Abstract
Schistosomiasis affects over 170 million people in Africa. Here we compare a novel, low-cost mobile phone microscope to a conventional light microscope for the label-free diagnosis of Schistosoma haematobium infections in a rural Ghanaian school setting. We tested the performance of our handheld microscope using 60 slides that were randomly chosen from an ongoing epidemiologic study in school-aged children. The mobile phone microscope had a sensitivity of 72.1% (95% confidence interval [CI]: 56.1–84.2), specificity of 100% (95% CI: 75.9–100), positive predictive value of 100% (95% CI: 86.3–100), and a negative predictive value of 57.1% (95% CI: 37.4–75.0). With its modest sensitivity and high specificity, this handheld and cost-effective mobile phone–based microscope is a stepping-stone toward developing a powerful tool in clinical and public health settings where there is limited access to conventional laboratory diagnostic support.
Introduction
Inadequate laboratory capacity is a key obstacle to the delivery of quality health care in resource-constrained settings, frequently resulting in undertreatment and overtreatment of infectious diseases based on clinical assessment alone. Laboratory infrastructure is typically clustered in urban settings and is relatively inaccessible in regions where significant portions of affected population reside.1 Many of the neglected tropical diseases (NTDs) in particular are more prevalent in rural areas, far from these diagnostic centers. Schistosomiasis, for example, affects over 200 million people worldwide with the vast majority of those affected residing in rural African settings.2 Chronic infection with Schistosoma haematobium can result in significant morbidity from hydronephrosis, renal failure, and infertility,3,4 in addition to mortality secondary to bladder cancer.5,6 Schistosoma haematobium may also be a risk factor for human immunodeficiency virus acquisition.7 To overcome limitations in laboratory capacity, patients or samples are often transferred to distant diagnostic centers; however, this approach may be inefficient as it consumes limited time and resources. Therefore, novel, simple, and inexpensive approaches to diagnose schistosomiasis and other NTDs are needed in both clinical and public health environments. One potential solution to these barriers is handheld and mobile phone microscopy,8 given the ease in which diagnostic services can be transferred directly to settings in need. Recent studies have evaluated handheld and mobile phone–based microscopes designed to diagnose pathogens of global health concern, with field testing conducted in resource-constrained public health settings.9,10 Here we describe the diagnostic operating characteristics of a novel mobile phone–based microscope for S. haematobium infection in a rural Ghanaian school.
Methods
Sample preparation and analysis.
This study was conducted in Sorodofo–Abaasa Village, in the Abura Asebu Kwamankese District of the Central Region of Ghana in July 2015, and integrated into an ongoing schistosomiasis epidemiologic study and local control efforts in the region. Ethical permission for this study was granted by the University of Cape Coast (Cape Coast, Ghana). The study was conducted at the village school, with consent from headmasters, pupils, and their parents. The school consists of 223 pupils (age 7–16), all of whom provided urine samples for the larger epidemiologic study. Sixty urine specimens were randomly chosen for evaluation, with the microscopist blind to demographic data or a prior history of schistosome infection. Urine samples were all collected between 10:00 and 14:00 and processed and examined on the day of collection. Ten milliliters of urine was pipetted from each urine sample and processed at the University of Cape Coast Hospital Laboratory. The urine samples were centrifuged at 5,000 rpm for 5 minutes and the supernatant was discarded, whereas the sediment transferred and plated to a standard glass microscope slide, then protected by a cover slip as per clinical standards.11 As a reference standard, the slide was first examined by an expert microscopist via conventional light microscopy with an Olympus CX21FS1-5 microscope (Olympus, Tokyo, Japan) using both ×10and ×20 objective lenses. Identification and quantification of S. haematobium eggs were recorded. All individuals who tested positive for S. haematobium infection via conventional microscopy were treated with praziquantel (single dose of 40 mg/kg) free of charge. A second expert microscopist examined the slides that same day using our mobile phone–based microscope and identified the presence or absence of S. haematobium eggs. The microscopist was blinded to prior parasitologic results acquired via conventional benchtop microscopy.
Design of the mobile phone microscope.
