Abstract
Soil-transmitted helminths (STHs) infect over one billion people worldwide. There is concern that chronic infection with STHs among school-aged children may detrimentally affect their development, including their health, cognition, and education. However, two recent Cochrane reviews examining the impact of deworming drugs for STH on nutrition, hemoglobin, and school performance found that randomized controlled trials (RCTs) in the literature provide an insufficient evidence base to draw reliable conclusions. This study uses a cluster-RCT to add to existing evidence by assessing the impact of a deworming intervention on nutrition, cognition, and school performance among schoolchildren in rural China. The intervention, implemented by local health practitioners in a setting with a baseline infection prevalence of 41.9% (95% confidence interval [CI] = 39.8%, 43.9%) and infection intensity of 599.5 eggs per gram of feces among positive-tested schoolchildren (95% CI = 473.2, 725.8), consisted of distributing a 400-mg dose of albendazole accompanied with educational training about STH infection, treatment, and prevention. The intervention was conducted twice over the course of the study—at baseline in May 2013 and later in November 2013. We found that the deworming intervention reduced both infection prevalence and infection intensity, but these declines in infection were not accompanied by an impact on outcomes of nutrition, cognition, or school performance. Our interpretation is that the impact of deworming was attenuated by the light infection intensity in our sample population. Evidence from future RCTs is needed to assess the effect of deworming on key outcomes in areas with moderate and severe worm infections.
Introduction
Soil-transmitted helminths (STHs)—Ascaris lumbricoides, Trichuris trichiura, Necator americanus, and Ancylostoma duodenale—infect more than one billion people around the world.1–3 Observational studies have found that chronic infection with STHs among school-aged children is associated with malnutrition and impaired growth,4–6 cognitive impairment,7,8 and lower school attendance.9 These associations suggest that, in theory, reducing STH infection in children has the potential to improve nutrition and growth (i.e., hemoglobin levels, weight, height), cognitive abilities (i.e., working memory, processing speed), and school performance (i.e., school attendance, standardized test scores).10,11
This causal model, however, has not been fully supported by the literature. A 2012 Cochrane systematic review examining the impact of deworming drugs for STH on nutrition, hemoglobin, and school performance found that the few existing randomized controlled trials (RCTs) in the literature provide insufficient evidence from which to draw reliable conclusions.12 The subsequent update of the systematic review by Cochrane in 2015 showed community deworming programs “probably have little effect on weight gain … and no effect on average cognition” with only 1,361 participants in two trials with low-quality evidence. Furthermore, there is “probably no effect on height or … the average hemoglobin” looking at 3,595 participants in seven trials with low-quality evidence. Lastly, there is “very limited evidence assessing an effect on school attendance and the findings are inconsistent and at risk for bias” (20,243 participants, in two trials and with very low–quality evidence. In addition to the high risks of recruitment bias noted by the authors of the Cochrane study, the current evidence base is limited by the following characteristics of existing studies: many trials are conducted with small sample sizes and are underpowered13,14; many trials measure only one or two specific outcomes, often with selective reporting of outcomes12,14,15; and many trials do not report infection intensity,12 which is measured by fecal egg counts, and which (if intensities are high or low) may have implications for the nature of the impact being measured. Furthermore, the majority of existing trials have been efficacy studies of individualized treatment, which tend to be researcher-implemented in a highly controlled setting.16 The authors of the Cochrane report strongly recommend “effectiveness” studies (also known as pragmatic trials), which are ideally cluster-RCTs that examine the impact of the deworming intervention under real-world settings.12,17
Beyond the questions raised by the Cochrane report, there has also been a recent debate between epidemiologists and economists about the educational benefits of deworming, stemming from a failed replication of Miguel and Kremer's influential 2004 study that showed significant positive spillover effects from deworming in western Kenya.9,18–22 Some researchers argue that the failure of the replication study adds new evidence to the nonimpact of deworming efforts,21,22 whereas others point to new studies that show sustained positive impacts from childhood deworming.23,24
In light of these two controversies—the inconclusive findings of the 2015 Cochrane report, and the debate over the Miguel and Kremer (2004) study—there is a clear need for new evidence on the impact of deworming efforts on the health and educational outcomes of schoolchildren. We hope that our study can help to fill the gap in the existing literature.9
We designed a cluster-RCT that is the first, to our knowledge, to simultaneously address the issues noted above: our trial involves a larger sample size with high statistical power (> 80% power) to detect a 0.2 standardized effect on STH prevalence; it examines the impact of deworming on a comprehensive list of nutrition, cognitive, and school performance outcomes; and it reports intensity of STH infections based on measurements of fecal egg counts at baseline and follow-up. † Our trial is an effectiveness study of a deworming program that was implemented by local health practitioners, thereby mirroring a real-world scenario that more pragmatically measures the impact of the intervention and may be a better way of informing policymaking.
China—the location of this study—currently lacks a regular STH control program, despite historically high rates of STH prevalence in rural areas.25,26 For example, more than 40% of school-aged children in rural areas of Guizhou Province in southwest China are infected with STH.8,25 The primary aim of this RCT is to examine the impact of a deworming intervention on STH infection prevalence, infection intensity, nutritional indicators, cognitive abilities, and school performance among school-aged children in rural Guizhou Province. In doing so, we also assess treatment compliance among children who were randomized to receive the intervention and offered treatment by public health officials. We assert that this study bolsters evidence to make stronger conclusions about the impacts of deworming in a “real-world” setting. Although the carefully measured outcomes in this study do not favor deworming, this timely and well-executed cluster-randomized trial improves our understanding of this widely used intervention in the context of a population with low to moderate prevalence of STH infection.
Methods
Study design and participants.
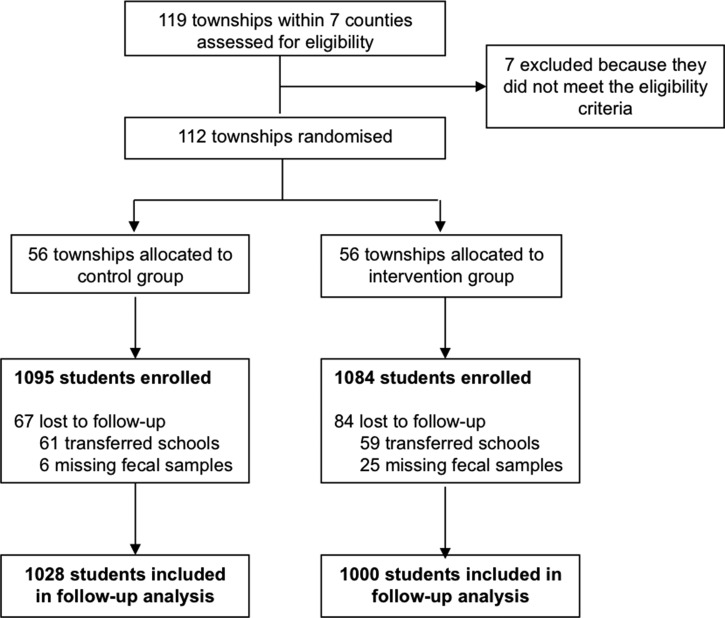
This cluster-RCT included a total of 2,240 sample children and spanned 112 townships in seven of the poorest rural counties in Qiandongnan Prefecture in Guizhou Province (Figure 1 ). The seven counties were randomly selected from the poorest half of counties in Qiandongnan based on per capita income, according to figures published by the Guizhou Provincial Bureau of Statistics.27 All townships within each of the selected counties were included in our sample, except for those which housed the county government; these townships were excluded because they are generally wealthier and more urbanized than the average rural township. A total of 112 townships met these selection criteria. In each township, we obtained from the central primary school a roster of all children 9–11 years of age attending any primary schools within the township for the 2013–2014 school year. We focused on this age group because school-aged children typically have the highest burden of STH infection,28 and specifically, elementary schoolchildren 9–11 years of age in our study area are old enough to take standardized examinations. We classified all 9- to 11-year-old children by their home village, and then we randomly selected 20 sample children from the home village with the largest number of children at that school. We excluded villages that housed the local township government, since these villages are typically wealthier and more urban than a typical village. If the first village we selected had fewer than 20 children in our age group attending the school, we randomly selected children from the next-largest village to fill in the gap. In total, then, 20 schoolchildren from either one or two villages in each township were randomly chosen for participation in the study. Overall, our sample population was composed of 2,240 children from 146 villages in 112 townships in seven rural counties.
