Abstract
There is growing consensus that multi-parametric MRI (mpMRI) is an effective modality in the detection of locally recurrent prostate cancer after prostatectomy and radiation therapy. The emergence of MR guided focal therapies, such as cryoablation, high-intensity focused ultrasound, and laser ablation, have made the use of mpMRI even more important, as the normal anatomy is inevitably altered and the detection of recurrence is made more difficult. The aim of this article is to review the utility of mpMRI in detecting recurrent prostate cancer in patients following radical prostatectomy (RP), radiation therapy (RT), and focal therapy (FT) and to discuss expected post-treatment mpMRI findings, the varied appearance of recurrent tumors, and their mimics.
Keywords: prostate cancer, mpMRI, prostatectomy, radiation therapy, focal therapy, biochemical recurrence
Introduction
Prostate cancer (PCa) is the most common non-cutaneous cancer in men with an incidence of 220,800 in US in 2015. The estimated number of deaths from PCa was 27,540 in 2015, accounting for 4.7% of all cancer deaths (1). Management options for stage I-III PCa include active surveillance or definitive therapy via radical prostatectomy (RP) or radiotherapy (RT) including brachytherapy, intensity modulated radiation therapy and stereotactic ablative radiotherapy. In addition, several prostate sparing focal therapy (FT) options have been utilized recently, including cryotherapy, microwave, laser, and high intensity focused ultrasound (HIFU) (2). The 10 year biochemical relapse free survival rates of men with organ confined disease following conventional definitive therapy can range from 65-80%; however, recurrence rates for focal therapies have not yet been accurately established (3-7).
Following definitive therapy, careful monitoring with sequential serum PSA is crucial to assess for recurrent disease. Criteria for biochemical recurrence varies according to therapy. Generally, serial increases in PSA exceeding the threshold of 0.2ng/dl define a biochemical recurrence after RP. RT generally results in a nadir of PSA, which is often greater than zero, within months of completion of therapy. Recurrence is defined as a rise of 2.0ng/dl above the nadir according to the commonly used Phoenix criteria. Thus, rises in PSA after therapy define the onset of biochemical recurrence (BCR).
Multi-parametric MRI (mpMRI) has begun to occupy an increasingly central role in the management of patients suspected of prostate cancer, as it generates both the best spatial resolution and soft tissue contrast for characterizing lesions in the prostate gland. The multiparametric approach which combines anatomic sequences (T1 and T2 weighted MRI) with functional imaging sequences, including diffusion weighted magnetic resonance imaging (DW MRI) and dynamic contrast enhanced MRI (DCE MRI) have proven successful in identifying clinically significant cancers while under-diagnosing low grade cancers that do not require treatment. The mpMRI can be fused to transrectal ultrasound (TRUS) to enable fusion image guided biopsy of MR-defined lesions (8). Recent studies have also suggested that mpMRI can also be used in the evaluation of recurrent or residual disease (9-11). However, treatment induced changes including distorted anatomy, fibrosis, artifacts from surgical clips, and alteration of the signal characteristics on MRI can complicate the interpretation. Therefore, it is essential to distinguish expected post-therapy changes from local recurrence.
The aim of this article is to review the utility of mpMRI in detecting recurrent PCa in patients following RP, RT, or FT. We begin by describing expected post-treatment mpMRI findings, then discuss the appearances of recurrent tumors as well as their mimics.
mpMRI of BCR following radical prostatectomy
RP is the most frequently utilized treatment option for patients with PCa. Approximately 40% of patients with localized PCa will select this definitive therapy (12-14). RP involves removal of the entire prostate gland and both seminal vesicles with the goal of negative surgical margins. The procedure is typically accompanied by a pelvic lymph node resection although the extent of this resection varies dramatically among surgeons (15). Following RP, PSA levels fall to undetectable levels (<0.01 ng/mL), within weeks.
Approximately 15 to 20% of patients experience biochemical recurrence (BCR) following RP (16-18). Positive surgical margins, high grade tumors, extra-prostatic extension of tumor, seminal vesicle invasion, increased tumor volume, perineural invasion, and PSA doubling time (PSADT) prior to and after surgery are all associated with increased risk of recurrence (12). The American Urological Association (AUA) and European Association of Urology (EAU) define BCR as a serum PSA ≥0.2ng/ml, with a second confirmatory level (19, 20). Of patients who demonstrate BCR, approximately one third will ultimately develop metastatic disease, and approximately one in five will die of PCa (21). Thus, successful detection and treatment of BCR becomes a potential “fire wall” in the prevention of metastatic disease.
The distinction between metastatic disease recurrence and locoregional residual or recurrent disease is important, as prognosis is significantly worse in the former. PSADT <4 months has been suggested as a predictor of distant metastases, while a PSADT >12 months is linked more to local failure; however, PSADT alone, as it depends on a serum assay, is neither sensitive nor specific for distinguishing metastatic from local recurrence (20, 22). Salvage RT with androgen deprivation therapy (ADT) is most commonly used in post-prostatectomy patients with residual locoregional recurrent disease (12, 23). However, this strategy is clearly insufficient for a patient with distant metastatic disease. Therefore, imaging is essential to distinguish between local and metastatic recurrence in BCR.
Normal MRI findings after RP reflect the extent of resection in this procedure (24). The bladder neck is anastomosed to extraprostatic distal urethra. Thus, the bladder neck has a conical shape that falls far more caudally than normal on sagittal images. In the axial plane, the tissue around the vesico-urethral anastomosis is low in signal on T2W MRI, reflecting postoperative scarring and fibrosis. Occasionally, the anastomosis may demonstrate intermediate T2W MRI signal which mimics recurrence, particularly if there was extensive hemorrhage at the time of surgery. Extensive fat stranding is often encountered surrounding the bladder base. Variations in the surgical approach may account for different patterns of fibrosis, as described by Allen et al. (25). The vas deferens and /or seminal vesicle which are supposed to be removed in a classical RP may be retained in part, in their normal location and are seen as low or intermediate intensity tubular structures on T1W MRI and T2W MRI (25). These structures can demonstrate enhancement on DCE MRI and restricted diffusion on DW MRI (26). On DCE MRI, no enhancement should be noted on early arterial phase, but low levels of uniform enhancement are often seen during the venous phase within the operative bed. Metallic clips, if present, can introduce susceptibility artifacts which greatly reduce the value of DWI MRI including the ADC map which is so useful in primary tumor detection. However, the use of surgical clips varies widely so that DWI MRI cannot be assumed to be of no value. Post-operative lymphoceles are common in patients who undergo lymph node resections, as they reflect accumulation of lymphatic fluid from damaged lymphatics. They can be recognized by their thin walled cystic nature, low signal intensity on T1W MRI, hyperintense signal on T2W MRI, and lack of enhancement on DCE MRI (27).
Recurrent tumors after RP can assume various shapes including uni- or multi-lobulated masses, semi-circumferential masses, or plaque-like soft tissue thickening in the surgical bed. Recurrent masses can occur anywhere within the prostatectomy bed including the bladder wall, the retrovesical space, the vesicourethral anastomosis, and the membranous urethra (28). The soft tissues may also be involved, including the lateral margins of the prostatectomy bed along the levator ani muscles (29). Recurrences may also be seen near or within seminal vesicles, or adjacent to the vas deferens (30). Signal characteristics of recurrent tumors are similar to those of the initial tumor; although they are often less conspicuous without a background of normal prostatic tissue for comparison. DCE MRI is the most useful MRI sequence, as residual/recurrent tumors will enhance in the early arterial phase while fibrosis will either not enhance or enhance very slowly and uniformly(11). Recurrent masses show early arterial and intense contrast enhancement compared to surrounding tissues and may washout earlier (27, 31, 32). Recurrent tumors demonstrate signal characteristics similar to those of muscle on T1W MRI, and are iso- to slightly hyperintense to adjacent pelvic muscles on T2W MRI. Recurrence, with its mild hyperintensity on T2W MRI, can often be distinguished from normal post-operative fibrotic changes which demonstrates low T2W MRI signal. DWI-MRI can be distorted by the presence of surgical clips and susceptibility artifacts. However, when distortion does not occur recurrences demonstrate restricted diffusion, with high grade tumors showing lower ADC values and high signal on high b-value DWI (23) (Figure 1).