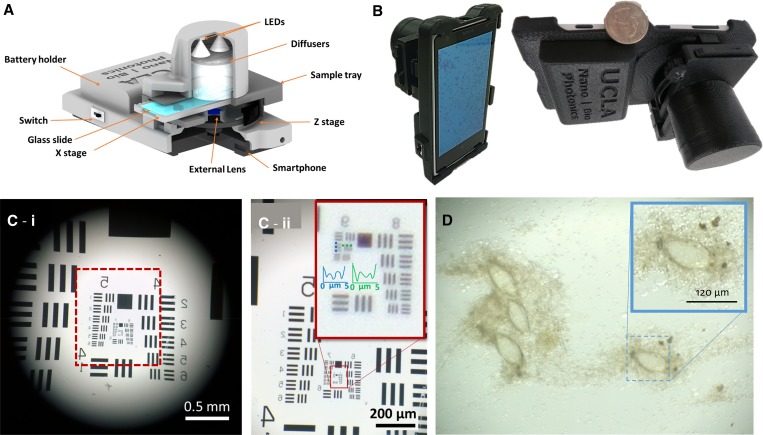
Our mobile phone microscope weighs ∼300 g (including the smart phone) and uses a 3D printed and custom-designed optomechanical attachment unit that is attached to the back camera unit of the smartphone (Figure 1A and B ). We used a Nokia Lumia 1020 (Microsoft, Redmond, WA) in our mobile phone microscope design, which uses an external lens in addition to the existing lens of the mobile phone camera, two “white” light-emitting diodes (LEDs) as light source, two polymer diffusers for uniform illumination of the sample, a custom-designed microscope slide tray, a z stage for manual adjustment of the focal plane, an x stage for lateral translation of the sample slide, two AA batteries to power up the illumination LEDs, and a switch to turn on/off the LEDs. This mobile microscope provides a half-pitch spatial resolution of 0.87 μm (Figure 1C) and a field of view that is larger than 4 mm2. After inserting the sample slide into the tray, LEDs are turned on and the regular smartphone camera application is used to capture transmission images of the samples (Figure 1D).
Figure 1.
(A) Schematic demonstrating the design of our mobile phone–based microscope. (B) Photos of the mobile phone microscope. (C) Quantification of spatial resolution using U.S. Air Force (USAF) test resolution chart. (c-i), an image of USAF chart taken using the mobile microscope; (c-ii), zoomed in region of USAF chart shown in c-i. (D) An image of Schistosoma haematobium eggs on the membrane captured using our mobile phone–based microscope.
Results
Sixty samples were randomly selected for evaluation; however, one sample was lost during laboratory processing. Forty-three samples were positive for S. haematobium for a prevalence of 72.9%. Of these, 35 (59.3%) were low-intensity (≤ 50 eggs per 10 mL urine) and eight (13.6%) were high-intensity (> 50 eggs per 10 mL urine) infections.11 Overall, our mobile phone microscope had a sensitivity of 72.1% (95% CI: 56.1–84.2), a specificity of 100% (95% CI: 75.9–100), positive predictive value of 100% (95% CI: 86.3–100), and negative predictive value of 57.1 (95% CI: 37.4–75.0). Our mobile phone microscope demonstrated a sensitivity of 65.7% (95% CI: 47.7–80.3) for low-intensity infection and 100% (95% CI: 59.8–100) for high-intensity infection (Table 1).
Table 1.
Diagnostic performance of a mobile phone microscope compared with conventional light microscopy for the diagnosis of Schistosoma haematobium infection
| Organism | Conventional microscope n (%) | Mobile phone microscope | |||
|---|---|---|---|---|---|
| N = 60 | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
| All samples with S. haematobium infection | 43 (72.9) | 72.1 (56.1–84.2) | 100.0 (75.9–100.0) | 100.0 (86.3–100.0) | 57.1 (37.4–75.0) |
| Low-intensity infection (≤ 50 eggs/10 mL urine) | 35 (59.3) | 65.7 (47.7–80.3) | NA | NA | NA |
| High-intensity infection (> 50 eggs/10 mL urine) | 8 (13.6) | 100.0 (59.8–100.0) | NA | NA | NA |
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Discussion
Mobile phone microscopy has the potential to bring quality diagnostics to settings without access to standard laboratory facilities and may have applications in both clinical and public health settings.8–10 Here, we demonstrated a modest sensitivity and excellent specificity of a novel mobile phone microscope design for the diagnosis of schistosomiasis in a school-based survey.