Figure 1.
Trial profile.
This study received ethical approvals from the Stanford University Institutional Review Board (Protocol ID 25027), and from the Sichuan University Ethical Review Board (Protocol ID 2013005-02). All participating children gave oral assent prior to baseline data collection, and the children's legal guardians gave written consent for their children's involvement in the study. Children who were found to have severe anemia were referred to the local hospital for treatment. All participants were provided with deworming medication at the conclusion of the study.
Randomization and masking.
Cluster randomization was conducted at the township level. All randomized selection and allocation was performed using a computerized random sequence generator. In each of the seven counties included in our study, we randomly assigned half of the townships within each county to the control group and the other half to the intervention group. To increase statistical power, we used baseline survey information to assign the sample townships in each county into two pairs, using an optimal matching algorithm. The optimal matching algorithm assigned sample townships into pairs by minimizing the total (Mahalanobis) distance within the matched pairs.29 The Mahalanobis distance measure was calculated using the following baseline covariates at the township level: prevalence of STH, per capita net income, prevalence of anemia, number of households with children between 3 and 18 years of age, and distance (km) to the nearest paved road.
After matching sample townships into pairs, we randomly assigned one township in each pair to either a control or intervention group. In our study, 56 of a total of 112 townships were randomly assigned to receive the intervention (intervention group). The remaining 56 townships were assigned to the control group, which did not receive the intervention. The risk of spillover effects was low, given that paired townships were separated by more than 50 minutes of driving time, on average. In addition, no two schools were in the same school district.
Trained enumerators and local health practitioners who assisted with baseline and follow-up surveys were not explicitly informed of the treatment assignment of participants, although blinding of participants themselves was not possible because of the nature of the intervention. Students in the intervention group, as well as their parents or teachers, were not told explicitly that the purpose of the study was to examine the effect of a trial intervention. The study team informed students that they were participating in a general study of health and education of rural pupils by the Chinese Academy of Sciences and the Chinese Centers for Disease Control and Prevention (CDC). Participants in the control group were not aware that they were in a randomized trial.
Procedures.
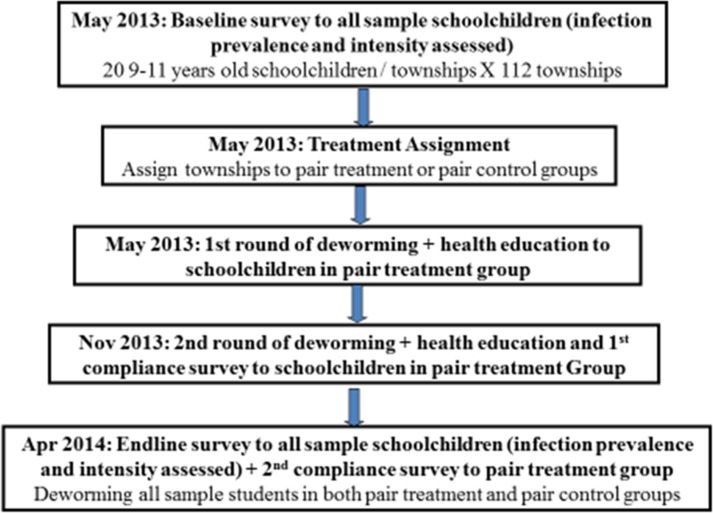
Figure 2 depicts the exact chronology of each project activity. Baseline surveying and fecal sample collection were performed in early May 2013. For each participating student, we obtained fecal samples for parasitological testing, administered a socioeconomic survey regarding individual and family characteristics, performed a physical examination to obtain measures of nutritional indices, and conducted standardized tests to assess cognitive abilities and school performance.
Figure 2.
Timeline of the randomized controlled trial.
For parasitological testing, the study team collected two fecal samples from each child in our sample: one fecal sample per day for two consecutive days. Samples were picked up once per day by the study team and were stored in a temperature-controlled cooler until collection. At the time of collection, members of the study team transported all fecal samples in a temperature-controlled cooler to the laboratory of the county branch of the CDC. A total of 2,179 children who produced at least one stool sample were included in our analysis. All fecal samples were tested on the same day that they were collected. Fecal samples were analyzed microscopically at the county CDC laboratory using the Kato-Katz thick-smear technique for A. lumbricoides (Ascaris), T. trichiura (Trichuris), and A. duodenale or N. americanus (hookworm).30 Two smears were taken from each of the two fecal samples collected from each child: one smear from each of the two samples was tested the same day on-site. The second smear from each sample was treated using a formaldehyde preservation technique and sent to the headquarters of the National Institute for Parasitic Diseases in Shanghai for quality control analysis and to perform egg counts for intensity of infection. Children were considered positive for STH infection if at least one of their fecal samples tested positive for one or more species of STH. Among fecal samples that tested positive for STH, we calculated fecal egg count by quantifying the geometric mean number of eggs per gram (epg) of feces in each sample. Categorization of infection intensity as light, moderate, or severe was assigned according to World Health Organization (WHO) classification, based on mean fecal egg count and STH species.31
The socioeconomic survey consisted of questions regarding the demographic characteristics and household conditions of children and parents. Students completed the survey in their classrooms under the supervision of trained enumerators from the Chinese Academy of Sciences and Guizhou University of Finance and Economics.
The physical examination measured three nutritional indicators: hemoglobin (Hb) concentrations, height, and weight. Hemoglobin levels were measured using HemoCue Hb 201 + systems (HemoCue Inc, Ängelholm, Sweden). Height and weight measurements were obtained following WHO standard protocol.32 The children were measured in light clothing without shoes, hats, or accessories. Weight was measured with a calibrated electronic scale recommended by professionals from the West China School of Public Health of Sichuan University. Body height was measured using a standard tape measure. The nursing team was trained to set up the weighing station on level ground to ensure accuracy of the equipment. Two nurses manned each measurement station, with one responsible for preparing subjects for measurement (removing shoes, offering instruction, positioning children, etc.) and the other responsible for conducting and recording the measurements.
Cognitive ability was assessed using a battery of four subtests from the Mandarin-language version of the latest Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) (Supplemental Appendix Table 1). The WISC-IV tests were culturally adapted, translated, and edited into simplified Chinese and validated for assessment among Chinese children in 2008.33 According to the literature, children's working memory and processing speed are the cognitive areas that are most likely to be affected by STH infection34,35; thus, we focused our efforts on measuring these two outcomes. In the WISC-IV, Working Memory Index (WMI) is assessed through two core subtests: Digit Span and Letter Number Sequencing. Processing Speed Index (PSI) is also assessed through two core subtests: Coding and Symbol Search. Trained examiners administered these four core subtests of cognitive ability to all children participating in the study on a one-on-one basis.
