefly, promastigotes of L. donovani (MHOM/IN/80/DD8) were harvested in stationary phase, washed, and the pellet was resuspended in the lysing solution (50 mM Tris/5 mM ethylenediaminetetraacetic acid/HCl) (1 mL of lysing solution per 109 parasites). After three cycles of freezing/thawing, the tube was placed on ice. Three pulses of 20 seconds at 40 W with Sonicator at 1-minute intervals were carried out. The tube was centrifuged, the supernatant was collected, and protein estimation was done by Bradford method. TSLA was stored at −80°C until further use.
Detection of anti-leishmanial antibodies in plasma.
Leishmania-specific antibodies were determined by ELISA as reported earlier.29,30 Briefly, 96-well polystyrene flat-bottom ELISA plates (NUNC MaxiSorp™, Brandby, Denmark) were coated with 100 μL of TSLA (10 μg/mL) in bicarbonate buffer (pH 9.0) overnight at 4°C. The plates were washed and blocked with 1% bovine serum albumin for 2 hours at 37°C and washed thrice with phosphate-buffered saline containing 0.1% Tween 20 (PBST). The plates were incubated for 2 hours with plasma (1:100) from asymptomatic Leishmania, healed VL (HVL), and healthy control groups. Wells were washed thrice with PBST and incubated with horseradish peroxidase-conjugated anti-human IgG (1:5,000) for TSLA for 2 hours at 37°C. Plates were washed and ortho-phenylenediamine substrate (SIGMAFAST OPD, St. Louis, MO) was added to produce a color reaction. The reaction was stopped by addition of 1 N H2SO4 and absorbance was measured at 492 nm using ELISA reader. All reactions were carried out at least in triplicate.
Ethics statement.
The study was approved by and carried out following the guidelines of the Ethical Committee of National Institute of Pathology (Indian Council of Medical Research), Safdarjung Hospital Campus, New Delhi. All patients or their guardians (in the case of minors) provided written informed consent for collection of samples and subsequent analysis.
Statistical analysis.
The data were analyzed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA). Statistical significance was determined by nonparametric Kruskal–Wallis test followed by the post hoc Dunn multiple comparison tests for more than two groups. The correlation was calculated by Spearman rank correlation test. Agreement between rK39 strip test and qPCR assay was estimated using Cohen's κ coefficient on SPSS software (IBM, Somers, NY). The statistical tests were two-tailed and P values < 0.05 were considered significant.
Results
Clinical characteristics.
The clinical characteristics of the study population are presented in Table 1. The majority of cases belonged to the age group 19–44 years, followed by pediatric cases aged ≤ 18 years and the group aged ≥ 45 years (Table 1). In the study, 36.17% (N = 89) of individuals reported a history of VL, whereas 63.82% (N = 157) had no previous VL episode. Among the study population, 76.82% (N = 189) had either household and/or neighborhood contacts of VL and/or PKDL.
Table 1.
Age–gender distribution of the study population
| Age (years) | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | N (%) | Mean ± SD | N (%) | Mean ± SD | |
| ≤ 18 | 36 (36.36) | 10.77 ± 17.34 | 38 (25.85) | 10.73 ± 17.41 | 74 (30.08) | 10.75 ± 17.31 |
| 19–44 | 42 (42.42) | 30.33 ± 17.24 | 74 (50.34) | 30.54 ± 17.33 | 116 (47.15) | 30.46 ± 17.24 |
| ≥ 45 | 21 (21.21) | 58.23 ± 17.45 | 35 (23.81) | 53.2 ± 17.41 | 56 (22.76) | 55.09 ± 17.41 |
| Total | 99 (40.24) | 29.14 ± 17.24 | 147 (59.75) | 30.81 ± 17.36 | 246 | 30.14 ± 17.24 |
SD = standard deviation.
Serological diagnosis.
The rK39 strip test was positive in 10.97% (N = 27) plasma samples and the rest 89.02% (N = 219) individuals were seronegative for the rK39 antigen. The majority of seropositive individuals, 81.48% (N = 22), had reported a history of VL and were HVL individuals. The remaining five seropositive individuals were exposed to either household or neighborhood contacts of VL. Among 219 seronegative individuals, 67 had reported a history of VL, with the range of time lapse following VL treatment as 1–20 years, (mean ± SD = 7.19 ± 3.51 years).