Figure 1.
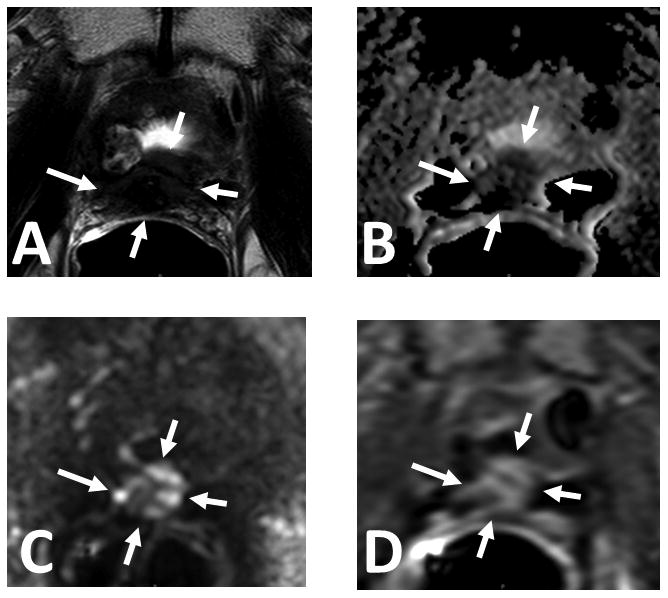
61 year old male with serum PSA = 16.39ng/mL and S/P radical prostatectomy. Axial T2W MRI (A), ADC map of DW MR (B), b2000 DW MRI (C) and DCE MRI (D) shows a soft tissue lesion in the prostatectomy bed within the anastomosis (arrows). Targeted biopsy revealed recurrent prostate cancer.
Mimics of recurrence can arise from fibrosis and surgical remnants. Retained seminal vesicles are seen in up to 20% of patients (33), and can mimic a recurrence. Normal retained seminal vesicles will appear as hyperintense fluid filled tubules in the superior aspect of the prostatectomy bed but will not show abnormal enhancement on DCE MRI, nor show restricted diffusion, distinguishing this finding from recurrent tumor. In the case where seminal vesicle invasion (SVI) has occurred but the SVs are nevertheless retained, they will show hypointense signal and early enhancement on ADC maps and DCE MRI, respectively. Prominent vascular structures in the surgical bed can also demonstrate rapid, intense enhancement and wash-out, in a pattern similar to recurrent tumor. This can be distinguished from recurrence on T2W MRI, where vessels will not show a soft tissue mass appearance. Residual normal prostatic tissue can also mimic a recurrence. While normal prostate tissue signal characteristics can help to distinguish this from cancer recurrence, a very long PSADT is expected with residual prostatic tissue in comparison with recurrence where the PSADT is expected to be shorter (11).
Clinical studies of mpMRI in the diagnosis of local PCa recurrence suggest it can be accurate for detection (34). The value of a multiparametric approach has been validated, although DCE and T2W scans play a larger role in the recurrence setting than they do in the primary setting. For instance, in an 80 patient cohort in the post RP setting (mean, median and range PSA of 1.17, 0.43 and 01-10.3ng/ml, respectively) sensitivity/specificity increased from 48-84% and 52-88%, respectively for T2W MRI alone to 71-100% and 74-100%, respectively for DCE plus T2W MRI (35, 36). Wassberg et al. analyzed 52 patients in the recurrence setting (mean PSA=2.2 [95% CI: 1.3-3.2]) with suspicious MRI findings and found that DCE added incremental value to T2W MRI alone especially for inexperienced readers (32).
The value of DW MRI is very variable in this context due to artifacts. However, when it can be successfully performed, it is valuable. For instance, it can be helpful to avoid the pitfall of misdiagnosing peri-prostatic vessels as enhancing nodules (37). Panebianco et al. evaluated a cohort of 262 patients with a high risk of local BCR following RP validated by either PSA reduction greater than 50% after salvage RT or biopsy. They evaluated how various combinations of sequences in mpMRI with endorectal coil performed in the detection of recurrence. By using a >50% decline in PSA following salvage radiotherapy as the gold standard for local recurrence in 126 patients (mean and range PSA of 1.3 and 0.5-1.7ng/ml, respectively) as validation, the combination of T2W MRI and DCE MRI showed 98% sensitivity, 94% specificity, and 93% accuracy in detecting local recurrence. They also demonstrated excellent results by combining T2W MRI with high b-value DW MRI of 3,000 s/mm2, showing 97% sensitivity, 95% specificity, and 92% accuracy. Using T2W MRI in combination with a lower b-value of 1,000 s/mm2 showed a sensitivity of 93%, specificity of 89%, and accuracy of 88%. The results were similar when TRUS biopsy was used as the gold standard. Thus, when feasible, the combination of either DW MRI and T2W MRI is comparable to DCE MRI and T2W MRI (23, 31, 37, 38). A study by Cha et al. also looked into the value of different mpMRI sequence combinations to detect BCR following RP. Forty-three patients with BCR (mean PSA=0.71 [0.20-3.51ng/ml]) and 14 patients who had no evidence of recurrence (mean PSA=0.02 [0.01-0.04ng/ml]) were included in this study. T2W MRI alone was compared to T2W+DCE MRI, T2W+DW MRI, and T2W+DCE+DW MRI. Median follow up time was 26 months for patients with BCR and 12 months for the control group. This study found that both DCE MRI and DW MRI add value to T2W MRI in detecting BCR after RP (38). Using two readers, the sensitivity was higher for T2W+DCE MRI (76-90%) than for T2W+DW MRI (46-49%). However, the specificity for T2W+DCE MRI was slightly lower compared to T2W+DW MRI (83-88% versus 87-90%, respectively). Combining T2W MRI with DCE MRI and DW MRI gave comparable sensitivities and specificities. The low sensitivity for T2W+DW MRI was explained by signal artifacts due to surgical clips thus, limiting its value (38).
The role of the endorectal coil in MRI for recurrence remains controversial with no head-to-head studies reported. Rischke et al. investigated the utility of non-endorectal coil mpMRI with DCE MRI in BCR patients, and found that mpMRI detected lesions in patients whose PSA levels were above 0.54ng/mL with an accuracy of 83%. However, this study was limited by a small patient population (n=33), its retrospective nature, and the lack of validation (39). In comparison, Cirillo et al. evaluated a cohort of 72 post RP patients (mean PSA=1.23±1.3ng/ml) using an endorectal coil MRI and found that DCE MRI and T2W resulted in a sensitivity of 84.1%, and a specificity of 89.3%. This study concluded that endorectal coil mpMRI improves the ability to detect recurrences and that the addition of DCE MRI improved the diagnostic performance of T2W MRI. However, this study was also limited by its patient population size and interobserver variability was not measured (28).
These studies suggest that the optimal method to evaluate for PCa recurrence in BCR following radical prostatectomy is to perform an endorectal coil mpMRI with at least DCE and T2W MRI, and to use DWI when artifacts are not present. As it cannot be reliably predicted when DW MRI will be limited by artifacts, one may include it as a matter of routine, understanding that it may not be useful in all cases. Overall, mpMRI yields relatively high sensitivity and specificity although it requires confirmation with biopsy as false positives are not infrequent.
mpMRI in BCR following Radiation Therapy
Radiation therapy (RT) targets high doses of ionizing radiation to the prostate through several forms of external beam radiation therapy (EBRT) and/or brachytherapy(40). In general, patients undergoing RT are older and have higher risk tumors than patients selected for RP. RT is also commonly combined with courses of ADT which can affect imaging.