Measures of school performance included attendance rates and scores on the Trends in International Mathematics and Science Study (TIMSS), an internationally used standardized test established by the International Association for the Evaluation of Educational Achievement to compare student educational achievement internationally.36 School attendance rates were obtained from reports recorded by homeroom teachers.
Intervention.
The intervention consisted of a distribution of a 400-mg albendazole dose (two tablets of 200 mg, per national Chinese treatment guidelines) accompanied by two educational pamphlets (one for children and one for parents) about STH infection, treatment, and prevention (Figure 3 ).37 Albendazole was manufactured by GlaxoSmithKline (GSK) and was purchased and shipped directly from the GSK warehouse to the county CDC. To mimic a real-world policy scenario, we consulted with health officials from the Chinese CDC to devise a plan for implementation. Health workers from local branches of the Chinese CDC were thus responsible for implementing the deworming intervention—distributing the albendazole and educational pamphlets—to children in the township schools randomized to receive the intervention. CDC health officials distributed albendazole in the classrooms twice over the course of the study—at baseline in May 2013, and 6 months later in November 2013—and instructed the children to take the tablets at home. (National policy within China forbids children from taking medication at school.)37 ‡ Follow-up surveys and fecal sample collection were performed in April 2014. All children who were randomized to the control group of our study received albendazole after the conclusion of the study in April 2014 (Figure 2).
Figure 3.
Covers of soil-transmitted helminth educational pamphlets.
Outcomes.
The primary outcomes analyzed were STH infection prevalence, stunting prevalence (height-for-age z-score [HAZ] < −2), underweight prevalence (weight-for-age z-score [WAZ] < −2), WMI, PSI, school attendance, and normalized TIMSS mathematics test scores. Secondary outcomes analyzed were infection intensity (fecal egg counts) and anemia prevalence.
Measurements of hemoglobin levels were used to determine anemia prevalence. Following WHO guidelines, anemia is defined as having a hemoglobin level of less than 115 g/L for children 5–11 years of age, and less than 120 g/L for children 12–13 years of age.38 Measurements of height and weight were used to construct body mass index-for-age z-scores and HAZ scores using WHO AnthroPlus, a software application of the WHO Reference 2007 for children 5–19 years of age that is used to monitor the growth of school-aged children and adolescents.39 WAZ scores were calculated using a SAS program (Center for Disease Control and Prevention, Atlanta, GA) for the 2000 U.S. CDC growth chart for children 0–20 years of age.40 Raw scores obtained from the core subtests of the WISC-IV were converted to age-scaled index scores using the tables of norms in the Mandarin version of the WISC-IV administration and scoring manual to produce the index scores for WMI and PSI that were analyzed in this study. Scores on the TIMSS were normalized by the distribution of the control group in both the baseline and the follow-up surveys, and school attendance reports were used to calculate attendance rates.
Treatment compliance rates were also analyzed. After each round of deworming treatment in May 2013 and November 2013, students in the intervention group were asked to fill out a brief survey regarding the number of albendazole pills that they took (zero, one, or two). Treatment compliance rates were obtained from the responses of students.
Statistical analysis.
Among the 10 outcomes of interest, the largest sample required to meet at least a 0.25 standardized effect was for worm prevalence, based on parameters from previous studies. With a sample of 100 townships (50 controls, 50 treatment) and 20 children per township, we estimated a 12% decline in worm prevalence at 80% power. We increased the sample size to 112 townships to account for potential attrition. We assumed a prevalence in the control group of 34% with a 95% plausible interval of 11–80%. Power calculations were performed with Optimal Design software from the University of Michigan (Stephen Raudenbush and team, University of Michigan, Ann Arbor, MI) using the option for a cluster-randomized trial with a binary outcome. Our sample size provided adequate power to detect meaningful effects on the other 11 outcomes of interest. Details are available from authors upon request.
To further increase the power of the trial, we used a pairwise matching randomization procedure (as discussed in the “randomization and masking” section above). While not explicitly accounted for in determining the required sample size, the power gains from matching are potentially substantial.41,42
All statistical analyses were performed using STATA 12.0 (STATA Corp., College Station, TX). P values below 0.05 were considered statistically significant. We report coefficients and 95% confidence intervals (CIs). Comparisons between the intervention and control groups for all outcomes by subgroup populations were assessed using a t test. Multivariate analyses for the continuous outcome measures—fecal egg count, HAZ, WAZ, WMI, PSI, as well as the normalized TIMSS mathematics test scores—were performed using STATA's multiple linear regression model and its estimation using ordinary least squares, taking into account the pairing nature of townships within county and data clustering at the township level. Multivariate analyses of binary outcome measures—STH prevalence, anemia prevalence, and school attendance—were performed using STATA's logistic regression model, also taking into account paired fixed effects and clustering at the township level. Following previous studies,8,25,43 we adjusted for the following two sets of additional covariates at baseline survey in the multivariate analyses to increase statistical precision: student individual characteristics (gender; age; boarding status; belonging to the Dong, Miao, or Shui minority groups) and household characteristics (number of siblings, number of durable assets, parental migration status, educational attainment of parents). We also included pair fixed effects at the township level. All P values were based on results from the adjusted model.
We supplemented our intention to treat (ITT) multivariable analyses (described above) by examining the average-treatment-effects-on-the-treated (ATT analysis) to measure the impact on outcomes among the subpopulation of children who were fully compliant with treatment, thereby controlling for any confounding due to noncompliance. For ATT analysis, we used an instrumental variable approach in which the treatment was used to instrument for observed compliance,44 thereby allowing us to measure the effect of treatment among the subpopulation of children who reported full compliance with treatment, and thus control for confounding due to noncompliance. ATT analyses for the continuous outcome measures were performed using STATA's ivreg model, for the binary outcome measures using STATA's ivprobit model. In estimating both models, we take into account the pairing nature of townships within each county by including the township pairing dummy variables as controls, and take data clustering at the township level into consideration by clustering the standard errors at the township level.
Additional analysis of the correlation between infection intensity and outcomes was determined by calculating pairwise correlation coefficients between fecal egg count and outcome measures at the baseline survey among samples with positive infection.
The trial was registered with the International Standard Randomized Controlled Trial Number (ISRCTN) Registry in April 2013 (trial number: ISRCTN97311712), prior to the start of study activities.
Role of the funding source.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Participants.
A total of 2,179 students were enrolled in our study at baseline in April 2013: 1,084 children in the intervention group and 1,095 children in the control group. Of the 2,179 students enrolled, 151 were lost to follow-up in May 2014: 84 students from the intervention group and 67 from the control group (Figure 1). Attrition was due either to students transferring to other schools or to missing fecal sample information. A total of 2,028 participants (93% of the enrolled sample) were included in the follow-up analysis: 1,000 children in the intervention group and 1,028 children in the control group. The groups were statistically identical on all outcome measures at the time of the baseline survey (Table 1).
Table 1.