The device used in this study shows great promise as it demonstrated significant ease of use, compared with other devices,12,13 and faster and more efficient throughput of specimens, although we did not record the time it took to examine individual slides in this study. Expert microscopists reported that device was uncomplicated to use, and effectively functioned to visualize and focus on eggs, and manipulate/translate slides. The diagnostic sensitivity was likely modest as the mobile phone microscope could only manipulate a slide in the x axis (while focusing in the z plane), and there were likely eggs that were not counted in the y direction as they were beyond the field of view of our design (∼4 mm2). Future iterations of the device will enable both x and y translation of slides that will very likely increase our diagnostic sensitivity. Despite these initial limitations, the demonstrated sensitivity was comparable to or considerably better than some of the earlier mobile microscope designs.9,13 Specificity was very high, and similar to other mobile phone–based microscopes.9,13,14
Weaknesses of this study include the relatively small sample size (N = 60), and that slides were only examined by expert microscopists with experience in mobile phone microscopy. In addition, slide preparation was performed in a hospital laboratory with electric centrifugation. To evaluate the true efficacy of these mobile phone–enabled devices, future studies should aim to process and analyze all specimens in field settings. This may include sample processing in the field with simple syringe filtration,11 or other devices such as electricity-free handheld centrifugation15 or gravity urine filtration.16 In addition, future studies will have samples analyzed by local microscopists with training on how to use mobile phone devices.
The next generation of mobile phone microscopes shows tremendous promise for practical applications in the field as they integrate novel technology, which might further improve the quality of care in resource-constrained settings. Computer vision and machine learning technology17–19 are being integrated on portable diagnostic devices. Such technology could conceivably automate the process of pathogen identification and quantification while removing the elements of human fatigue, poor training, and error. Given the paucity of skilled microscopists, machine learning technology could enable better access to care, as many health-care technicians would conceivably require training for sample processing, rather than training on both sample processing and microscopy analysis. Other future directions include integrating these novel diagnostic devices into routine public health practice, as has been recently studied with schistosomiasis,9 malaria,10 and Loa loa control efforts.20
Mobile phone–enabled imaging, sensing, and diagnostics technologies have the potential to bring diagnostic testing to areas most in need, and to support clinical and public health practice in underserviced locations. The mobile phone microscope evaluated here is a stepping-stone toward enabling better quality care for schistosomiasis control efforts, among other potential applications that it can serve in global health.
Footnotes
Financial support: IIB is supported by Grand Challenges Canada 0631-01-10 (www.grandchallenges.ca) and a grant from the MSH UHN AMO Innovation Fund.
Authors' addresses: Isaac I. Bogoch, Divisions of General Internal Medicine and Infectious Diseases, Toronto General Hospital, Toronto, ON, Canada, E-mail: isaac.bogoch@uhn.ca. Hatice C. Koydemir, Derek Tseng, and Aydogan Ozcan, Department of Electrical Engineering, University of California Los Angeles, Los Angeles, CA, E-mails: hceylan@ucla.edu, delike@gmail.com, and ozcan@ucla.edu. Richard K. D. Ephraim and Evans Duah, Department of Medical Laboratory Sciences, University of Cape Coast, Cape Coast, Ghana, E-mails: rephraim@ucc.edu.gh and evans.duah@stu.ucc.edu.gh. Joseph Tee, Volta River Authority Corp, Accra, Ghana, E-mail: tjoseph_2001@yahoo.co.uk. Jason R. Andrews, Division of Infectious Diseases and Geographic Medicine, Stanford University, Stanford, CA, E-mail: jandr@stanford.edu.
