Abstract
Exosomes are nano-sized, membrane-bound vesicles released from cells that transport cargo including DNA, RNA, and proteins, between cells as a form of intercellular communication. In addition to their role in intercellular communication, exosomes are beginning to be appreciated as agents of immunoregulation that can modulate antigen presentation, immune activation, suppression and surveillance. This article summarizes how these multifaceted functions of exosomes may promote development and/or progression of chronic inflammatory lung diseases including asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis. The potential of exosomes as a novel therapeutic are also discussed.
Keywords: Chronic Lung Diseases, Exosomes, Extracellular Vesicles, Immunoregulation, Inflammation
Introduction
Inflammation plays an important role in the pathogenesis of respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis (PF) (1-8). Common among these diseases are the dysregulated inflammatory processes that contribute to their maintenance and progression. Pulmonary cells that are known to modulate inflammation include, but are not limited to, airway epithelial cells (1, 2, 8-10), dendritic cells (DCs) (1, 2, 9), macrophages (1, 8, 11, 12), CD4+ and CD8+ T lymphocytes (1, 8, 13), NK cells (14-16), and myeloid derived regulatory cells (MDRCs) (17-22). Recent studies provide evidence that extracellular vesicles (EVs) promote the pathogenesis of these diseases by promoting inflammation and immune activation (23-30).
Exosomes are membrane-bound EVs that are 50-150nm in diameter and are generated from the endosome. Genetic material, proteins, and metabolites have been shown to be packaged in exosomes and transferred to cells both locally and distally (31-35). The precise mechanisms of protein packaging into exosomes are starting to be elucidated, and relatively recent studies have shown that higher order oligomerization, as well as specific signaling sequences, have been linked to proteins destined to exosomes (36-40). Not surprisingly, in vitro studies have shown that human immunodeficiency virus (HIV) gag and env proteins can be packaged in exosomes, suggesting a shared pathway or “hijacking” of exosome biogenesis mechanisms in the host (36, 37, 41-43).
In asthmatics, exosomes have been isolated from human Bronchoalveolar Lavage Fluid (BALF) (44), and these have been shown to promote inflammation and immune activation (25, 27, 45). COPD is also a chronic inflammatory disease with similarities in pulmonary symptoms and physiology to asthma; however, with dissimilar mechanisms of inflammation (1, 2). An initial airway insult, such as cigarette smoke, activates both alveolar type II epithelial cells and macrophages to promote inflammation, as well as Th1 and T cytotoxic type 1 (Tc1) responses (12, 46). Together these cells, via the elaboration of proteases (47, 48), pro-inflammatory cytokines (1, 2, 46, 49, 50), and microRNAs (miRNAs) (23, 51), promote the destruction of the alveolar wall resulting in emphysema, and fibrosis of the small airways that contribute to airway narrowing. Increasing evidence suggest that miRNAs packaged in exosomes contribute to inflammation in many disease contexts (24, 52, 53). Only a limited number of studies have investigated the potential for exosomes to package the cellular mediators that drive COPD-associated inflammation.
Inflammation also plays a role in pulmonary fibrosis (PF). Activated alveolar type II epithelial cells can secrete pro-fibrotic factors such as transforming growth factor-β (TGF-β) and platelet derived growth factor (54). These growth factors promote local fibroblast recruitment as well as their transition to myofibroblasts. Although direct evidence for exosomes is lacking in PF, exosomal TGF-β from cancer cells has been shown to promote myofibroblast differentiation (55). Thus with evidence that supports the potential for exosomes to alter gene programs (55), and induce differentiation or de-differentiation of target cells, it is likely that exosomes participate in the process of tissue repair and fibrosis (56).
History and Life Cycle of Exosomes
Membrane-bound EVs were first described in the calcification of extracellular collagen (57), and then in cancer cells (58). These EVs contained cytoplasmic content, such as proteins and lipids, from the cell it originated from (59). The classification and nomenclature for EVs have evolved as different types of vesicles were discovered with diverse biochemical properties, and newer techniques allowed for characterization of important molecular cargo in the exosome including the proteome and identification of the cellular origins of exosomes (60-63).
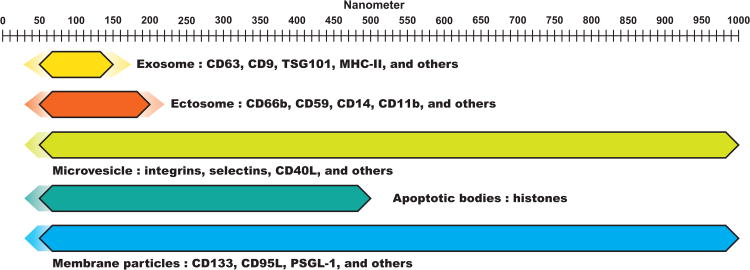
In 1981, the term exosome was used for the first time to describe membrane-bound EVs. Exosomes are now recognized as endosome-derived membrane particles that generally range in size from 50-150nm in diameter (Figure 1) (43, 64-67). The inward budding of endosomes generates multiple intraluminal vesicles (ILVs) inside, and the vesicle-filled endosome is eventually referred to as a multi-vesicular body (MVB). MVBs can either fuse with lysosomes, or mature into a lysosome, resulting in the degradation of cargo, or fuse with the plasma membrane (PM), releasing newly synthesized exosomes into the extracellular space (64, 66, 68-74). Gould et al. referred to this method of exosome biogenesis as the delayed mode (41). They have also described the immediate mode of biogenesis in the context of the Trojan exosome hypothesis and HIV retroviral budding. This mode refers to the biogenesis of exosomes directly from the plasma membrane due to residual endosomal lipid content left from previous MVB fusion (41, 43). The same group has suggested that activated T lymphocytes adopt the immediate mode of biogenesis of exosomes, while delayed mode of biogenesis is observed in macrophages (43).