Analysis of parasite load in blood samples.
Of 246 healthy individuals, 21.54% (N = 53) were found positive by qPCR for amplification of parasite DNA and therefore considered to have asymptomatic Leishmania infection. The median value of parasite load/mL of blood was 7.7 (range = 1–65). Among these, 12.82% (N = 5) were exclusively exposed to household VL contacts, and 27.11% (N = 32) had neighborhood VL and/or PKDL contacts. Overall, 81.81% (45/53) individuals living in the endemic area had come in contact with VL and/or PKDL either in the household and/or neighborhood (Table 2). Among these qPCR-positive individuals, 37.73% (N = 20) had parasitemia levels below 5 parasites/mL, followed by 32.07% (N = 17) individuals with parasitemia between 5 and 10 parasites/mL, 20.75% (N = 11) individuals between 11 and 25 parasites/mL, 5.67% (N = 3) individuals between 26 and 50 parasites/mL, and the rest 3.77% (N = 2) with parasitemia above 50 parasites/mL (Table 2). Besides, two healthy individuals without a history of VL who reacted positive to rK39 strip test were found negative by qPCR and were categorized as asymptomatic Leishmania cases. In addition, we observed eight overlapping cases that were found positive by both qPCR and rK39 test. Also, all the 20 individuals from the nonendemic area were found negative by qPCR.
Table 2.
Clinico-epidemiological characteristics of the study population living in VL-endemic regions in two districts in the state of West Bengal, India
| Household contacts, n (%) | Neighbor contacts, n (%) | Both, n (%) | None, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|---|---|
| Percent contacts of either VL and/or PKDL in different study groups | Asymptomatic | 5 (12.82) | 32 (27.11) | 8 (25) | 10 (17.54) | 55 (22.35) | |
| EHCs | 28 (71.79) | 59 (50) | 10 (31.25) | 21 (36.84) | 118 (47.96) | ||
| HVL | 6 (15.38) | 27 (22.88) | 14 (43.75) | 26 (45.61) | 73 (29.67) | ||
| Total | 39 (15.85) | 118 (47.96) | 32 (13) | 57 (23.17) | 246 | ||
| PCR | rK39 strip test | Total | Kappa value, κ | ||||
| Positive | Negative | ||||||
| Comparative analysis of rK39, PCR results | Positive | 8 (29.62) | 45 (20.54) | 53 (21.54) | κ = 0.089, P = 0.13 | ||
| Negative | 19 (70.37) | 174 (79.45) | 193 (78.45) | ||||
| Total | 27 (10.97) | 219 (89.02) | 246 | ||||
| Analysis of parasite load in blood samples | Range of parasite load/mL blood from healthy individuals with or without history of VL from endemic and nonendemic areas, evaluated using qPCR technique | ||||||
| Positive | Negative | < 5 | 5–10 | 11–25 | 26–50 | > 50 | |
| H/VL (N = 89) | 16 | 73 | 7 | 5 | 2 | 1 | 1 |
| Rest (N = 157) | 37 | 120 | 13 | 12 | 9 | 2 | 1 |
| NEHCs (N = 20) | 0 | 20 | |||||
EHC = endemic healthy control; HVL = healed visceral leishmaniasis; H/VL = healthy individuals with history of VL; NEHCs = nonendemic healthy individuals; PCR = polymerase chain reaction; PKDL = post-kala-azar dermal leishmaniasis; VL = visceral leishmaniasis.
Among the total asymptomatic Leishmania group (N = 55), the mean age of male (mean ± SD = 35.36 ± 16.26) was comparable to that of female (mean ± SD = 31.41 ± 16.13). The majority of asymptomatic Leishmania cases belonged to age group19–44 years (60%, N = 33), followed by the group aged ≥ 45 years (23.63%, N = 13) and the pediatric cases aged ≤ 18 years (16.36%, N = 9) (Table 3).
Table 3.