Baseline demographic and household characteristics of study participants
| Control (N = 1,095), 95% CI† | Intervention (N = 1,084), 95% CI | P value | |
|---|---|---|---|
| Individual characteristics | |||
| Age | 10.61 (10.56 to 10.66) | 10.56 (10.50 to 10.61) | 0.391 |
| Female (%) | 43.38 (40.44 to 46.32) | 48.99 (46.00 to 51.97) | 0.044* |
| Boarding at school (%) | 27.38 (24.74 to 30.02) | 24.84 (22.26 to 27.41) | 0.680 |
| Dong ethnic minority (%) | 47.03 (44.07 to 49.99) | 43.54 (40.59 to 46.50) | 0.675 |
| Miao ethnic minority (%) | 36.07 (33.22 to 38.92) | 37.73 (34.84 to 40.62) | 0.830 |
| Shui ethnic minority (%) | 2.92 (1.92 to 3.92) | 4.43 (3.20 to 5.65) | 0.597 |
| Household characteristics | |||
| Number of siblings | 1.13 (1.07 to 1.18) | 1.24 (1.18 to 1.29) | 0.129 |
| Pieces of durable assets | 8.45 (8.27 to 8.63) | 8.30 (8.11 to 8.50) | 0.581 |
| Parents are migrant workers (%) | 31.6 (28.85 to 34.34) | 28.53 (25.84 to 31.22) | 0.340 |
| Mother attended secondary school (%) | 7.28 (5.75 to 8.82) | 6.74 (5.25 to 8.24) | 0.690 |
| Father attended secondary school (%) | 12.26 (10.32 to 14.19) | 10.99 (9.13 to 12.85) | 0.428 |
| Sanitation and hygiene | |||
| Washes hands before eating (%) | 84.63 (82.49 to 86.77) | 84.24 (82.08 to 86.41) | 0.854 |
| Washes hands after using toilet (%) | 87.75 (85.81 to 89.68) | 85.74 (83.66 to 87.83) | 0.318 |
| Drinks boiled water only (%) | 5.21 (3.89 to 6.52) | 8.39 (6.74 to 10.05) | 0.013* |
| Wears shoes while playing outside (%) | 32.33 (29.55 to 35.10) | 33.30 (30.49 to 36.11) | 0.771 |
| House has dirt floor (%) | 17.08 (14.85 to 19.31) | 14.30 (12.21 to 16.39) | 0.303 |
| House has dirt-based latrine (%) | 19.58 (17.23 to 21.93) | 23.38 (20.86 to 25.90) | 0.160 |
| Family uses feces as fertilizer (%) | 65.48 (62.66 to 68.30) | 62.08 (59.19 to 64.98) | 0.270 |
| Infection prevalence | |||
| Any STH infection (%) | 41.10 (38.18 to 44.01) | 42.62 (39.67 to 45.57) | 0.779 |
| Ascaris infection (%) | 30.50 (27.77 to 33.23) | 31.09 (28.33 to 33.85) | 0.891 |
| Trichuris infection (%) | 23.29 (20.78 to 25.80) | 24.35 (21.80 to 26.91) | 0.847 |
| Hookworm infection (%) | 1.00 (0.41 to 1.60) | 0.74 (0.23 to 1.25) | 0.568 |
| Ascaris and Trichuris coinfection (%) | 12.97 (10.98 to 14.96) | 12.92 (10.92 to 14.91) | 0.989 |
| Infection intensity (among samples with positive infection) | |||
| Ascaris infection (epg) | 728.32 (526.90 to 929.75) | 1,065.04 (741.17 to 1,388.92) | 0.151 |
| Trichuris infection (epg) | 55.90 (38.29 to 73.50) | 71.79 (48.01 to 95.58) | 0.562 |
| Hookworm infection (epg) | 17.33 (−16.83 to 51.50) | 18.00 (−70.94 to 106.94) | 0.967 |
CI = confidence interval; STH = soil-transmitted helminth.
Bolded values indicate significance at 95% CI.
CI denotes confidence interval. P values adjusted for clustering at the township level.
Prevalence and intensity of infection.
Table 2 compares infection prevalence and infection intensity for the intervention and control groups from baseline to follow-up. There was a significant between-group (treatment versus control) difference in infection prevalence at follow-up (P = 0.026), with the prevalence of any STH infection at 31.4% (95% CI = 28.6–34.2%) in the control group and 27.7% (95% CI = 24.9–30.4%) in the intervention group (adjusted odds ratio in the intervention schools, 0.71; 95% CI = 0.52–0.96).
Table 2.
Infection prevalence and intensity in control and intervention groups
| Variable | Control group | Intervention group | Intervention effect (95% CI†) | |||
|---|---|---|---|---|---|---|
| Unadjusted | P value | Adjusted‡ | P value | |||
| Infection prevalence (%) | ||||||
| Baseline | 41.10 (38.18, 44.01) | 42.62 (39.67, 45.57) | 1.06 (0.69 to 1.65) | 0.779 | 1.15 (0.93 to 1.43) | 0.192 |
| Follow-up | 31.40 (28.56 to 34.23) | 27.66 (24.89 to 30.43) | 0.84 (0.52 to 1.35) | 0.464 | 0.71 (0.52 to 0.96) | 0.026* |
| Infection intensity§ (epg) | ||||||
| Baseline | 493.68 (357.83 to 629.53) | 702.71 (491.07 to 914.34) | 209.02 (−167.29 to 585.33) | 0.272 | 115.29 (−101.48 to 332.06) | 0.293 |
| Follow-up | 533.32 (390.69 to 675.95) | 299.93 (193.98 to 405.89) | −233.39 (−489.36 to 22.58) | 0.073 | −209.78 (−383.16 to −36.39) | 0.018* |
CI = confidence interval.
Bolded values indicate significance at 95% CI.
CI denotes confidence interval.
Values were adjusted for individual characteristics (gender, age, boarding status, minority identification) and household characteristics (siblings, durable assets, parental migrant worker status, parental education levels), as well as township pair-fixed effects. Coefficients for infection prevalence are reported as an odds-ratio, as well as township pair-fixed effects. In the case of follow-up, values were also adjusted for the baseline value of the dependent variable. Coefficients for infection prevalence are reported as odds-ratio.
Infection intensity calculated as average fecal egg count among samples with positive infection.
At baseline, the mean fecal egg count, assessed as the geometric mean epg of feces among positive-tested samples, was 490 epg (95% CI = 360–630) in the control group and 700 epg (95% CI = 490–910) in the intervention group. According to WHO categorization, both groups had light-intensity infection, defined as a mean egg count of 1–4,999 epg for Ascaris and 1–999 epg for Trichuris.45 There was no significant between-group difference in mean fecal egg count at baseline (P = 0.293). At follow-up, the intensity of STH infection was 530 epg (95% CI = 390 to680) in the control group and 300 epg (95% CI = 190–410) in the intervention group. STH infection intensity from baseline to follow-up increased by 40 epg in the control group, whereas it decreased by 400 epg in the intervention group. There was a significant between-group difference in infection intensity at follow-up (P = 0.018).
Nutritional indicators.
Table 3 uses ITT analysis to compare nutritional indicators, cognitive abilities, and school performance for the intervention and control groups from baseline to follow-up. At baseline, the mean hemoglobin level was 126.2 g/L in the control group (95% CI = 125.4–126.9) and 126.3 g/L in the intervention group (95% CI = 125.6–127.1). There was no significant between-group difference at follow-up (P = 0.623).
Table 3.