Figure 1.
Graphical representation of various extracellular vesicles, their sizes, and related surface proteins. Color gradients indicate reported variability in size.
Exosomes can be isolated from bodily fluids such as blood (75), urine (62), semen (31), breast milk (76), and bronchoalveolar lavage fluid (BALF) (44). Many purification methods for exosomes exists, but differential centrifugation with or without a gradient has been described and well established (77). Additionally, commercial exosome purification kits that do not require multiple lengthy centrifugation steps are also available (78). Because exosomes are endosomally-derived, they are enriched in tetraspanins, such as CD63, CD9, CD81, and CD82, which are the markers currently used to characterize exosomes (79, 80).
Homeostatic Roles of Exosomes
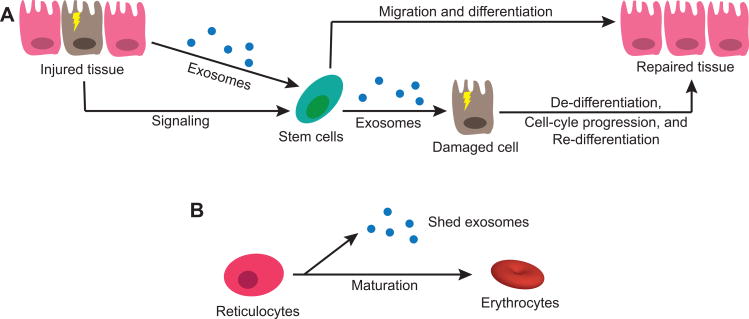
Exosomes are necessary for normal cellular function and homeostatic regulation of the host tissues and organs (31, 34, 60, 81-85). Perhaps the best recognized function of exosomes is in intercellular communication and signaling (32). Exosomes can signal to immune cells and modulate their function (64, 86-92). Injured cells can signal resident progenitor cells or bone-marrow stem cells via exosomes (Figure 2A), inducing migration and differentiation to the sites of tissue injury (81). Radiation-injured whole lung cells have been shown to secrete microvesicles that have the ability to induce lung-specific gene and protein expression in ex vivo co-cultured bone marrow cells (93). This process is proposed to be bidirectional (Figure 2A), where stem cells or progenitor cells can secrete EVs that also contain miRNA and mRNA which induce de-differentiation of damaged tissue cells, proliferation, re-differentiation of proliferated cells, and ultimately tissue repair (81). Supporting this concept, in vitro studies have demonstrated that embryonic stem cells can secrete EVs that reprogram hematopoietic progenitor cells through the transfer of mRNA (94). Additionally, conditioned medium from mesenchymal stem cells promoted tissue regeneration, similar to the effects seen with stem cell therapy (95). The results from these studies provide an explanation as to why tissue repair occurs despite low stem cell engraftment at the site of injury, as EVs and other secreted factors may play a central role in tissue regeneration and remodeling instead of direct cell engraftment and differentiation.
Figure 2.
Homeostatic roles for exosomes and extracellular vesicles. (A) Injured tissue cells secrete exosomes and membrane particles that promote migration and differentiation of resident stem cells (progenitor cells). Tissue damage can also be “sensed” by resident or bone marrow stem cells, which can then secrete exosomes or membrane particles that may promote de-differentiation, cell-cycle progression, and tissue repair. (B) Exosomes are also important in the maturation process of various cell types, for example in the maturation of reticulocytes into erythrocytes through shedding of proteins and surface receptors by exosomes.
Exosomes have been shown to directly modulate differentiation as well as metabolic homeostasis through the transfer of miRNAs. Myotube-derived exosomes containing miRNA suppressed expression of Sirtuin-1, which is involved in regulating metabolism and myogenesis (34). Differential miRNA content was found at varying stages of differentiation, and furthermore, not all cytoplasmic miRNAs were packaged in exosomes, suggesting a selective packaging mechanism of these miRNAs. Of note, in a diseased state, the exosome content is drastically different from those obtained from normal skeletal muscle cells. Exosomes from the insulin-resistant skeletal muscle cells promoted disease progression by altering gene expression of nearby cells (60).
In the context of cellular maturation, exosomes and other EVs have both been shown to be involved in transferring epididymal proteins to spermatozoa, to promote the production of functional male gametes (31). EVs secreted from the prostate gland epithelial cells, or prostasomes, have been found in post-ejaculatory semen with potential roles such as antimicrobial and protease functions (31). Reticulocytes also utilize exosomes (Figure 2B) to dispose the transferrin receptor in the process of maturation into functional red blood cells (96). This process of exosome-mediated disposal of cellular proteins is also seen in cells that have active autophagy and lysosome pathways (97, 98).
Autophagy and lysosome pathways degrade damaged organelles and proteins as a stress response to re-establish homeostasis (84, 99-102). Exosomes have been suggested to play a role in the secretion of cellular “garbage” as a compensatory mechanism to faulty autophagy pathways due to aging or disease (84). A signaling function may be implicated as nearby cells may sense packaged autophagosomal content, and aid in clearance and homeostasis of cellular and organismal level function. Interestingly, cells that experienced radiation-induced stress secreted exosomes in a p53-dependent fashion (103). This study also showed that TSAP6, a downstream target gene of p53, is necessary for exosome production. Constitutive expression of TSAP6 was achieved by transfection in H1299 cells, a human non-small cell lung cancer epithelial cell line, which lacks p53 and does not produce exosomes. Under constitutive expression of TSAP6, production of exosomes was observed even in the absence of irradiation. This suggests that cells undergoing a stress-response may upregulate exosome production as a form of danger signal.