Age–gender distribution of the identified asymptomatic Leishmania population
| Age (years) | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| N (%) | Mean ± SD | N (%) | Mean ± SD | N (%) | Mean ± SD | |
| ≤ 18 | 4 (21.04) | 13 ± 17.83 | 5 (13.88) | 9 ± 17.36 | 9 (16.36) | 10 ± 16.29 |
| 19–44 | 10 (52.63) | 30.7 ± 16.03 | 23 (63.88) | 29.52 ± 16.19 | 33 (60) | 29.87 ± 16.2 |
| ≥ 45 | 5 (26.31) | 62.6 ± 16.53 | 8 (22.22) | 50.87 ± 16.57 | 13 (23.63) | 55.31 ± 17.08 |
| Total | 19 (34.54) | 35.36 ± 16.26 | 36 (65.45) | 31.41 ± 16.13 | 55 | 32.78 ± 16.11 |
SD = standard deviation.
Level of anti-leishmanial IgG.
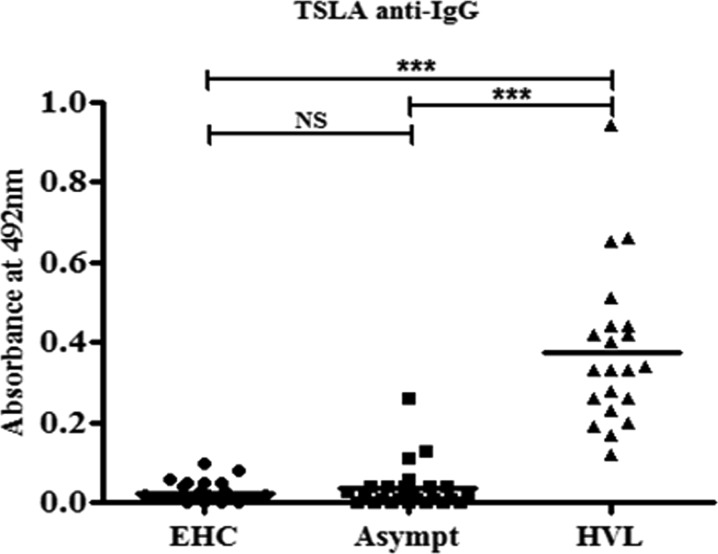
Based on results of serological and molecular tests and the history of VL, the study population was categorized into three groups—1) endemic healthy controls (EHCs): healthy individuals from VL-endemic regions who reported no history of VL and were negative by both qPCR and rK39 strip test. 2) HVL group: healthy individuals who reported a history of VL and tested negative for Leishmania DNA by qPCR. 3) Asymptomatic Leishmania group: healthy individuals from VL-endemic regions who test positive by rK39 strip test (for cases with no history of VL) and/or by qPCR assay. The host immune response was evaluated in terms of humoral anti-leishmanial IgG marker in different study groups. Plasma samples from EHCs (N = 25), HVL (N = 21), and asymptomatic Leishmania (N = 25) groups were tested for the presence of anti-TSLA IgG. Total anti-TSLA IgG was found significantly high in HVL (mean ± SD, OD492, 0.38 ± 0.196, P < 0.001) compared with asymptomatic Leishmania (mean ± SD, OD492, 0.04 ± 0.058) and EHCs (mean ± SD, OD492, 0.04 ± 0.027) (Figure 2 ), whereas anti-TSLA IgG level was comparable in asymptomatic Leishmania and EHCs group (P > 0.05). In addition, every individual in HVL group and 12% (N = 3) of the asymptomatic Leishmania group had anti-TSLA IgG absorbance level above cut-off (0.111). The cut-off value was calculated as mean + 3 SD of the EHCs group.
Figure 2.
Humoral response in the study population. Anti-leishmanial (total soluble Leishmania antigen [TSLA]) IgG in the plasma of endemic healthy controls (EHCs) (N = 25), asymptomatic Leishmania (N = 25), and healed visceral leishmaniasis (HVL) (N = 21) individuals was evaluated using enzyme-linked immunosorbent assay as mean ± standard deviation, OD492 level. The bars represent the mean for the different groups. Data were analyzed between groups by the nonparametric Kruskal–Wallis test followed by the post hoc Dunn multiple comparison tests. NS = not significant; P < 0.05 is considered statistically significant. *** P < 0.001.
Association of parasite load and anti-leishmanial immunoglobulin.