Intention to treat (ITT) analysis of differences in outcomes of nutrition, cognitive abilities, and school performance between control and intervention groups
| Variable | Control group | Intervention group | Intervention effect (95% CI†) | |||
|---|---|---|---|---|---|---|
| Unadjusted | P value | Adjusted‡ | P value | |||
| Nutritional indicators | ||||||
| Hemoglobin levels | ||||||
| Baseline | 126.17 (125.43 to 126.91) | 126.33 (125.59 to 127.07) | 0.16 (−1.73 to 2.05) | 0.864 | 0.24 (−0.67 to 1.14) | 0.604 |
| Follow-up | 132.16 (131.37 to 132.95) | 131.73 (130.92 to 132.54) | −0.43 (−2.53 to 1.68) | 0.690 | −0.33 (−1.64 to 0.98) | 0.623 |
| Anemia prevalence (%) | ||||||
| Baseline | 16.62 (14.41 to 18.83) | 16.14 (13.95 to 18.34) | 0.97 (0.70 to 1.34) | 0.833 | 0.93 (0.79 to 1.10) | 0.385 |
| Follow-up | 9.98 (8.14 to 11.82) | 11.58 (9.61 to 13.55) | 1.18 (0.79 to 1.76) | 0.413 | 1.25 (0.91 to 1.72) | 0.174 |
| % Stunted (HAZ < −2) | ||||||
| Baseline | 26.98 (24.32 to 29.65) | 29.66 (26.89 to 32.42) | 1.14 (0.86 to1.52) | 0.368 | 1.10 (0.92 to 1.31) | 0.291 |
| Follow-up | 23.48 (20.85 to 26.11) | 27.63 (24.84 to 30.42) | 1.24 (0.93 to 1.67) | 0.148 | 1.15 (0.85 to 1.55) | 0.367 |
| % Underweight (WAZ < −2) | ||||||
| Baseline | 24.11 (21.57 to 26.65) | 28.90 (26.20 to 31.61) | 1.28 (1.01 to 1.63) | 0.045* | 1.29 (1.09 to 1.54) | 0.004* |
| Follow-up | 21.37 (18.85 to 23.88) | 24.19 (21.56 to 26.82) | 1.17 (0.92 to 1.51) | 0.204 | 0.77 (0.56 to 1.06) | 0.113 |
| Cognitive abilities | ||||||
| Processing Speed Index Score | ||||||
| Baseline | 86.21 (85.44 to 86.99) | 86.09 (85.31 to 86.87) | −0.12 (−2.47 to 2.23) | 0.919 | 0.16 (−1.28 to 1.59) | 0.827 |
| Follow-up | 88.18 (87.37 to 88.99) | 88.83 (88.01 to 89.65) | 0.65 (−1.47 to 2.77) | 0.545 | 0.63 (−0.22 to 1.49) | 0.143 |
| Working Memory Index Score | ||||||
| Baseline | 78.68 (78.08 to 79.27) | 78.51 (77.92 to 79.10) | −0.16 (−1.60 to 1.28) | 0.822 | −0.05 (−0.98 to 0.89) | 0.922 |
| Follow-up | 78.23 (77.61 to 78.86) | 78.50 (77.86 to 79.14) | 0.27 (−1.18 to 1.72) | 0.715 | 0.51 (−0.09 to 1.11) | 0.093 |
| School performance | ||||||
| School attendance rate (%) | ||||||
| Baseline | 86.73 (84.65 to 88.81) | 87.32 (85.29 to 89.35) | 1.05 (0.68 to 1.64) | 0.818 | 1.08 (0.75 to 1.56) | 0.692 |
| Follow-up | 86.13 (83.81 to 88.45) | 85.30 (82.95 to 87.66) | 0.93 (0.57 to 1.54) | 0.790 | 0.86 (0.55 to 1.33) | 0.496 |
| Normalized TIMSS score | ||||||
| Baseline | 0.00 (−0.06 to 0.06) | −0.04 (−0.10 to 0.02) | −0.04 (−0.22 to 0.14) | 0.633 | 0.01 (−0.1 to 0.11) | 0.912 |
| Follow-up | 0.00 (−0.06 to 0.06) | −0.07 (−0.14 to −0.01) | −0.07 (−0.24 to 0.10) | 0.412 | −0.04 (−0.09 to 0.02) | 0.190 |
CI = confidence interval; HAZ = height-for-age z-score; ITT = intention to treat; TIMSS = Trends in International Mathematics and Science Study; WAZ = weight-for-age z-score.
Bolded values indicate significance at 95% CI.
CI denotes confidence interval.
Values were adjusted for individual characteristics (gender, age, boarding status, minority identification) and household characteristics (siblings, durable assets, parental migrant worker status, parental education levels), as well as township pair-fixed effects. In the case of follow-up, values were also adjusted for the baseline value of the dependent variable. Coefficients for anemia prevalence, % stunted, % underweight, and school attendance rate are reported as an odds ratio.
At baseline, anemia prevalence was 16.6% in the control group (95% CI = 14.4–18.8%) and 16.1% in the intervention group (95% CI = 14.0–18.3%). There was no significant between-group difference at follow-up (P = 0.174).
At baseline, the prevalence of stunting, defined as HAZ < −2, was 27.0% (95% CI = 24.3–29.7%) in the control group and 29.7% (95% CI = 26.9–32.4%) in the intervention group. The prevalence of stunting decreased slightly in both groups between baseline and follow-up, but there was no significant between-group difference at follow-up (P = 0.367).
At baseline, the prevalence of children who were underweight, defined as WAZ < −2, was 24.1% (95% CI = 21.6–26.7%) in the control group and 28.9% (95% CI = 26.2–31.6%) in the intervention group. The prevalence of underweight children decreased slightly in both groups between baseline and follow-up, but there was no significant between-group difference at follow-up (P = 0.113).
Cognitive abilities.
At baseline, the mean PSI score was 86.2 points (95% CI = 85.4–87.0 points) in the control group and 86.1 points (95% CI = 85.3–86.9 points) in the intervention group. The PSI score increased slightly in both groups between baseline and follow-up, but there was no significant between-group difference at follow-up (P = 0.143).
At baseline, mean WMI score was 78.7 points (95% CI = 78.1–79.3 points) in the control group and 78.5 points (95% CI = 77.9–79.1 points) in the intervention group. The WMI remained level between baseline and follow-up, with no significant between-group difference at follow-up (P = 0.093).
School performance.
At baseline, the school attendance rate was 86.7% (95% CI = 84.7–88.8%) in the control group and 87.3% (95% CI = 85.3–89.4%) in the intervention group. These rates remained fairly stable between baseline and follow-up, with no significant between-group difference at follow-up (P = 0.496).
At baseline, the normalized score on the TIMSS math assessment was 0.00 (by normalization) in the control group (95% CI = −0.06 to 0.06) and −0.04 in the intervention group (95% CI = −0.10 to 0.02). At follow-up, the normalized score on the TIMSS math assessment was 0.00 (by normalization) in the control group (95% CI = −0.06 to 0.06) and −0.07 in the intervention group (95% CI = −0.14 to −0.01). There was no significant between-group difference at follow-up (P = 0.190).
Treatment compliance.
Treatment compliance was assessed among children in the intervention group after both the first and second rounds of deworming (May 2013 and November 2014, respectively). Of 1,000 sample children in the intervention group who were included in the follow-up analysis, 52.2% reported taking the complete dose of two 200 mg albendazole pills in both rounds of deworming, and 75.9% reported taking at least one of the two 200 mg albendazole pills in both rounds. When assessing compliance survey responses in the second round of deworming in November 2013 only, we found that 63.4% of children took the complete dose of two 200 mg albendazole pills and 82.1% took at least one of the two pills. Our data show that compliers have significantly lower infection prevalence at the endline than do noncompliers (23.0% versus 33.5%, P = 0.000), but that there is no significant difference in endline infection intensity between compliers and noncompliers (269.6 versus 325.2, P = 0.607).
ATT analysis.