Exosome Mediated Immune Regulation
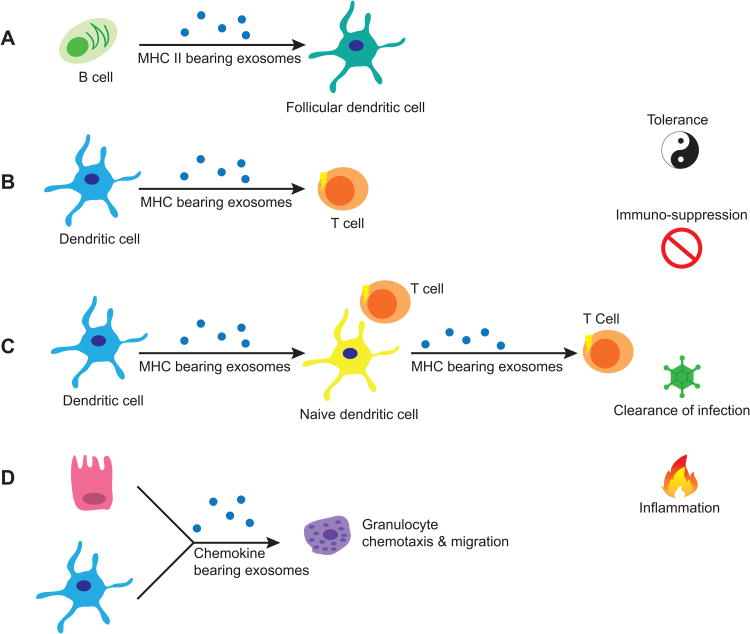
Exosomes can modulate the activity of immune cells by playing a role in development, recruitment, activation, and suppression of the immune system (64, 82, 86-92, 104). For example, follicular DCs (FDCs) were identified to acquire peptide-bound major histocompatibility complex class II (pMHC-II) molecules from EVs secreted by B lymphocytes (Figure 3A) (105). FDCs are accessory cells in germinal centers that present antigens to B lymphocytes and have been shown to lack the expression of MHC-II molecules (106). B cell derived exosomes with pMHC-II found on FDCs were shown to stimulate CD4+ T cells, aiding in their development (105). This observation suggests the ability for exosomes with pMHC-II to engage T lymphocytes and to modulate immune memory, expanding the repertoire of antigens.
Figure 3.
Exosomes in immune regulation. (A) Follicular dendritic cells (FDCs) gain MHC class II molecules from exosomes shed by B cells in the germinal centers. (B) Dendritic cells (DCs) can secrete exosomes bearing MHC molecules that potentially directly activate T cells. (C) Primed dendritic cells can secrete peptide-loaded MHC molecules that can be taken-up by naïve DCs which can then activate nearby T cells by displaying the loaded MHC molecule from exosome on its cell surface. Naïve DCs can also re-package the loaded MHC into new exosomes, thus amplifying the effect. (D) Both epithelial cells and DCs can secrete chemokine-containing exosomes which can recruit granulocytes and other inflammatory mediators.
Peripheral DCs have been shown to produce exosomes with pMHC-II after antigen uptake and processing (67). These DC-derived exosomes, or dexosomes, have been shown to activate T cell with assistance from DCs and B cells (107, 108). Dexosomes with pMHC-II can be transferred to other dendritic cells (cross-dressing), or endocytosed and re-presented on the cell surface or in a new dexosome (Figure 3C) (104, 109, 110). Although dexosomes have been primarily linked with T cell activity, preliminary human studies in our laboratory have shown the potential for other MHC-II expressing cells to generate exosomes capable of directly activating T cells and modulating their polarization (unpublished data). Admyre et al. have reported evidence for exosome-mediated direct stimulation of CD8+ T cells (Figure 3B) (45). In this study, human peripheral CD8+ T cells were co-cultured with exosomes purified from autologous monocyte-derived DCs pulsed with immunogenic peptides from Epstein-Barr virus, cytomegalovirus, and influenza virus. They inferred direct stimulation by measuring IFN-γ production and demonstrated that this production was dependent on MHC-I mediated stimulation. As mentioned briefly, there are reports indicating that exosomes alone do not provide a potent activation signal and that they require the assistance of B cells or DCs (107, 108). Furthermore, recent evidence suggests that exosome-mediated immunotherapy in cancer is more efficient if whole antigen is present, independent of MHC/peptide complex, thus requiring APCs to help elicit a potent immune response (111). However, the observed results may be dependent on exosome concentration, the type of surface protein present, the presence of whole or processed antigen, and the level of protein expression. Lastly, since the physiological distribution of exosomes throughout tissues and parenchymal fluids is unclear, direct activation of T cells by exosomes, and exosome-APC mediated T cell activation are both plausible.
Exosomes secreted from macrophages and DCs have been shown to enhance migration of polymorphonuclear cells in vitro (Figure 3D) (112). These exosomes also contained enzymes that synthesize leukotrienes, which are lipid mediators of inflammation, suggesting a pro-inflammatory role for exosomes. Immune modulation by exosomes is not limited to APC-derived exosomes. Epithelial-derived exosomes in the airway can drive proliferation of monocytes, and enhance chemotaxis (28). Th2 cytokines were found to stimulate epithelial production of exosomes, and these exosomes induced monocyte proliferation. Furthermore, monocytes treated with epithelial-derived exosomes, in the presence of monocyte chemoattractant protein 1 (MCP-1), enhanced their migration.