The American Society for Therapeutic Radiology and Oncology (ASTRO) defines BCR after EBRT using the “Phoenix Criteria” as an increase in PSA of ≥2ng/mL above the nadir PSA (41). BCR occurs in about 25% of patients treated with RT, and is more common in patients with high risk tumors (42, 43). Risk factors for BCR include high Gleason score, clinical tumor stage T3b or T4, and a post treatment PSADT <3 months (43). The time to reach a PSA nadir is usually 18 months after treatment, though determining the PSA nadir may be complicated by a transient PSA rise, known as a PSA “bounce”, that occurs at a median of 12-18 months after treatment (44-46). A shorter PSADT and a faster time to BCR portend a worse prognosis and BCR before 18 months after treatment doubles the risk of death from PCa (43, 47). Among those who experience BCR, the median time to metastases is 5.4 years and median time to PCa specific mortality is 10.5 years (43).
As metastatic disease is a relative contraindication to local definitive salvage therapy following BCR, differentiating between local and metastatic recurrence following BCR is an essential step in the workup of a patient with BCR after RT. As mentioned earlier, metastatic disease is more likely with a rapidly rising PSA, (DT<6 months) and a moderately rising PSA (PSADT 6 mo-1 year) likely indicates local recurrence (48). However, PSA levels fluctuate significantly following RT and false positive rates can be as high as 32% with PSA alone (49). Most recurrences after RT for localized PCa occur within the prostate, with one series of 2,694 patients demonstrating that 55.3% of patients with BCR had biopsy confirmed local recurrence (42). Imaging, particularly mpMRI, therefore, is a key tool in detecting cancer recurrence within the prostate.
Irradiated prostatic tissue shows characteristic changes on MRI. On T2W MRI, radiation-induced glandular atrophy and fibrosis manifest as diffusely decreased signal in the entire gland. Although the treated prostate is heterogeneous with diffuse hypo-isointense signal features, the zonal anatomy is still distinguishable (50). The entire prostate gland and seminal vesicles decrease in size (51). The mean membranous urethral length decreases by 2-4 mm after RT, with even more shortening after brachytherapy (52). Bladder, rectal wall, perirectal fascia, and pelvic sidewall muscles also demonstrate increased signal intensity on T2W MRI with the levator ani muscles more affected after brachytherapy and the obturator internus more affected after EBRT (27, 52). Fatty replacement of bone marrow can be visualized as hyperintense signal changes on T1W MRI and hypointense changes on T2W MRI in the region of the treatment port (27, 50).
On T2W MRI, recurrent PCa after RT appears as a nodular lesion which hypointense relative to normal prostatic tissue. The nodule most commonly appears in the same location as the pre-treatment tumor, with only 4-9% of lesions recurring in a previously unidentified area (53, 54). This fact is very helpful in determining the presence of recurrence. Nodular recurrence may demonstrate growth relative to the atrophic gland and present with a capsular bulge, especially in lesions located at the periphery of the prostate gland. DCE MRI adds information about tumor contrast uptake, vascularity, and permeability which should be combined with the anatomical information from T2W MRI. As the surrounding benign tissue atrophies, vascularity also decreases, whereas the recurrent tumor can grow and create a more vascular network (55, 56). With this increased neovascularity, recurrent prostate lesions demonstrate early and high peak enhancement on DCE MRI relative to the treated prostate (57).
DW MRI reflects the restriction of proton diffusion through malignant tissues in the prostate. This restricted water proton movement can be identified on an apparent diffusion coefficient (ADC) map, calculated from multiple b-value DW MRI. Prostatic tumors demonstrate an increased ADC value as a normal response to RT, whereas benign and normal areas undergo a slightly decreased ADC value shift when compared to their pre-radiotherapy ADC (58). Recurrent lesions demonstrate low signal on ADC maps and hyperintensity on high b-value DW MRI. Evaluation of the transition zone is challenged by heterogeneous signals from BPH nodules on ADC, thus ADC maps must be interpreted in the context of anatomical information from T2W MRI. False positives on ADC may be from prostatitis, hemorrhage, dysplasia, and high-grade prostatic intraepithelial neoplasia (59),(60).
The sensitivity and specificity of T2W MRI alone to detect recurrence after EBRT range widely. Sala et al. in a retrospective cohort of 45 patients with BCR after definitive EBRT demonstrated a sensitivity for T2W MRI ranging between 36-75% and specificity of 65-81% with salvage prostatectomy as the standard (61). Westphalen et al. investigated if time since therapy was a factor on T2W MRI accuracy by evaluating 25 patients imaged within three years of therapy and 34 imaged more than three years after therapy. Accuracy for the overall group was similar to the findings of Sala et al. for the sensitivity of T2W MRI as 62-74% and specificity from 64-68%. Logistic regression demonstrated no difference in accuracy between those imaged early or later than 3 years post therapy (p=0.86) (62). Much of the variation in accuracy is due to the limitations of T2W MRI as a single parameter, as the contrast resolution between malignant lesions and normal prostatic tissue decreases in the irradiated gland.
In head-to-head comparisons with T2W MRI, DCE MRI consistently performed with a higher level of accuracy and reproducibility (63, 64). Preliminary studies by Rouviere et al. compared the accuracy of DCE MRI to T2W MRI in the peripheral zone and found that DCE MRI performed better than T2W MRI with a sensitivity of 70-74% and specificity of 73-85% (p<0.001). (55). They also reported improved reproducibility with DCE MRI among three readers achieving a kappa value of 0.63-0.70 for DCE MRI versus 0.18-0.39 for T2W MRI (55). Haider et al. found similar benefits of DCE MRI over T2W MRI with sensitivity improving to 72% compared to only 38% on T2W MRI (p=0.005) (65).
In imaging recurrence after RT, DW MRI holds particular promise in identifying recurrence when combined with T2W MRI (66). Kim et al. prospectively evaluated a series of 36 patients with BCR after RT with T2W MRI and DW MRI and found an improvement in sensitivity from 25 to 62% (p<0.001). The ADC values were significantly lower in recurrent cancer than in treated but benign tissue (p<0.01) (67). Other studies have tended to confirm these findings (68) (69). The value of ERC in DW MRI was implicated further in a study by Morgan et al. who used it to obtain ADC maps and T2W MRI in 24 patients with BCR after EBRT and found a sensitivity of 94% and specificity of 75% (69). Thus, it is apparent that the combination of parameters improves the detection of recurrent cancer(70) (71). A meta-analysis by Wu et al. evaluated the pooled sensitivity and specificity of these studies to detect recurrence after EBRT to be 82% and 74%, respectively on a patient level. Additionally, DCE MRI was shown to be particularly accurate with the highest individual pooled sensitivity of 90% on a patient based analysis and 71% on a sextant based analysis among all parameters (72). All these results together suggest the optimal method to evaluate for PCa recurrence in a patient treated with EBRT is a multi-parametric approach with T2W+DCE+DW MRI for optimal accuracy and agreement between readers.
Some disagreement has recently arisen in the literature about the relative value of DCE MRI in the multi-parametric approach (73). Some studies show a clear improvement in diagnostic value by the addition of DCE-MRI while others do not. (74) (73). It is unclear how much the technique of DCE MRI matters in its diagnostic utility. For instance, until recently there have been few standards regarding the rate of injection of contrast media, the temporal and spatial resolution of the DCE MRI sequence and the method of evaluation (75). Hopefully, future studies will try to standardize their methods so that direct comparisons will be more meaningful.
The evaluation of a patient with BCR after brachytherapy is similar to EBRT, but is complicated by artifacts related to permanent seed implants and more variability in PSA after treatment (46, 76). Regardless of which radionuclide the seeds are loaded with, they are composed of small rice-sized metal containers that can distort the magnetic field if they are closely packed. These seeds show gradual peripheral migration in the prostate as the gland atrophies from treatment effects. The prostate shows similar changes to EBRT with T2W MRI showing diffusely hypointense signal with blurred anatomical margins. Depending on time from treatment, usually before six to eight weeks post treatment, hemorrhage may be hyperintense on T1W MRI. Recurrence appears as hypointense nodules on T2W MRI with diffusion restriction on DW MRI and rapid contrast uptake on DCE MRI (Figure 2).