The results of the ATT analysis mirror those of the primary ITT analysis of the full sample of children: the intervention had a significant impact on reducing STH infection prevalence and infection intensity, but no impact on any of the other measured outcomes. The ATT analysis shows that children who reported being compliant with the deworming treatment experienced significantly greater reductions in both infection prevalence (P = 0.011) and infection intensity (P = 0.019) (see Supplemental Appendix Table 2 for results). The point estimates generated by the ATT analysis are greater than those generated by the primary ITT analysis (−0.39 versus −0.21 for infection prevalence, and −370 versus −210 for infection intensity), indicating that our compliance variable is at least in part accurately measuring student behavior. As with the ITT analysis, there is no evidence of a significant impact of deworming on the measured outcomes of hemoglobin levels (P = 0.622), anemia prevalence (P = 0.185), stunting prevalence (P = 0.335), underweight prevalence (P = 0.174), PSI score (P = 0.142), WMI score (P = 0.093), school attendance rate (P = 0.491), or normalized TIMSS score (P = 0.187).
Correlation between infection prevalence and intensity and primary outcomes.
We conducted an additional analysis to assess the correlation between infection prevalence and intensity and primary outcome variables among participants in our sample at baseline (Table 4). We find that there is a strong correlation (P < 0.05) between infection prevalence and all primary outcome variables. We also find that there is a strong correlation (P < 0.05) between fecal egg counts and outcomes of cognition and school performance. Higher fecal egg counts, indicating more severe infection intensity, are associated with a lower PSI (R = −0.12, P = 0.007), a lower WMI (R = −0.13, P = 0.004), and lower TIMSS mathematics test scores (R = −0.14, P = 0.002).
Table 4.
Correlation between infection prevalence and intensity (fecal egg counts) and student outcomes at baseline
| Outcome variables | Correlation coefficient (P value) | |
|---|---|---|
| Prevalence (full sample) | Intensity (sample with infection at baseline) | |
| Prevalence | Correlation coefficient | |
| % Anemic | 0.0492* (0.0216) | 0.0125 (0.7823) |
| % Stunted | 0.1246* (0.0000) | 0.0844 (0.0640) |
| % Underweight | 0.0963* (0.0000) | 0.0862 (0.0566) |
| Processing Speed Index | −0.1790* (0.0000) | −0.1217* (0.0070) |
| Working Memory Index | −0.1691* (0.0000) | −0.1288* (0.0043) |
| School attendance | −0.0755* (0.0006) | −0.0279 (0.5452) |
| Normalized TIMSS score | −0.1984* (0.0000) | −0.1416* (0.0017) |
CI = confidence interval; TIMSS = Trends in International Mathematics and Science Study.
Bolded values indicate significance at 95% CI.
Brackets contain P values.
Discussion
Our cluster-RCT assessed the impact of a deworming intervention on nutritional indicators, cognitive abilities, and school performance. Our results show that the intervention significantly reduced both infection prevalence and infection intensity relative to the control group. These declines in infection, however, were not accompanied by an impact on outcomes of nutritional indicators, cognitive abilities, or school performance.
The impact of the deworming intervention had the greatest observable effect on infection intensity. The intervention group experienced a 57.3% reduction in mean fecal egg counts, whereas the control group experienced a 0.1% increase in mean fecal egg counts. Examining the effect of deworming on infection intensity holds significant implications for epidemiologic surveillance of STH infection.
Regarding infection prevalence, reinfection dynamics often cause levels of STH prevalence to persist in a population, a factor that should be considered when evaluating the impact of future deworming interventions. The pattern of rapid reinfection after antihelminthic treatment (in our case, we tested the follow-up fecal samples 6 months after treatment) has been consistently identified in geographically diverse populations in both children and adults,46 and was previously confirmed in our study area.8,25 In our scenario, we surmise that children in the intervention group were cleared of STH infection after each of the two rounds of antihelminthic treatment, but experienced high rates of reinfection within the subsequent months leading up to postintervention evaluation. In a 2012 article by Jia and others, helminth reinfections occur rapidly after treatment, particularly for A. lumbricoides and T. trichiura.47 Hence, there is a need for frequent anthelmintic drug administrations to maximize the benefit of preventive chemotherapy. Integrated control approaches emphasizing health education and environmental sanitation are needed to interrupt transmission of STH. Additionally, natural fluctuations in infection in the environment, as previously observed in other populations,47–49 may have caused the decrease in infection prevalence in the control group and further attenuated the between-group difference in prevalence reduction. Incomplete compliance with the deworming treatment may have also served to lessen the between-group difference in prevalence reduction.
The most recent Cochrane review (July 2015) about treating schoolchildren for worms states: “In trials that treat only children known to be infected, deworming drugs may increase weight gain (low quality evidence), but we do not know if there is an effect on cognitive functioning or physical well-being (very low quality evidence).” Our study adds to the quality of evidence to help make informed decisions about treating children in helminth-endemic areas. The design (RC cluster), and measures (growth and cognitive impacts) of our study address some of the key shortcomings delineated in the 2012 and 2015 Cochrane Deworming reviews which cite a need for more and better quality (GRADE) studies with randomized cluster design. While there was no observed impact in our study on any of the nutrition, cognition, or educational outcomes, our results add better evidence to prior but not convincing studies that did not find strong evidence for any improvement in nutritional indicators, cognitive abilities, or school performance from deworming interventions.12 The value and impact of deworming programs is an important question to more clearly define, since many health programs include mass drug administration for STH infections.
One explanation for our results may be that the impact of deworming was attenuated by the low levels of infection intensity in our sample population. The WHO classifies fecal egg counts of 1–4,999 epg of feces for Ascaris and 1–999 epg for Trichuris as “light-intensity infections.” The mean fecal egg count in our sample population (in which the vast majority of infections were with Ascaris and Trichuris) at baseline was 490 epg in the control group and 700 epg in the intervention group, placing our sample population in the low range of light-intensity infections. In our study, it is possible that due to low baseline levels of infection intensity (especially hookworm) in our sample population, we observed no significant impact of deworming on the nutrition, cognition, and education outcome variables. Varying levels of infection intensity and kind of infection likely would explain the variance in the results of the deworming trials included in the 2012 Cochrane review. It is possible that studies finding an impact of deworming on nutrition, cognitive abilities, and school performance outcomes were more likely to be conducted in areas of (moderate to) high-intensity STH infection, whereas studies that found no impact on key outcomes were conducted in areas of light-intensity infection. If our study design was to be replicated within a population with a higher intensity infection, in particular with a higher intensity of hookworm than we observed, impacts may be significant. Unfortunately, this is only speculation because among the trials included in the 2012 Cochrane systematic review on deworming drugs and their effects on key outcomes, the majority of studies (36 of 41) failed to report infection intensity in their sample populations; the few studies that did report intensity had exceptionally small sample sizes, used different antihelminthic treatments (i.e., albendazole, pyrantel, mebendazole), and had a high degree of variability in study design.12 In summary, because most research teams did not report infection intensities, it is unknown if the baseline STH intensities explain observed differences in these studies.
Our findings have three major implications that inform us about the value of our deworming intervention. First, we find no empirical evidence that the intervention can be justified on the basis of improvement of nutritional indicators, cognitive abilities, or school performance. The question remains: should the Chinese government allocate resources for deworming in areas with light-intensity STH infection? If resources are not severely limited, deworming, which is a relatively inexpensive endeavor, will reduce STH infections. Moreover, although the degree of rise may be too small to detect in a study with the low STH intensity we observe in this study, it may rise to the level of significance in studies conducted in areas of high STH intensity. However, the results of this effectiveness study indicate that the intervention is unlikely to produce a rapid impact on nutritional outcomes in areas with light-intensity infections and low prevalence. Thus, if resources are scarce, public health efforts might best be concentrated on other interventions—though the final decision ought to be supported by detailed cost-benefit analysis.