The elevated level of parasite load in asymptomatic Leishmania group was measured for correlation with anti-leishmanial IgG level in plasma samples. We obtained a relatively weak negative correlation between parasite load and anti-TSLA IgG level (r = −0.229, P = 0.270) as represented in Figure 3 . Also, the study demonstrated a poor agreement between qPCR and serological tests (κ = 0.089, P = 0.13), and 29.62% and 20.54% of the study population were qPCR positive in seropositive and seronegative groups, respectively (Table 2).
Figure 3.
Correlation between parasite load and anti-leishmanial IgG level in the asymptomatic Leishmania group. The correlation was calculated by Karl Pearson correlation test.
Discussion
In the Indian subcontinent, humans are considered the only known reservoir for the Leishmania parasites9,10; therefore, apart from the control measures of VL, such as early diagnosis and treatment of active cases of VL and PKDL, and integrated vector management, the assessment of asymptomatic Leishmania infections in the endemic population needs attention. The study using mathematical modeling indicated that the asymptomatic Leishmania cases constitute a silent pool of parasites driving the epidemic,12 but their infectivity to sand flies has not been yet established. In addition, several prospective studies have reported the ratio of incident asymptomatic Leishmania infections to incident clinical cases as 8.9:1 in India and Nepal13,18 and 4:1 in Bangladesh,31 demonstrating that a fraction of these does get converted to symptomatic VL that might facilitate further transmission in their ambience. The knowledge of the actual estimate of Leishmania infection in healthy individuals in an endemic area is difficult to assess due to various reasons such as nonavailability of appropriate diagnostic methods in primary health centers, the lack of skilled personnel, the willingness of residents to participate in epidemiological studies, and other practical issues.
For diagnosis of active VL, the direct microscopic examination and/or culture of tissue aspirates from bone marrow or spleen are considered the gold standard techniques.11 However, these are ethically inappropriate and impractical for screening large healthy population for Leishmania infection. Therefore, procedures based on minimal invasive sampling, such as serologic analysis or qPCR assay, are more suitable for this purpose. Besides, some investigators used leishmanin skin test (LST), an another useful method for screening subclinical infection in the healthy population,32 that measures a delayed-type hypersensitivity reaction to Leishmania antigens. Further, LST becomes positive later after infection and persist much longer than antileishmanial antibodies. In addition, seropositivity indicates more recent infection and has been related to disease progression.33,34 The present study is the first report to assess the magnitude of the asymptomatic Leishmania infection in villages of Malda and Murshidabad districts in the state of West Bengal, India. Herein, molecular and serological methods were combined to identify Leishmania infection in healthy individuals living in VL-endemic areas. We screened the study population initially with rK-39 strip test, which is simple, easy, and cost-effective point-of-care test. The observed seropositivity at baseline was 3.25%, which was comparatively lower than other reports from Bihar, 5.6%,14 13.79%,35 and 13%.36 These differences could be attributed to variations in size of the study population, ethnicity, and/or the selection of the endemic areas under study. Besides, we observed two seropositive healthy individuals who reported no history of VL. Interestingly, they were found negative for parasite DNA by qPCR assay, which could be due to degradation and clearance of Leishmania DNA after infection, corresponding to the development of protective immunity.19
Using highly sensitive quantitative PCR method, the observed positivity at baseline was 21.54%, proportionally lower than one earlier study (34.78%)19 but higher than other studies from Bihar, India that investigated asymptomatic Leishmania cases using conventional PCR with 10%24 and 7.2%14 Leishmania infection in the healthy population. Similar observation was also made in the adjoining country, Nepal, another VL-endemic area, that demonstrated 12.5% asymptomatic Leishmania cases.37 The higher positivity observed in the studies that used qPCR (present study and Sudarshan and others19) may be due to the higher sensitivity of qPCR compared with that of conventional PCR used in other studies.
Notably, combining both molecular and serological methods have slightly increased the baseline prevalence rate of asymptomatic Leishmania infection to 22.35% in the current study. Another study from Bihar reported the lower prevalence rate of 9.8%.14 The varied rates of asymptomatic Leishmania infections could be multifactorial such as methods used for diagnosis, variation in sample size, geographical areas/climatic conditions, the risk level of population, and immune and socioeconomic status.38 Among molecular and serological methods, we observed poor agreement (κ = 0.089), the finding in line with two earlier reports.13,19 Therefore, the deployment of combined molecular and serological methods rather than one proved superior for screening the human population for the asymptomatic Leishmania infection and that positive serology may not stand necessarily be true for a molecular method of Leishmania detection.