Figure 2.
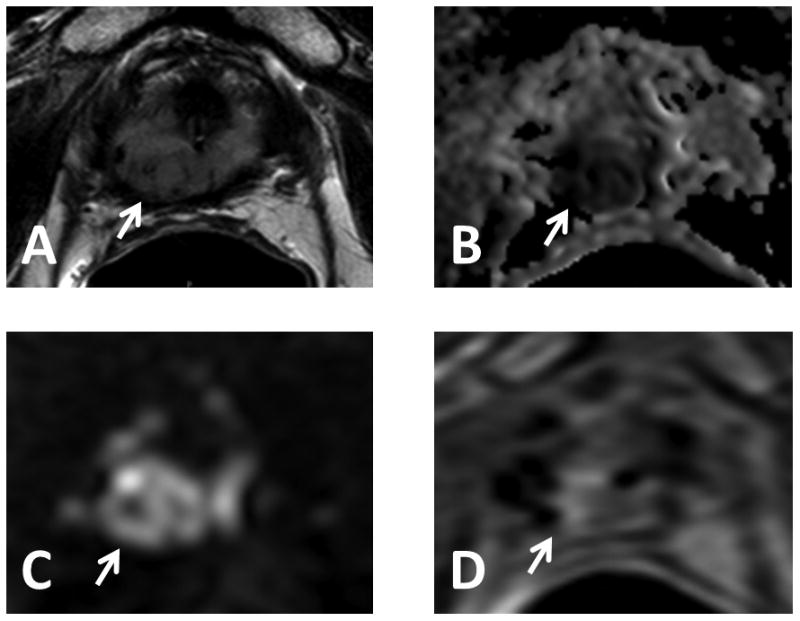
77 year old male with serum PSA = 11.97ng/mL S/P brachytherapy. Axial T2W MRI (A), ADC map of DW MR (B), b2000 DW MRI (C) and DCE MRI (D) show a lesion in the right distal apical peripheral zone (arrows). Low signal artifacts are related to retained seeds. Targeted biopsy revealed Gleason 4+4 recurrent prostate cancer.
Studies on the efficacy of mpMRI for detection of recurrent PCa after brachytherapy are limited. Tamada et al. evaluated 16 men with BCR after brachytherapy with mpMRI. T2W MRI, DCE MRI, DW MRI, and mpMRI showed a sensitivity of 27%, 50%, 68%, and 77%, respectively and a specificity of 92% for mpMRI on a sector based analysis (77). However, in reality very few studies have been performed in this cohort of patients perhaps reflecting a relatively low rate of recurrence due to patient selection for brachytherapy.
mpMRI in focal therapy
Focal therapy (FT) of PCa is sometimes used to treat prostate cancer as an alternative to RP or RT. FT is not standard of care and is generally considered to be experimental. Nonetheless, it is popular among patients because it promises to treat the tumor while minimizing damage to the remainder of the gland thus reducing the known side effects of prostate cancer treatment including urinary incontinence and erectile dysfunction (7). FT generally is an image guided procedure and is used in conjunction with probes that kill cells by various physical interventions including cryoablation, high intensity focused ultrasound (HIFU), and laser ablation (78). MR guidance is a good but not perfect guidance tool. MRI can miss lesions or underestimate their volume. Meanwhile, it can be difficult to assess the margins of treatment at the time of their application. Therefore, it is common to undertreat portions of the tumor leading to recurrence (79).
The definition of BCR following FT is controversial (80, 81). By definition, much of the normal prostate is preserved and produces PSA therefore a non-zero nadir is expected. A number of studies have compiled definitions of BCR after FT and generally use an approach similar to the Phoenix criteria, namely a rise of 2ng/ml from PSA nadir (82, 83).
Each of the various FT methods has unique methods of killing cancer cells. Cryotherapy alternates extreme temperatures to cause coagulative necrosis after several freeze-thaw cycles. Focal cryoablation is successful in 60-80% of patients, as determined by their five to ten year biochemical failure free survival rate (84-87). Naturally, the rate of recurrence has much to do with patient selection and most patients undergoing FT have low grade tumors. The exact “kill zone” is typically underestimated on imaging, where the “ice ball” typically is larger than the “kill zone”. Moreover, the intense freeze-thaw cycles of this therapy method make image interpretation of post-cryoablation changes difficult. MRI after cryoablation is limited since the prostatic fossa is often completely disrupted and therefore, difficult to interpret (88). Recurrences appear similar to primary tumors on T2W, DW-MRI and DCE-MRI. However, the symmetry that is expected on a normal prostate MRI is lost after FT (11, 89) (Figure 3). Though biochemical outcomes after cryoablation seem promising, further research is needed to assess its value and more accurately define the role of mpMRI in PCa recurrence after focal cryoablation.
Figure 3.
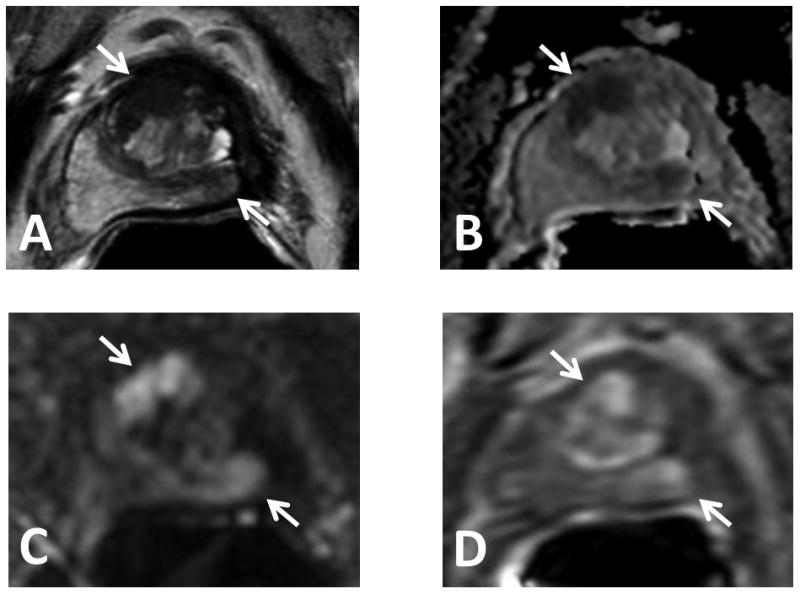
74 year old male with serum PSA = 3.68ng/mL S/P cryotherapy. Note the distortion of the gland on all sequences. Axial T2W MRI (A), ADC map of DW MR (B), b2000 DW MRI (C) and DCE MRI (D) show a lesion in the midline apical-base anterior transition zone and in the midline to the left mid-base peripheral zone of the prostate (arrows). Targeted biopsy revealed Gleason 3+3 and Gleason 3+4 recurrent prostate cancer in the midline apical-base anterior transition zone and left mid-base peripheral zone lesions, respectively.
High intensity focused ultrasound (HIFU) is a thermoablative technique that uses extremely high temperatures to heat tumor tissue by focused ultrasound to cause coagulative necrosis within the tumor. Among 160 patients with a median follow up time of 72 months in a study by Mearini et al., the biochemical-recurrence-free rate for HIFU treatment was 70% and 41% for low and intermediate risk disease, respectively (90). Post HIFU MRI can also be challenging due to the heterogeneity and diffuse hypointensity of the prostate gland on T2W MRI (88, 91, 92). DCE-MRI can demonstrate rim enhancement around the site that was ablated due to reparative tissue. Within 6 months of treatment T2W MRI remains heterogeneous with hypointense signal intensity at the treatment site. Nonetheless, it is possible to identify recurrences by their hypointense T2W MRI signal, restricted diffusion on ADC maps of DW MRI, and early focal enhancement on DCE MRI (11, 89). Among the sequences, DCE-MRI tends to be the most reliable in this context with sensitivity/specificity of 80-87% and 63-68% (93).