Second, we emphasize the significance of measuring and reporting fecal egg counts to categorize infection intensity in the study population. Most deworming trials do not report infection intensity (although they most likely do measure it as part of standard laboratory procedures); however, specification of the level of STH infection intensity in the area allows for more accurate characterization of the sample population, and would provide greater consistency when comparing trial results conducted among different populations and geographical areas.
Finally, while our evidence shows that the intervention had no impact on nutritional indicators, cognitive abilities, or school performance in this lightly infected population, the impact on populations with moderate-to-high levels of infection intensity is an area for further investigation. Our additional descriptive analysis identified a significant correlation between higher levels of infection intensity and worse measures on key outcomes of cognition and school performance (Table 4). Our findings reveal the opportunity for future RCTs to examine whether the effect of deworming is empirically associated with baseline infection intensity in the targeted population. These trials should be conducted in settings with varying baseline levels of STH infection intensity in the population, involve uniform implementation of the intervention, and maintain consistency in the measurement and reporting of a comprehensive range of outcomes for rigorous comparison.
Study limitations.
The majority of fecal samples from the children were not produced on-site at the time of collection. Thus, it is possible that there was a delay of up to a few hours before children delivered their samples to refrigeration facilities at the school or village clinic. This may have caused an underestimate of total STH prevalence, especially with respect to hookworm; therefore, estimates presented in this article can be considered a lower bound for actual infection prevalence and infection intensity in our study population. In addition, this study is focused on examining short-run impacts of deworming; more differences may be detected looking across a period longer than 2 years. Studies are also needed that follow children with no STH infections for longer intervals of time compared with controls with higher levels of infection intensities. Finally, for reasons related to official regulations, we were unable to observe if the sample children actually took the deworming medication. This may have led to lower treatment compliance rates; however, we control for this limitation with our ATT analysis.
Strengths of our study include the following: our cluster-RCT had a sample size that allowed more than 80% statistical power; we measured and reported the effect of deworming on a comprehensive list of outcomes regarding nutrition, cognitive abilities, and school performance; we quantified the intensity of STH infection at baseline and follow-up; and our trial offered a robust assessment of the effectiveness of a deworming intervention under real-world conditions, as opposed to an efficacy study of targeted and researcher-implemented treatment.
In conclusion, this randomized-controlled trial conducted in rural Guizhou, China, found that in a population of schoolchildren with light-intensity Ascaris, Trichuris, and hookworm infection, a biannual deworming intervention reduced STH infection prevalence and intensity in the population, but had no impact on outcomes of nutrition, cognitive abilities, or school performance. The results of this effectiveness trial are relevant to developing an effective strategy to reduce STH infection and improve the health of children in China and other countries with high STH prevalence. The main implications of our study include the following: 1) in areas with light-intensity STH infection, limited resources might best be concentrated on targeting other, more impactful, public health issues; 2) future deworming studies should quantify and report infection intensity (fecal egg counts) for accurate epidemiological characterization of the sample population; and 3) evidence from future RCTs is needed to assess the effect of deworming on key outcomes in populations with moderate- and high-intensity STH infections.
Supplementary Material
ACKNOWLEDGMENTS
Footnotes
Financial support: We acknowledge financial support from the National Natural Science Foundation of China (grant nos. 71473240 and 71333012), as well as the International Initiative for Impact Evaluation (3IE, grant no. PW2.04.02.02).
Authors' addresses: Chengfang Liu and Renfu Luo, China Center for Agricultural Policy, School of Advanced Agricultural Sciences, Peking University, Beijing, China, E-mails: cfliu.ccap@pku.edu.cn and luorf.ccap@pku.edu.cn. Louise Lu, Yale University School of Medicine, New Haven, CT, and Freeman Spogli Institute, Stanford University, Stanford, CA, E-mail: louise.lu@yale.edu. Linxiu Zhang, Center for Chinese Agricultural Policy, Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing, China, E-mail: lxzhang.ccap@igsnrr.ac.cn. Sean Sylvia, School of Economics, Renmin University of China, Beijing, China, E-mail: sean.sylvia@gmail.com. Alexis Medina and Scott Rozelle, Freeman Spogli Institute, Stanford University, Stanford, CA, E-mails: amedina5@stanford.edu and rozelle@stanford.edu. Darvin Scott Smith, Stanford University School of Medicine, Stanford, CA, E-mail: ssmith@stanford.edu. Yingdan Chen and Tingjun Zhu, National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention, Shanghai, China, E-mails: cyingdan@126.com and ztjren@163.com.
Statistical power calculations were conducted using the code of clustersampsi in Stata12. According to our sampling strategy, 19–20 students 9–11 years of age were randomly sampled in each township (cluster). Data from the baseline survey of the project show that the intracluster correlation (ICC) for the Working Memory Index is 0.102. To detect an effect size of 0.20 standard deviations with 80% power and 95% significance level, when we run the code “clustersampsi, mu1(0) mu2(.2) alpha(.05) beta(.8) rho(0.102) m(19)” in Stata, we find that 60 clusters are required per experimental arm. For the Processing Speed Index, the ICC is 0.188. When we run the code “clustersampsi, mu1(0) mu2(.2) alpha(.05) beta(.8) rho(0.188) m(19)” in Stata, we find that 90 clusters are required per experimental arm.
It should be noted that the approach examined in this study of distributing medicine at school but instructing children to take it at home is different from the standard deworming approach used by the WHO and in many other countries, where children are typically observed taking the medication in the classroom. Ministry of Education regulations forbid children from taking any form of medication en masse at school, including deworming tablets. As a result, after consultation with local governments and international experts in the field of parasitic disease, we decided to distribute deworming tablets to children at school and instruct them to take the tablets at home. This is the policy-relevant approach in our study area, and in all of China.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors. 2012;5:81. doi: 10.1186/1756-3305-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012;142:13727. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 4.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 5.Hall A, Hewitt G, Tuffrey V. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4:118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suchdev PS, Davis SM, Bartoces M, Ruth LJ, Worrell CM, Kanyi H, Odero K, Wiegand RE, Njenga SM, Montgomery JM, Fox LM. Soil-transmitted helminth infection and nutritional status among urban slum children in Kenya. Am J Trop Med Hyg. 2014;90:299–305. doi: 10.4269/ajtmh.13-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, McGarvey ST. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72:540–548. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Luo R, Yi H, Zhang L, Li S, Bai Y, Medina A, Rozelle S, Smith S, Wang G, Wang J. Soil-transmitted helminths in southwestern China: a cross-sectional study of links to cognitive ability, nutrition, and school performance among children. PLoS Negl Trop Dis. 2015;9:e0003877. doi: 10.1371/journal.pntd.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- 10.Vercruysse J, Behnke JM, Albonico M, Ame SM, Angebault C, Bethony JM, Engels D, Guillard B, Nguyen TV, Kang G, Kattula D, Kotze AC, McCarthy JS, Mekonnen Z, Montresor A, Periago MV, Sumo L, Tchuenté LA, Dang TC, Zeynudin A, Levecke B. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl Trop Dis. 2011;5:e948. doi: 10.1371/journal.pntd.0000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 12.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance (Review) Cochrane Database Syst Rev. 2012;11:CD000371. doi: 10.1002/14651858.CD000371.pub5. [DOI] [PubMed] [Google Scholar]