In the current study, a range of parasite load was observed in peripheral blood of the screened asymptomatic Leishmania cases. The cases with high parasitemia are more likely to turn into full-blown VL disease as compared with those with lower parasitemia, as reflected by similar study.19 Further, routine follow-up is required to know the conversion rate of asymptomatic Leishmania cases or their status of infection. Besides, among individuals with a history of VL, 17.97% were found positive by qPCR, in line with two other reports, one from India 23%39 and another one from Nepal 26.1%.37 Persistence of Leishmania DNA and DAT antibodies has been demonstrated immediately after VL treatment both in Sudan and India,40,41 whereas during their follow-up DAT remained positive but PCR became negative in the majority of HVL cases. Considering the time lapse following VL treatment (1–20 years), the PCR-positive cases might represent re-infection. Seronegative individuals having positive qPCR test could occur if the individual was bitten by a Leishmania-infected sand fly, either immune response has not yet developed, or antibody levels are not high enough to be detectable by the methods used. Similar findings were made by other investigators from India, where out of 1,068 EHCs seronegative for Leishmania antigen, 31.8% (N = 340) were found positive by qPCR.19
Besides, the host immunity to TSLA was evaluated in terms of humoral response in different study groups to identify a marker for asymptomatic Leishmania infection. As expected, elevated levels of anti-TSLA IgG were observed in HVL group compared with asymptomatic Leishmania and EHCs, whereas it was found comparable between asymptomatic Leishmania and EHCs groups. Further, only 12% asymptomatic Leishmania cases showed observable IgG level, indicating that level of anti-TSLA IgG in plasma samples does not hold promise for identifying asymptomatic Leishmania cases from HVL in the endemic areas. In addition, the weak negative correlation between anti-TSLA IgG level and parasite load for the asymptomatic Leishmania group implies that the humoral responses do not mirror the parasite load. Therefore, anti-TSLA IgG level is not a good choice for identifying asymptomatic Leishmania cases from HVL and healthy individuals for Leishmania infection in the VL-endemic areas.
There were a few limitations in the study. First is the selection of study area as only two VL-endemic districts were selected. Hence, the observed prevalence of the asymptomatic Leishmania cases cannot be generalized to all VL-endemic areas in the state of West Bengal. Second, not all villagers were willing to participate and so did not attend the camp for clinical examination and investigations.
Although the qPCR method is clearly more sensitive and specific than serological methods, neither approach is perfect. It is evident from our study as well from other studies14,19 that some asymptomatic Leishmania cases were found negative by qPCR but had positive serology test. Hence, a combination of both, qPCR to detect parasitemia and serology to identify individuals with high titers, may be an appropriate approach for early monitoring of Leishmania infection in the healthy population who might serve as a reservoir for the disease transmission.
In conclusion, the study identified 22.35% asymptomatic Leishmania individuals among the recruited individuals living in the two VL-endemic districts (Malda and Murshidabad) of West Bengal. Besides, the qPCR method was more sensitive than the serological method in the assessment of asymptomatic Leishmania individuals. Deployment of combined molecular and serological methods proved better approach that effectively estimated the asymptomatic Leishmania infection in healthy individuals living in the endemic regions, and may contribute to early case detection. Furthermore, the knowledge of quantitative estimation of asymptomatic Leishmania individuals in the endemic area will be useful to take appropriate measures for the sustainable elimination of VL from Indian subcontinent.
Footnotes
Financial support: The work received financial assistance from The National Academy of Sciences, India.
Disclosure: Himanshu Kaushal is registered PhD student at the Department of Biological Sciences, Birla Institute of Technology and Science, Pilani, India.