Focal laser ablation (FLA) uses laser energy, delivered via optical fibers, to ablate tumor tissues. Data on recurrence after FLA is limited. Post treatment appearance on MRI shows heterogeneous T2W MRI signal intensity due to intravascular coagulation from laser light exposure (94). Ablated lesions exhibit a hypointense defect on T2W MRI indicating fibrosis, low signal intensity on ADC maps which is caused by a signal void (absence of water), and hypovascularity on DCE MRI (89). Recurrence post FLA can be detected by hypointense T2W MRI signal, restricted diffusion, and early hyper-enhancement on DCE MRI (11). Most recurrences are near the edges of the ablated region (11), because the “MR margin” often underestimates the true margin of the tumor, a treatment margin of at least 9mm around the visible lesion is recommended (79). A phase I trial of FLA was performed by Oto et al. indicating the feasibility of focal laser ablation. In this study, DCE MRI demonstrated non-enhancing focal defect within the ablated region in eight of nine patients. At six months post FLA treatment, MRI guided biopsy revealed Gleason 3+3 in two patients. However, it was retrospectively determined that the two recurring patients did not have adequate ablation coverage of their suspicious lesions (95). Currently, University of Chicago, University of Toronto, and the National Cancer Institute are conducting phase II trials of focal laser ablation to determine its oncological efficacy.
Currently, the literature on recurrence after cryoablation, HIFU, and FLA are limited to single-institutional, and retrospective studies (96). In an attempt to standardized FT trials, Van den Bos et al. in 2014 proposed a standard template for designing the follow-up of FT trials (97). Using the Delphi consensus method, they concluded that follow-up should include mpMRI with T1W MRI, T2W MRI, DW MRI with b-values>1000 and ADC maps, and DCE MRI sequences in addition to TRUS-MRI fusion guided biopsies (98). Since long term data on the effects of FT recurrence are limited (7), larger multi-institutional studies are needed to establish the criteria of biochemical recurrence following FT and the accuracy of mpMRI for detecting recurrence in post FT treatment of PCa.
Conclusion
In conclusion, mpMRI is useful in detecting recurrent PCa in patients following RP, RT, or FT. Early detection is crucial in the management of treatment success and patient survival. Although every kind of PCa therapy will leave its unique changes within the pelvis, making imaging difficult to interpret, recent advances in MRI have proven mpMRI to be the most effective modality to detect local recurrent PCa after RP and RT even while PSA levels are low. Although the literature is still sparse, several conclusions can be made. Since recurrences tend to be small, MRI usually benefits from the use of an endorectal coil. While T2W MRI is usually positive it is less sensitive than other techniques. DCE-MRI is consistently the most helpful although DW MRI can also be helpful after RT. Unlike RP and RT, the definition of BCR after FT is still controversial and thus, the use of MRI after FT is still done as a routine rather than in response to specific rises in PSA. While mpMRI allows effective and fast detection of recurrence it always requires confirmation preferably with biopsy or PSA response after focal treatment. Lastly, there needs to be more research on the medical impact of discovering recurrent disease and the initiation of treatment in cases of biochemically recurrence.
References
- 1.Society AC. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Trewartha D, Carter K. Advances in prostate cancer treatment. Nature reviews Drug discovery. 2013;12(11):823–4. doi: 10.1038/nrd4068. [DOI] [PubMed] [Google Scholar]
- 3.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. The Journal of urology. 2001;166(2):416–9. [PubMed] [Google Scholar]
- 4.Zietman AL, Coen JJ, Dallow KC, Shipley WU. The treatment of prostate cancer by conventional radiation therapy: an analysis of long-term outcome. International journal of radiation oncology, biology, physics. 1995;32(2):287–92. doi: 10.1016/0360-3016(95)00123-G. [DOI] [PubMed] [Google Scholar]
- 5.Kuban DA, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. International journal of radiation oncology, biology, physics. 2003;57(4):915–28. doi: 10.1016/s0360-3016(03)00632-1. Epub 2003/10/25. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester JE, Blasko JC, Grimm PD, Meier R, Malmgren JA. Ten-year biochemical relapse-free survival after external beam radiation and brachytherapy for localized prostate cancer: the Seattle experience. International journal of radiation oncology, biology, physics. 2003;57(4):944–52. doi: 10.1016/s0360-3016(03)00739-9. [DOI] [PubMed] [Google Scholar]
- 7.Sankineni S, Wood BJ, Rais-Bahrami S, Walton Diaz A, Hoang AN, Pinto PA, et al. Image-guided focal therapy for prostate cancer. Diagnostic and interventional radiology. 2014;20(6):492–7. doi: 10.5152/dir.2014.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama. 2015;313(4):390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abd-Alazeez M, Ramachandran N, Dikaios N, Ahmed HU, Emberton M, Kirkham A, et al. Multiparametric MRI for detection of radiorecurrent prostate cancer: added value of apparent diffusion coefficient maps and dynamic contrast-enhanced images. Prostate cancer and prostatic diseases. 2015;18(2):128–36. doi: 10.1038/pcan.2014.55. [DOI] [PubMed] [Google Scholar]
- 10.Valeria Panebianco FB, Sciarra Alessandro, Ciardi Antonio, Indino Elena Lucia, Papalia Rocco, Gallucci Michele, Tombolini Vincenzo, Gentile Vincenzo, Catalano Carlo. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urologic oncology: Seminars and Original Investigations. 2015;33(1):17.e1–e7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Notley M, Yu J, Fulcher AS, Turner MA, Cockrell C, Nguyen D. Diagnosis of Recurrent Prostate Cancer and Its Mimics at Multiparametric Prostate MRI. The British journal of radiology. 2015:20150362. doi: 10.1259/bjr.20150362. Epub 2015/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamis S, Varkarakis IM. Defining prostate cancer risk after radical prostatectomy. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2014;40(5):496–504. doi: 10.1016/j.ejso.2014.02.221. [DOI] [PubMed] [Google Scholar]
- 13.Petit JH, Chen MH, Loffredo M, Sussman B, Renshaw AA, D'Amico AV. Prostate-specific antigen recurrence and mortality after conventional dose radiation therapy in select men with low-risk prostate cancer. Cancer. 2006;107(9):2180–5. doi: 10.1002/cncr.22243. [DOI] [PubMed] [Google Scholar]
- 14.Hricak H, Schoder H, Pucar D, Lis E, Eberhardt SC, Onyebuchi CN, et al. Advances in imaging in the postoperative patient with a rising prostate-specific antigen level. Seminars in oncology. 2003;30(5):616–34. doi: 10.1016/s0093-7754(03)00359-2. [DOI] [PubMed] [Google Scholar]
- 15.Lopes Dias J, Lucas R, Magalhaes Pina J, Joao R, Costa NV, Leal C, et al. Post-treated prostate cancer: normal findings and signs of local relapse on multiparametric magnetic resonance imaging. Abdominal imaging. 2015 doi: 10.1007/s00261-015-0473-1. [DOI] [PubMed] [Google Scholar]
- 16.Shikanov S, Kocherginsky M, Shalhav AL, Eggener SE. Cause-specific mortality following radical prostatectomy. Prostate cancer and prostatic diseases. 2012;15(1):106–10. doi: 10.1038/pcan.2011.55. [DOI] [PubMed] [Google Scholar]
- 17.Moul JW. Prostate specific antigen only progression of prostate cancer. The Journal of urology. 2000;163(6):1632–42. [PubMed] [Google Scholar]
- 18.Laufer M, Pound CR, Carducci MA, Eisenberger MA. Management of patients with rising prostate-specific antigen after radical prostatectomy. Urology. 2000;55(3):309–15. doi: 10.1016/s0090-4295(99)00465-3. [DOI] [PubMed] [Google Scholar]