- 13.Ebenezer R, Gunawardena K, Kumarendran B, Pathmeswaran A, Jukes MCH, Drake LJ, de Silva N. Cluster-randomised trial of the impact of school-based deworming and iron supplementation on the cognitive abilities of schoolchildren in Sri Lanka's plantation sector. Trop Med Int Health. 2013;8:942–951. doi: 10.1111/tmi.12128. [DOI] [PubMed] [Google Scholar]
- 14.Yap P, Wu F-W, Du Z-W, Hattendorf J, Chen R, Jiang JY, Kriemler S, Krauth SJ, Zhou XN, Utzinger J, Steinmann P. Effect of deworming on physical fitness of school-aged children in Yunnan, China: a double-blind, randomized, placebo-controlled trial. PLoS Negl Trop Dis. 2014;8:e2983. doi: 10.1371/journal.pntd.0002983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awasthi S, Peto R, Read S, Richards SM, Pande V, Bundy D. DEVTA (Deworming and Enhanced Vitamin A) team Population deworming every 6 months with albendazole in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet. 2013;381:1478–1486. doi: 10.1016/S0140-6736(12)62126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicotra CG, Black RE, Boerma JT, Bryce J. Measuring impact in the Millennium Development Goal era and beyond: a new approach to large-scale effectiveness evaluations. Lancet. 2011;377:85–95. doi: 10.1016/S0140-6736(10)60810-0. [DOI] [PubMed] [Google Scholar]
- 17.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. Criteria for Distinguishing Effectiveness from Efficacy Trials in Systematic Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. [PubMed] [Google Scholar]
- 18.Aiken AM, Davey C, Hargreaves JR, Hayes RJ. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a pure replication. Int J Epidemiol. 2015;44:1572–1580. doi: 10.1093/ije/dyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davey C, Aiken AM, Hayes RJ, Hargreaves JR. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: a statistical replication of a cluster quasi-randomized stepped-wedge trial. Int J Epidemiol. 2015;44:1581–1592. doi: 10.1093/ije/dyv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks JH, Kremer M, Miguel E. The case for mass treatment of intestinal helminths in endemic areas. PLoS Negl Trop Dis. 2015;9:e0004214. doi: 10.1371/journal.pntd.0004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hicks JH, Kremer M, Miguel E. Commentary: deworming externalities and schooling impacts in Kenya: a comment on Aiken et al. (2015) and Davey et al. (2015) Int J Epidemiol. 2015;44:1593–1596. doi: 10.1093/ije/dyv129. [DOI] [PubMed] [Google Scholar]
- 22.Humphreys M. What Has Been Learned from the Deworming Replications: A Nonpartisan View. 2015. http://www.macartan.nyc/comments/worms2/ Available at. Accessed November 25, 2016.
- 23.Ozier O. Exploiting Externalities to Estimate the Long-Run Effect of Early Childhood Deworming. Washington, DC: The World Bank; 2014. Policy Research Working Paper 7052. [Google Scholar]
- 24.Baird S, Hicks JH, Kremer M, Miguel E. Worms at work: long-run impacts of a child health investment. Q J Econ. 2016;2016:1637–1680. doi: 10.1093/qje/qjw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhang L, Luo R, Wang G, Chen Y, Medina A, Eggleston K, Rozelle S, Smith DS. Soil-transmitted helminth infections and correlated risk factors in preschool and school-aged children in rural southwest China. PLoS One. 2012;7:e45939. doi: 10.1371/journal.pone.0045939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Ohtsuka R, He Y, Yuan L, Yamauchi T, Sleigh AC. Impact of parasitic infections and dietary intake on child growth in the schistosomiasis-endemic Dongting Lake region, China. Am J Trop Med Hyg. 2005;72:534–539. [PubMed] [Google Scholar]
- 27.Guizhou Provincial Bureau of Statistics . Guizhou Statistical Yearbook 2011. Beijing, China: China Statistics Press; 2012. [Google Scholar]
- 28.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P. Chapter 24: Helminth infections: soil-transmitted helminth infections and schistosomiasis. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. 2nd edition. Washington, DC: The World Bank; 2006. [PubMed] [Google Scholar]
- 29.Moore Ryan T. Block Tools: Blocking, Assignment, and Diagnosing Interference in Randomized Experiments. 2012. Technical Report. [Google Scholar]
- 30.Ash LR, Orihel TC, Savioli L, Sin MA, Montresor A, Renganathan E. Training Manual on Diagnosis of Intestinal Parasites. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 31.World Health Organization . Helminth Control for School-Age Children. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 32.de Onis M, Blossner M, Borghi E, Morris R, Fronglio EA. Methodology for estimating regional and global trends in child malnutrition. Int J Epidemiol. 2004;33:1260–1270. doi: 10.1093/ije/dyh202. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) Administration and Scoring Manual. Zhuhai, China: King-may Psychological Assessment Ltd.; 2008. (Chinese version) [Google Scholar]
- 34.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. Parasitic helminthic infection and cognitive function in school children. Proc R Soc Lond B Biol Sci. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- 35.Jardim-Botelho A, Raff S, De Ávila Rodrigues R, Hoffman HJ, Diemert DJ, Corrêa-Oliveira R, Bethony JM, Gazzinelli MF. Hookworm, Ascaris lumbricoides infection and polyparasitism associated with poor cognitive performance in Brazilian schoolchildren. Trop Med Int Health. 2008;13:994–1004. doi: 10.1111/j.1365-3156.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 36.Mullis IV, Martin MO, Gonzalez EJ, Chrostowski SJ. TIMSS 2003 International Mathematics Report: Findings from IEA's Trends in International Mathematics and Science Study at the Fourth and Eighth Grades. Boston, MA: International Association for the Evaluation of Educational Achievement, TIMSS, and PIRLS International Study Center, Lynch School of Education, Boston College; 2004. [Google Scholar]
- 37.Ministry of Health . Technical Guidelines for Soil-Transmitted Helminth Disease Control. Beijing, China: 2010. (2010 Version) [in Chinese] [Google Scholar]
- 38.World Health Organization . Iron Deficiency Anemia: Assessment, Prevention and Control, A Guide for Programme Managers. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 39.World Health Organization WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World's Children and Adolescents. 2009. http://www.who.int/growthref/tools/en/ Available at.
- 40.Centers for Disease Control and Prevention (CDC) A SAS Program for the 2000 CDC Growth Charts (Ages 0 to <20 Years) 2014. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm Available at.
- 41.Bruhn M, McKenzie D. In pursuit of balance: randomization in practice in development field experiments. Am Econ J Appl Econ. 2009;1:200–232. [Google Scholar]
- 42.Greevy R, Lu B, Silber JH, Rosenbaum P. Optimal multivariate matching before randomization. Biostat. 2004;5:263–275. doi: 10.1093/biostatistics/5.2.263. [DOI] [PubMed] [Google Scholar]
- 43.Shang Y. Doctoral Thesis, National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention. Shanghai, China: 2011. Burden of Diseases on Soil-Transmitted Helminth Infections among School-Age Children in China [in Chinese] [Google Scholar]
- 44.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–455. [Google Scholar]
- 45.World Health Organization . Helminth Control in School-Age Children: A Guide for Managers of Control Programmes. 2nd edition. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 46.Holland CV. Predisposition to ascariasis: patterns, mechanisms and implications. Parasitology. 2009;136:1537–1547. doi: 10.1017/S0031182009005952. [DOI] [PubMed] [Google Scholar]
- 47.Jia T-W, Melville S, Utzinger J, King CH, Zhou X-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabrera BD. Reinfection and infection rates of ascariasis in relation to seasonal variation in the Philippines. Southeast Asian J Trop Med Public Health. 1984;15:394–401. [PubMed] [Google Scholar]
- 49.Pan CT, Ritchie LS, Hunter GW., 3rd Reinfection and seasonal fluctuations of Ascaris lumbricoides among a group of children in an area where night soil is used. J Parasitol. 1954;40:603–608. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.