Airway Disease and Exosomes
COPD is a chronic unresolved inflammatory disease that results in persistent airway remodeling, fibrosis of small airways, and the destruction of the alveolar cavity (1, 2, 48, 50). Cigarette smoke and other irritants stimulate both airway epithelial cells and macrophages to release cytokines and growth factors that promote the chronic inflammation, in COPD (30, 50, 113). TGF-β (49, 114) and fibroblast growth factor (FGF) secreted by epithelial cells promote fibroblast proliferation and fibrosis of small airways (50). Activated macrophages promote inflammation by recruiting neutrophils, Th1 and Tc1 subsets (1, 12, 46), and by secreting proteases (47, 48), which together with Tc1 cells aid in the destruction of the alveolus (12, 47, 48). Additionally, IL-1β levels are markedly increased in the airway of COPD patients, which further promotes inflammation and induces production of matrix metalloproteases by macrophages (47, 48). IL-1β has been reported in exosomes in both humans and mice (115, 116). The presence of CYR61/CTGF/NOV family 1 (CCN1) has been reported in exosomes from lung epithelial cells exposed to cigarette smoke extract (30). CCN1 is a matrix-associated CCN family protein with multiple signaling functions that affect cell survival and growth (117-119). Soluble forms of CCN1 have previously been shown to stimulate IL-8 production via the Wnt pathway, which can recruit inflammatory cells into the lung parenchyma (113). Thus exosome-bound CCN1 may have similar effects in enhancing recruitment of inflammatory mediators. As is the case with most pulmonary diseases, a number of miRNAs have been found to be involved in COPD, including miR-15b, miR-223, miR-1274a, miR-424, mir-210 (23, 51). These miRNAs could be packaged in exosomes and alter the cellular function of recipient cells. Particularly, miR-210 expression has been demonstrated to be elevated in EVs from human lung tissue post-cigarette smoke exposure (23). These miR-210 containing EVs also inhibited autophagy by blocking ATG7 and promoted differentiation of fibroblasts into myofibroblasts.
Asthma is a heterogeneous disease that results in narrowing of the airway, clinically manifesting as wheezing and difficulty in breathing (1, 8, 11). Allergic asthma (Figure 4A) initiates with activation of DCs by an allergen, such as ragweed pollen (1, 2, 13). Primed DCs will then activate CD4+ T cells while promoting their polarization to a Th2 profile through the secretion of CCL17 and CCL22 cytokines (1, 13). Th2 cells produce IL-4 and IL-13, which can further drive Th2 differentiation of nearby T helper cells, and stimulate plasma B cells to secrete IgE. Admyre et al. in 2003, isolated exosomes for the first time from BALF of healthy human subjects, and demonstrated surface expression of proteins such as HLA-DR, CD63, CD86, and CD54 (44). These findings suggest the potential for airway exosomes to present antigen to the adaptive arm of the immune system and mediate co-stimulation. They further showed that T cells can be stimulated to produce Th2 cytokines by pMHC-II on the surface of exosomes derived from B cells of patients with birch pollen allergy (25). These studies intimate an important potential for exosomes to fuel allergic airway inflammation.
Figure 4.
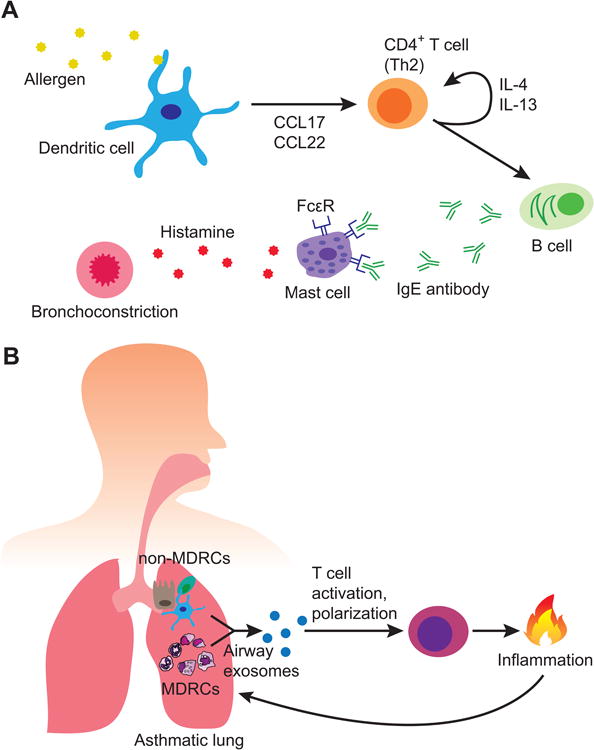
(A) Classical mechanism of allergic asthma. An initial stimulus, such as pollen, is processed by dendritic cells and Th2 promoting cytokines released. Th2 polarized CD4+ T cells secrete a milieu of cytokines including IL-4 and IL-13, further promoting Th2 skewing of nearby CD4+ T cells. This stimulates plasma B cells to secrete IgE, a key mediator of allergic asthma. IgE induces histamine production by mast cells resulting in bronchoconstriction, which manifests clinically as wheezing or dyspnea. (B) Exosomes may have the potential to directly activate T cells and influence their activity, as well as mediate inflammation through leukotriene synthesis. Additionally, exosomes secreted by MDRCs may preferentially promote the development of T cell subsets, such as Th17 cells, which can drive inflammation. The inflammatory environment can promote bronchial epithelial cell production of exosomes, creating a vicious cycle of disease maintenance.
In asthmatics, the expression levels of CD81, CD36 and HLA-DR on airway exosomes were significantly higher as compared to healthy subjects (25). These findings are particularly interesting as CD36 is a pattern-recognition scavenger protein capable of promoting sterile inflammation by assembling toll-like receptor 4 (TLR4) and TLR6 into a complex (120). CD36 is a membrane glycoprotein known to bind phospholipids, lipoproteins, oxidized lipids, fatty acids, and apoptotic cells (121). CD36 has been reported to associate with CD9, a tetraspanin molecule enriched on exosomes, on the cell surface to regulate oxidized lipoprotein uptake (122, 123). Tocopherols are transported by lipoproteins (124), and particularly α-tocopherol has been shown to modulate CD36 expression (125). The roles of α- and γ-tocopherol in modulating inflammation have been reported in pulmonary inflammation (126-128). Together, the transfer of CD36+ exosomes to target cells may putatively facilitate asthma progression by promoting inflammation through TLR complex formation (120), or by increased uptake of tocopherols known to aid in inflammation (125-128). More detailed experimental analyses are needed to draw conclusions on the role of CD36+ exosomes, and its associated mechanisms. Lastly, exosomes from BALF of asthmatics were found to also contain functional leukotriene producing enzymes. Co-culture of these exosomes with bronchial epithelial cells resulted in leukotriene and IL-8 production, suggesting yet another mechanism for exosome driven inflammation (Figure 4B) (27).