- 19.Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D'Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. The Journal of urology. 2007;177(2):540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. European urology. 2011;59(1):61–71. doi: 10.1016/j.eururo.2010.10.039. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 21.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. Jama. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 22.Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clinic proceedings. 2001;76(6):576–81. doi: 10.4065/76.6.576. [DOI] [PubMed] [Google Scholar]
- 23.Panebianco V, Barchetti F, Musio D, De Felice F, Proietti C, Indino EL, et al. Advanced imaging for the early diagnosis of local recurrence prostate cancer after radical prostatectomy. BioMed research international. 2014;2014:827265. doi: 10.1155/2014/827265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes Dias J, Lucas R, Magalhães Pina J, João R, Costa N, Leal C, et al. Post-treated prostate cancer: normal findings and signs of local relapse on multiparametric magnetic resonance imaging. Abdominal imaging. 2015;40(7):2814–38. doi: 10.1007/s00261-015-0473-1. [DOI] [PubMed] [Google Scholar]
- 25.Allen SD, Thompson A, Sohaib SA. The normal post-surgical anatomy of the male pelvis following radical prostatectomy as assessed by magnetic resonance imaging. European radiology. 2008;18(6):1281–91. doi: 10.1007/s00330-008-0867-3. [DOI] [PubMed] [Google Scholar]
- 26.Rouviere O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? European radiology. 2010;20(5):1254–66. doi: 10.1007/s00330-009-1647-4. Epub 2009/11/19. [DOI] [PubMed] [Google Scholar]
- 27.Vargas HA, Wassberg C, Akin O, Hricak H. MR imaging of treated prostate cancer. Radiology. 2012;262(1):26–42. doi: 10.1148/radiol.11101996. Epub 2011/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cirillo S, Petracchini M, Scotti L, Gallo T, Macera A, Bona MC, et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. European radiology. 2009;19(3):761–9. doi: 10.1007/s00330-008-1174-8. [DOI] [PubMed] [Google Scholar]
- 29.De Visschere PJ, Vargas HA, Ost P, De Meerleer GO, Villeirs GM. Imaging treated prostate cancer. Abdominal imaging. 2013;38(6):1431–46. doi: 10.1007/s00261-013-9998-3. [DOI] [PubMed] [Google Scholar]
- 30.Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004;231(2):379–85. doi: 10.1148/radiol.2312030011. [DOI] [PubMed] [Google Scholar]
- 31.Roy C, Foudi F, Charton J, Jung M, Lang H, Saussine C, et al. Comparative sensitivities of functional MRI sequences in detection of local recurrence of prostate carcinoma after radical prostatectomy or external-beam radiotherapy. AJR American journal of roentgenology. 2013;200(4):W361–8. doi: 10.2214/ajr.12.9106. Epub 2013/03/26. [DOI] [PubMed] [Google Scholar]
- 32.Wassberg C, Akin O, Vargas HA, Shukla-Dave A, Zhang J, Hricak H. The incremental value of contrast-enhanced MRI in the detection of biopsy-proven local recurrence of prostate cancer after radical prostatectomy: effect of reader experience. AJR American journal of roentgenology. 2012;199(2):360–6. doi: 10.2214/ajr.11.6923. Epub 2012/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sella T, Schwartz LH, Hricak H. Retained seminal vesicles after radical prostatectomy: frequency, MRI characteristics, and clinical relevance. AJR American journal of roentgenology. 2006;186(2):539–46. doi: 10.2214/AJR.04.1770. [DOI] [PubMed] [Google Scholar]
- 34.Alfarone A, Panebianco V, Schillaci O, Salciccia S, Cattarino S, Mariotti G, et al. Comparative analysis of multiparametric magnetic resonance and PET-CT in the management of local recurrence after radical prostatectomy for prostate cancer. Critical reviews in oncology/hematology. 2012;84(1):109–21. doi: 10.1016/j.critrevonc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Kitajima K, Hartman RP, Froemming AT, Hagen CE, Takahashi N, Kawashima A. Detection of Local Recurrence of Prostate Cancer After Radical Prostatectomy Using Endorectal Coil MRI at 3 T: Addition of DWI and Dynamic Contrast Enhancement to T2-Weighted MRI. AJR American journal of roentgenology. 2015;205(4):807–16. doi: 10.2214/ajr.14.14275. Epub 2015/09/24. [DOI] [PubMed] [Google Scholar]
- 36.Silverman JM, Krebs TL. MR imaging evaluation with a transrectal surface coil of local recurrence of prostatic cancer in men who have undergone radical prostatectomy. AJR American journal of roentgenology. 1997;168(2):379–85. doi: 10.2214/ajr.168.2.9016212. [DOI] [PubMed] [Google Scholar]
- 37.Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, et al. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. European radiology. 2013;23(6):1745–52. doi: 10.1007/s00330-013-2768-3. [DOI] [PubMed] [Google Scholar]
- 38.Cha D, Kim CK, Park SY, Park JJ, Park BK. Evaluation of suspected soft tissue lesion in the prostate bed after radical prostatectomy using 3T multiparametric magnetic resonance imaging. Magnetic resonance imaging. 2015;33(4):407–12. doi: 10.1016/j.mri.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Rischke HC, Schafer AO, Nestle U, Volegova-Neher N, Henne K, Benz MR, et al. Detection of local recurrent prostate cancer after radical prostatectomy in terms of salvage radiotherapy using dynamic contrast enhanced-MRI without endorectal coil. Radiation oncology. 2012;7:185. doi: 10.1186/1748-717X-7-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D'Amico AV, et al. Prostate cancer, version 2.2014. Journal of the National Comprehensive Cancer Network : JNCCN. 2014;12(5):686–718. doi: 10.6004/jnccn.2014.0072. Epub 2014/05/09. [DOI] [PubMed] [Google Scholar]
- 41.Roach M, 3rd, Hanks G, Thames H, Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. International journal of radiation oncology, biology, physics. 2006;65(4):965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. European urology. 2015;67(6):1009–16. doi: 10.1016/j.eururo.2014.09.028. Epub 2014/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Kollmeier M, et al. Anatomical Patterns of Recurrence Following Biochemical Relapse in the Dose Escalation Era for Prostate Patients Undergoing External Beam Radiotherapy. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.06.100. Epub 2015/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickles T. Prostate-specific antigen (PSA) bounce and other fluctuations: which biochemical relapse definition is least prone to PSA false calls? An analysis of 2030 men treated for prostate cancer with external beam or brachytherapy with or without adjuvant androgen deprivation therapy. International journal of radiation oncology, biology, physics. 2006;64(5):1355–9. doi: 10.1016/j.ijrobp.2005.10.008. Epub 2006/01/13. [DOI] [PubMed] [Google Scholar]
- 45.Horwitz EM, Levy LB, Thames HD, Kupelian PA, Martinez AA, Michalski JM, et al. Biochemical and clinical significance of the posttreatment prostate-specific antigen bounce for prostate cancer patients treated with external beam radiation therapy alone: a multiinstitutional pooled analysis. Cancer. 2006;107(7):1496–502. doi: 10.1002/cncr.22183. Epub 2006/09/01. [DOI] [PubMed] [Google Scholar]
- 46.Caloglu M, Ciezki JP, Reddy CA, Angermeier K, Ulchaker J, Chehade N, et al. PSA bounce and biochemical failure after brachytherapy for prostate cancer: a study of 820 patients with a minimum of 3 years of follow-up. International journal of radiation oncology, biology, physics. 2011;80(3):735–41. doi: 10.1016/j.ijrobp.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Buyyounouski MK, Pickles T, Kestin LL, Allison R, Williams SG. Validating the interval to biochemical failure for the identification of potentially lethal prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(15):1857–63. doi: 10.1200/jco.2011.35.1924. Epub 2012/04/18. [DOI] [PubMed] [Google Scholar]
- 48.Sartor CI, Strawderman MH, Lin XH, Kish KE, McLaughlin PW, Sandler HM. Rate of PSA rise predicts metastatic versus local recurrence after definitive radiotherapy. International journal of radiation oncology, biology, physics. 1997;38(5):941–7. doi: 10.1016/s0360-3016(97)00082-5. Epub 1997/07/15. [DOI] [PubMed] [Google Scholar]