Authors' addresses: Himanshu Kaushal, Sujit Kumar Bhattacharya, Sandeep Verma, and Poonam Salotra, Molecular Parasitology Laboratory, National Institute of Pathology, New Delhi, India, E-mails: hkarya@gmail.com, sujitkbhattacharya@yahoo.com, sanip_verma6@yahoo.co.in, and salotra@vsnl.com.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, de Boer M. WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zijlstra EE, Khalil EA, Kager PA, El-Hassan AM. Post-kala-azar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br J Dermatol. 2000;143:136–143. doi: 10.1046/j.1365-2133.2000.03603.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramesh V, Singh R, Salotra P. Short communication: post-kala-azar dermal leishmaniasis-an appraisal. Trop Med Int Health. 2007;12:848–851. doi: 10.1111/j.1365-3156.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 4.Rahman KM, Islam S, Rahman MWM, Kenah E, Ghalib CM, Galive CM, Zahid MM, Maguire J, Rahman MWM, Haque R, Luby SP, Bern C. Increasing incidence of post-kala-azar dermal leishmaniasis in a population-based study in Bangladesh. Clin Infect Dis. 2010;50:73–76. doi: 10.1086/648727. [DOI] [PubMed] [Google Scholar]
- 5.Mondal D, Nasrin KN, Huda MM, Kabir M, Hossain MS, Kroeger A, Thomas T, Haque R. Enhanced case detection and improved diagnosis of PKDL in a Kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis. 2010;4:e832. doi: 10.1371/journal.pntd.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- 7.Kesari S, Bhunia GS, Kumar V, Jeyaram A, Ranjan A, Das P. A comparative evaluation of end-emic and non-endemic region of visceral leishmaniasis (Kala-azar) in India with ground survey and space technology. Mem Inst Oswaldo Cruz. 2011;106:515–523. doi: 10.1590/s0074-02762011000500001. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Rinzin N, Chusak P, Dash AP, Chowdhury R, Tobgay T, Narain JP. Occurrence & significance of Kala-Azar in Bhutan. Indian J Med Res. 2010;132:337–338. [PubMed] [Google Scholar]
- 9.WHO . Post-Kala-Azar Dermal Leishmaniasis: A Manual for Case Management and Control. Kolkata, India: Report of a WHO Consultative Meeting, July 2–3, 2012; 2012. [Google Scholar]
- 10.Bhattacharya SK, Dash AP. Treatment of visceral leishmaniasis: options and choice. Lancet Infect Dis. 2016;16:142–143. doi: 10.1016/S1473-3099(15)00528-9. [DOI] [PubMed] [Google Scholar]
- 11.WHO . Control of the Leishmaniases. Geneva, Switzerland: WHO Technical Report Series 949, March 22–26, 2010; 2010. [Google Scholar]
- 12.Stauch A, Sarkar RR, Picado A, Ostyn B, Sundar S, Rijal S, Boelaert M, Dujardin J-CC, Duerr H-PP. Visceral leishmaniasis in the Indian subcontinent: modelling epidemiology and control. PLoS Negl Trop Dis. 2011;5:e1405. doi: 10.1371/journal.pntd.0001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostyn B, Gidwani K, Khanal B, Picado A, Chappuis F, Singh SP, Rijal S, Sundar S, Boelaert M. Incidence of symptomatic and asymptomatic Leishmania donovani infections in high-endemic foci in India and Nepal: a prospective study. PLoS Negl Trop Dis. 2011;5:e1284. doi: 10.1371/journal.pntd.0001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das VNR, Siddiqui NA, Verma RB, Topno RK, Singh D, Das S, Ranjan A, Pandey K, Kumar N, Das P. Asymptomatic infection of visceral leishmaniasis in hyperendemic areas of Vaishali district, Bihar, India: a challenge to kala-azar elimination programmes. Trans R Soc Trop Med Hyg. 2011;105:661–666. doi: 10.1016/j.trstmh.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Sharma MC, Gupta AK, Das VNR, Verma N, Kumar N, Saran R, Kar SK. Leishmania donovani in blood smears of asymptomatic persons. Acta Trop. 2000;76:195–196. doi: 10.1016/s0001-706x(00)00068-1. [DOI] [PubMed] [Google Scholar]
- 16.Srividya G, Kulshrestha A, Singh R, Salotra P. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res. 2012;110:1065–1078. doi: 10.1007/s00436-011-2680-1. [DOI] [PubMed] [Google Scholar]