Although evidence for the involvement of exosomes in PF has not been established, in vitro and in vivo murine models of organ fibrosis point to the potential for its role in the disease (56). Injured kidney epithelial cells have been shown to produce TGF-β containing exosomes which activate fibroblasts to engage in fibrotic repair in vitro (55). MicroRNAs have been known to play a role in PF (129, 130), and fibrosis of various other organs, including liver, heart, and kidney (131). Since exosomes can convey microRNA to other cells, it would not be surprising if they are involved in PF. With regards to PF, notably, miR-21 has been shown to be upregulated in a TGF-β dependent manner in both patients and bleomycin-induced IPF mouse models (129). Expression of miR-21 results in the inhibition of apoptosis and tumor suppressor genes, thus allowing proliferation and differentiation of fibroblasts into myofibroblasts. MicroRNAs are not the only regulators of fibrotic process. Th2 cells have been shown to promote fibrosis through its characteristic cytokines (IL-4, IL-5 and IL-13) (132), each further stimulating Th2 differentiation, inflammation, and fibrotic repair. Particularly, the secretion of IL-13 by both Th2 cells and eosinophils, which have been recruited by IL-5, have detrimental effects on the severity of disease. In conjunction with findings that show the capacity for exosomes to mediate inflammation, promote fibrotic repair in vitro, and drive T cell proliferation, it is feasible for exosomes to function as mediators of PF.
Lastly, mechanical stress or loss of complacency in the lung epithelium has been demonstrated to alter gene expression of fibroblasts (133, 134). Varying degrees of matrix stiffness, on which lung fibroblasts were grown, influenced their protein expression and proliferative capabilities (133, 134). Furthermore, mechanical stimulation of bronchial epithelial cells resulted in the secretion of tissue factor-bearing exosomes (29). These findings demonstrate the significance of mechano-complacency of tissue matrices in significantly affecting gene expression, secretome profiles, and exosome production in the lung.
Exosomes as a Therapeutic Tool
While recent advances in nanoparticle technology have made the development of slow-release drugs possible with promising results (135), a major hurdle with silicon-based nanoparticle technology is hepatotoxicity caused by accumulation of nanoparticles in the liver (135, 136). This toxicity makes dosing regimens hard to determine despite requiring only minuscule amounts. As of today, exosomes have not been associated with similar toxicity, although detailed toxicological studies may be necessary. And because of their versatile nature, various groups have demonstrated the ability to engineer exosomes for therapeutic purposes (137-142).
Several pre-clinical studies on the use of exosomes as a novel therapeutic have demonstrated encouraging translational potential (137-140). A recombinant chimeric neurotropic protein expressed on the surface of DC-derived exosomes has been shown to traverse the blood-brain-barrier (BBB), enabling these exosomes to target neurons (138). Although inorganic nanoparticles have been reported to cross the BBB, their engineering and synthesis have been problematic (135). The progress in engineering exosomes to target the CNS provides hope for developing systemic drugs that can target the CNS against diseases such as Alzheimer's, Parkinson's, amyotrophic lateral sclerosis, and glioblastoma multiforme.
Exosomes also have therapeutic potential as a mucosal vaccine. Prado et al. have shown that exosomes purified from the BALF of mice that were intranasally immunized with the olive pollen antigen (Ole e) can protect naïve mice from Ole e induced allergic response (137). The authors observed lower IgE titer and Th2 cytokine production with the administration of tolerogenic exosomes. Furthermore, the authors state that because exosomes are relatively stable, lower doses are needed to observe its effects. These findings pave an exciting future for exosome-based immunotherapy.
Two phase I clinical trials have reported the safety of patient-derived dexosome therapy against cancer (141, 142). The trials used patient DCs pulsed with melanoma-associated antigen 3 (MAGE-A3) to generate therapeutic dexosomes for the treatment of metastatic melanoma and advanced NSCLC. Interestingly, both studies showed partial response, higher activation of NK cells, and stabilization of disease progression in a subset of patients treated with the dexosomes. These studies demonstrated safety and feasibility of using exosomes as a novel therapeutic, and further optimization of the therapy may enable activation of greater immune populations and efficacy in larger subsets of patients.
Conclusions and Perspectives
Exosomes are produced by virtually all cell types, and can be isolated from a variety of bodily fluids (31, 44, 62, 64, 67, 68, 75, 76). Proteomics data analyses of exosomes purified from bodily fluids have been deposited in databases such as ExoCarta (143), and the NIH Urinary Exosome Protein Database (61, 62). From the early characterization efforts to functional studies being conducted now, exosomes have been discovered to not only contain diverse proteins, but are also multifaceted in their functional roles ranging from the development of healthy cells, tissues and organs, to promoting immunosuppression, and inflammation (23-31, 33-35, 45, 56, 76, 81-85, 93, 94, 104, 105, 107, 110, 112, 137). Exosomes are also important in immune modulation, for example, the ability for exosomes with class-II molecules to cross-present or cross-dress antigens to T lymphocytes and other DCs. Furthermore, the exosome biogenesis pathway has been proposed to be “hijacked” by enveloped viruses, like HIV, to evade the host immune system (41, 42). These findings and putative propositions put together demonstrate exosomes as versatile couriers of bioactive cargo that can cross biological barriers (138) and deliver their payload to distant targets.