- 49.Denham JW, Kumar M, Gleeson PS, Lamb DS, Joseph D, Atkinson C, et al. Recognizing false biochemical failure calls after radiation with or without neo-adjuvant androgen deprivation for prostate cancer. International journal of radiation oncology, biology, physics. 2009;74(2):404–11. doi: 10.1016/j.ijrobp.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Sugimura K, Carrington BM, Quivey JM, Hricak H. Postirradiation changes in the pelvis: assessment with MR imaging. Radiology. 1990;175(3):805–13. doi: 10.1148/radiology.175.3.2343132. Epub 1990/06/01. [DOI] [PubMed] [Google Scholar]
- 51.Coakley FV, Teh HS, Qayyum A, Swanson MG, Lu Y, Roach M, 3rd, et al. Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology. 2004;233(2):441–8. doi: 10.1148/radiol.2332032086. Epub 2004/09/18. [DOI] [PubMed] [Google Scholar]
- 52.Marigliano C, Donati OF, Vargas HA, Akin O, Goldman DA, Eastham JA, et al. MRI findings of radiation-induced changes in the urethra and periurethral tissues after treatment for prostate cancer. European journal of radiology. 2013;82(12):e775–81. doi: 10.1016/j.ejrad.2013.09.011. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arrayeh E, Westphalen AC, Kurhanewicz J, Roach M, 3rd, Jung AJ, Carroll PR, et al. Does local recurrence of prostate cancer after radiation therapy occur at the site of primary tumor? Results of a longitudinal MRI and MRSI study. International journal of radiation oncology, biology, physics. 2012;82(5):e787–93. doi: 10.1016/j.ijrobp.2011.11.030. Epub 2012/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jalloh M, Leapman MS, Cowan JE, Shinohara K, Greene KL, Roach Iii M, et al. Patterns of Local Failure following Radiation Therapy for Prostate Cancer. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.04.111. doi:http://dx.doi.org/10.1016/j.juro.2015.04.111. [DOI] [PubMed] [Google Scholar]
- 55.Rouviere O, Valette O, Grivolat S, Colin-Pangaud C, Bouvier R, Chapelon JY, et al. Recurrent prostate cancer after external beam radiotherapy: value of contrast-enhanced dynamic MRI in localizing intraprostatic tumor--correlation with biopsy findings. Urology. 2004;63(5):922–7. doi: 10.1016/j.urology.2003.12.017. Epub 2004/05/12. [DOI] [PubMed] [Google Scholar]
- 56.Franiel T, Ludemann L, Taupitz M, Bohmer D, Beyersdorff D. MRI before and after external beam intensity-modulated radiotherapy of patients with prostate cancer: the feasibility of monitoring of radiation-induced tissue changes using a dynamic contrast-enhanced inversion-prepared dual-contrast gradient echo sequence. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;93(2):241–5. doi: 10.1016/j.radonc.2009.08.016. Epub 2009/09/15. [DOI] [PubMed] [Google Scholar]
- 57.Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. BioMed research international. 2014;2014:316272. doi: 10.1155/2014/316272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song I, Kim CK, Park BK, Park W. Assessment of response to radiotherapy for prostate cancer: value of diffusion-weighted MRI at 3 T. AJR American journal of roentgenology. 2010;194(6):W477–82. doi: 10.2214/ajr.09.3557. Epub 2010/05/22. [DOI] [PubMed] [Google Scholar]
- 59.Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(26):4104–9. doi: 10.1200/jco.2007.11.9610. Epub 2007/09/11. [DOI] [PubMed] [Google Scholar]
- 60.Grant K, Lindenberg ML, Shebel H, Pang Y, Agarwal HK, Bernardo M, et al. Functional and molecular imaging of localized and recurrent prostate cancer. European journal of nuclear medicine and molecular imaging. 2013;40(Suppl 1):S48–59. doi: 10.1007/s00259-013-2419-6. Epub 2013/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sala E, Eberhardt SC, Akin O, Moskowitz CS, Onyebuchi CN, Kuroiwa K, et al. Endorectal MR imaging before salvage prostatectomy: tumor localization and staging. Radiology. 2006;238(1):176–83. doi: 10.1148/radiol.2381052345. Epub 2005/12/24. [DOI] [PubMed] [Google Scholar]
- 62.Westphalen AC, Kurhanewicz J, Cunha RM, Hsu IC, Kornak J, Zhao S, et al. T2-Weighted endorectal magnetic resonance imaging of prostate cancer after external beam radiation therapy. International braz j urol : official journal of the Brazilian Society of Urology. 2009;35(2):171–80. doi: 10.1590/s1677-55382009000200007. discussion 81-2. Epub 2009/05/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kara T, Akata D, Akyol F, Karcaaltincaba M, Ozmen M. The value of dynamic contrast-enhanced MRI in the detection of recurrent prostate cancer after external beam radiotherapy: correlation with transrectal ultrasound and pathological findings. Diagnostic and interventional radiology. 2011;17(1):38–43. doi: 10.4261/1305-3825.dir.3079-09.1. Epub 2010/08/13. [DOI] [PubMed] [Google Scholar]
- 64.Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, et al. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR American journal of roentgenology. 2012;198(6):1277–88. doi: 10.2214/ajr.12.8510. Epub 2012/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haider MA, Chung P, Sweet J, Toi A, Jhaveri K, Menard C, et al. Dynamic contrast-enhanced magnetic resonance imaging for localization of recurrent prostate cancer after external beam radiotherapy. International journal of radiation oncology, biology, physics. 2008;70(2):425–30. doi: 10.1016/j.ijrobp.2007.06.029. Epub 2007/09/21. [DOI] [PubMed] [Google Scholar]
- 66.Tsien C, Cao Y, Chenevert T. Clinical applications for diffusion magnetic resonance imaging in radiotherapy. Seminars in radiation oncology. 2014;24(3):218–26. doi: 10.1016/j.semradonc.2014.02.004. Epub 2014/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. Journal of magnetic resonance imaging : JMRI. 2009;29(2):391–7. doi: 10.1002/jmri.21645. Epub 2009/01/24. [DOI] [PubMed] [Google Scholar]
- 68.Hara T, Inoue Y, Satoh T, Ishiyama H, Sakamoto S, Woodhams R, et al. Diffusion-weighted imaging of local recurrent prostate cancer after radiation therapy: comparison with 22-core three-dimensional prostate mapping biopsy. Magnetic resonance imaging. 2012;30(8):1091–8. doi: 10.1016/j.mri.2012.04.022. Epub 2012/07/24. [DOI] [PubMed] [Google Scholar]
- 69.Morgan VA, Riches SF, Giles S, Dearnaley D, deSouza NM. Diffusion-weighted MRI for locally recurrent prostate cancer after external beam radiotherapy. AJR American journal of roentgenology. 2012;198(3):596–602. doi: 10.2214/ajr.11.7162. Epub 2012/02/24. [DOI] [PubMed] [Google Scholar]
- 70.Arumainayagam N, Kumaar S, Ahmed HU, Moore CM, Payne H, Freeman A, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU international. 2010;106(7):991–7. doi: 10.1111/j.1464-410X.2010.09291.x. Epub 2010/03/17. [DOI] [PubMed] [Google Scholar]
- 71.Kim CK, Park BK, Park W, Kim SS. Prostate MR imaging at 3T using a phased-arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdominal imaging. 2010;35(2):246–52. doi: 10.1007/s00261-008-9495-2. Epub 2009/01/09. [DOI] [PubMed] [Google Scholar]
- 72.Wu LM, Xu JR, Gu HY, Hua J, Zhu J, Chen J, et al. Role of magnetic resonance imaging in the detection of local prostate cancer recurrence after external beam radiotherapy and radical prostatectomy. Clinical oncology. 2013;25(4):252–64. doi: 10.1016/j.clon.2012.11.010. Epub 2013/01/15. [DOI] [PubMed] [Google Scholar]
- 73.Donati OF, Jung SI, Vargas HA, Gultekin DH, Zheng J, Moskowitz CS, et al. Multiparametric prostate MR imaging with T2-weighted, diffusion-weighted, and dynamic contrast-enhanced sequences: are all pulse sequences necessary to detect locally recurrent prostate cancer after radiation therapy? Radiology. 2013;268(2):440–50. doi: 10.1148/radiol.13122149. Epub 2013/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akin O, Gultekin DH, Vargas HA, Zheng J, Moskowitz C, Pei X, et al. Incremental value of diffusion weighted and dynamic contrast enhanced MRI in the detection of locally recurrent prostate cancer after radiation treatment: preliminary results. European radiology. 2011;21(9):1970–8. doi: 10.1007/s00330-011-2130-6. Epub 2011/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.(ACR) ACoR. PI-RADSv2: Prostate Imaging and Reporting and Data System 2015. [10/16/2015]. Available from: http://www.acr.org/∼/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf.