References
- 1.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis. 2006;42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 2.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjetland EF, Poggensee G, Helling-Giese G, Richter J, Sjaastad A, Chitsulo L, Kumwenda N, Gundersen SG, Krantz I, Feldmeier H. Female genital schistosomiasis due to Schistosoma haematobium. Clinical and parasitological findings in women in rural Malawi. Acta Trop. 1996;62:239–255. doi: 10.1016/s0001-706x(96)00026-5. [DOI] [PubMed] [Google Scholar]
- 4.Magak P, Chang-Cojulun A, Kadzo H, Ireri E, Muchiri E, Kitron U, King CH. Case-control study of posttreatment regression of urinary tract morbidity among adults in Schistosoma haematobium-endemic communities in Kwale County, Kenya. Am J Trop Med Hyg. 2015;93:371–376. doi: 10.4269/ajtmh.15-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand M, Weinberg RW, Castle WM. Relation between carcinoma of the bladder and infestation with Schistosoma haematobium. Lancet. 1967;1:1249–1251. doi: 10.1016/s0140-6736(67)92714-6. [DOI] [PubMed] [Google Scholar]
- 6.Bedwani R, Renganathan E, El Kwhsky F, Braga C, Abu Seif HH, Abul Azm T, Zaki A, Franceschi S, Boffetta P, La Vecchia C. Schistosomiasis and the risk of bladder cancer in Alexandria, Egypt. Br J Cancer. 1998;77:1186–1189. doi: 10.1038/bjc.1998.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustinduy A, King C, Scott J, Appleton S, Sousa-Figueiredo JC, Betson M, Stothard JR. HIV and schistosomiasis co-infection in African children. Lancet Infect Dis. 2014;14:640–649. doi: 10.1016/S1473-3099(14)70001-5. [DOI] [PubMed] [Google Scholar]
- 8.Ozcan A. Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools. Lab Chip. 2014;14:3187–3194. doi: 10.1039/c4lc00010b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulibaly JT, Ouattara M, D'Ambrosio MV, Fletcher DA, Keiser J, Utzinger J, N'Goran EK, Andrews JR, Bogoch II. Accuracy of mobile phone and handheld light microscopy for the diagnosis of schistosomiasis and intestinal protozoa infections in Côte d'Ivoire. PLoS Negl Trop Dis. 2016;10:e0004768. doi: 10.1371/journal.pntd.0004768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulibaly JT, Ouattara M, Keiser J, Bonfoh B, N'Goran EK, Andrews JR, Bogoch II. Evaluation of malaria diagnoses using a handheld light microscope in a community-based setting in rural Côte d'Ivoire. Am J Trop Med Hyg. 2016;95:831–834. doi: 10.4269/ajtmh.16-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Expert Committee WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 12.Cybulski JS, Clements J, Prakash M. Foldscope: origami-based paper microscope. PLoS One. 2014;9:e98781. doi: 10.1371/journal.pone.0098781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ephraim RKD, Duah E, Cybulski JS, Prakash M, D'Ambrosio MV, Fletcher DA, Keiser J, Andrews JR, Bogoch II. Diagnosis of Schistosoma haematobium infection with a mobile phone-mounted Foldscope and a reversed-lens CellScope in Ghana. Am J Trop Med Hyg. 2015;92:1253–1256. doi: 10.4269/ajtmh.14-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogoch II, Coulibaly JT, Andrews JR, Speich B, Keiser J, Stothard JR, N'goran EK, Utzinger J. Evaluation of portable microscopic devices for the diagnosis of Schistosoma and soil-transmitted helminth infection. Parasitology. 2014;141:1811–1818. doi: 10.1017/S0031182014000432. [DOI] [PubMed] [Google Scholar]
- 15.Brown J, Theis L, Kerr L, Zakhidova N, O'Connor K, Uthman M, Oden ZM, Richards-Kortum R. A hand-powered, portable, low-cost centrifuge for diagnosing anemia in low-resource settings. Am J Trop Med Hyg. 2011;85:327–332. doi: 10.4269/ajtmh.2011.10-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ephraim RKD, Duah E, Andrews JR, Bogoch II. Ultra-low-cost urine filtration for Schistosoma haematobium diagnosis: a proof-of-concept study. Am J Trop Med Hyg. 2014;91:544–546. doi: 10.4269/ajtmh.14-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Sencan I, Wong J, Dimitrov S, Tseng D, Nagashima K, Ozcan A. Cost-effective and rapid blood analysis on a cell-phone. Lab Chip. 2013;13:1282–1288. doi: 10.1039/c3lc41408f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linder E, Grote A, Varjo S, Linder N, Lebbad M, Lundin M, Diwan V, Hannuksela J, Lundin J. On-chip imaging of Schistosoma haematobium eggs in urine for diagnosis by computer vision. PLoS Negl Trop Dis. 2013;7:e2547. doi: 10.1371/journal.pntd.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koydemir HC, Gorocs Z, Tseng D, Cortazar B, Feng S, Chan RYL, Burbano J, McLeod E, Ozcan A. Rapid imaging, detection and quantification of Giardia lamblia cysts using mobile-phone based fluorescent microscopy and machine learning. Lab Chip. 2015;15:1284–1293. doi: 10.1039/c4lc01358a. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, Switz N, Kamgno J, Pion S, Boussinesq M, Nutman TB, Fletcher DA. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med. 2015;7:286re4. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]