In the context of airway diseases, exosomes are beginning to be appreciated in the pathogenesis of lung cancer, PF, COPD and asthma. Exosomes can transfer miRNAs that alter gene expression of target cells resulting in a diseased phenotype, such as miR-210 in COPD (23). They can also carry pro-inflammatory cytokines such as IL-1β (115, 116), which contributes to inflammation associated with both COPD and fibrosis. In asthma, exosomes have been shown to contain MHC-II molecules (27), and clinical ex vivo experiments in allergy have demonstrated the potential for patient exosomes to promote T cell proliferation (25). These findings, along with data that shows presence of co-stimulatory molecules on human BALF exosomes (44), suggest capacity for exosomes to directly modulate T cell activity and influence differentiation. Previous work from our laboratory demonstrated the effects of cigarette smoke on how MDRCs can be skewed to enhance Th2 polarization and proliferation (17). In light of several lines of evidence that show cigarette smoke induces altered exosomal content (30), the potential for pro-inflammatory MDRCs to produce pathogenic exosomes and promote asthma exists and thus necessitates detailed examination of the role of exosomes from various cellular players of asthma.
Not only are these discoveries and postulations exciting, but provide a platform for development of novel therapies. Already, several pre-clinical and phase I clinical trials have been conducted with exosome-based treatments (137-142). Dexosome-based vaccines have a promising future, as not only can they effectively activate the adaptive immune system, but their signal can be amplified by uptake and re-distribution of antigen presenting exosomes. The studies mentioned in this review have illustrated the ability of researchers to usurp the natural ability of APCs to load antigenic peptides on class-I and -II molecules and package them into exosomes for therapeutic use (108, 139, 141, 142). Early studies have already confirmed the ability to express recombinant proteins, or over-express miRNA in host cells to “design” exosomes (138, 140). Furthermore, targeting of exosomes to select cell-types has been demonstrated by engineering chimeric cell-tropic surface proteins on exosomes (138). As more mechanistic research is conducted on exosome biogenesis and cargo sorting, we will be able to develop nifty tools to engineer exosomes for therapeutic purposes.
Acknowledgments
The authors would like to acknowledge their funding sources: FAMRI YCSA 2010 (JSD), Parker B Francis Foundation (JSD), R01HL128502 (JSD), and P01HL114470 (VJT).
Footnotes
Disclosures/Conflict of Interest: The authors declare no conflict of interest.
Contributions of Authors: KPH and JSD outlined the review and together drafted and completed the manuscript. SRD and VJT reviewed and edited the manuscript. DC provided intellectual discussion and critical comments of the manuscript.
References
- 1.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 4.Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016;375(9):871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 5.Porto BN, Stein RT. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King CS, Nathan SD. Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. Lancet Respir Med. 2016 doi: 10.1016/S2213-2600(16)30222-3. [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 8.Boushey HA, Fahy JV. Basic mechanisms of asthma. Environ Health Perspect. 1995;103 Suppl 6:229–233. doi: 10.1289/ehp.95103s6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 10.Nakagami Y, Favoreto S, Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA, et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J Immunol. 2008;181(3):2203–2210. doi: 10.4049/jimmunol.181.3.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372(9643):1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1(1):59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 13.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 14.Korsgren M. NK cells and asthma. Curr Pharm Des. 2002;8(20):1871–1876. doi: 10.2174/1381612023393738. [DOI] [PubMed] [Google Scholar]
- 15.Lunding L, Wegmann M. NK cells in asthma exacerbation. Oncotarget. 2015;6(24):19932–19933. doi: 10.18632/oncotarget.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souzdaltseva TV, Makarova TV, Vechkanova NN. NK Cells and IgE Level in Peripheral Blood in Aspirin-Induced and Allergic Bronchial Asthma. Russ J Immunol. 2000;5(3):315–319. [PubMed] [Google Scholar]
- 17.Wang Y, Jin TH, Farhana A, Freeman J, Estell K, Zmijewski JW, et al. Exposure to cigarette smoke impacts myeloid-derived regulatory cell function and exacerbates airway hyper-responsiveness. Lab Invest. 2014;94(12):1312–1325. doi: 10.1038/labinvest.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshane JS, Redden DT, Zeng M, Spell ML, Zmijewski JW, Anderson JT, et al. Subsets of airway myeloid-derived regulatory cells distinguish mild asthma from chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;135(2):413–424 e415. doi: 10.1016/j.jaci.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshane J, Zmijewski JW, Luther R, Gaggar A, Deshane R, Lai JF, et al. Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 2011;4(5):503–518. doi: 10.1038/mi.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora M, Poe SL, Oriss TB, Krishnamoorthy N, Yarlagadda M, Wenzel SE, et al. TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 2010;3(6):578–593. doi: 10.1038/mi.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arora M, Poe SL, Ray A, Ray P. LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation. Int Immunopharmacol. 2011;11(7):827–832. doi: 10.1016/j.intimp.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray P, Arora M, Poe SL, Ray A. Lung myeloid-derived suppressor cells and regulation of inflammation. Immunol Res. 2011;50(2-3):153–158. doi: 10.1007/s12026-011-8230-1. [DOI] [PubMed] [Google Scholar]
- 23.Fujita Y, Araya J, Ito S, Kobayashi K, Kosaka N, Yoshioka Y, et al. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. J Extracell Vesicles. 2015;4:28388. doi: 10.3402/jev.v4.28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita Y, Kosaka N, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol Med. 2015;21(9):533–542. doi: 10.1016/j.molmed.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120(6):1418–1424. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 26.Admyre C, Telemo E, Almqvist N, Lotvall J, Lahesmaa R, Scheynius A, et al. Exosomes - nanovesicles with possible roles in allergic inflammation. Allergy. 2008;63(4):404–408. doi: 10.1111/j.1398-9995.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- 27.Torregrosa Paredes P, Esser J, Admyre C, Nord M, Rahman QK, Lukic A, et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67(7):911–919. doi: 10.1111/j.1398-9995.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- 28.Kulshreshtha A, Ahmad T, Agrawal A, Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J Allergy Clin Immunol. 2013;131(4):1194–1203. 1203 e1191–1114. doi: 10.1016/j.jaci.2012.12.1565. [DOI] [PubMed] [Google Scholar]