- 17.Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I, Rousseau D, Kubar J. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 1999;37:1953–1957. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topno RK, Das VNR, Ranjan A, Pandey K, Singh D, Kumar NN, Siddiqui NA, Singh VP, Kesari S, Kumar NN, Bimal S, Kumar AJ, Meena C, Kumar R, Das P. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in Bihar, India. Am J Trop Med Hyg. 2010;83:502–506. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudarshan M, Singh T, Singh AK, Chourasia A, Singh B, Wilson ME, Chakravarty J, Sundar S. Quantitative PCR in epidemiology for early detection of visceral leishmaniasis cases in India. PLoS Negl Trop Dis. 2014;8:e3366. doi: 10.1371/journal.pntd.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman LAC, Dyson L, Courtenay O, Chowdhury R, Bern C, Medley GF, Hollingsworth TD. Quantification of the natural history of visceral leishmaniasis and consequences for control. Parasit Vectors. 2015;8:521. doi: 10.1186/s13071-015-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Rutte EA, Coffeng LE, Bontje DM, Hasker EC, Postigo JAR, Argaw D, Boelaert MC, De Vlas SJ. Feasibility of eliminating visceral leishmaniasis from the Indian subcontinent: explorations with a set of deterministic age-structured transmission models. Parasit Vectors. 2016;9:24. doi: 10.1186/s13071-016-1292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R, Subba Raju BV, Jain RK, Salotra P. Potential of direct agglutination test based on promastigote and amastigote antigens for serodiagnosis of post-kala-azar dermal leishmaniasis. Clin Diagn Lab Immunol. 2005;12:1191–1194. doi: 10.1128/CDLI.12.10.1191-1194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salotra P, Raina A, Ramesh V. Western blot analysis of humoral immune response to Leishmania donovani antigens in patients with post-kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1999;93:98–101. doi: 10.1016/s0035-9203(99)90197-9. [DOI] [PubMed] [Google Scholar]
- 24.Salotra P, Sreenivas G, Pogue GP, Lee N, Nakhasi HL, Ramesh V, Negi NS. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J Clin Microbiol. 2001;39:849–854. doi: 10.1128/JCM.39.3.849-854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreenivas G, Ansari NA, Kataria J, Salotra P. Nested PCR assay for detection of Leishmania donovani in slit aspirates from post-kala-azar dermal leishmaniasis lesions. J Clin Microbiol. 2004;42:1777–1778. doi: 10.1128/JCM.42.4.1777-1778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundar S, Singh RK, Maurya R, Kumar B, Chhabra A, Singh V, Rai M. Serological diagnosis of Indian visceral leishmaniasis: direct agglutination test versus rK39 strip test. Trans R Soc Trop Med Hyg. 2006;100:533–537. doi: 10.1016/j.trstmh.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Verma S, Kumar R, Katara GK, Singh LC, Negi NS, Ramesh V, Salotra P. Quantification of parasite load in clinical samples of leishmaniasis patients: IL-10 level correlates with parasite load in visceral leishmaniasis. PLoS One. 2010;5:e10107. doi: 10.1371/journal.pone.0010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushal H, Bras-Gonçalves R, Negi NS, Lemesre J-L, Papierok G, Salotra P. Role of CD8+ T cells in protection against Leishmania donovani infection in healed visceral leishmaniasis individuals. BMC Infect Dis. 2014;14:653. doi: 10.1186/s12879-014-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salotra P, Sreenivas G, Nasim AA, Subba Raju BV, Ramesh V. Evaluation of enzyme-linked immunosorbent assay for diagnosis of post-kala-azar dermal leishmaniasis with crude or recombinant k39 antigen. Clin Diagn Lab Immunol. 