- 76.Thompson A, Keyes M, Pickles T, Palma D, Moravan V, Spadinger I, et al. Evaluating the Phoenix definition of biochemical failure after (125)I prostate brachytherapy: Can PSA kinetics distinguish PSA failures from PSA bounces? International journal of radiation oncology, biology, physics. 2010;78(2):415–21. doi: 10.1016/j.ijrobp.2009.07.1724. Epub 2010/02/06. [DOI] [PubMed] [Google Scholar]
- 77.Tamada T, Sone T, Jo Y, Hiratsuka J, Higaki A, Higashi H, et al. Locally recurrent prostate cancer after high-dose-rate brachytherapy: the value of diffusion-weighted imaging, dynamic contrast-enhanced MRI, and T2-weighted imaging in localizing tumors. AJR American journal of roentgenology. 2011;197(2):408–14. doi: 10.2214/ajr.10.5772. Epub 2011/07/26. [DOI] [PubMed] [Google Scholar]
- 78.Mottet N. Guidelines on Prostate Cancer: European Associate of Urology 2015. 2015 [updated March 2015; cited 2015 Dec 4]. Available from: http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf.
- 79.Le Nobin J, Rosenkrantz AB, Villers A, Orczyk C, Deng FM, Melamed J, et al. Image Guided Focal Therapy for Magnetic Resonance Imaging Visible Prostate Cancer: Defining a 3-Dimensional Treatment Margin Based on Magnetic Resonance Imaging Histology Co-Registration Analysis. The Journal of urology. 2015;194(2):364–70. doi: 10.1016/j.juro.2015.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Association AU. Best Practice Policy Statement on: Cryosurgery for the Treatment of Localized Prostate Cancer 2008. [cited 2015 November 6]. Available from: https://www.auanet.org/common/pdf/education/clinical-guidance/Cryosurgery.pdf.
- 81.Marshall S, Taneja S. Focal therapy for prostate cancer: The current status. Prostate international. 2015;3(2):35–41. doi: 10.1016/j.prnil.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ganzer R, Robertson CN, Ward JF, Brown SC, Conti GN, Murat FJ, et al. Correlation of prostate-specific antigen nadir and biochemical failure after high-intensity focused ultrasound of localized prostate cancer based on the Stuttgart failure criteria - analysis from the @-Registry. BJU international. 2011;108(8 Pt 2):E196–201. doi: 10.1111/j.1464-410X.2011.10091.x. Epub 2011/02/22. [DOI] [PubMed] [Google Scholar]
- 83.Pitman M, Shapiro EY, Hruby GW, Truesdale MD, Cheetham PJ, Saad S, et al. Comparison of biochemical failure definitions for predicting local cancer recurrence following cryoablation of the prostate. The Prostate. 2012;72(16):1802–8. doi: 10.1002/pros.22541. Epub 2012/05/24. [DOI] [PubMed] [Google Scholar]
- 84.Levy DA, Ross AE, ElShafei A, Krishnan N, Hatem A, Jones JS. Definition of biochemical success following primary whole gland prostate cryoablation. The Journal of urology. 2014;192(5):1380–4. doi: 10.1016/j.juro.2014.05.003. Epub 2014/05/13. [DOI] [PubMed] [Google Scholar]
- 85.de Castro Abreu AL, Bahn D, Leslie S, Shoji S, Silverman P, Desai MM, et al. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU international. 2013;112(3):298–307. doi: 10.1111/bju.12151. Epub 2013/07/06. [DOI] [PubMed] [Google Scholar]
- 86.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JN., Jr Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001;57(3):518–23. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 87.Scheenen TW, Rosenkrantz AB, Haider MA, Futterer JJ. Multiparametric Magnetic Resonance Imaging in Prostate Cancer Management: Current Status and Future Perspectives. Investigative radiology. 2015;50(9):594–600. doi: 10.1097/RLI.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 88.Martino P, Scattoni V, Galosi AB, Consonni P, Trombetta C, Palazzo S, et al. Role of imaging and biopsy to assess local recurrence after definitive treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU) World journal of urology. 2011;29(5):595–605. doi: 10.1007/s00345-011-0687-y. Epub 2011/05/10. [DOI] [PubMed] [Google Scholar]
- 89.Muller B, Sankineni S, Elbuluk O, Grant K, Rais-Bahrami S, Walton-Diaz A, Agarwal H, Bernardo M, Wood B, Pinto P, Choyke P, Turkbey B. Multi-Parametric MRI Findings of Post-Treatment Changes in the Prostate Gland [Poster] RSNA2014. [cited 2015 Dec 25]. Available from: http://archive.rsna.org/2014/14019036.html.
- 90.Mearini L, D'Urso L, Collura D, Nunzi E, Muto G, Porena M. High-intensity focused ultrasound for the treatment of prostate cancer: A prospective trial with long-term follow-up. Scandinavian journal of urology. 2015;49(4):267–74. doi: 10.3109/21681805.2014.988174. [DOI] [PubMed] [Google Scholar]
- 91.Niaf E, Lartizien C, Bratan F, Roche L, Rabilloud M, Mege-Lechevallier F, et al. Prostate focal peripheral zone lesions: characterization at multiparametric MR imaging--influence of a computer-aided diagnosis system. Radiology. 2014;271(3):761–9. doi: 10.1148/radiol.14130448. Epub 2014/03/07. [DOI] [PubMed] [Google Scholar]
- 92.Kirkham AP, Emberton M, Hoh IM, Illing RO, Freeman AA, Allen C. MR imaging of prostate after treatment with high-intensity focused ultrasound. Radiology. 2008;246(3):833–44. doi: 10.1148/radiol.2463062080. [DOI] [PubMed] [Google Scholar]
- 93.Kim CK, Park BK, Lee HM, Kim SS, Kim E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR American journal of roentgenology. 2008;190(5):1180–6. doi: 10.2214/ajr.07.2924. Epub 2008/04/24. [DOI] [PubMed] [Google Scholar]
- 94.Kulik M, Nedelcu C, Martin F, Lebdai S, Rousselet MC, Azzouzi AR, et al. Post-treatment MRI aspects of photodynamic therapy for prostate cancer. Insights into imaging. 2014;5(6):697–713. doi: 10.1007/s13244-014-0359-8. Epub 2014/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, Stadler WM, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267(3):932–40. doi: 10.1148/radiol.13121652. Epub 2013/02/27. [DOI] [PubMed] [Google Scholar]
- 96.Palermo G, Foschi N, D'Agostino D, Sacco E, Bassi P, Pinto F. Local relapse of prostate cancer after primary definitive treatment: the management. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2015 Epub 2015/09/12. [PubMed] [Google Scholar]
- 97.van den Bos W, Muller BG, Ahmed H, Bangma CH, Barret E, Crouzet S, et al. Focal therapy in prostate cancer: international multidisciplinary consensus on trial design. European urology. 2014;65(6):1078–83. doi: 10.1016/j.eururo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Muller BG, van den Bos W, Brausi M, Futterer JJ, Ghai S, Pinto PA, et al. Follow-up modalities in focal therapy for prostate cancer: results from a Delphi consensus project. World journal of urology. 2015;33(10):1503–9. doi: 10.1007/s00345-014-1475-2. Epub 2015/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]