- 29.Park JA, Sharif AS, Tschumperlin DJ, Lau L, Limbrey R, Howarth P, et al. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J Allergy Clin Immunol. 2012;130(6):1375–1383. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon HG, Kim SH, Gao J, Quan T, Qin Z, Osorio JC, et al. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am J Physiol Lung Cell Mol Physiol. 2014;307(4):L326–337. doi: 10.1152/ajplung.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003;24(2):149–154. doi: 10.1002/j.1939-4640.2003.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 32.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113 Pt 19:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, Tahara H. The role of exosomes and microRNAs in senescence and aging. Adv Drug Deliv Rev. 2013;65(3):368–375. doi: 10.1016/j.addr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Forterre A, Jalabert A, Chikh K, Pesenti S, Euthine V, Granjon A, et al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014;13(1):78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J Lipid Res. 2010;51(8):2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol. 2007;5(6):e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gan X, Gould SJ. Identification of an inhibitory budding signal that blocks the release of HIV particles and exosome/microvesicle proteins. Mol Biol Cell. 2011;22(6):817–830. doi: 10.1091/mbc.E10-07-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J Biol Chem. 2011;286(51):44162–44176. doi: 10.1074/jbc.M111.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen B, Wu N, Yang JM, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286(16):14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JM, Gould SJ. The cis-acting signals that target proteins to exosomes and microvesicles. Biochem Soc Trans. 2013;41(1):277–282. doi: 10.1042/BST20120275. [DOI] [PubMed] [Google Scholar]
- 41.Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A. 2003;100(19):10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem. 2003;278(52):52347–52354. doi: 10.1074/jbc.M309009200. [DOI] [PubMed] [Google Scholar]
- 43.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22(4):578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 45.Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral human T cells detected by ELISPOT. Eur J Immunol. 2006;36(7):1772–1781. doi: 10.1002/eji.200535615. [DOI] [PubMed] [Google Scholar]
- 46.Hodge G, Nairn J, Holmes M, Reynolds PN, Hodge S. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin Exp Immunol. 2007;150(1):22–29. doi: 10.1111/j.1365-2249.2007.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro SD. Elastolytic metalloproteinases produced by human mononuclear phagocytes. Potential roles in destructive lung disease. Am J Respir Crit Care Med. 1994;150(6 Pt 2):S160–164. doi: 10.1164/ajrccm/150.6_Pt_2.S160. [DOI] [PubMed] [Google Scholar]
- 48.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 49.Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD) Am J Respir Crit Care Med. 2001;163(6):1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 50.Kranenburg AR, De Boer WI, Van Krieken JH, Mooi WJ, Walters JE, Saxena PR, et al. Enhanced expression of fibroblast growth factors and receptor FGFR-1 during vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;27(5):517–525. doi: 10.1165/rcmb.4474. [DOI] [PubMed] [Google Scholar]
- 51.Osei ET, Florez-Sampedro L, Timens W, Postma DS, Heijink IH, Brandsma CA. Unravelling the complexity of COPD by microRNAs: it's a small world after all. Eur Respir J. 2015;46(3):807–818. doi: 10.1183/13993003.02139-2014. [DOI] [PubMed] [Google Scholar]
- 52.Levanen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131(3):894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122(8):2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 56.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, et al. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24(3):385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A. 1970;67(3):1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black PH. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- 59.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 60.Forterre A, Jalabert A, Berger E, Baudet M, Chikh K, Errazuriz E, et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9(1):e84153. doi: 10.1371/journal.pone.0084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aalberts M, van Dissel-Emiliani FM, van Adrichem NP, van Wijnen M, Wauben MH, Stout TA, et al. Identification of distinct populations of prostasomes that differentially express prostate stem cell antigen, annexin A1, and GLIPR2 in humans. Biol Reprod. 2012;86(3):82. doi: 10.1095/biolreprod.111.095760. [DOI] [PubMed] [Google Scholar]
- 66.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147(3):599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 69.Mobius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T, et al. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50(1):43–55. doi: 10.1177/002215540205000105. [DOI] [PubMed] [Google Scholar]
- 70.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 71.Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 72.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25(1):1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 74.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5(4):317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 75.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 76.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 77.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3 doi: 10.1002/0471143030.cb0322s30. Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 78.Lane RE, Korbie D, Anderson W, Vaidyanathan R, Trau M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep. 2015;5:7639. doi: 10.1038/srep07639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273(32):20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 80.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 81.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 82.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, et al. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci U S A. 2015;112(19):E2497–2506. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baixauli F, Lopez-Otin C, Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol. 2014;5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aswad H, Forterre A, Wiklander OP, Vial G, Danty-Berger E, Jalabert A, et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57(10):2155–2164. doi: 10.1007/s00125-014-3337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simhadri VR, Reiners KS, Hansen HP, Topolar D, Simhadri VL, Nohroudi K, et al. Dendritic cells release HLA-B-associated transcript-3 positive exosomes to regulate natural killer function. PLoS One. 2008;3(10):e3377. doi: 10.1371/journal.pone.0003377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S, et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One. 2009;4(3):e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, Minami M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett. 2003;89(2-3):125–131. doi: 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 89.Luketic L, Delanghe J, Sobol PT, Yang P, Frotten E, Mossman KL, et al. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J Immunol. 2007;179(8):5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- 90.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26(19):4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195(10):1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128(7):1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 93.Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25(9):2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 95.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18(9):2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 96.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, Fisher EM, et al. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179(3):485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29(10):2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28(19):3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 104.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 105.Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165(3):1259–1265. doi: 10.4049/jimmunol.165.3.1259. [DOI] [PubMed] [Google Scholar]