2002;9:370–373. doi: 10.1128/CDLI.9.2.370-373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chamakh-Ayari R, Bras-Gonçalves R, Bahi-Jaber N, Petitdidier E, Markikou-Ouni W, Aoun K, Moreno J, Carrillo E, Salotra P, Kaushal H, Negi NS, Arevalo J, Falconi-Agapito F, Privat A, Cruz M, Pagniez J, Papierok G-MM, Rhouma FBH, Torres P, Lemesre J-LL, Chenik M, Meddeb-Garnaoui A. In vitro evaluation of a soluble Leishmania promastigote surface antigen as a potential vaccine candidate against human leishmaniasis. PLoS One. 2014;9:e92708. doi: 10.1371/journal.pone.0092708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bern C, Haque R, Chowdhury R, Ali M, Kurkjian KM, Vaz L, Amann J, Wahed MA, Wagatsuma Y, Breiman RF, Williamson J, Secor WE, Maguire JH. The epidemiology of visceral leishmaniasis and asymptomatic leishmanial infection in a highly endemic Bangladeshi village. Am J Trop Med Hyg. 2007;76:909–914. [PubMed] [Google Scholar]
- 32.Gadisa E, Custodio E, Cañavate C, Sordo L, Abebe Z, Nieto J, Chicharro C, Aseffa A, Yamuah L, Engers H, Moreno J, Cruz I. Usefulness of the rK39-immunochromatographic test, direct agglutination test, and leishmanin skin test for detecting asymptomatic Leishmania infection in children in a new visceral leishmaniasis focus in Amhara State, Ethiopia. Am J Trop Med Hyg. 2012;86:792–798. doi: 10.4269/ajtmh.2012.11-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoodi M, Khamesipour A, Dowlati Y, Rafati S, Momeni AZ, Emamjomeh M, Hejazi H, Modabber F. Immune response measured in human volunteers vaccinated with autoclaved Leishmania major vaccine mixed with low dose of BCG. Clin Exp Immunol. 2003;134:303–308. doi: 10.1046/j.1365-2249.2003.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha PK, Bimal S, Pandey K, Singh SK, Ranjan A, Kumar N, Lal CS, Barman SB, Verma RB, Jeyakumar A, Das P, Bhattacharya M, Sur D, Bhattacharya SK. A community-based, comparative evaluation of direct agglutination and rK39 strip tests in the early detection of subclinical Leishmania donovani infection. Ann Trop Med Parasitol. 2008;102:119–125. doi: 10.1179/136485908X252278. [DOI] [PubMed] [Google Scholar]
- 35.Sinha PK, Bimal S, Pandey K, Singh SK, Ranjan A, Kumar N, Lal CS, Barman SB, Verma RB, Jeyakumar A, Das P, Bhattacharya M, Sur D, Bhattacharya SK. A community-based, comparative evaluation of direct agglutination and rK39 strip tests in the early detection of subclinical Leishmania donovani infection. Ann Trop Med Parasitol. 2008;102:119–125. doi: 10.1179/136485908X252278. [DOI] [PubMed] [Google Scholar]
- 36.Gidwani K, Kumar R, Rai M, Sundar S. Longitudinal seroepidemiologic study of visceral leishmaniasis in hyperendemic regions of Bihar, India. Am J Trop Med Hyg. 2009;80:345–346. [PubMed] [Google Scholar]
- 37.Bhattarai NR, Van der Auwera G, Khanal B, De Doncker S, Rijal S, Das ML, Uranw S, Ostyn B, Praet N, Speybroeck N, Picado A, Davies C, Boelaert M, Dujardin J-C. PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for Kala-Azar. Trop Med Int Health. 2009;14:404–411. doi: 10.1111/j.1365-3156.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- 38.Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends Parasitol. 2006;22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava P, Gidwani K, Picado A, Van der Auwera G, Tiwary P, Ostyn B, Dujardin JC, Boelaert M, Sundar S. Molecular and serological markers of Leishmania donovani infection in healthy individuals from endemic areas of Bihar, India. Trop Med Int Health. 2013;18:548–554. doi: 10.1111/tmi.12085. [DOI] [PubMed] [Google Scholar]
- 40.Zijlstra EE, Nur Y, Desjeux P, Khalil EA, El-Hassan AM, Groen J. Diagnosing visceral leishmaniasis with the recombinant K39 strip test: experience from the Sudan. Trop Med Int Health. 2001;6:108–113. doi: 10.1046/j.1365-3156.2001.00680.x. [DOI] [PubMed] [Google Scholar]
- 41.Maurya R, Singh RK, Kumar B, Salotra P, Rai M, Sundar S. Evaluation of PCR for diagnosis of Indian kala-azar and assessment of cure. J Clin Microbiol. 2005;43:3038–3041. doi: 10.1128/JCM.43.7.3038-3041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]