- 106.Gray D, Kosco M, Stockinger B. Novel pathways of antigen presentation for the maintenance of memory. Int Immunol. 1991;3(2):141–148. doi: 10.1093/intimm/3.2.141. [DOI] [PubMed] [Google Scholar]
- 107.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3(12):1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 108.Naslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190(6):2712–2719. doi: 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- 109.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179(3):1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 110.Campana S, De Pasquale C, Carrega P, Ferlazzo G, Bonaccorsi I. Cross-dressing: an alternative mechanism for antigen presentation. Immunol Lett. 2015;168(2):349–354. doi: 10.1016/j.imlet.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 111.Hiltbrunner S, Larssen P, Eldh M, Martinez-Bravo MJ, Wagner AK, Karlsson MC, et al. Exosomal cancer immunotherapy is independent of MHC molecules on exosomes. Oncotarget. 2016 doi: 10.18632/oncotarget.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esser J, Gehrmann U, D'Alexandri FL, Hidalgo-Estevez AM, Wheelock CE, Scheynius A, et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J Allergy Clin Immunol. 2010;126(5):1032–1040. 1040 e1031–1034. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 113.Moon HG, Zheng Y, An CH, Kim YK, Jin Y. CCN1 secretion induced by cigarette smoking extracts augments IL-8 release from bronchial epithelial cells. PLoS One. 2013;8(7):e68199. doi: 10.1371/journal.pone.0068199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marwick JA, Kirkham P, Gilmour PS, Donaldson K, Mac NW, Rahman I. Cigarette smoke-induced oxidative stress and TGF-beta1 increase p21waf1/cip1 expression in alveolar epithelial cells. Ann N Y Acad Sci. 2002;973:278–283. doi: 10.1111/j.1749-6632.2002.tb04649.x. [DOI] [PubMed] [Google Scholar]
- 115.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179(3):1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 116.Pizzirani C, Ferrari D, Chiozzi P, Adinolfi E, Sandona D, Savaglio E, et al. Stimulation of P2 receptors causes release of IL-1beta-loaded microvesicles from human dendritic cells. Blood. 2007;109(9):3856–3864. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 117.Jim Leu SJ, Sung JS, Chen MY, Chen CW, Cheng JY, Wang TY, et al. The matricellular protein CCN1 suppresses lung cancer cell growth by inducing senescence via the p53/p21 pathway. J Cell Biochem. 2013;114(9):2082–2093. doi: 10.1002/jcb.24557. [DOI] [PubMed] [Google Scholar]
- 118.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 119.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 120.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res. 1998;39(4):777–788. [PubMed] [Google Scholar]
- 122.Huang W, Febbraio M, Silverstein RL. CD9 tetraspanin interacts with CD36 on the surface of macrophages: a possible regulatory influence on uptake of oxidized low density lipoprotein. PLoS One. 2011;6(12):e29092. doi: 10.1371/journal.pone.0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rocha-Perugini V, Sanchez-Madrid F, Martinez Del Hoyo G. Function and Dynamics of Tetraspanins during Antigen Recognition and Immunological Synapse Formation. Front Immunol. 2015;6:653. doi: 10.3389/fimmu.2015.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goulinet S, Chapman MJ. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17(4):786–796. doi: 10.1161/01.atv.17.4.786. [DOI] [PubMed] [Google Scholar]
- 125.Devaraj S, Hugou I, Jialal I. Alpha-tocopherol decreases CD36 expression in human monocyte-derived macrophages. J Lipid Res. 2001;42(4):521–527. [PubMed] [Google Scholar]
- 126.Geiser M, Lay JC, Bennett WD, Zhou H, Wang X, Peden DB, et al. Effects of ex vivo gamma-tocopherol on airway macrophage function in healthy and mild allergic asthmatics. J Innate Immun. 2013;5(6):613–624. doi: 10.1159/000350234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ekstrand-Hammarstrom B, Osterlund C, Lilliehook B, Bucht A. Vitamin E down-modulates mitogen-activated protein kinases, nuclear factor-kappaB and inflammatory responses in lung epithelial cells. Clin Exp Immunol. 2007;147(2):359–369. doi: 10.1111/j.1365-2249.2006.03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kolleck I, Sinha P, Rustow B. Vitamin E as an antioxidant of the lung: mechanisms of vitamin E delivery to alveolar type II cells. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S62–66. doi: 10.1164/rccm.2206019. [DOI] [PubMed] [Google Scholar]
- 129.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207(8):1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43(9):479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vettori S, Gay S, Distler O. Role of MicroRNAs in Fibrosis. Open Rheumatol J. 2012;6:130–139. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208(7):1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tschumperlin DJ, Shively JD, Kikuchi T, Drazen JM. Mechanical stress triggers selective release of fibrotic mediators from bronchial epithelium. Am J Respir Cell Mol Biol. 2003;28(2):142–149. doi: 10.1165/rcmb.2002-0121OC. [DOI] [PubMed] [Google Scholar]
- 134.Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2013;48(4):422–430. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. doi: 10.1016/s1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 136.Liu T, Li L, Fu C, Liu H, Chen D, Tang F. Pathological mechanisms of liver injury caused by continuous intraperitoneal injection of silica nanoparticles. Biomaterials. 2012;33(7):2399–2407. doi: 10.1016/j.biomaterials.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 137.Prado N, Marazuela EG, Segura E, Fernandez-Garcia H, Villalba M, Thery C, et al. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181(2):1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 138.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 139.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 140.Koppers-Lalic D, Hogenboom MM, Middeldorp JM, Pegtel DM. Virus-modified exosomes for targeted RNA delivery; a new approach in nanomedicine. Adv Drug Deliv Rev. 2013;65(3):348–356. doi: 10.1016/j.addr.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3(1):10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9(21):4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]